Abstract
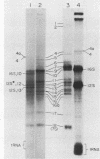
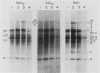
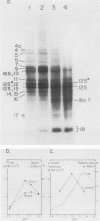
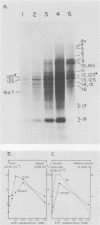
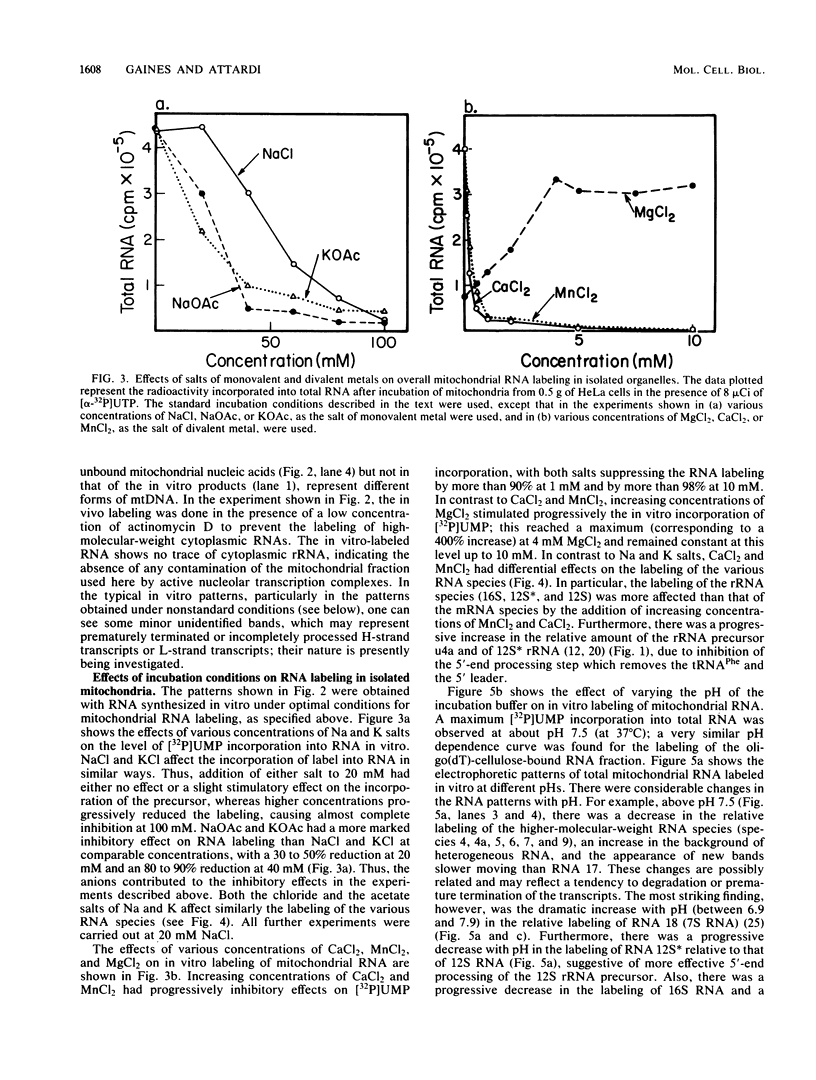
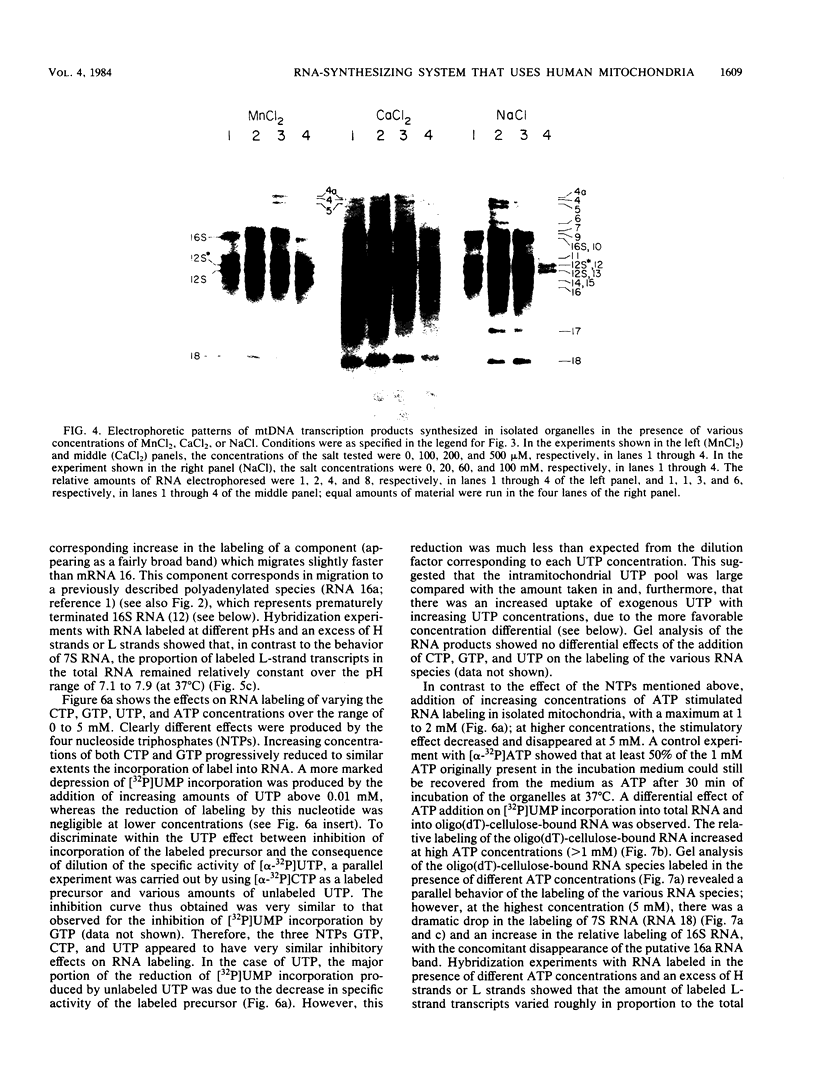
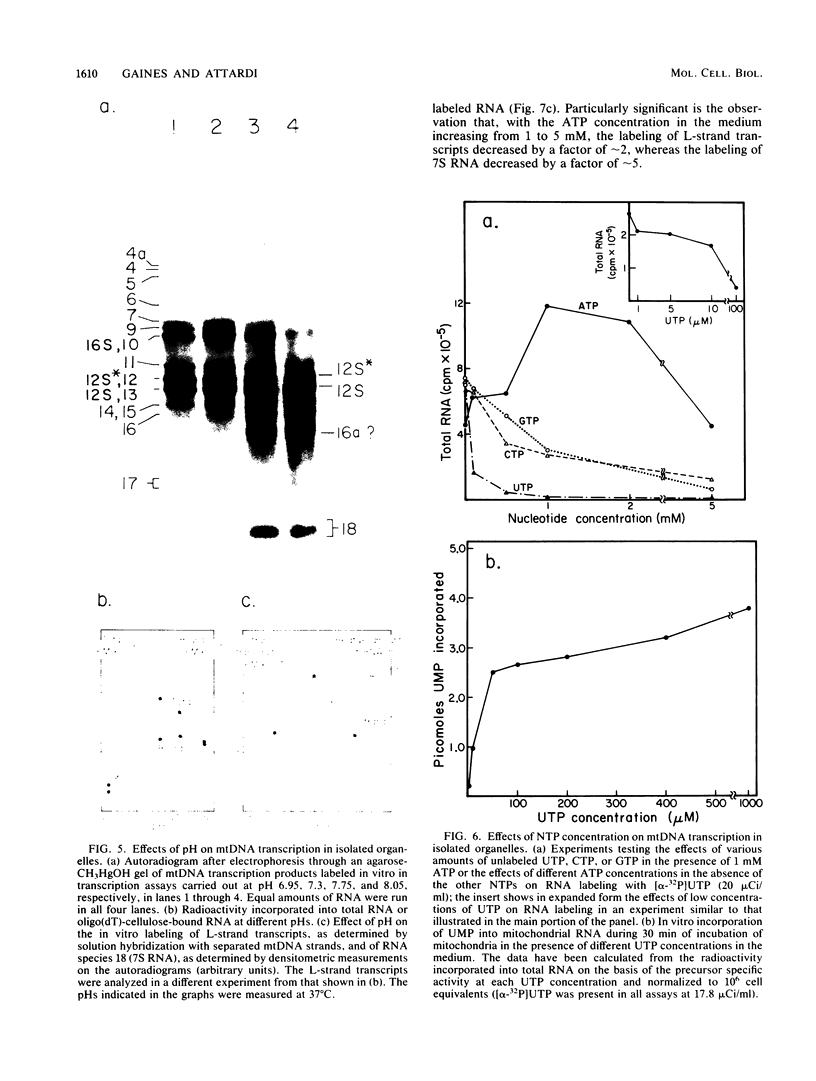
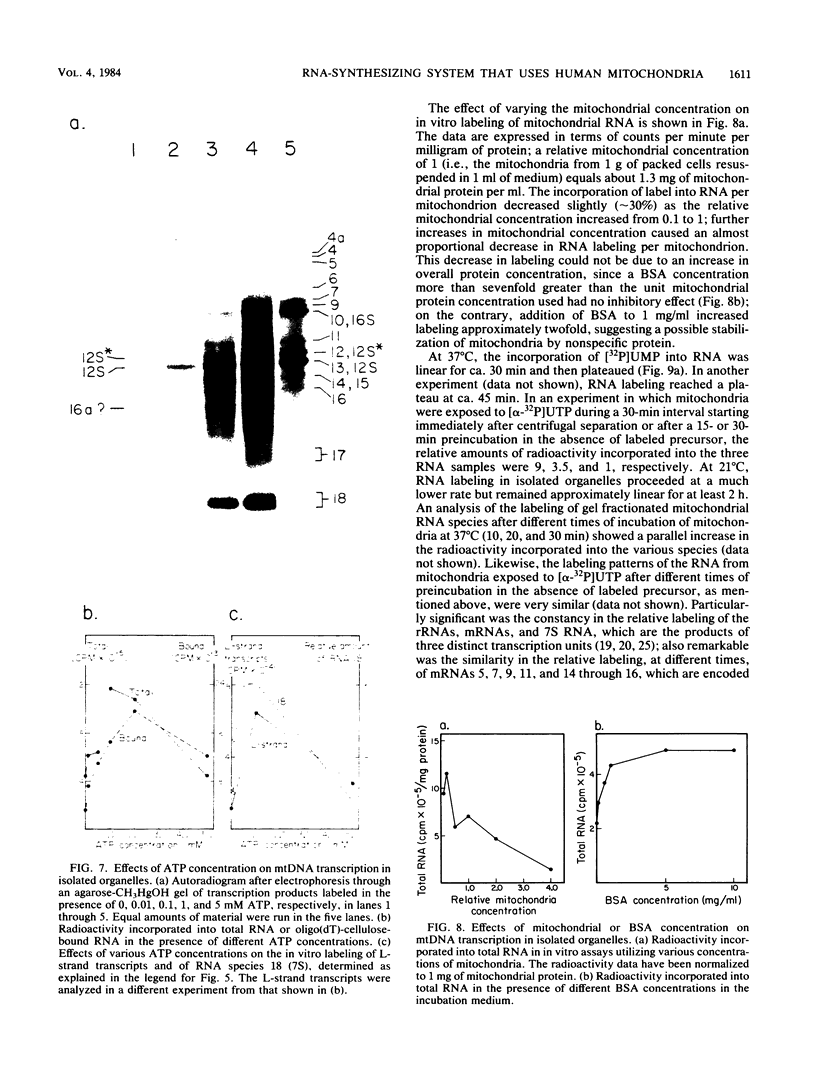
A highly efficient RNA-synthesizing system with isolated HeLa cell mitochondria has been developed and characterized regarding its requirements and its products. In this system, transcription is initiated and the transcripts are processed in a way which closely reproduces the in vivo patterns. Total RNA labeling in isolated mitochondria proceeds at a constant rate for about 30 min at 37 degrees C; the estimated rate of synthesis is at least 10 to 15% of the in vivo rate. Polyadenylation of the mRNAs is less extensive in this system than in vivo. Furthermore, compared with the in vivo situation, rRNA synthesis in vitro is less efficient than mRNA synthesis. This is apparently due to a decreased rate of