Abstract
Cerebral angiostrongyliasis due to Angiostrongylus cantonensis continues to affect human health and productivity in Thailand. The dietary habits of the populace have been an important contributing factor, particularly in the northeast of the country where the disease is endemic and the indigenous people enjoy a local undercooked snail dish called “koi-hoi”. Hundreds of cases of disease continue to be reported annually. Because of the difficulty in obtaining a definitive diagnosis, immunological methods have played an important role in the confirmation of A. cantonensis infection. Although enzyme-linked immunosorbent assay (ELISA) and immunoblot are test formats that have been used over the past decade, modern molecular approaches, such as PCR-based diagnostic techniques, are being developed and assessed as additional tests for the diagnosis of cerebral angiostrongyliasis. This short review focuses on the history, incidence, and laboratory diagnosis of angiostrongyliasis in Thailand.
Keywords: Angiostrongyliasis, Angiostrongylus cantonensis, Diagnosis, Eosinophilic meningitis, Laboratory testing, Thailand
Past and Present Angiostrongyliaisis in Thailand
The rat lungworm, Angiostrongylus cantonensis, is the primary cause of eosinophilic meningitis or eosinophilic meningoencephalitis. In Thailand, the disease was first recognized in 1955 and documented in 1957.1 Many cases were recorded in the 1960s, starting in 1961 with two patients that developed eosinophilic meningitis after eating undercooked Pila snails.2 The number of cases in the 1960s increased rapidly from those two cases to 572 cases in 1966. Of the 1164 cases from 1955 to 1966, 912 were from the northeastern provinces.2–10
Between 1965 and 1968, typical cases of eosinophilic meningitis were investigated throughout the country.2 Among the 484 cases investigated, there was no apparent difference in the age distribution of patients from different geographic areas.2 The youngest patient was 2 years old and the oldest 65 years old. Most of the patients belonged to the 20–39 age groups. Males were affected 2.6 times more frequently than females, with 348 males and 136 females. More than half of the patients were farmers. Laborers made up the second largest group of cases. Other cases included students, housewives, government officials, military men, and merchants.
During 1981–1984, 30 cases of eosinophilic meningitis in children were reported in the northeastern province of Khon Kaen.11 The patients ranged from 6 to 14 years old, comprising 18 boys and 12 girls. The cases were reported throughout the year and there was no significant seasonal pattern in their occurrence. Two-thirds of the patients had a history of eating snails and raw food. One of them had ocular A. cantonensis infection. Twenty-nine of the children recovered completely but one died.
More recently, in 1991, an additional three cases of eosinophilic myelomeningoencephalitis were recorded.12 During 1995–2005, 654 cases were treated at Srinagarind Hospital, Khon Kaen.13
Despite the difficulty of recovering A. cantonensis from infected patients, worms have been recovered from the cerebrospinal fluid (CSF) of a number of Thai patients,10,14–16 and from the brain of at least ten fatal cases following brain biopsy.9,11,17–19 Eleven living adult worms were recovered from the CSF of an 8 month old girl with a two week history of chronic fever and seizures.15 In a fatal case at Siriraj Hospital, Bangkok, in 1990, many fifth stage larvae were detected in the brain.17 The infection was attributed to eating raw or partially cooked livers from monitor lizards preceding the onset of symptoms. Unfortunately, no leftover liver was available to confirm the presence of infective larvae, but a subsequent study of 22 monitor lizards from five provinces in Thailand showed that 96% (21 out of 22) were infected with A. cantonensis, with most of the larvae in the liver.20
Fatal cases are relatively rare. Five fatal cases were recorded in Thailand in the 1960s,9 four in the period 1974–1977,19 one in 1981–1984,11 and one in 1990.17 Many live and dead fourth and fifth stage larvae were present in the meninges and brain tissue of these fatal cases.
