Abstract
Associative learning can enable cues from the environment to stimulate feeding in the absence of physiological hunger. How learned cues are integrated with the homeostatic regulatory system is unknown. Here we examined whether the underlying mechanism involves the hypothalamic orexigenic neuropeptide regulators orexin/hypocretin (ORX) and melanin-concentrating hormone (MCH). We used a Pavlovian conditioning procedure to train food-restricted rats to associate a discrete cue, a tone, with food pellets distinct from their regular lab chow diet. Rats in the conditioned group (Paired) received presentations of a tone immediately prior to food delivery, while the rats in the control group (Unpaired) received random presentations of the same number of tones and food pellets. After conditioning rats were allowed ad libitum access to lab chow for at least 10 days before testing. At test sated rats were presented with the tones in their home cages, and then one group was allowed to consume food pellets, while another group was left undisturbed until sacrifice for Fos induction analysis. The tone cue stimulated food consumption in this setting; rats in the Paired group consumed larger amounts of food pellets than rats in the Unpaired group. To examine Fos induction we processed the brain tissue using fluorescent immunohistochemistry methods for combined detection of Fos and characterization of ORX and MCH neurons. We found a greater percentage of ORX and Fos double-labeled neurons in the Paired compared to the Unpaired condition, specifically in the perifornical area. In contrast, there were very few MCH neurons with Fos induction in both the Paired and Unpaired conditions. Thus, the food-cue selectively induced Fos in ORX but not in MCH neurons. These results suggest a role for ORX in cue-induced feeding that occurs in the absence of physiological hunger.
Keywords: appetite, conditioning, non-homeostatic feeding, motivation, obesity, orexin
INTRODUCTION
Learning plays an important role in the control of food consumption. Through associative learning, arbitrary cues from the environment can become signals for food (Pavlov, 1927). Food signals, and associated anticipatory mechanisms, function to prepare an organism for the signaled meal and as such aid in the physiological control of eating (e.g., Woods, 1991; Berridge, 2004). Learned food-cues also acquire an ability to stimulate eating in the absence of physiological hunger in animals and humans (e.g., Weingarten, 1983; Birch et al., 1989, for review see Petrovich, 2011).
This ability of food-cues to stimulate eating on demand, in conditions where calories and nutrients are available, may have been adaptive when food availability was uncertain. However, it is becoming maladaptive in contemporary environments that are rich in readily available, palatable, high-calorie foods. In our world, food-cues, which are plentiful in the form of food advertisements and other food reminders, are relentless appetite stimulants that ultimately encourage overeating and contribute to obesity (for reviews see Hill et al., 2003; Volkow and Wise, 2005; Small, 2009; Berthoud, 2011). Nevertheless, how learned food-cues are integrated with physiological signals to control food intake is currently unknown.
To begin to delineate the critical brain systems involved in cue-driven feeding, here we examined whether the underlying mechanism involves recruitment of lateral hypothalamic neurons that express orexigenic neuropeptide regulators. Prior evidence showed that the lateral hypothalamus is a necessary node in the cue-induced feeding network (Petrovich et al., 2002; for review see Petrovich, 2011); however, specific neuronal mediators are unknown. Two orexigenic neuropeptides are expressed in the lateral hypothalamus: orexin/hypocretin (ORX) and melanin-concentrating hormone (MCH) (Nahon et al., 1989; Broberger et al., 1998; Elias et al., 1998; Sakurai et al., 1998; Swanson et al., 2005; Morton et al., 2006; Hahn, 2010). Interestingly, ORX and MCH are expressed in separate neuronal populations (Broberger et al., 1998; Elias et al., 1998; Peyron et al., 1998; Swanson et al., 2005; Hahn, 2010), and their functions in the control of food intake are also distinct.
Converging evidence has established a critical regulatory function for MCH in energy homeostasis (e.g., Qu et al., 1996; Shimada et al., 1998; Ludwig et al., 2001), and as such MCH would be an effective substrate for cue-induced feeding. In contrast, ORX’s role in feeding is more complex. ORX stimulates feeding (e.g., Sakurai et al., 1998; Rodgers, 2000; Clegg et al., 2002), but it is also important for other motivated behaviors driven by food and drug rewards (e.g., Harris et al., 2005; Thorpe et al., 2005; Nair et al., 2008; Borgland et al., 2009; Choi et al., 2010; Sharf et al., 2010a; for reviews see Cason et al., 2010; Sharf et al., 2010b), and it is critical for wakefulness and arousal (e.g., de Lecea et al., 1998; Chemelli et al., 1999; Mieda et al., 2004; Berridge et al., 2010; Boutrel et al., 2010). These diverse functions have been conceptualized to reflect the role of ORX in coordinating the current motivational state with adaptive physiological and behavioral responses (e.g., Yamanaka et al., 2003; for reviews see Willie et al., 2001; Saper, 2006; Tsujino and Sakurai, 2009), a role which is supported by its widespread connectional network and extensive receptor distribution (e.g., Peyron et al., 1998; Marcus et al., 2001; Baldo et al., 2003; Yoshida et al., 2006). There is also evidence that distinct ORX subsystems might mediate arousal and reward-seeking functions (e.g., Harris and Aston-Jones, 2006). As such, ORX might be an important motivational and appetite substrate for cue-driven feeding.
Thus, here we examined whether a learned food-cue functionally activates ORX- or MCH-producing neurons. We identified immediate early gene (IEG) c-fos protein product (Fos) induction in ORX- and MCH-identified neurons using a fluorescent double-label immunohistochemistry method. We used our recently developed behavioral preparation, which allowed us to map food-cue Fos induction independent of the training context, and in the absence of food (Reppucci and Petrovich, 2012a). This is important because the training context, as well as food presentation and consumption, could stimulate Fos induction in the brain areas of interest.
In brief, here we trained rats to associate a discrete cue, a tone (conditioned stimulus, CS) with food pellets (unconditioned stimulus, US) distinct from their regular chow diet. Then during testing sated rats were given tone (CS) presentations in their home cages. Following CSs, one group of rats was left undisturbed until sacrifice to examine cue-induced Fos expression in ORX and MCH neurons (brain analysis group), while another group of rats was given access to food pellets to assess the cue’s effects on feeding (food consumption group).
EXPERIMENTAL PROCEDURES
Subjects
Forty experimentally naïve, male Long–Evans rats approximately 2 months of age (Charles River Laboratories; Raleigh, NC, USA), were individually housed and maintained on a 12-h light/dark cycle (lights on at 6:00). Upon arrival, subjects were allowed 1 week to acclimate to the colony room, during which time they had ad libitum access to standard laboratory chow and water and were handled daily. All housing and testing procedures were in compliance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and approved by the John Hopkins University and Boston College Institutional Animal Care and Use Committees.
