Abstract
Skeletal bone consists of hydroxyapatite (HA) [Ca10(PO4)6(OH)2] and collagen type I, both of which are osseoconductive. The goal of osseointegration of orthopedic and dental implants is the rapid achievement of a mechanically stable long-lasting fixation between bone and an implant surface. In this study, we evaluated the mechanical fixation and tissue distribution surrounding implants coated with three surfaces: plasma-sprayed HA coating, thinner coating of electrochemical-assisted deposition of HA, and an identical thin coating with a top layer of mineralized collagen. Uncoated plasma-sprayed titanium (Ti-6Al-4V) served as negative control. The electrochemical-assisted deposition was performed near physiological conditions. We used a canine experimental joint replacement model with four cylindrical implants (one of each treatment group) inserted in the humeri cancellous metaphyseal bone in a 1 mm gap. Observation time was 4 weeks. The mechanical fixation was quantified by push-out test to failure, and the peri-implant tissue formation by histomorphometric evaluation. HA coatings deposited by plasma spray technique or electrochemically, increased the mechanical fixation and bone ongrowth, but there was no statistical difference between the individual HA applications. Addition of collagen to the mineralized phase of the coating to create a more bone natural surface did not improve the osseoconductive effect of HA.
Keywords: hydroxyapatite, electrochemistry, implants, osseointegration, materials testing
INTRODUCTION
Early implant fixation securing stability is critical in obtaining good long-term success of total hip replacement.1 This is particulary important when bone healing is compromised or initial stability is more delicate.
In the last decades, coatings of hydroxyapatite (HA) have been introduced as a means to improve the fixation of implants. HA is highly osseoconductive and the positive effect is well documented both experimentally and clinically.2–4 The osseoconductive properties of HA coatings depend on crystallinity, solubility and stability,5 and on thickness and electrical polarization of the HA surface,6 which all vary with method of deposition. Traditionally, HA has been coated on a core metal implant by the technique of plasma spraying. This technique demands high temperatures, results in coating thickness above 50 µm, has a potential risk of debonding from the implant, may cause partial decomposition with formation of heterogeneous calcium phosphates,7 and may result in large variations in coating thickness especially on porous surfaces. In this case, HA will fill small pores rather than follow the contours of the surface.
The principle of the electrochemical-assisted calcium phosphate deposition on implants is based on the pH-dependent solubility of calcium phosphate. Under cathodic polarization of the implant the hydrolysis of water leads to an increase of the pH close to the sample surface, so that the solubility limit is reached and calcium phosphate precipitation takes place. It possesses several theoretical advantages over the plasma-sprayed HA. Because it is not a line-of-sight method it achieves full coverage of the entire porous coating of an implant, evoking a complete topographical stimulus. This could be important to the creation of an enhanced biological seal against potential migration of polyethylene wear debris from the joint surface of a joint replacement prosthesis into the peri-implant space.8–10 The osseoconductive effect of HA deposited electrochemically on implants has shown promise to enhance the potential for implant integration.11–16 In the dental field it has only recently been introduced,11,13,14,17 but in orthopedics its use is infrequent. In one in vivo study by Costa et al.,13 dental screw implants of pure titanium were coated with a 4–8 µm HA by electrophoresis followed by at 800°C for 2 h. Implants were inserted in rabbit tibiae for 8 or 12 weeks and histomorphometric analysis showed significantly greater bone-implant contact for the HA-coated implants, compared with the titanium implants. Thus far, HA layers have been produced with electrochemical methods only under conditions with temperatures (60–800°C) and composition fare from physiological.
Another alternative to the traditional plasma-sprayed HA coating is biomimetic coatings. The concept of the biomimetic coatings is to combine the osseoconductivity of HA with biological molecules that stimulate osteoblast activity. In vitro and in vivo studies have shown a positive response of collagen type I on osteoblast adhesion and bone integration of implants.14,18–23 Therefore, the fixation of titanium implants might be enhanced by using a composite coating, which combines collagen type I with HA deposited electrochemically under physiological nondegrading conditions.
In this study, we evaluated the mechanical fixation and tissue response to implants coated with the well-documented plasma-sprayed HA coating, and two new alternative thinner HA coatings: one with electrochemical-assisted deposited HA and one biomimetic coating combining cold electrochemical-assisted deposition of HA and mineralized collagen type I. All three HA coatings were placed on a porous coated plasma-sprayed titanium implant. The similar plasma-sprayed titanium implant served as control without any HA and with the core surface coating as prothesis for clinical applications. We used a well established experimental canine model for joint replacement with cylindrical implants inserted in the humerus cancellous metaphyseal bone. We hypothesized that all HA coatings would achieve improved mechanical fixation and osseointegration compared with the titanium control implant. We expected to find similar fixation and osseointegration between the traditional plasma-sprayed HA and the two electrodeposited HA coatings.
METHODS
Implants
Four different implant surfaces were investigated: (1) plasma spray titanium alloy (P-Ti) serving as control; (2) plasma spray HA coating (P-HA); (3) electrochemical-assisted HA coating (E-HA); and (4) electrochemical-assisted HA mineralized-collagen coating (E-HA-Col). The P-HA implants were fabricated by Biomet Inc., Warsaw, IN, USA, and the two electrochemical-assisted implants by Biomet Deutschland GmbH, Berlin, Germany.