Ocular angiostrongyliasis has been documented occasionally in Thailand. Three cases with young adult A. cantonensis recovered from the anterior chamber of the eye were recorded in the 1960s.21–23 A case associated with eosinophilic meningitis was reported in 1971,24 and a pediatric case in 1985.11 Three cases of intravitreal infection have been reported.25,26 In a woman who had eaten raw snails, the worm was located by the use of an intravitreal cryoprobe and was successfully removed via the pars plana with vitreous foreign body forceps.26 Signs of meningitis were present in two men, in each of whom a small motile worm was found in the vitreous cavity.25 In one case there was also a dead, disintegrated worm in the inferior portion of the vitreous cavity. Another seven cases (four men and three women) with intraocular angiostrongyliasis were documented in Srinagarind Hospital, Khon Kaen, between January 1995 and April 2005.13 There is no evidence that surgical and laser interventions improve the course of the ocular disease. Visual outcome depends only on initial visual defects/acuity.
The first ever reported case of eosinophilic meningitis associated with sensorineural hearing loss involved a 59 year old woman who had chronic headache, neck stiffness, and left-side hearing loss.27 Her condition, including hearing, improved after treatment with prednisolone.
Epidemiology in Thailand 2000–2009
According to recent statistics from the National Surveillance System, Department of Disease Control, Ministry of Public Health, Thailand,28 for the period 2000 to October 2009, the rate of reported cases declined sharply from about 2.24 per 100,000 of the population (1,386 reported cases) in 2000 to 0.2–0.3 per 100,000 in 2005–2009, with 172 cases reported in 2009. The fatality rate varied among years. The rate was 0.07 per 100,000 of the population in 2000, 0.19 in 2002, and 0.78 in 2007. There were no fatal cases in 2001, 2003–2006, and 2008–2009 (Figure 1).
Figure 1.
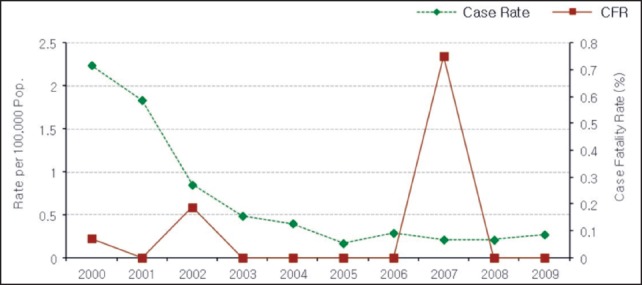
Total annual reported cases and fatal cases of eosinophilic meningitis per 100,000 of the population in Thailand, 2000–October 2009.28
The reported cases over the years are in a broad range of age-categories, as reflected, for example, by the statistics for January–October 2009 (Figure 2). The highest rate of reported cases is in the 35–44 age group, with 0.41 per 100,000 of the population. This is followed by the 15–24 and 45–54 age groups with 0.39 and 0.37 per 100,000 of the population, respectively. Overall, there are more males than females among the reported cases, with a ratio of 1.77 to 1. Farmers constituted the majority of the reported cases, accounting for 70% (120/172). Thai farmers eat a local undercooked snail dish called ‘koi-hoi’, usually as an appetizer when drinking alcoholic beverages after their daily farming activities. Daily-paid workers constituted 15% (25/172) and pre-school children 7% (12/172) of the cases.
Figure 2.
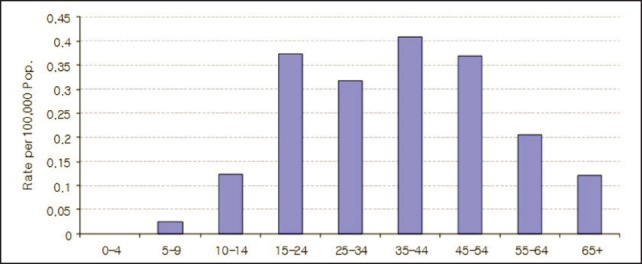
Reported cases of eosinophilic meningitis per 100,000 of the population by age-group in Thailand, January–October 2009.28
Cases of eosinophilic meningitis are reported year-round. However, during 2009, the number of cases rose steadily from January to the end of the year (Figure 3), the reason for this remaining unknown.