Apparatus
The behavioral training was conducted in two sets of behavioral chambers (Set 1: 30 × 24 × 30 cm, Set 2: 30 × 28 × 30 cm; Coulbourn Instruments, Allentown, PA, USA) located in rooms that were different from the colony housing rooms, and different from the rooms used for testing (see Behavioral procedure section). All chambers had aluminum tops and sides, a transparent Plexiglas back and front, a recessed receptacle for food (“food cup”, 3.2 × 4.2 cm), and grid floors. One set of chambers had a black Plexiglas panel placed on top of the grid floor, and were enclosed in isolation cubicles (79 × 53 × 53 cm; Coulbourn Instruments, Allentown, PA, USA) composed of monolithic rigid foam walls, which isolate chambers from ambient sound and light. All chambers were dimly illuminated, and a ventilation fan provided masking noise (55–60 dB) during training sessions. Stimulus presentation was controlled by software (LabView: National Instruments, Austin, TX, USA or GraphicState 3.0: Coulbourn Instruments, Allentown, PA, USA). Video cameras recorded behavior during training.
Behavioral procedure
Experimental design is outlined in Fig. 1. Before behavioral training, rats were gradually reduced to 85% of their ad libitum weight. Additionally, prior to the start of the conditioning protocol all rats received one magazine training session in which they learned to eat from the food cup. During this session rats received 16 trials of food pellet delivery (45-mg pellets, formula 5TUL; Test Diets, Richmond, IN, USA); no other stimuli were presented. After the magazine session, rats received 10 training sessions (one session per day, excluding weekends) each approximately 32 min in length. For half of the rats (conditioned group, Paired), these sessions each consisted of eight presentations of the CS, a 10-s tone (1.5–2 kHz, 75 dB), immediately followed by delivery of the US, two food pellets, into the food cup. For the other half of the rats (control group, Unpaired), the sessions consisted of the same number of tone and food presentations as the Paired group, but delivered in a non-conditional random order. After the last training session, rats had ad libitum access to standard laboratory chow for 10–16 days to allow rats to reach at least 110% of their pre-training body weight. During this time, rats were habituated to a new testing room on two occasions. For the first habituation rats were brought to the testing room and left undisturbed in their home cages for 15 min. The second habituation was the same except that lab chow was removed immediately prior to transporting the rats to the testing room to acclimate them to the food removal that would occur prior to testing. Additionally, a group of rats that would be given food after testing was habituated to the glass dish (107 × 87 × 70 mm) that would be used for food pellet presentation. For these rats, on three occasions that were separate from testing room habituation, the empty glass dishes were left for one hour in the rats’ home cages in the colony room.
Fig. 1.
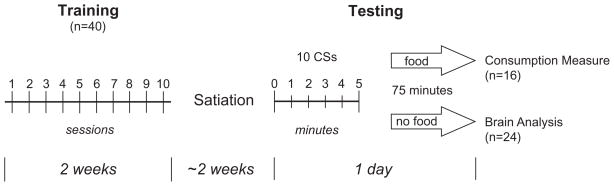
Experimental design. The behavioral training consisted of 10 sessions over the course of two weeks. Sessions were conducted in behavioral chambers, and each consisted of eight pairings of a tone with food pellet delivery (conditioned group, Paired; n=20) or the same number of tones and food pellets presented at random (control group, Unpaired; n=20). Rats were maintained on a food-restricted feeding regimen throughout training. Following training rats were allowed ad libitum access to chow (satiation), and then tested while sated. During testing rats remained in their home cages and were given 10 presentations of the tone over 5 min. One group of rats (food consumption group; n=8/experimental condition) was then allowed to consume food pellets ad libitum for 75 min, while a second group (brain analysis group; ORX: n=12/experimental condition, MCH: n=4/experimental condition) was left undisturbed for 75 min until sacrifice.
On test day rats were transported to the testing room, and all food was removed from the cage just prior to transport. During the test, rats remained in their home cages and were given 10 presentations of the CS (10-s tone) over 5 min. After the test one group of rats (n=24; brain analysis group) was immediately returned to the colony room and left undisturbed without access to any food for 75 min, at which time the animals were sacrificed. The second group of rats (n=16; food consumption group) was given 20 g of chow in the wire cage top and 20 g of food pellets in the glass dish immediately following the test, and returned to the colony room. After 75 min all uneaten chow and pellets were removed, weighed, and the amount consumed was calculated.
All training and testing was conducted during the light phase, approximately between 9:00 and 15:00, with the exact time counterbalanced across groups. This period of the light–dark cycle corresponds to the lowest baseline Fos induction in orexin neurons (Estabrooke et al., 2001). The experiment was completed in three replications. The training procedure was identical for all replications. One replication was used solely for the food consumption analysis, and the two other replications were used for the brain analysis. Each replication had an equal number of rats in the conditioned and control groups. In the first replication, subjects (n=16) completed their behavioral training at Johns Hopkins University, and the brain tissue was transported to Boston College for processing and analysis (ORX+Fos). The other two replications were completed at Boston College, and one set of subjects (n=8) was also used for brain tissue processing and analysis (ORX+Fos and MCH+Fos), while the other set (n=16) was used in the food consumption test.
Behavioral observations
Conditioned responses (CRs) were assessed from videos recorded during the last training session. The expression of food cup behavior was the primary measure of conditioning. Food cup behavior included nose pokes into the recessed food cup, and standing in front of and facing the food cup. Observations were paced by a metronome set to 48 beats per minute and thus were made every 1.25 s by observers “blind” with respect to the training condition of the animals observed. At each observation (indicated by a beat from the metronome), only one behavior was recorded (“food cup” or “none”). Food cup behavior was scored during the 10-s tone (CS) and during the 10 s immediately preceding the tone (Pre-CS). The percentage of time rats spent expressing food cup behavior during these two periods was then calculated.