transcription initiation at the rRNA promoter and probably a tendency also for premature termination of the nascent rRNA chains. The 5'-end processing of rRNA also appears to be slowed down, and it is very sensitive to the incubation conditions, in contrast to mRNA processing. It is suggested that the lower efficiency and the lability of rRNA synthesis and processing in isolated mitochondria may be due to cessation of import from the cytoplasm of ribosomal proteins that play a crucial role in these processes. The formation of the light-strand-coded RNA 18 (7S RNA) is affected by high pH or high ATP concentration differently from the overall light-strand transcription. The dissociation of the two processes may have important implications for the mechanism of formation and the functional role of this unusual RNA species. The high efficiency, initiation capacity, and processing fidelity of the in vitro RNA-synthesizing system described here make it a valuable tool for the analysis of the role of nucleocytoplasmic-mitochondrial interactions in organelle gene expression.
Full text
PDF
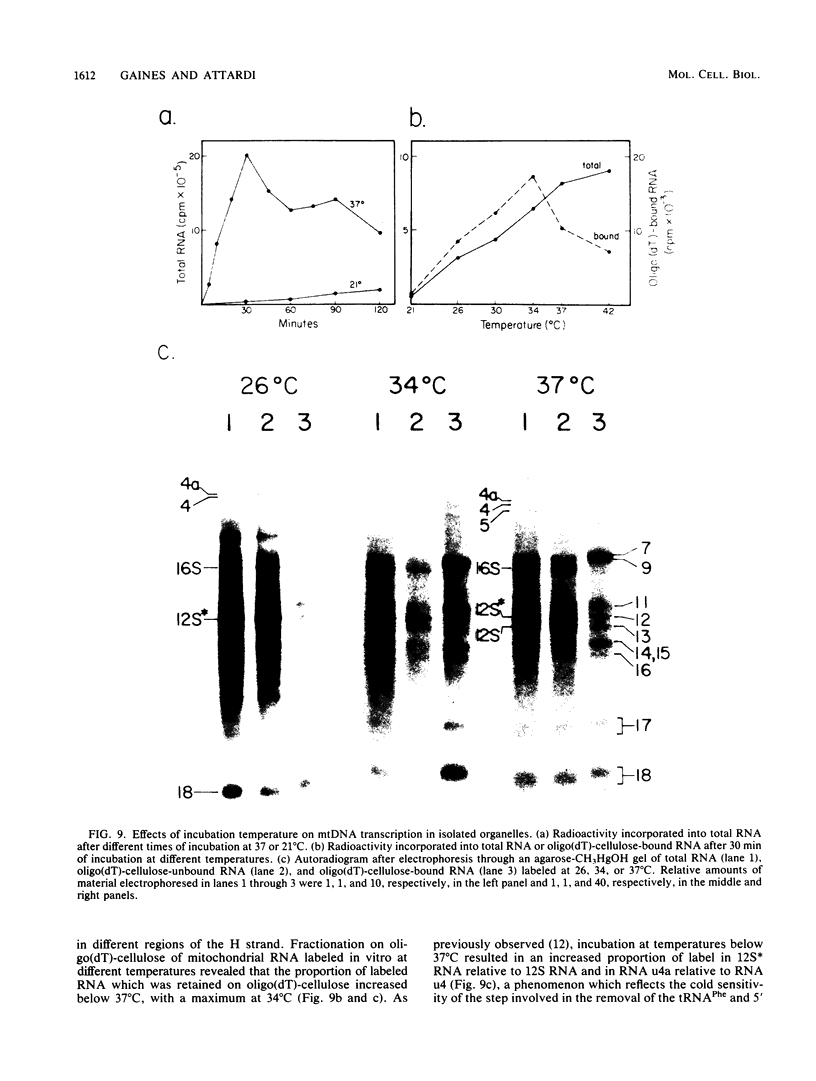

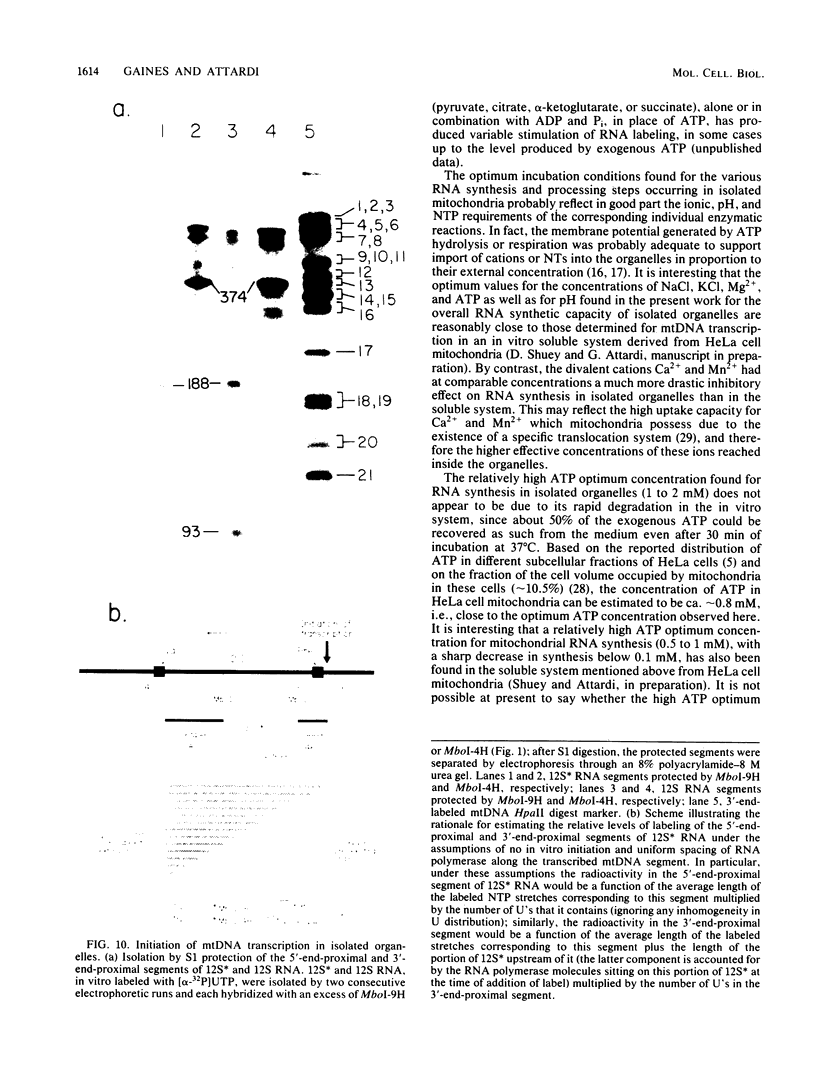



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Merkel C., Gelfand R., Attardi G. Fractionation of mitochondrial RNA from HeLa cells by high-resolution electrophoresis under strongly denaturing conditions. J Mol Biol. 1978 Jan 5;118(1):1–25. doi: 10.1016/0022-2836(78)90241-3. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Attardi B., Attardi G. Expression of the mitochondrial genome in HeLa cells. I. Properties of the discrete RNA components from the mitochondrial fraction. J Mol Biol. 1971 Jan 28;55(2):231–249. doi: 10.1016/0022-2836(71)90194-x. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Moffett G. L., Mathews C. K. Selective expansion of mitochondrial nucleoside triphosphate pools in antimetabolite-treated HeLa cells. J Biol Chem. 1982 Aug 25;257(16):9300–9304. [PubMed] [Google Scholar]