Figure 3.
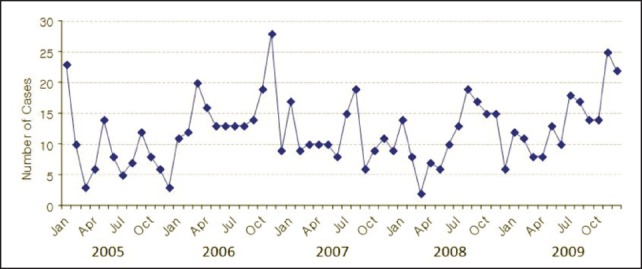
Monthly reported cases of eosinophilic meningitis in Thailand, 2005–2009.28
The highest rate of annual reported cases was 23.08 per 100,000 in the northeast region, where Loei province accounted for more than half of the total reported cases in Thailand during January–October 2009 (Figure 4). Rates in all other provinces were much lower, with the second highest rate (1.23 per 100,000) in Phayao province and all other rates <1 per 100,000 (Figure 4).
Figure 4.
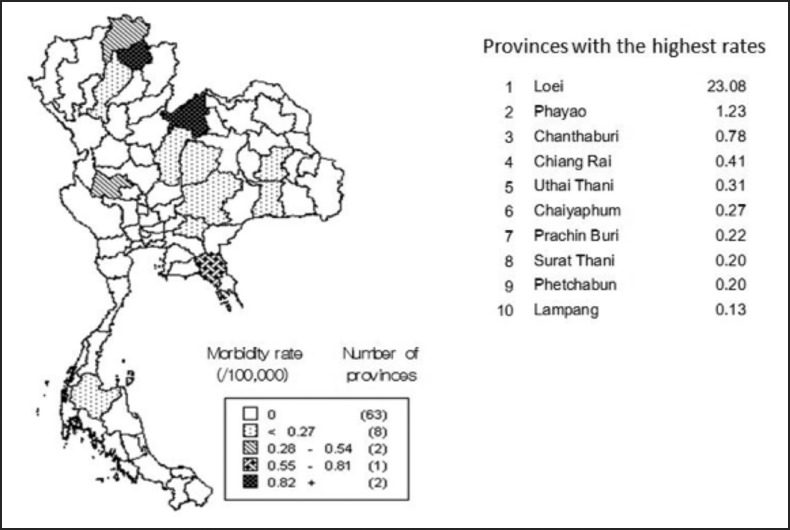
Reported cases of eosinophilic meningitis per 100,000 population in the ten provinces with the highest rates, Thailand, 2009.28
Laboratory Diagnosis
Eosinophilic meningitis caused by A. cantonensis is generally diagnosed based on clinical presentations and laboratory findings. Typically there is pleocytosis in the CSF with an eosinophil count of 26–75%, accompanied by a peripheral eosinophilia of 5–65%.18 In Thai patients, presumptive preliminary diagnoses are usually made based on the person's specific eating habits, particularly among people living in the parasite endemic areas, the northeastern and central parts of the country.
Although eosinophilic meningitis is an indication of the infection, the various clinical presentations caused by A. cantonensis must be differentiated from those caused by other related helminths (ie, Gnathostoma spp., Paragonimus spp., and Taenia solium metacestodes) that are also endemic in Thailand.29
Immunological testing has been very helpful in supporting the clinical diagnosis. With the introduction of purified homologous antigens for detection of the parasitic infection, the problem of cross-reactivity that occurs in immunodiagnostic methods has been eliminated. A 31 kDa glycoprotein from A. cantonensis has been used as a highly specific antigen for immunodiagnosis.30,31 In the Department of Parasitology at Siriraj Hospital, the immunoblot technique currently used for diagnosis of angiostrongyliasis has improved antibody detection. A standard ELISA using crude antigens is used for screening and all ELISA-positive samples are tested by immunoblot for routine confirmation. A serum reacting with a specific 31 kDa band is indicative of angiostrongyliasis.