Histological procedures
Rats were briefly anesthetized with isoflurane, then deeply anesthetized with an intraperitoneal injection of tribromoethanol (375 mg/kg) or Nembutal (100 mg/kg) depending on the institution where the surgery was performed (see Subject section). Rats were then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M borate buffer. Brains were extracted and post-fixed overnight in the solution used for perfusion with 12% sucrose. The brains were then rapidly frozen in hexanes cooled in dry ice, and stored at −80 °C. Brains were sliced in 30-μm sections using a Leica SM200R sliding microtome and were collected accordingly into four series. Two adjacent tissue series were collected into trays containing a cryoprotectant solution (0.025 M sodium phosphate buffer with 30% ethylene glycol and 20% glycerol) and stored at −20 °C until immunohistochemical processing. A third series was collected into a 0.02 M potassium phosphate-buffered saline (KPBS) solution, mounted onto slides (SuperFrost Plus; Fisher Scientific, Pittsburgh, PA, USA), and stained with Thionin (Simmons and Swanson, 1993) for identification of brain structures as defined in Swanson’s rat brain atlas (Swanson, 2004). Brain perfusions, collection, slicing, and storage were counterbalanced across the experimental conditions (Paired, Unpaired). Additionally, tissue processing was conducted in pairs (Paired, Unpaired) in the same staining tray to balance for possible minute solution differences.
Fos detection in ORX neurons
For visualization of Fos protein induction in ORX neurons, one series of brain tissues (n=24) underwent fluorescent double immunohistochemical processing for combined identification of ORX and Fos. The brain sections that encompass the lateral hypothalamic area with ORX neurons (Swanson, 2004; Swanson et al., 2005; Hahn, 2010) were identified and rinsed from cryoprotectant storage solution with several washes in KPBS. The tissue was incubated for 72 h at 4 °C in a solution of KPBS containing 0.3% Triton X-100 (Sigma–Aldrich, St. Louis, MO, USA), 2% normal donkey serum (017-000-121; Jackson ImmunoResearch, West Grove, PA, USA), and the primary antibodies: anti-c-fos antibody raised in rabbit (1:5000; SC-52; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and anti-orexin-A antibody raised in goat (1:2000; SC-8070; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
After rinses in KPBS, tissue was incubated for one hour in the dark in a KPBS solution containing 0.3% Triton X-100, 2% normal donkey serum, and the secondary fluorescence antibodies: Alexa 488 anti-rabbit raised in donkey (1:200; A21206; Invitrogen, Carlsbad, CA, USA) and Alexa 546 anti-goat raised in donkey (1:200; A11056; Invitrogen, Carlsbad, CA, USA). Tissue was then mounted in semi-darkness onto slides (SuperFrost Plus), left to dry, coverslipped with Vectashield HardSet Mounting Medium with DAPI (4′,6-diamidino-2-phenylindole; H-1500; Vector Labs, Burlingame, CA, USA), and stored at 4 °C.
Fos detection in MCH neurons
An adjacent series of brain tissue was processed for visualization of Fos induction in MCH neurons in one set of brains (n=8). All processing methods were as described above, except that anti-pro-MCH antibody raised in goat (1:1000; SC-14507; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as the primary antibody, in place of the anti-orexin-A antibody.
Image acquisition and analysis
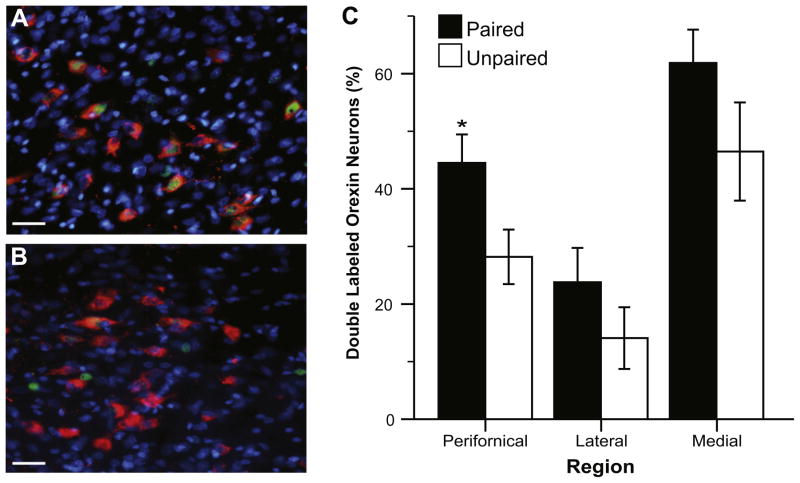
Processed tissue was imaged using the 20× objective on a Zeiss Axioplan II fluorescence microscope with attached Hamamatsu camera (Bridgewater, NJ, USA). Images of the tissue were taken bilaterally at the best available representation of anatomical level 29 (Swanson, 2004) for three different locations within the lateral hypothalamus (Fig. 2A): directly above fornix (perifornical), medial to fornix (medial), and lateral to fornix (lateral). Sampling in the perifornical area for ORX and MCH neurons was in the same location directly above the fornix as shown in Fig. 2A; this corresponds to the suprafornical region defined by Swanson (Swanson, 2004; Swanson et al., 2005). Medial and lateral sampling locations were determined by each peptide’s distribution, and thus were somewhat topographically distinct within the regions shown in Fig. 2A. Because of broader distribution of MCH neurons in the lateral area, two images within the lateral region (one dorsal and one ventral) were taken in a majority of the brains (two lateral images: n=5, single lateral image: n=3; balanced across experimental conditions). Additionally, the specific location for each image within the outlined borders of the sampling regions shown in Fig. 2A was determined for each brain by the exact location of a dense peptide-positive group. It should be noted that we observed a similar pattern of ORX and MCH distribution in our Long–Evans rats as has been previously reported in Sprague–Dawley rats (Swanson et al., 2005; Hahn, 2010). Images were pseudocolored with red for neuropeptide (ORX or MCH), green for Fos, and blue for DAPI (nuclear counterstain), then stacked using Improvision OpenLab (PerkinElmer, Waltham, MA, USA) imaging software (Fig. 2B).
Fig. 2.