The implants used for control and HA coatings were cylindrical plasma-sprayed Ti-6Al-4V, with a nominal diameter of 6 mm and a length of 10 mm. A mean surface roughness (Ra) of 47 µm and a maximum roughness depth (Ry) 496 µm have previously been reported.2 The HA plasma spray implants (P-HA) were prepared using same standard HA plasma spraying technique, as for the manufacturers clinical applications. Their specifications were according to manufacturer a 50-µm thick HA coating (Ca/P ratio 1.67), a mean surface roughness (Ra) of 41 µm, a maximum roughness depth (Ry) of 445 µm, and 62% HA. The electrochemical-assisted HA deposition (E-HA) was done by a time (75 min), temperature (36°C), and pH (6.4) controlled process in an electrolyte solution consisting of 1.67 mM CaCl2 and 1 mM NH4H2PO4 in equal volumes with the sample polarized in cathode galvanostatic mode (−75 A/m2) (as by Rössler with time modification).24 The layer consisted of 70% crystalline HA with the balance being amorphous and a thickness of 5 µm (Ca/P ratio 2.0). Coating preparations, characteristics, and appearances are as described in detail by Rössler et al.24 and Sewing et al.25 The mineralized collagen coating was based on the HA layer of the E-HA implant. An additional and exactly similar mineralization step was applied (t = 15 min) after adsorption of fibrillar collagen (bovine collagen type I, Fluka Seelze, Germany). The collagen coating was applied by incubating implants in collagen supernatant at 4°C for 15 min, as described and characterized in detail by Geissler et al. and Roessler et al.19,26 As a result of the process the surface layer of the coating is formed by a network of completely mineralized collagen fibrils. The addition of collagen increased coating thickness with less than 10%, whereas Ca/P ratio and crystallinity were unchanged. Data on the three HA coating preparations and characteristics were provided by manufacturer of the coatings.
Study design
An experimental canine study was done after approval by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation. We used eight skeletally mature purpose bred American Hound Dogs with a mean age of 15.2 months (range 12.4–20.7) and mean weight 22.9 kg (range 20.5–26.7).
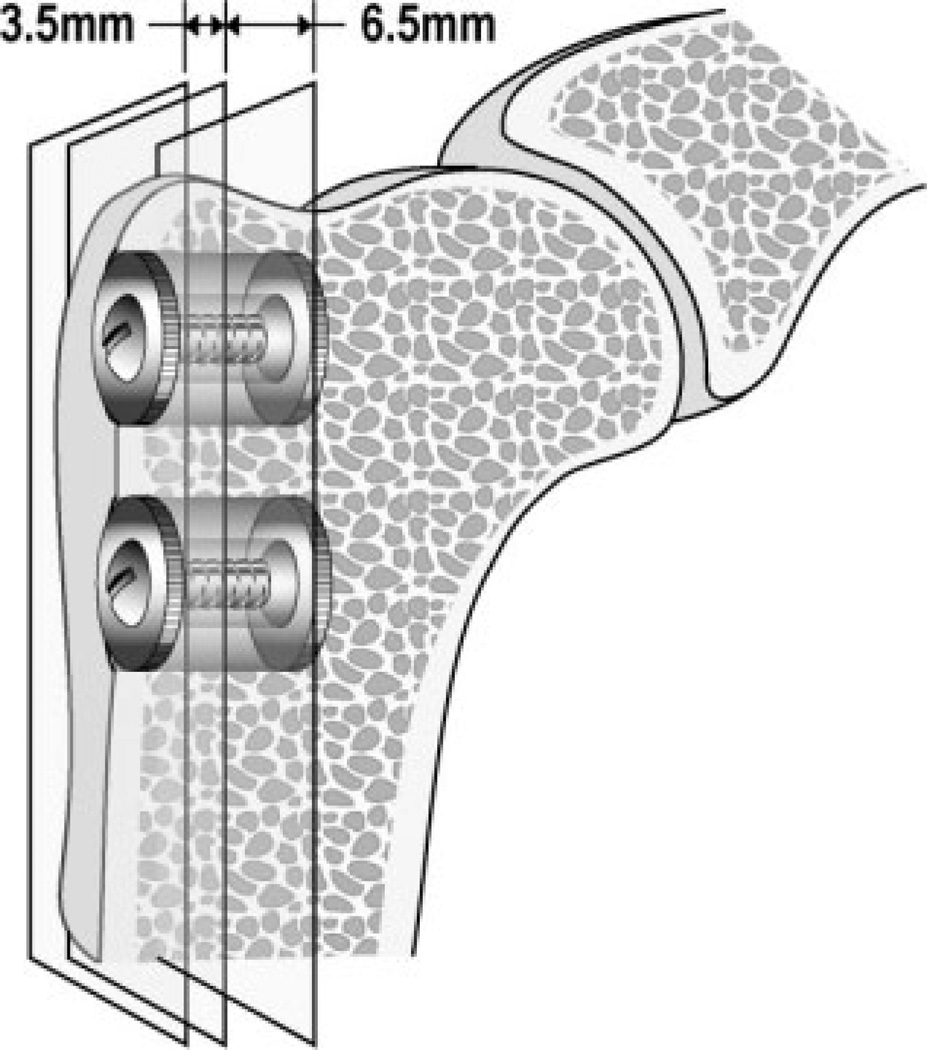
Each animal received two implants in the extra-articular bone of each proximal humerus; four total implants per animal (Fig. 1). Implants were altered between the proximal and distal implant bed with the two electrochemical-assisted HA implants on one side and plasma-sprayed HA and titanium contralaterally. The implants were surrounded by a 1.0 mm annular gap in cancellous bone. The observation period was 4 weeks.27
Figure 1.