Nevertheless, the current enzyme immunoassay format is time-consuming because of the need for multiple reagent additions and long washing and incubation steps. A more user-friendly, rapid, filtration-based immunogold assay is under evaluation. An initial non-enzymatic, dot immunogold filtration assay (DIGFA) with crude antigen preparation was used to detect specific immunoglobulin G (IgG) antibody against A. cantonensis in infected patients and was found to have a diagnostic sensitivity of 91% and specificity of 98% for human angiostrongyliasis.32 In a subsequent collaborative study between Siriraj Hospital and the Institute of Parasitic Diseases, Zhejiang Academy of Medical Sciences, Hangzhou, People's Republic of China (with Dr. Xiao-Xian Gan), a modified rapid dot-immunogold test using purified 31 kDa antigen of A. cantonensis to enhance test sensitivity has performed well on clinical samples at Siriraj Hospital. This test is now being validated on serum samples collected from various areas in Thailand where A. cantonensis is endemic. As the test is rapid (3 min), easy to perform, and needs no special equipment, it is possible that a DIGFA test will soon replace the 2 hr immunoblot test for support of clinical diagnosis of human angiostrongyliasis. The approach is also promising in terms of future diagnostic test kits.
As an alternative to the antibody or antigen detection assay, a conventional PCR technique for the detection of A. cantonensis DNA in clinical CSF samples has been developed that unequivocally demonstrated the presence of parasites in patients.33 Primers were designed based on a mRNA sequence encoding a 66 kDa native protein of the A. cantonensis adult worm present in CSF samples from Thai patients with serologically confirmed angiostrongyliasis. Primers produced an amplified fragment of approximately 300 base pairs in four out of ten patients studied. The nucleotide sequences shared 98.8–99.2% similarity with the reference sequence of A. cantonensis.33 Although preliminary results are encouraging, more clinical samples from angiostrongyliasis patients and other clinically related parasitic infections with their full clinical information are still needed for evaluation of this PCR approach for diagnostic use, including its specificity and sensitivity.
With the rising need for more economical, reliable, and rapid diagnostic tools, the development of new diagnostic tests, such as those in a chromatographic test format, that can differentiate parasitic causes of eosinophilic meninigitis, including that caused by G. spinigerum and by T. solium metacestodes, are overdue. Since multiple parasite infections via food-borne pathways are common, particularly in endemic communities in northeastern Thailand, this need is even more urgent. Development of these tests will also enhance large-scale epidemiological studies, given the potential to screen multiple parasites in patient specimens at one time.
Acknowledgements
Comments on the manuscript by Emeritus Professor Dr. H. S. Yong, University of Malaya, are gratefully acknowledged. This paper represents a contribution to the Rat Lungworm Disease Scientific Workshop held at the Ala Moana Hotel, Honolulu, Hawai‘i, in August 2011. Funding for the workshop and for this publication was provided by the National Institute of Food and Agriculture, United States Department of Agriculture, through Award No. 2011-65213-29954.
Conflict of Interest
The author identifies no conflict of interest.