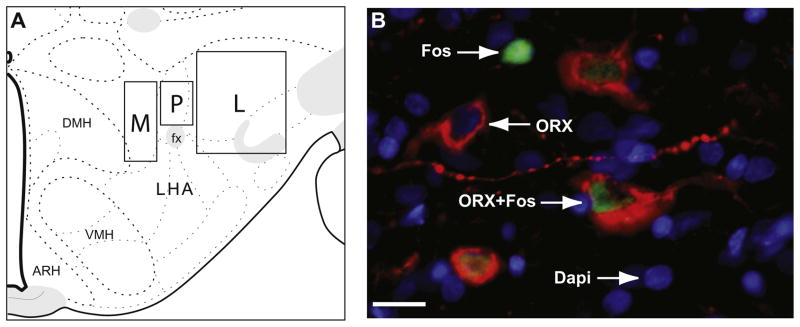
Image acquisition and analysis. (A) Representation of the sampling regions is shown on a modified rat brain atlas template (level 29, Swanson, 2004). Images were taken within the outlined regions shown, and the exact location within each region was determined by the peptide distribution. Images were taken in three sampling regions: directly above fornix (perifornical, P), medial to fornix (M), and lateral to fornix (L). (B) Image shows representative types of labeled neurons. Arrows point to a representative of each type of labeled neuron: single-labeled Fos (green), single-labeled neuropeptide (red, in this example ORX), and double-labeled neuropeptide and Fos. Nuclear counterstain, DAPI, is shown in blue. In panel B, Scale bar=15 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
All neuron counting was conducted from the triple-merged images, however, single images were consulted as needed to confirm the cell and stain types. Three types of neurons were identified and counted: single-labeled neuropeptide-positive, single-labeled Fos-positive, and double-labeled neuropeptide-positive and Fos-positive neurons (Fig. 2B). Single-labeled neuropeptide-positive neurons were characterized by distinct cytoplasmic staining, single-labeled Fos protein-positive neurons were characterized by distinct nuclear staining, and double-labeled neurons (neuropeptide+Fos) had both cytoplasmic-neuropeptide and nuclear-Fos labeling (Fig. 2B). A neuropeptide-containing neuron was counted as positive only if both the cell body and the nucleus were clearly visible in the image. Fos-labeled neurons were counted as positive if the Fos-labeling intensity was clearly above the background and contained within the nucleus. Nuclear counterstain, DAPI, was used in all images to identify nuclei. Cell identification and counts analysis was conducted by an experimenter unaware of the experimental condition of the images observed.
Total counts from images of the left and right hemispheres were pooled to calculate the total number of Fos-positive neurons, the total number of neuropeptide-positive neurons, and the total number of double-labeled (neuropeptide+Fos) neurons within each sampling region (perifornical, medial, and lateral) for each brain. To perform a more accurate analysis of the degree of ORX and MCH neuron recruitment the percentage of the total number of neuropeptide-specific neurons that were double-labeled with Fos, and the percentage of the total number of Fos-positive neurons that were double-labeled with a neuropeptide were then calculated for each sampling region. Additionally, the total number of Fos-positive neurons and total number of neuropeptide-positive for each of the three regions was expressed per brain side, and was calculated by averaging total numbers for the left and right hemispheres. Due to poor tissue quality or tissue damage two brains were excluded from all ORX analyses, and only unilateral counts were available for three ORX brains.
Statistics
Data were analyzed using analysis of variances (ANOVAs), t-Tests, Mann–Whitney tests, and Wilcoxon matched-pairs tests when appropriate in SPSS. In all cases, p<0.05 was considered significant.
RESULTS
Training
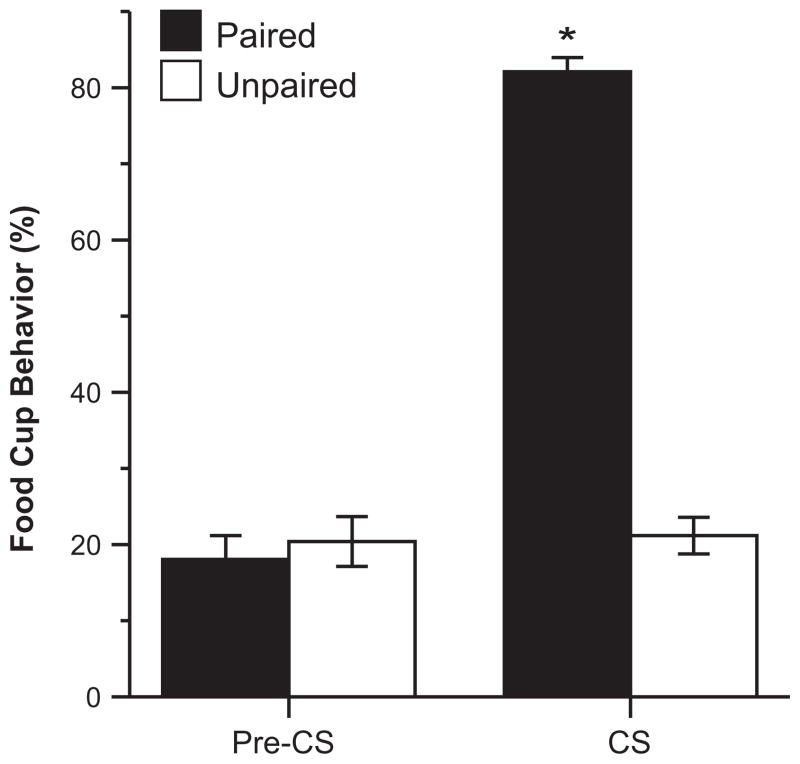
Learning was assessed during the last training session. Conditioning of rats in the Paired group was clearly evident from observations of the CRs directed toward the food cup during the presentation of the CS. To confirm that learning was similar across all three replications, 2 × 3 (test group × replication) ANOVAs were conducted. There were no differences in the expression of CRs between replications during the Pre- CS or CS time periods (p>0.05, all). There was a significant effect of test group during the presentation of the CS (F(1,39)=384.38, p<0.001); rats in the Paired group expressed high levels of CRs during the CS compared to their behavior during the Pre-CS period, while the CRs of the rats in the Unpaired condition were low during both periods (Fig. 3). A repeated measures ANOVA collapsed for replication showed a significant effect of Pre-CS vs. CS time period (F(1,38)=442.50, p<0.001), a significant effect of training group (F(1,38)=68.65, p<0.001), and significant time period by test group interaction (F(1,38)=421.43, p<0.001).
Fig. 3.
Conditioned responses during the last training session. Mean (±SEM) percent of the total time rats expressed food cup behavior during the Pre-CS and CS periods; *p<0.05.
Testing
Testing was conducted in the home cages and consisted of presentations of the tone, CS, in the absence of any food. Following the tone test rats in the food consumption group were immediately given food to examine CS effects on consumption, while rats in the brain analysis group were left undisturbed until sacrifice.