Test implant device of titanium alloy (Ti6Al4V) (6 mm × 10 mm) inserted in proximal humerus, extraarticular, unloaded, in cancellous bone, and in a 1-mm gap. Vertical planes indicating section lines during preparation.
Surgery
Surgery was performed under sterile conditions and general anesthesia. The implantation site was exposed through an anterolateral skin incision and blunt dissection medial to the deltoid muscle fascia. The periosteum was elevated at the implant insertion area. Over a 2.5-mm guide wire, a cannulated drill (Ø 8.0 mm) was used to drill a 12-mm deep cylindrical cavity, perpendicular to the bone surface and a distance of 2 and 17 mm from the greater tuberosity. To avoid thermal trauma to the bone, the speed was less than 2 Hz. The implant was mounted with an 8-mm top and bottom washer, thereby creating a central positioning in the circumferential gap of 1.0 mm. The overlying soft tissue was closed in layers. X-ray was obtained postoperatively to ensure correct implant position. Prophylactic antibiotic (Rocephin, Roche Pharmaceuticals) was administered preoperatively and for the first 3 days. All animals were allowed unrestricted weight bearing after surgery and daily exercise periods. The animals were fed a nutritionally complete and balanced commercial standard diet. The animals were daily observed for changes in weight bearing and sign of pain or infection. At the end of the 4-week observation period, the proximal humeri with the implants in situ were removed and stored at −20°C until specimen preparation and mechanical testing.
Specimen preparation
Specimen preparation was done blinded. Sections were cut perpendicular to the implant axis (Fig. 1), using a water-cooled diamond band saw and implant based alignment post (Exact Appertebau, Nordenstedt, Germany). The exterior 3.5-mm section was used for mechanical testing and stored until testing at −20°C. The interior 6.5-mm section was used for undecalcified histomorphometric evaluation. The specimen was dehydrated in graded ethanol (70–100%) containing 0.4% basic fuchsine, (Merck, Darmstadt, Germany) followed by embedding in polymethylmethacrylate (PMMA, Merck, Hohenbrunn, Germany). The vertical section technique was applied with random rotation of the specimen blocks,28 and serially sectioned to 20 µm parallel to the implant axis using a microtome (Leiden Microtome KDG-95-TM, Leiden, Holland). Four serial specimens were obtained from the center of the implant. After specimen sectioning, the surface was counterstained with 2% light green (BDH Laboratory Supplies, Poole, England).29 The staining technique stains bone green and nonmineralized tissue red. This makes it possible to visualize mineralized bone, fibrous tissue, and marrow-like tissue.
Mechanical testing
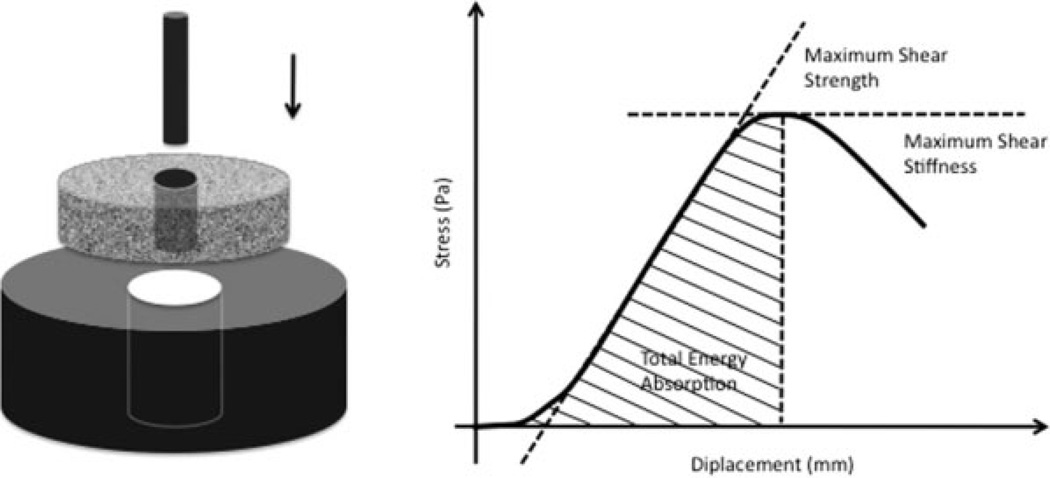
Implants were tested to failure by axial push-out test (Instron Universal Test Machine, Instron Ltd., High Wycombe, U.K.) (Fig. 2). Testing was done blinded. The specimens were placed on a metal support jig and the implant centered over a 7.4-mm circular opening. Centralizing the implant over the opening assured a 0.7 mm distance between the implant and support jig. A preload of 2N defined the contact position for starting the test. A displacement velocity of 5 mm/min was used, and load-displacement data were recorded. Data are presented as ultimate shear strength (MPa) determined from the maximum force applied until failure of the bone-implant interface, as apparent shear stiffness (MPa/mm) obtained from the slope of the straight line part of the load-displacement curve, and total energy absorption (J/m2) calculated as the area under the load-displacement curve until failure. All push-out parameters were normalized by the cylindrical surface area of the implant section tested (π × diameter × length).
Figure 2.
Mechanical testing. Left: Axial push-out test with specimen placed on metal platform with central opening. Specimen thickness = 3.5 mm, implant θ = 6 mm, support hole θ = 7.4 mm, preload = 2N, Displacement velocity 5 mm/min. Right: Load (Pa) displacement (mm) curve enables calculation of ultimate shear strength (MPa), apparent shear stiffness (MPa/mm), and total energy absorption (J/m2).