- Boerner P., Mason T. L., Fox T. D. Synthesis and processing of ribosomal RNA in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6379–6390. doi: 10.1093/nar/9.23.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner P., Mason T. L., Fox T. D. Synthesis and processing of ribosomal RNA in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6379–6390. doi: 10.1093/nar/9.23.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., Attardi G. Mapping of nascent light and heavy strand transcripts on the physical map of HeLa cell mitochondrial DNA. Nucleic Acids Res. 1980 Jun 25;8(12):2605–2625. doi: 10.1093/nar/8.12.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Attardi G. The sequences of the small ribosomal RNA gene and the phenylalanine tRNA gene are joined end to end in human mitochondrial DNA. Cell. 1980 Mar;19(3):775–784. doi: 10.1016/s0092-8674(80)80053-5. [DOI] [PubMed] [Google Scholar]
- Crews S., Ojala D., Posakony J., Nishiguchi J., Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979 Jan 18;277(5693):192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Montoya J., Timko K. D., Attardi G. Sequence analysis and precise mapping of the 3' ends of HeLa cell mitochondrial ribosomal RNAs. J Mol Biol. 1982 May 5;157(1):1–19. doi: 10.1016/0022-2836(82)90510-1. [DOI] [PubMed] [Google Scholar]
- Gaines G., Attardi G. Intercalating drugs and low temperatures inhibit synthesis and processing of ribosomal RNA in isolated human mitochondria. J Mol Biol. 1984 Feb 5;172(4):451–466. doi: 10.1016/s0022-2836(84)80017-0. [DOI] [PubMed] [Google Scholar]
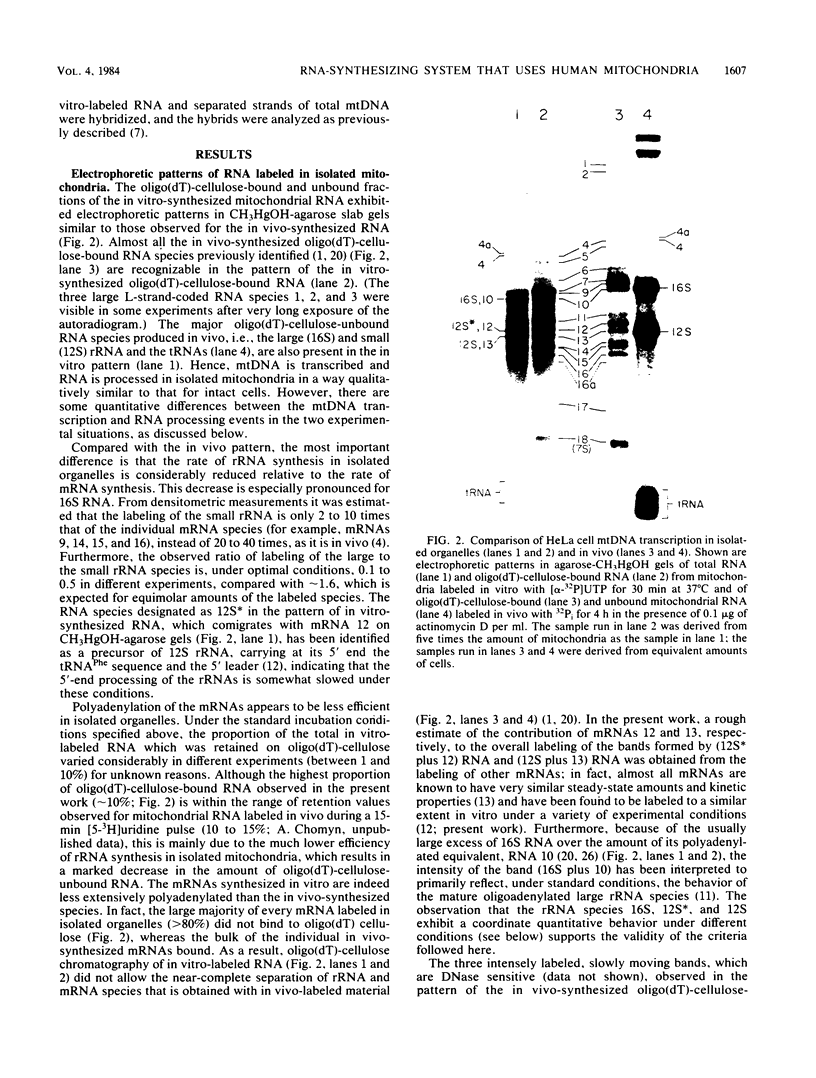
- Gelfand R., Attardi G. Synthesis and turnover of mitochondrial ribonucleic acid in HeLa cells: the mature ribosomal and messenger ribonucleic acid species are metabolically unstable. Mol Cell Biol. 1981 Jun;1(6):497–511. doi: 10.1128/mcb.1.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Ching E., Attardi G. Isolation, subunit composition, and site of synthesis of human cytochrome c oxidase. Biochemistry. 1980 May 13;19(10):2023–2030. doi: 10.1021/bi00551a003. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- Hirsch M., Penman S. Mitochondrial polyadenylic acid-containing RNA: localization and characterization. J Mol Biol. 1973 Nov 5;80(3):379–391. doi: 10.1016/0022-2836(73)90410-5. [DOI] [PubMed] [Google Scholar]