Food consumption test
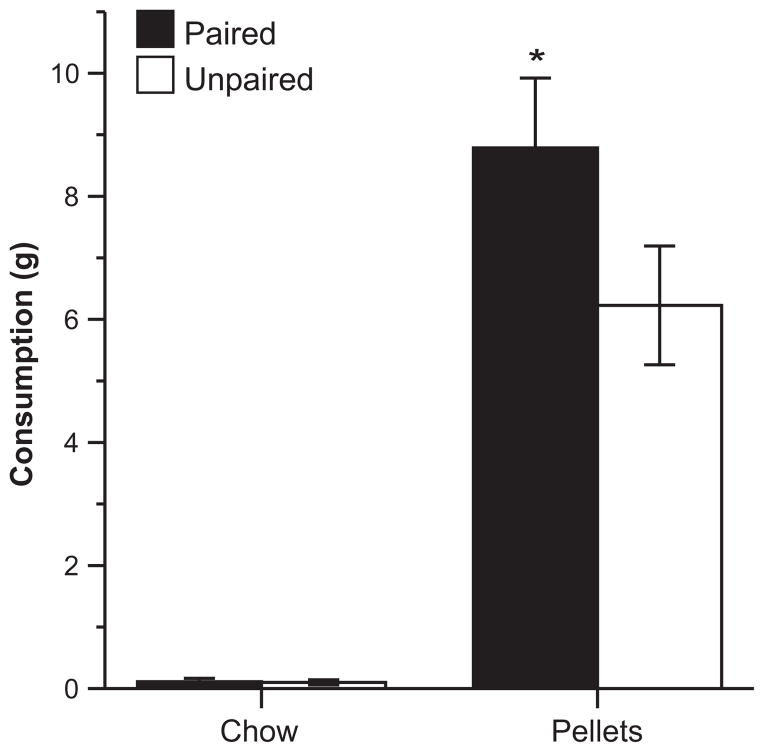
Rats in the Paired group ate more food pellets than rats in the Unpaired group during the food consumption test (Fig. 4), which is in agreement with our previous work using similar procedures (Reppucci and Petrovich, 2012a,b). Shapiro–Wilk tests indicated that the consumption data were not normally distributed (pellets: p=0.058, chow: p<0.001), thus nonparametric tests were used for all food consumption analysis measures. A Mann–Whitney test confirmed that the Paired group ate significantly more pellets than the Unpaired group (U=10, p<0.05). Both groups ate little chow, and there were no differences in chow consumption between the groups according to a Mann–Whitney test (p>0.05). Rats in both groups showed a strong preference for the pellets over chow, and Wilcoxon matched-pairs tests confirmed that both the Paired and Unpaired groups consumed significantly more pellets than chow (P: Z=2.37, p<0.05; U: Z=2.52, p<0.05). The chow consumption measurement for one subject (6.54 g) was much greater than (more than six standard deviations above) the mean consumption of all other subjects (0.11 g), and thus data from this subject were excluded from the food consumption analysis.
Fig. 4.
Food consumption test. Mean (±SEM) consumption in grams of food pellets and chow following CS presentations. *Indicates a significant difference (p<0.05) in food pellet consumption between groups.
Rats in the Paired and Unpaired groups had similar body weights prior to the beginning of the training (P: 313±4 g, U: 313±4 g), while maintained at 85% body weight during training (P: 272±3 g, U: 274±4 g), and following the satiation period prior to testing (P: 380±6 g, U: 383±4 g). Independent samples t-Tests confirmed there were no significant differences in body weight between groups at any time point (p>0.05 for all).
Fos induction in ORX neurons
We examined IEG c-fos protein product expression in ORX neurons within three areas of the lateral hypothalamus: perifornical, medial, and lateral. Overall we found a greater percentage of double-labeled ORX neurons (ORX+Fos) in the Paired compared to the Unpaired condition at all three sampling locations (Fig. 5). Two-way (Replication by Test Group) ANOVAs showed that this increase was statistically significant for the perifornical region (F(1,23)=6.35, p<0.05), but not for the medial or lateral areas (p>0.05, both). There was no effect or interaction with Replication for any region (p>0.05, all); the data from both replications used in the analysis were statistically similar.
Fig. 5.
Fos induction in ORX neurons. Images show Fos induction (green) in ORX neurons (red) in the perifornical area following CS presentation in the Paired (A) and Unpaired (B) condition. Nuclear counterstain, DAPI is shown in blue. Scale bar=30 μm. (C) Graph shows the proportion of ORX neurons that were co-labeled with Fos in each sampling region. Data are shown as mean (±SEM) percent of ORX neurons that were double-labeled (ORX+Fos). *Indicates a significant difference (p<0.05) between groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We also analyzed the percent of the total number of Fos-positive neurons that were double-labeled with ORX and found that roughly half of the Fos-positive neurons in our sampling areas were labeled with ORX; an independent samples t-Test showed that there were no significant differences between groups (P: 46.85± 3.62%, U: 53.80±5.71%; p>0.05). This indicates that although rats in the Paired group had a higher percentage of activated ORX neurons, the percentage of activated non-ORX neurons in the ORX-dense sampling area was similar between the Paired and Unpaired groups.
We also conducted independent samples t-Tests to confirm that there were no differences between groups in the average number of ORX neurons per side for any sampling region (p>0.05, all). Data from the sampling regions were then collapsed to calculate the total average number of ORX neurons per side, and a two-way (Replication by Test Group) ANOVA confirmed there were no group differences (P: 57.91±3.26, U: 59.36±4.81), and indicated that there was no effect or interaction with Replication on the number of ORX-positive neurons (p>0.05). Thus, training did not alter baseline levels of neurons expressing ORX, and we sampled from a similar number of ORX neurons across experimental conditions.
Fos induction in MCH neurons
We found very low and sporadic Fos induction in MCH neurons; less than 6% of MCH neurons were double labeled in any image in either group (Fig. 6). For this reason MCH was assessed using data from one replication. Additionally, the counts from the three sampling regions for each brain were pooled together (perifornical+ medial+lateral) and the total percent of MCH-double-labeled neurons per brain was compared between groups. Independent samples t-Tests showed there were no significant differences in the percentage of double-labeled MCH neurons (MCH+Fos) between groups (P: 2.27±0.91%, U: 3.12±0.84%; p>0.05). Similarly, we found that only a very small percentage of Fos-positive neurons were double-labeled with MCH; an independent samples t-Test showed that there was no significant difference between groups (P: 2.45±0.91%, U: 6.54±1.57%; p>0.05). As a control, we conducted an independent samples t-Test which confirmed that there were no differences between groups in the average number of total MCH neurons per side (P: 96.88±14.32, U: 121.88±16.59; p>0.05). Thus, training did not alter baseline levels of neurons expressing MCH, and we sampled from a similar number of MCH neurons between groups.
Fig. 6.