Histological analysis
Bone ongrowth at implant surface and peri-implant bone density was quantified by static histomorphometry. Computer assisted histomorphometry was performed blinded in random specimen order with light microscope (Olympus BX51 TF, Olympus Denmark) and image analysis system (CAST-Grid, Version 2.1.4, Olympus Denmark) at magnification 400×. Stereological methods were applied using systematic uniform random sampling in order to obtain unbiased estimates.30 Tissue ongrowth was defined as tissue directly at the implant surface, and was determined using the linear intercept technique with random placement of sine weighted lines. Peri-implant tissue density was achieved using point counting in divided zones of 0–500 µm and 500–1000 µm from the implant surface with random point disposition. Each zone was evaluated in independent counting sessions. Bone ongrowth and peri-implant density is expressed as tissue percentage and tissue volume percentage respectively.
Statistical analysis
The STATA Intercooled 8.0 statistical software was used. As results did not follow a normal distribution a nonparametric analysis was carried out. The variance between groups was tested using ANOVA on ranks. Then the differences between the implant types were analyzed using the Wilcoxon sign ranks test. Two tailed p < 0.05 were considered statistically significant.
RESULTS
Surgery
During surgery, one animal was excluded as an incorrect drill size was inadvertently used. All animals were ambulated the same day of surgery and fully weight bearing after 1 day of recovery. There were no postoperative complications, weight loss, or clinical infections. No bacterial growth was detected in joint fluid culture obtained at euthanasia. All animals completed the 4-week observation period.
Mechanical testing
Results of the push-out test are summarized in Table I. The mechanical fixation was significantly higher for plasma-sprayed HA compared with titanium and comparing HA with collagen (p < 0.05). Electrochemical-deposited HA showed significantly increased mechanical fixation compared with titanium, but not to HA with collagen. No difference between plasma-sprayed and electrochemically deposited HA was observed. The fixation of the titanium control implant was inferior as four of seven implants were clinically loose before mechanical testing.
TABLE I.
Mechanical Testing
| Ultimate Shear Strength (MPa) |
Apparent Shear Stiffness (MPa/mm) |
Total Energy Absorption (J/m2) |
|
|---|---|---|---|
| P-Ti | 0.0 (0.0–0.3) | 0.0 (0.0–1.3) | 0 (0–45) |
| P-HA | 2.1 (1.5–3.2)a,b | 7.8 (4.2–17.9)a,b | 535 (213–574)a,b |
| E-HA | 2.0 (0.7–3.4)a | 9.0 (2.7–16.6)a | 339 (92–618)a |
| E-HA-Col | 0.5 (0.1–2.1) | 2.5 (0.6–8.9) | 73 (7–456) |
Data presented as median and interquartile ranges. n = 7.
P-Ti, plasma spray titanium alloy; P-HA, plasma spray hydroxyapatite coating; E-HA, electrochemical-assisted hydroxyapatite coating; E-HA-Col, electrochemical-assisted hydroxyapatite mineralised collagen coating.
p < 0.05 compared with P-Ti.
p < 0.05 compared with E-HA-Col.
Histology
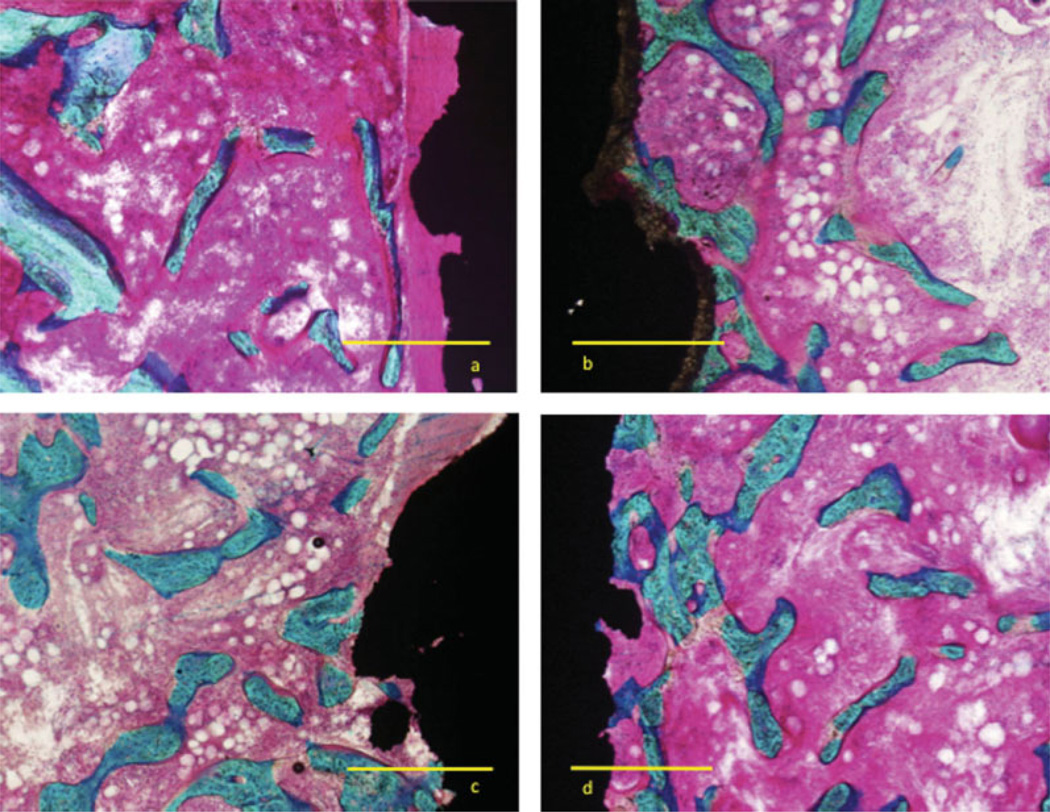
At 4 weeks, all implants displayed bone formation expanding into most of the gap [Fig. 3(a–d)]. The titanium implants more often showed a dense fibrous band along the implant surface with strands of new bone running along fibrous tissue and bounded by marrow. The bone formation of the P-HA displayed trabeculae radiating perpendicular to the implant making surface contact as either a pseudopodia projection or a more flattened configuration. The two electrochemically HAs displayed bone contact as punctual contacts or conglomerate of these with less radiating character of the gap trabeculae. No apparent visual difference in trabecular size was apparent among implants. The HA coating of the P-HA was still apparent with a thickness of 30–50 µm, but not visible for the electrochemical-HA coatings.