References
- 1.Khwanmitra S, Bodhidatta A, Punyagupta S. Eosinophilic meningitis. Report of four cases. J Med Assoc Thailand. 1957;40:341–343. [Google Scholar]
- 2.Punyagupta S, Bunnag T, Juttiyudata P, Rosen L. Eosinophilic meningitis in Thailand. Epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am J Trop Med Hyg. 1970;19:950–958. [PubMed] [Google Scholar]
- 3.Benjapongse W. Meningo-encephalomyelitis with eosinophilia in cerebrospinal fluid. Vejasarn Med J. 1964;13:173–184. [Google Scholar]
- 4.Buranasin P, Manunpichu K, Tulyaluk P, Settachan D, Juttijudata P, Punyagupta S. The preliminary study of eosinophilic meningitis in Nakornrajsima hospital and review of 50 cases. Vejasarn Med J. 1965;14:1–13. [Google Scholar]
- 5.Chularerk P, Suyarnsethakorn P. Angiostrongylus cantonensis (Chen) as a possible cause of a outbreak of meningo-encephalitis in the 4th military circle of the Royal Thai Army. Royal Thai Army Med J. 1965;18:623–630. [Google Scholar]
- 6.Hongladarom T, Indarakoses A. Eosinophilic meningo-encephalitis caused by Pila snail ingestion in Bangkok. J Med Assoc Thailand. 1966;49:1–9. [Google Scholar]
- 7.Jittayasothorn K, Setasuban P, Keschamrus N. Eosinophilic meningo-encephalitis in Udorn. Vejasarn Med J. 1965;14:1–8. [Google Scholar]
- 8.Punyagupta S. Eosinophilic meningoencephalitis in Thailand: summary of nine cases and observations on Angiostrongylus cantonensis as a causative agent and Pila ampullacea as a new intermediate host. Am J Trop Med Hyg. 1965;14:370–374. doi: 10.4269/ajtmh.1965.14.370. [DOI] [PubMed] [Google Scholar]
- 9.Tangchai P, Nye BW, Beaver PC. Eosinophilic meningo-encephalitis caused by angiostrongyliasis in Thailand. Autopsy report. Am J Trop Med Hyg. 1967;16:454–461. doi: 10.4269/ajtmh.1967.16.454. [DOI] [PubMed] [Google Scholar]
- 10.Tantibhedyangur P. A family of meningitis most probably caused by Angiostrongylus cantonensis. J Pediatr Soc Thailand. 1963;2:77–96. [Google Scholar]
- 11.Panamonta O. Eosinophilic meningitis in children. Bull Dept Med Services. 1985;10:265–269. [Google Scholar]
- 12.Witoonpanich R, Chuahirun S, Soranastaporn S, Rojanasunan P. Eosinophilic myelomeningo-encephalitis caused by Angiostrongylus cantonensis: a report of three cases. Southeast Asian J Trop Med Public Health. 1991;22:262–267. [PubMed] [Google Scholar]
- 13.Sawanyawisuth K, Kithaweesi K, Limpawattana P, et al. Intraocular angiostrongyliasis: clinical findings, treatments and outcomes. Trans R Soc Trop Med Hyg. 2007;101:497–501. doi: 10.1016/j.trstmh.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Bunnag T, Benjapongse W, Noeypatimanond S, Punyagupta S. The recovery of Angiostrongylus cantonensis in the cerebrospinal fluid of a case of eosinophilic meningitis. J Med Assoc Thailand. 1969;52:665–672. [Google Scholar]
- 15.Laopornpichayanuwat S. Angiostrongylus cantonensis in the cerebrospinal fluid of a female child with eosinophilic meningitis in Thailand. Siriraj Hosp Gaz. 2000;52:553–558. [Google Scholar]
- 16.Nitidandhaprabhas P, Harnsomburana K, Thepsitthar P. Angiostrongylus cantonensis in the cerebro-spinal fluid of an adult male patient with eosinophilic meningitis in Thailand. Am J Trop Med Hyg. 1975;24:711–712. doi: 10.4269/ajtmh.1975.24.711. [DOI] [PubMed] [Google Scholar]
- 17.Eamsobhana P, Tungtrongchitr A. Angiostrongyliasis in Thailand. In: Arizono N, Chai JY, Nawa Y, Takahashi T, editors. Food-borne Helminthiasis in Asia. Chiba, Japan: The Federation of Asian Parasitologists; 2005. pp. 183–197. [Google Scholar]