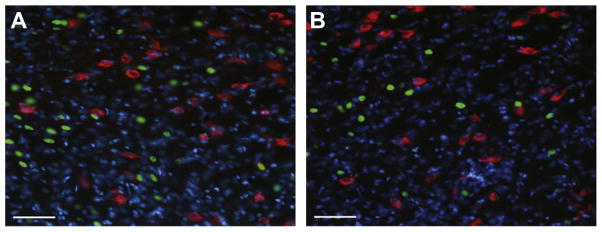
Fos induction in MCH neurons. Images show Fos induction (green) in MCH neurons (red) in the perifornical area following CS presentation in the Paired (A) and Unpaired (B) condition. Nuclear counterstain, DAPI is shown in blue. There was very low Fos-induction within MCH neurons for either condition, however there was substantial Fos induction in non-MCH neurons particularly in the Paired condition. Scale bar=60 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Total Fos induction
We compared the total number of Fos-positive neurons within our sampling areas across ORX and MCH stained tissue, and found that even though Fos induction was much lower in MCH neurons compared to ORX neurons, the total number of Fos-positive neurons was similar in samples from both sets of tissues (Figs. 5 and 6, Table 1). Paired t-Tests showed no differences in the total number of Fos-positive neurons for any sampling region between ORX and MCH stained tissue for either group (p>0.05, all), demonstrating that the low occurrence of MCH+Fos double-labeled neurons was not due to overall lower Fos induction in the MCH compared to ORX tissues. For both ORX and MCH stained tissues, we found a larger number of Fos-positive neurons in the Paired compared to the Unpaired group, and independent samples t-Tests showed that this increase neared significance within the perifornical region in both sets of tissues (ORX stained tissue, t(20)=2.08, p=0.05; MCH stained tissue, t(6)=2.06, p=0.09).
Table 1.
Total number of Fos-positive neurons. Data are presented as the mean ± SEM average total number of Fos-positive neurons per hemisphere for each of the three sampling locations (perifornical, medial, lateral) in the ORX and MCH-stained tissue
| Sampling region | Group | ORX series | MCH series |
|---|---|---|---|
| Perifornical | Paired | 23.82 ± 3.38+ | 31.25 ± 3.82+ |
| Unpaired | 14.73 ± 2.78 | 20.63 ± 3.49 | |
| Medial | Paired | 27.86 ± 5.33 | 32.25 ± 6.70 |
| Unpaired | 21.32 ± 5.00 | 20.75 ± 5.05 | |
| Lateral | Paired | 11.68 ± 2.20 | 18.63 ± 6.92 |
| Unpaired | 7.86 ± 2.43 | 18.63 ± 5.30 |
P vs. U, p<0.10.
DISCUSSION
Our aim here was to determine whether cue-induced feeding mechanisms involve recruitment of hypothalamic orexigenic peptide systems. We examined whether a learned food-cue functionally activates (as measured by Fos induction) lateral hypothalamic neurons that express orexigenic neuropeptides, ORX or MCH, in the absence of food or physiological hunger (food-deprivation). We found that exposure to food-cues selectively recruited ORX but not MCH neurons.
We used our recently developed cue-induced feeding protocol (Reppucci and Petrovich, 2012a,b), which is suitable for IEG mapping because its design minimizes non-specific IEG induction. In this preparation rats are tested in their home cages to eliminate IEG induction due to exposure to the training context, which alone could serve as a CS and has been shown to modulate food intake (Le Merrer and Stephens, 2006; Petrovich et al., 2007a,b; Boggiano et al., 2009; Bouton, 2011). Furthermore, during testing the cue presentation and food consumption are temporally separated; food access is provided immediately after cue presentations. This is important since exposure to the sensory properties of the food (e.g. sight, smell, taste) as well as the act of consumption could potentially stimulate Fos induction in the areas of interest. Thus, the preparation allowed us to examine the brain substrates recruited by the food-cue alone. Importantly, in this setting the cue stimulates intake once the food is provided (current results, Reppucci and Petrovich, 2012a).
Using this preparation, we found Fos induction in ORX neurons, but not in MCH neurons in the conditioned rats compared to the controls following CS presentations. There were more double-labeled ORX and Fos neurons in each of the three sampling regions (medial, perifornical, and lateral) in the conditioned compared to the control group, and this increase was statistically significant within the perifornical region. In contrast, we found very low and sporadic Fos induction in MCH neurons, less than 6% of MCH neurons were double-labeled in any region for either group, and there were no significant differences between groups. The lack of Fos induction in MCH neurons was not due to a non-specific decrease of Fos expressing neurons in the MCH-stained tissue. We examined the total Fos induction and found it to be similar between the tissue processed for ORX+Fos and the adjacent tissue processed for MCH+Fos. Thus, our results show that the CS selectively recruited ORX, but not MCH neurons under the same conditions in the same animals.
The dissociation in recruitment between ORX and MCH neurons found here is in agreement with prior rodent studies that also examined Fos induction in these neurons. Microinjections of the GABA-A receptor agonist muscimol into the nucleus accumbens, a manipulation well known to stimulate feeding (Stratford and Kelley, 1997), recruited ORX but not MCH neurons in the absence of food (Zheng et al., 2003; Baldo, 2004), and similar to the current findings, ORX recruitment was found in the perifornical region (Zheng et al., 2003). In another behavioral model, morphine withdrawal precipitated by an opioid receptor antagonist also induced Fos in ORX but not in MCH neurons (Georgescu et al., 2003). Similarly, in a dehydrationanorexia model, rehydration and subsequent feeding (reversal of dehydration-induced anorexia) induced Fos in ORX but not MCH neurons (Watts and Sanchez- Watts, 2007).
Collectively, prior and current studies found minimal, non-selective Fos induction in MCH neurons in the same settings that produced substantial and selective Fos induction in ORX neurons. These studies used different behavioral preparations, however, all were conducted under non food-deprived conditions (except for dehydration-anorexia (Watts and Sanchez-Watts, 2007)), and thus it is possible that the lack of MCH recruitment observed was due to the physiological state. Fasting dramatically upregulates MCH expression (Qu et al., 1996), and as such might be required for MCH recruitment. Thus, MCH might be important during the acquisition phase of cue-induced feeding, which occurs under food-restricted conditions. In agreement with this hypothesis, recent evidence from experiments with MCH receptor (MCH-1R) deficient mice and experiments with pharmacological manipulation using an MCH-1R antagonist showed that functional MCH-1R signaling was necessary for cue-induced feeding (Johnson et al., 2011).