Figure 3.
Micrograph four weeks after insertion of plasma-sprayed titanium implant inserted in cancellous bone in 1-mm gap. Coating (a) plasma-spray titanium, (b) plasma-spray HA, (c) electrochemically deposited HA, and (d) electrochemically deposited HA mineralized collagen. Images showing area with bone ongrowth. Bar equals 500 µm. Basic fuchsine/Light Green stain. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Histomorphometry
Results from histomorphometry are summarized in Table II. All HA coatings showed significantly increased bone ongrowth compared with the titanium control (p < 0.05). The bone density at the interface of the two electrochemically deposited HAs was significantly increased by 6-fold compared with the plasma-sprayed titanium, and 17-fold for the plasma-sprayed HA. There was no significant difference in bone ongrowth in the three HAs. The surface of titanium implants was dominated by fibrous tissue anchorage.
TABLE II.
Histomorphometry
| Bone | Marrow | Fibrous tissue | |
|---|---|---|---|
| Ongrowth | |||
| P-Ti | 3 (0–3) | 33 (21–50) | 56 (45–76) |
| P-HA | 51 (20–62)a | 33 (10–35) | 15 (2–71) |
| E-HA | 18 (5–25)a | 54 (32–68)b | 21 (17–64)a |
| E-HA-collagen | 21 (13–27)a | 53 (36–69)b | 21 (1–55) |
| 0–500 µm | |||
| P-Ti | 11 (10–16) | 71 (52–77) | 13 (12–17) |
| P-HA | 18 (11–27) | 65 (61–69) | 9 (1–27) |
| E-HA | 24 (11–28)a | 67 (51–75) | 11 (4–16) |
| E-HA-collagen | 19 (14–32) | 67 (58–79) | 4 (1–12)a |
| 500–1000 µm | |||
| P-Ti | 11 (6–18) | 80 (72–88) | 0.1 (0–0.7) |
| P-HA | 13 (10–29) | 81 (69–85) | 0 (0–0.2) |
| E-HA | 19 (14–22)a | 75 (72–83) | 0 (0–0.5) |
| E-HA-collagen | 19 (14–27) | 76 (62–79) | 0.3 (0–2) |
Density (percentage) of bone, marrow-like and fibrous tissue at the implant surface, concentric zone 0–500 µm and 500–1000 µm.
Median, interquartile ranges. n = 7.
P-Ti, plasma spray titanium alloy; P-HA, plasma spray hydroxyapatite coating; E-HA, electrochemical-assisted hydroxyapatite coating; E-HA-Col, electrochemical-assisted hydroxyapatite mineralised collagen coating.
p < 0.05 compared with titanium.
p < 0.05 compared with HA plasma.
In the peri-implant concentric zones of 0–500 and 500–1000 µm, only electrodeposited HA had significantly higher bone volume than titanium implants. We did not observe any significant differences in the HAs.
DISCUSSION
Skeletal bone consists of HA [Ca10(PO4)6(OH)2] and collagen type I as the major inorganic and structural organic component, both being osseoconductive. The goal of osseointegration of orthopedic implants is the rapid achievement of a mechanically stable and long-lasting fixation between living bone and the implant surface. The electrochemical-assisted deposition of HA on implants may be more favorable than the plasma-sprayed HA in being three-dimensional in coverage, more preservative of implant roughness, less thick reducing potential coating failure and third-body wear, and being simple and inexpensive in manufacturing.24
The aim of our study was to determine whether a positive bone response was observed around implants with various coatings. It was theorized that a more bone-like osseoconductive composition at the implant surface could be made by using HA with a physiologic biomimetic coating process like the electrochemical-assisted deposition, and also by the addition of the main organic bone component collagen type I to the HA. A noncoated plasma-sprayed titanium implant was used as control, and compared with plasma-sprayed HA coating similar to clinical applications and two new coatings with HA electrodeposited. The electrodeposited HA coatings consisted of (1) HA alone and (2) with mineralized collagen added. We quantified the mechanical fixation by push-out test to failure and the peri-implant tissue formation by histomorphometric evaluation. We hypothesized that the deposition of HA electrochemically acquired improved fixation compared with uncoated implants and similar to plasmasprayed HA. The hypothesis was confirmed in all cases except for mechanical testing on E-HAcollagen.