- Montoya J., Christianson T., Levens D., Rabinowitz M., Attardi G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7195–7199. doi: 10.1073/pnas.79.23.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J., Gaines G. L., Attardi G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell. 1983 Aug;34(1):151–159. doi: 10.1016/0092-8674(83)90145-9. [DOI] [PubMed] [Google Scholar]
- Montoya J., Ojala D., Attardi G. Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs. Nature. 1981 Apr 9;290(5806):465–470. doi: 10.1038/290465a0. [DOI] [PubMed] [Google Scholar]
- Newman D., Martin N. Synthesis of RNA in isolated mitochondria from Saccharomyces cerevisiae. Plasmid. 1982 Jan;7(1):66–76. doi: 10.1016/0147-619x(82)90028-2. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Expression of the mitochondrial genome in HeLa cells. XIX. Occurrence in mitochondria of polyadenylic acid sequences, "free" and covalently linked to mitochondrial DNA-coded RNA. J Mol Biol. 1974 Jan 15;82(2):151–174. doi: 10.1016/0022-2836(74)90338-6. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Fine mapping of the ribosomal RNA genes of HeLa cell mitochondrial DNA. J Mol Biol. 1980 Apr;138(2):411–420. doi: 10.1016/0022-2836(80)90296-x. [DOI] [PubMed] [Google Scholar]
- Ojala D., Crews S., Montoya J., Gelfand R., Attardi G. A small polyadenylated RNA (7 S RNA), containing a putative ribosome attachment site, maps near the origin of human mitochondrial DNA replication. J Mol Biol. 1981 Aug 5;150(2):303–314. doi: 10.1016/0022-2836(81)90454-x. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., England J. M., Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol. 1977 Aug;74(2):468–491. doi: 10.1083/jcb.74.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. In vitro transcription of human mitochondrial DNA. Identification of specific light strand transcripts from the displacement loop region. J Biol Chem. 1983 Jan 25;258(2):1268–1275. [PubMed] [Google Scholar]