To our knowledge, the current study is the first to show that a discrete learned cue for food induces Fos in ORX neurons in the absence of food or physiological hunger. Nevertheless, these findings are in agreement with prior work with contextual cues associated with food (Harris et al., 2005; Harris and Aston-Jones, 2006; Choi et al., 2010). Harris, Aston-Jones and colleagues demonstrated Fos induction in ORX neurons after exposure to a food-associated environment in the conditioned place preference task (Harris et al., 2005; Harris and Aston-Jones, 2006). These studies also showed topographically distinct recruitment of ORX neurons located lateral to the fornix, but not in the ORX neurons located in the perifornical or the area medial to the fornix. Interestingly, the opposite pattern was found in response to footshock, suggesting that reward seeking and arousal are supported by distinct ORX subsystems (Harris and Aston-Jones, 2006; Cason et al., 2010). Here we found that a discrete CS most potently induced Fos in the perifornical area. The discrepancy might be due to the difference in behavioral preparations, or other methodological differences. Nevertheless, in agreement with our current findings another recent study showed Fos induction in ORX neurons in the perifornical area after placement into a context previously associated with food consumption (Choi et al., 2010).
The recruitment of the perifornical area has intriguing implications for cue-induced feeding mechanisms. This region of the lateral hypothalamus is a potent stimulation site for feeding and motivation. Feeding can be induced in sated rats by electrical stimulation in this region, as well as by direct infusions of neuropeptides, glutamatergic agonists, and GABA antagonists (e.g., Stanley et al., 1993; Gillard et al., 1998; Lee and Stanley, 2005, for reviews see Wise, 1974; Stanley et al., 2011). The perifornical area is also a site where the motivation to self-stimulate for electrical reward (“brain stimulation reward”) (Olds and Milner, 1954) is sensitive to physiological hunger and energy state. Food deprivation enhances brain stimulation reward when electrode placement is in the perifornical area (Blundell and Herberg, 1968; Carr and Wolinsky, 1993), while the opposite effect was produced by leptin (Fulton et al., 2000), a hormone released in proportion to body fat that acts in the brain as a satiety signal (for reviews see Friedman and Halaas, 1998; Morton et al., 2006). Rats worked harder to obtain the same electrical reward when chronically food restricted and this effect was attenuated by intracerebroventricular leptin injections (Fulton et al., 2000).
Notably, the perifornical region is the most effective hypothalamic site for neuropeptide Y (NPY) induced feeding in sated animals (Stanley et al., 1985, 1993). NPY is one of the most potent brain orexigens (Leibowitz, 1994), and importantly, it acts in part by changing the motivation to eat (Flood and Morley, 1991; Ammar et al., 2000). ORX neurons receive input from NPY neurons located in the arcuate nucleus of the hypothalamus (Elias et al., 1998), and ORX-induced feeding requires functioning NPY receptor signaling (Dube et al., 2000; Jain et al., 2000; Yamanaka et al., 2000; also see Campbell et al., 2003) for non-NPY ligand on Y4) receptors. There is also evidence for a reciprocal role, where NPY-driven mechanisms might be assisted by the ORX system. NPY-induced feeding is mediated in part by the ORX system (Niimi et al., 2001), and an increase in ORX expression precedes an increase in NPY expression in response to sleep deprivation and subsequent hyperphagia (Martins et al., 2010). ORX input to NPY neurons in the arcuate nucleus is supported by anatomical evidence (Horvath et al., 1999; Marcus et al., 2001). Relevant for cue-driven mechanisms, the NPY–ORX bidirectional microcircuitry is well positioned to mediate information sharing between the lateral hypothalamic area, which is under telencephalic influence (see below), and energy-sensing signals from the arcuate nucleus. Nevertheless, whether the ORX–NPY microcircuitry mediates cue-driven motivation to consume food remains to be determined.
How the food-cue information might be conveyed to ORX neurons, and specific inputs that may drive Fos induction in these neurons are not known. Our prior work has shown that the basolateral amygdala and the ventromedial prefrontal cortex, and their communication with the lateral hypothalamus are critical for cue-induced feeding (Holland et al., 2002; Petrovich et al., 2002, 2005, 2007b; for review Petrovich, 2011). However, future work is needed to determine whether these areas are integrated with ORX neurons specifically.
Interestingly, the regions of the basolateral area of the amygdala (basomedial and basolateral nuclei) that send direct projections to the lateral hypothalamus do not reach the perifornical area, and instead innervate the ventrolateral region of the lateral hypothalamus (Petrovich et al., 2001, 2005; Reppucci and Petrovich, 2011). On the other hand, the ventromedial prefrontal cortex innervates the perifornical area, and thus might be able to directly influence ORX neurons (Floyd et al., 2001; Reppucci and Petrovich, 2012b). A recent study offers an intriguing possibility for the ventromedial prefrontal cortex influence on hypothalamic orexigenic substrates. Stimulation of μ-opioid system within the ventromedial prefrontal cortex was shown to drive palatable food (carbohydrate) intake in sated rats (Mena et al., 2011). Nevertheless, whether that system acts via the ORX system remains to be determined.
Another intriguing possibility for the role of the ventromedial prefrontal cortex glutamatergic input is in learning plasticity. In this regard, the NMDA-receptor mediated feeding in the lateral hypothalamus was shown to be sensitive to tyrosine kinase inhibition, and phosphorylation changes of tyrosine kinases in the perifornical region were found during food deprivation (Khan et al., 2004). However, whether similar changes might contribute to plasticity underlying the learned food-cue’s ability to stimulate feeding, and the source of the critical glutamatergic input remains to be determined.
The current finding of ORX neuron recruitment by a food-cue offers appealing functional implications for cue-induced feeding mechanisms. Indeed, this result suggests that ORX recruitment may be part of the CS-driven anticipatory motivational mechanism that primes an organism to eat. This hypothesis is in agreement with ORX stimulatory effects on feeding and arousal, and its proposed role in regulating coordinated behavioral responses according to the current motivational state (for reviews see Willie et al., 2001; Saper, 2006; Boutrel et al., 2010). The distribution of ORX neuron outputs and ORX-receptors is extensive (e.g., Peyron et al., 1998; Marcus et al., 2001; Baldo et al., 2003), and allows for communication with forebrain and brainstem areas critical for feeding, arousal, and motivation (Swanson, 2000; Grill and Kaplan, 2002).
Less is known, however, about the specific connections of the perifornical ORX neurons, although there is evidence for selective connections with the hypothalamic and brainstem regions regulating homeostatic and arousal functions (e.g., Zheng et al., 2005; Yoshida et al., 2006). Interestingly, a population of orexin neurons within this region sends projections to multiple sympathetic systems (Geerling et al., 2003), suggesting a possible role of these neurons in integrating conditioned cues, feeding, and autonomic function. A recent comprehensive analysis of afferent and efferent connections of the suprafornical region, which corresponds well with the perifornical sampling region in the current study, revealed that this region is preferentially connected with the ingestive behavior network (Hahn and Swanson, 2010). Importantly, that connectional network includes all functional divisions of the nervous system, underscoring an integrative role for this area (Hahn and Swanson, 2010). Nevertheless, future research is necessary to determine the role ORX might play in cue-induced feeding and the efferent systems through which that role is exerted.