We used an established experimental canine model2,31 being advantageous in representing the cancellous bone region critical for ingrowth in cementless fixation of orthopedic prosthesis in humans. This model evaluates the biological response to the implant surface. The response is quantified by (1) unbiased stereological histomorphometry and (2) mechanical testing at the implant interface of the combined static bone adherent to the implant surface and of the dynamic interlocking of bone along the porous surface during mechanical testing. The canine was chosen as animal testing model, since its bone architecture most closely resembles human.32 The proximal humerus was chosen because of its environment abundant with cancellous bone and implants were altered between the proximal and distal implant bed to minimize site differences. We evaluated the bone healing at the implant surface during the early stages of implant fixation/bone regeneration, choosing an observation time of 4 weeks based on previous studies.27 The surface coatings were all compared within each animal. This reduced sampling size and influence of interindividual factors on bone healing. The implants were inserted in a 1-mm gap based on previous experience.2,27,33 This approaches clinical practice in which implants may have as little as 20% direct contact with bone early after implantation despite intended press-fit insertion34,35 and also variable bone growth (10–13%) into the available space of the porous coating.36,37 Although the study is limited by the implants not being axially weight bearing, the setting reflects the load weight transmission in periarticular bone, weeks after implantation of orthopedic prosthesis. The testing of the mechanical forces on the implants was done by push-out test to failure on a support jig with a clearance of the jig-hole of 0.7 mm. To compare failure loads experimental conditions need to be well controlled in respect to support boundary conditions. The applied clearance was comparable with recommended.2,38,39
In contrast to our hypothesis, only traditional plasma-sprayed HA and electrochemically deposited HA had a significantly improved mechanical fixation compared with the titanium controls. The mechanical fixation of the HA collagen coating was unexpectedly lower. We did observe a rather high degree of variation in all three groups of HA-coated implants, as is typical with in vivo studies. It is however clear that the majority of the HA-collagen implants had poorer fixation than implants with the two other HA coatings. Our ability to implant all four coatings within one animal allows a mean for reducing the statistical consequences of high variation; however, it cannot be totally eliminated. Although the E-HA and P-HA coatings demonstrated differences in surface roughness, we did not observe an influence mechanically.
The differences in tissue distribution between titanium and HA-coated implants was primarily at the interface. The data suggest that the traditional plasma-sprayed HA coating more effectively stimulates bone ongrowth compared with the electrochemically deposited HA coatings (p = 0.06). In the gap, the bone volume was generally higher for all HA coatings compared with the titanium control, but the difference was only statistically significant for electrochemically deposited HA. No major differences in tissue distribution between the three HA coatings were found in the gap. The addition of collagen I to the HA coating did not provide any advantages in terms of stimulation of bone growth.
The histomorphometrical results did not provide an explanation for the lower mechanical fixation of the HA collagen. The failure might have occurred in the interface between HA of the mineralized collagen rather than between the coating and the bone trabeculae. We could, however, not visualize a microstructure of the surface coating during histomorphometry suggesting that it may have been decomposed during the 4-week study period. Another explanation might be that the new bone growth is of different maturity and thereby different mechanical competence. The osseoconductivity of a HA coating depends on it crystallinity and stability.5 Porter et al.40 established that new bone formation on plasma-sprayed HA implants is accelerated in the presence of the HA, and that the rate of the earliest stage bone formation (3 h to14 days) is influenced by the solubility of the HA. Coatings with high crystallinity exhibited delayed new bone formation. In a study by Wang et al.15 bone apposition at 6 h, 7 and 14 days was examined on plasma-sprayed and electrochemically deposited HA coating (85°C, pH 6.0, cathode 1.4V, 2 h, 0.61 mM Ca(NO3)2 mM + 0.36 mM NH4H2PO4). SEM showed that the plasma-sprayed coatings had higher bone apposition ratios than the titanium controls and electrodeposited HAs after 7 days. During the first 7 days, the elektroHA coating made almost no contribution to bone apposition exhibiting the same apposition ratio as bare titanium. Plasma-spray HA with the higher solubility may contribute a much higher local concentration of calcium and phosphorus ions at day 7, which could assist in and accelerate local mineralization of new bone or be involved in cell signaling. At day 14, both HA coatings exhibited similar bone apposition ratios and higher than titanium. In our study, the HA and the addition of collagen to HA alters the coatings morphology and composition.24–26 The differences in coating applications may affect coating kinetics and onset times for mineralization. Finally, at mechanical testing implant fixation is a result of a combined static implant-bone adhesion and dynamic bone-implant interlocking during testing. Although all HA coatings in theory might show the same interlocking-bone fixation, the addition of collagen might be the reason for the overall more poor fixation by impairing the bone adhesion.
Very few in vivo studies have examined the osseointegration of implant surfaces with a thin coating of electrochemically deposited HA, most of these at temperatures or acidity at the extreme11–13,15,17 and a limited number under physiological conditions14,41 or as composite collagen coating.14,22,23,41 Schmidmaier et al.16 investigated the osseointegration of electrochemically deposited HA-coated titanium Kirschner wires inserted in a nonweight-bearing rat model with axial placement in femur. The coating was applied, graded with cathode–anode transition with a thickness of 2 µm (no further process specifications available). Push-out test and histology at 2 months revealed a 3-fold higher fixation and bone ongrowth of the HA-coated wires compared with the uncoated controls. These findings are in accordance with our implant study. In addition to its rare usage in orthopedic devices, the electrochemical deposition of HA has been used as a method of stimulating bone integration of dental implants. When inserted in the mandible11,41 and tibia,13,17 these threaded implants show increased bone-in-contact percentage at the interface. No data on mechanical testing are available on these studies. The addition of collagen to the HA coating did increase osseointegration.41 Schliephake et al.14 evaluated titanium rectangular implants with polished surfaces coated with various coatings of electrodeposited HA and collagen: coating of collagen, with mineralized collagen, with sequential coating of HA + collagen and with HA alone. The sequential HA + collagen process corresponds with the electrochemically HA collagen coating process used in our study. The implants were inserted press-fit in the canine mandible with observation times of 1 and 3 months. Histomorphometry at the cancellous bone implant level after 1 month showed significantly higher bone implant contact in the HA-coated implants compared with the control titanium implants and the collagen coated surfaces, but after 3 months these differences were balanced. These results are consistent with our implant study at 4 weeks of observation.