- 18.Punyagupta S. Angiostrongyliasis: clinical features and human pathology. In: Cross JH, editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. NAMRU-2-SP-44. Taipei, Taiwan: U. S. Army Medical Research Unit No. 2; 1979. pp. 138–150. [Google Scholar]
- 19.Sonakul D. Pathological findings in four cases of human angiostrongyliasis. Southeast Asian J Trop Med Public Health. 1978;9:220–227. [PubMed] [Google Scholar]
- 20.Radomyos P, Tungtrongchitr A, Praewanich R. Occurrence of the infective stage of Angiostrongylus cantonensis in the yellow tree monitor (Varanus bengalensis) in five provinces of Thailand. Southeast Asian J Trop Med Public Health. 1994;25:498–500. [PubMed] [Google Scholar]
- 21.Prommindaroj K, Leelawongs N, Pradatsundarasar A. Human angiostrongyliasis of the eyes in Bangkok. Am J Trop Med Hyg. 1962;11:759–761. doi: 10.4269/ajtmh.1962.11.759. [DOI] [PubMed] [Google Scholar]
- 22.Ketsuwan P, Pradatsundarasar A. Third case of ocular angiostrongyliasis in Thailand. J Med Assoc Thailand. 1965;48:799–805. doi: 10.4269/ajtmh.1966.15.50. [DOI] [PubMed] [Google Scholar]
- 23.Ketsuwan P, Pradatsundarasar A. Second case of ocular angiostrongyliasis in Thailand. Am J Trop Med Hyg. 1966;15:50–51. doi: 10.4269/ajtmh.1966.15.50. [DOI] [PubMed] [Google Scholar]
- 24.Kanchanaranya C, Punyagupta S. A case of ocular angiostrongyliasis associated with eosinophilic meningitis. Am J Ophthal. 1971;71:931–934. doi: 10.1016/0002-9394(71)90268-6. [DOI] [PubMed] [Google Scholar]
- 25.Patikulsila D, Ittipunkul N, Theerakittikul B. Intravitreal angiostrongyliasis: report of 2 cases. J Med Assoc Thailand. 2003;86:981–985. [PubMed] [Google Scholar]
- 26.Singalavanija A, Wangspa S, Teschareon S. Intravitreal angiostongyliasis. Aust NZ J Ophthalmol. 1986;14:381–384. doi: 10.1111/j.1442-9071.1986.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 27.Chotmongkol V, Yimtae K, Intapan PM. Angiostrongylus eosinophilic meningitis associated with sensorineural hearing loss. J Laryngol Otol. 2004;118:57–58. doi: 10.1258/002221504322731664. [DOI] [PubMed] [Google Scholar]
- 28.Karnjanapiboonwong A. Annual Epidemiological Surveillance Report 2009. Bangkok: Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health; 2010. Eosinophilic meningitis; pp. 27–32. [Google Scholar]
- 29.Jaroonvesama N. Differential diagnosis of eosinophilic meninigitis. Parasitol Today. 1988;4:262–266. doi: 10.1016/0169-4758(88)90146-9. [DOI] [PubMed] [Google Scholar]
- 30.Eamsobhana P. Immunological studies on the rat lung-worm Angiostrongylus cantonensis (Nematoda: Metastrongylidae) Kuala Lumpur: University of Malaya; 1994. PhD Thesis. [Google Scholar]
- 31.Eamsobhana P, Yong HS. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) Int J Infec Dis. 2009;13:425–431. doi: 10.1016/j.ijid.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Gan XX, Wang Y, Guo JX, Yang FZ, Zhang LL, Eamsobhana P. Rapid detection of specific IgG antibody for Angiostrongylus cantonensis by dot immunogold filtration assay. Chin J Zoonoses. 2007;23:345–347. [Google Scholar]
- 33.Eamsobhana P, Wanachiwanawin D, Dechkum N, Parsartvit A, Yong HS. Molecular diagnosis of eosinophilic meningitis due to Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) by PCR-DNA sequencing of cerebrospinal fluids of patients. Mem Inst Oswaldo Cruz. 2013;108(1):116–118. doi: 10.1590/S0074-02762013000100020. [DOI] [PMC free article] [PubMed] [Google Scholar]