CONCLUSION
The present results indicate that a learned food-cue selectively recruits ORX, but not MCH neurons, in a preparation that stimulates feeding in sated rats. These results suggest a role for ORX in cue-induced feeding that occurs in the absence of physiological hunger.
Acknowledgments
We thank Pari Mody, Katherine Gildersleeve, Brandon Davenport, and Meghana Kuthyar for technical assistance, and Professor Michela Gallagher for generous space and equipment support for the first replication of the study. This research was supported in part by NIH grants MH67252 and DK085721 to G.D.P.
Abbreviations
- ANOVA
analysis of variance
- CR
conditioned response
- CS
conditioned stimulus
- DAPI
4′,6-diamidino-2-phenylindole
- IEG
immediate early gene
- KPBS
potassium phosphate-buffered saline
- MCH
melanin-concentrating hormone
- MCH-1R
MCH receptor1
- NPY
neuropeptide Y
- ORX
orexin/hypocretin
- US
unconditioned stimulus
References
- Ammar AA, Sederholm F, Saito TR, Scheurink AJW, Johnson AE, Södersten P. NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1627–R1633. doi: 10.1152/ajpregu.2000.278.6.R1627. [DOI] [PubMed] [Google Scholar]
- Baldo BA. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAa receptor-mediated inhibition of the nucleus accumbens shell, but not exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlaping distribution of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Herberg LJ. Relative effects of nutritional deficit and deprivation period on rate of electrical self-stimulation of lateral hypothalamus. Nat Neurosci. 1968;219:627–628. doi: 10.1038/219627a0. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes. 2009;33:693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103:51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, de Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/Orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, ffrench-Mullen JMH, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Wolinsky TD. Chronic food restriction and weight loss produce opioid facilitation of perifornical hypothalamic self-stimulation. Brain Res. 1993;607:141–148. doi: 10.1016/0006-8993(93)91499-i. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee G, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzerald ME, Benoit SC. The role of orexin- A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, Horvath TL, Kalra PS, Kalra SP. Evidence of NPY receptor involvement in food intake elicited by orexin A in sated rats. Peptides. 2000;21:1557–1560. doi: 10.1016/s0196-9781(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Increased food intake by neuropeptide Y is due to an increased motivation to eat. Peptides. 1991;12:1329–1332. doi: 10.1016/0196-9781(91)90215-b. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nat Neurosci. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard ER, Khan AM, Grewal RS, Mouradi B, Wolfsohn SD, Stanley BG. The second messenger cAMP elicits eating by an anatomically specific action in the perifornical hypothalamus. J Neurosci. 1998;18:2646–2652. doi: 10.1523/JNEUROSCI.18-07-02646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience. 2003;122:541–550. doi: 10.1016/j.neuroscience.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Hahn JD. Comparison of melanin-concentrating hormone and hypocretin-orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2010;468:12–17. doi: 10.1016/j.neulet.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev. 2010;64:14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nat Neurosci. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;161:751–756. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Psychol Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic intercation between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MR, Horvath TL, Kalra PS, Kalra SP. Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regil Pept. 2000;87:19–24. doi: 10.1016/s0167-0115(99)00102-0. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Sherwood A, Adamantidis A, Millan M, Gallagher M, Holland PC. Melanin concentrating hormone influences cue driven food intake under conditions of satiety. Society for Neuroscience Abstracts. 2011 [Google Scholar]
- Khan AM, Cheung HH, Gillard ER, Palarca JA, Welsbie DS, Gurd JW, et al. Lateral hypothalamic signaling mechanisms underlying feeding stimulation: differential contributions of Src family tyrosine kinases to feeding triggered either by NMDA injection or by food deprivation. J Neurosci. 2004;24:10603–10615. doi: 10.1523/JNEUROSCI.3390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26:7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Stanley BG. NMDA receptors mediate feeding elicited by neuropeptide Y in the lateral and perifornical hypothalamus. Brain Res. 2005;1063:1–18. doi: 10.1016/j.brainres.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Specificity of hypothalamic peptides in the control of behavioral and physiological procesesses. Ann N Y Acad Sci. 1994;739:12–35. doi: 10.1111/j.1749-6632.1994.tb19804.x. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaistis JW, Kulkarni R, Kokkotoy E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martins PJF, Marques MS, Tufik S, D’Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am J Physiol Endocrinol Metab. 2010;298:E726–E734. doi: 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed region of frontal cortex. J Neurosci. 2011;31:3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nat Neurosci. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi M, Sato M, Taminato T. Neuropeptide Y in central control of feeding and interactions with Orexin and Leptin. Endocrine. 2001;14:269–273. doi: 10.1385/ENDO:14:2:269. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Petrovich GD. Learning and the motivation to eat: forebrain circuitry. Physiol Behav. 2011;104:582–589. doi: 10.1016/j.physbeh.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007a;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007b;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nat Neurosci. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Characterization of the basolateral amygdala pathways to the medial prefrontal cortex and lateral hypothalamus in rats. Society for Neuroscience Abstracts 2011 [Google Scholar]
- Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012a;59:437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Neuroanatomical evidence for a topographically organized forebrain network composed of the amygdala, medial prefrontal cortex, and lateral hypothalamus in rats. Society for Neuroscience Abstracts 2012b [Google Scholar]
- Rodgers RJ. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96:71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–252. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010a;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010b;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nat Neurosci. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. The Nissl stain. In: Wouterlood FG, editor. Neuroscience Protocols. Amsterdam, New York: Elsevier; 1993. pp. 93-050-12-01–93-050-12-07. [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes. 2009;33:S44–S48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s) Brain Res. 1993;604:304–317. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104:40–46. doi: 10.1016/j.physbeh.2011.04.046. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A laboratory guide with printed and electronic templates for data, models and schematics. Amsterdam: Elsevier; 2004. Brain maps: structure of the rat brain. [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology. 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol. 2007;502:768–782. doi: 10.1002/cne.21316. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wise RA. Lateral hypothalamic electrical stimulation: does it make animals “hungry”? Brain Res. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai A, Matsuzaki I, et al. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell T. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol. 2003;284:R1436–1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]