In conclusion, this study showed that HA coatings, deposited either by plasma spray technique or electrochemically, increases the mechanical fixation and bone ongrowth compared with an uncoated titanium control in a canine cancellous bone implant model. The plasma spraying technique seemed to stimulate more bone ongrowth. Otherwise, only minor (non-significant) histomorphometric differences between the two HA applications was found. The addition of collagen to the mineralized phase of the HA coating did not improve the osseoinductive effect of the HA. Further studies are necessary to evaluate the fixation of an orthopedic implant with electrochemically deposited HA in weight bearing conditions. Also long-term investigations are necessary to evaluate the electrochemically deposited HA’s solubility effect on long-term fixation.
References
- 1.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 2.Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand Suppl. 1993;255:1–58. doi: 10.3109/17453679309155636. [DOI] [PubMed] [Google Scholar]
- 3.Geesink RG. Osteoconductive coatings for total joint arthroplasty. Clin Orthop Relat Res. 2002;395:53–65. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Karrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated, and porous-coated stems with roentgen stereophotogrammetric analysis. J Bone Joint Surg Am. 1994;76:1692–1705. doi: 10.2106/00004623-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Dhert WJ. Retrieval studies on calcium phosphate-coated implants. Med Prog Technol. 1994;20:143–154. [PubMed] [Google Scholar]
- 6.Nakamura S, Kobayashi T, Yamashita K. Numerical osteobonding evaluation of electrically polarized hydroxyapatite ceramics. J Biomed Mater Res A. 2004;68:90–94. doi: 10.1002/jbm.a.10124. [DOI] [PubMed] [Google Scholar]
- 7.Zyman Z, Weng J, Liu X, Zhang X, Ma Z. Amorphous phase and morphological structure of hydroxyapatite plasma coatings. Biomaterials. 1993;14:225–228. doi: 10.1016/0142-9612(93)90027-y. [DOI] [PubMed] [Google Scholar]
- 8.Rahbek O, Overgaard S, Lind M, Bendix K, Bunger C, Soballe K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles. An experimental study in dogs. J Bone Joint Surg Br. 2001;83:441–447. doi: 10.1302/0301-620x.83b3.10667. [DOI] [PubMed] [Google Scholar]
- 9.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. [PubMed] [Google Scholar]
- 10.Bobyn JD, Jacobs JJ, Tanzer M, Urban RM, Aribindi R, Sumner DR, Turner TM, Brooks CE. The susceptibility of smooth implant surfaces to periimplant fibrosis and migration of polyethylene wear debris. Clin Orthop Relat Res. 1995;311:21–39. [PubMed] [Google Scholar]
- 11.Ishizawa H, Fujino M, Ogino M. Histomorphometric evaluation of the thin hydroxyapatite layer formed through anodization followed by hydrothermal treatment. J Biomed Mater Res. 1997;35:199–206. doi: 10.1002/(sici)1097-4636(199705)35:2<199::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Ban S, Maruno S, Arimoto N, Harada A, Hasegawa J. Effect of electrochemically deposited apatite coating on bonding of bone to the HA-G-Ti composite and titanium. J Biomed Mater Res. 1997;36:9–15. doi: 10.1002/(sici)1097-4636(199707)36:1<9::aid-jbm2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Costa CA, Sena LA, Pinto M, Muller CA, Cavalcanti JH, Soares GA. In vivo characterization of titanium implants coated with synthetic hydroxyapatite by electrophoresis. Braz Dent J. 2005;16:75–81. doi: 10.1590/s0103-64402005000100013. [DOI] [PubMed] [Google Scholar]
- 14.Schliephake H, Scharnweber D, Dard M, Robetaler S, Sewing A, Huttmann C. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. J Biomed Mater Res A. 2003;64:225–234. doi: 10.1002/jbm.a.10363. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Eliaz N, Xiang Z, Hsu HP, Spector M, Hobbs LW. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials. 2006;27:4192–4203. doi: 10.1016/j.biomaterials.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Schmidmaier G, Wildemann B, Schwabe P, Stange R, Hoffmann J, Sudkamp NP, Haas NP, Raschke M. A new electrochemically graded hydroxyapatite coating for osteosynthetic implants promotes implant osteointegration in a rat model. J Biomed Mater Res. 2002;63:168–172. doi: 10.1002/jbm.10130. [DOI] [PubMed] [Google Scholar]
- 17.Badr NA, El Hadary AA. Hydroxyapatite-electroplated cptitanium implant and its bone integration potentiality: An in vivo study. Implant Dent. 2007;16:297–308. doi: 10.1097/ID.0b013e31805d7dc4. [DOI] [PubMed] [Google Scholar]
- 18.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 19.Geissler U, Hempel U, Wolf C, Scharnweber D, Worch H, Wenzel K. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J Biomed Mater Res. 2000;51:752–760. doi: 10.1002/1097-4636(20000915)51:4<752::aid-jbm25>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Rossler S, Ogami T, Scharnweber D, Worch H. Biomimetic coating functionalized with adhesion peptides for dental implants. J Mater Sci: Mater Med. 2001;12:871–877. doi: 10.1023/a:1012807621414. [DOI] [PubMed] [Google Scholar]
- 21.Bernhardt R, van den DJ, Bierbaum S, Beutner R, Scharnweber D, Jansen J, Beckmann F, Worch H. Osteoconductive modifications of Ti-implants in a goat defect model: Characterization of bone growth with SR muCT and histology. Biomaterials. 2005;26:3009–3019. doi: 10.1016/j.biomaterials.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Rammelt S, Schulze E, Bernhardt R, Hanisch U, Scharnweber D, Worch H, Zwipp H, Biewener A. Coating of titanium implants with type-I collagen. J Orthop Res. 2004;22:1025–1034. doi: 10.1016/j.orthres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Rammelt S, Schulze E, Witt M, Petsch E, Biewener A, Pompe W, Zwipp H. Collagen type I increases bone remodelling around hydroxyapatite implants in the rat tibia. Cells Tissues Organs. 2004;178:146–157. doi: 10.1159/000082245. [DOI] [PubMed] [Google Scholar]
- 24.Rossler S, Sewing A, Stolzel M, Born R, Scharnweber D, Dard M, Worch H. Electrochemically assisted deposition of thin calcium phosphate coatings at near-physiological pH and temperature. J Biomed Mater Res A. 2003;64:655–663. doi: 10.1002/jbm.a.10330. [DOI] [PubMed] [Google Scholar]
- 25.Sewing A, Lakatos M, Scharnweber D, Roessler S, Born R, Dard M, Worch H. Influence of Ca/P ratio on electrochemical assisted deposition of hydroxyapatite on titanium. Key Eng Mater. 2004;254:419–422. [Google Scholar]
- 26.Roessler S, Born R, Scharnweber D, Worch H, Sewing A, Dard M. Biomimetic coatings functionalized with adhesion peptides for dental implants. J Mater Sci: Mater Med. 2001;12:871–877. doi: 10.1023/a:1012807621414. [DOI] [PubMed] [Google Scholar]
- 27.Soballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bunger C. Hydroxyapatite coating enhances fixation of porous coated implants. A comparison in dogs between press fit and noninterference fit. Acta Orthop Scand. 1990;61:299–306. doi: 10.3109/17453679008993521. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard S, Soballe K, Jorgen H, Gundersen G. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: Optimizing the stereological vertical-section design. J Orthop Res. 2000;18:313–321. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 29.Gotfredsen K, Budtz-Jorgensen E, Jensen LN. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol. 1989;64:121–127. doi: 10.3109/10520298909106984. [DOI] [PubMed] [Google Scholar]
- 30.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen TB, Rahbek O, Overgaard S, Soballe K. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation. An experimental study in dogs. J Orthop Res. 2004;22:653–658. doi: 10.1016/j.orthres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 33.Soballe K, Hansen ES, Brockstedt-Rasmussen H, Hjortdal VE, Juhl GI, Pedersen CM, Hvid I, Bunger C. Gap healing enhanced by hydroxyapatite coating in dogs. Clin Orthop Relat Res. 1991;272:300–307. [PubMed] [Google Scholar]
- 34.Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS. The anatomic basis of femoral component design. Clin Orthop Relat Res. 1988;235:148–165. [PubMed] [Google Scholar]
- 35.Schimmel JW, Huiskes R. Primary fit of the Lord cementless total hip. A geometric study in cadavers. Acta Orthop Scand. 1988;59:638–642. doi: 10.3109/17453678809149415. [DOI] [PubMed] [Google Scholar]
- 36.Engh CA, Zettl-Schaffer KF, Kukita Y, Sweet D, Jasty M, Bragdon C. Histological and radiographic assessment of well functioning porous-coated acetabular components. A human postmortem retrieval study. J Bone Joint Surg Am. 1993;75:814–824. doi: 10.2106/00004623-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Urban RM, Jacobs JJ, Sumner DR, Peters CL, Voss FR, Galante JO. The bone-implant interface of femoral stems with non-circumferential porous coating. J Bone Joint Surg Am. 1996;78:1068–1081. doi: 10.2106/00004623-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Dhert WJ, Verheyen CC, Braak LH, de W, Jr, Klein CP, de GK, Rozing PM. A finite element analysis of the push-out test: Influence of test conditions. J Biomed Mater Res. 1992;26:119–130. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 39.Harrigan TP, Kareh J, Harris WH. The influence of support conditions in the loading fixture on failure mechanisms in the push-out test: A finite element study. J Orthop Res. 1990;8:678–684. doi: 10.1002/jor.1100080509. [DOI] [PubMed] [Google Scholar]
- 40.Porter AE, Hobbs LW, Rosen VB, Spector M. The ultrastructure of the plasma-sprayed hydroxyapatite-bone interface predisposing to bone bonding. Biomaterials. 2002;23:725–733. doi: 10.1016/s0142-9612(01)00177-6. [DOI] [PubMed] [Google Scholar]
- 41.Schliephake H, Scharnweber D, Roesseler S, Dard M, Sewing A, Aref A. Biomimetic calcium phosphate composite coating of dental implants. Int J Oral Maxillofac Implants. 2006;21:738–746. [PubMed] [Google Scholar]