Abstract
Cells mobilize diverse signaling pathways to protect against stress-mediated injury. Ras family GTPases play critical roles in this process, controlling the activation and integration of multiple regulatory cascades. p38 mitogen-activated protein kinase (MAPK) signaling serves as a critical fulcrum in this process, regulating networks that stimulate cellular apoptosis but also promote cell survival. However, this functional dichotomy is incompletely understood, particularly regulation of p38-dependent survival. Here, we discuss our recent evidence that the Rit GTPase associates with and is required for stress-mediated activation of a scaffolded p38-MK2-HSP27-Akt pro-survival signaling cascade. Drosophila lacking D-Ric, a Rit homologue, are susceptible to a variety of environmental stresses, while embryonic fibroblasts derived from Rit knockout mice display blunted stress-dependent signaling and decreased viability. Conversely, expression of constitutively active Rit triggers p38-Akt-dependent cell survival. Together, our studies establish Rit as the central regulator of an evolutionarily conserved, p38-dependent signaling cascade that functions as a critical survival mechanism in response to stress.
Keywords: Ras, GTPases, MAPK, Akt, HSP27, stress, ROS, cell survival
The ability to resist injury following stress exposure is critical to cellular survival. The stress-activated JNK and p38 MAPK cascades have traditionally been viewed as promoting signaling networks which antagonize cell proliferation and survival.1 However, it is now clear that JNK/p38 pathways function in a context-specific manner to modulate cell proliferation, differentiation, migration and survival.2,3 The cellular balance between the extent and duration of the various MAPK cascades play a critical role in regulating cell fate, although the molecular mechanisms controlling whether stress-activated MAPK signaling, particularly p38 activation, results in cell death or recovery remain incompletely understood.2-4
Ras-related small GTP-binding proteins function as guanine nucleotide regulated molecular switches that govern physiological processes by regulating diverse effector pathways, including prominent roles in the control of MAPK signaling cascades.5 While the physiological role for many Ras family GTPases is known, the cellular function for a number of “orphan” GTPases remain to be determined.5 This includes Rit, the founding member of a novel branch of the Ras subfamily, sharing closest homology with Rin (Ras‐like in neurons) and the Drosophila Ric (Ras‐related protein that interacts with calmodulin) GTPases. The conservation of the Rit GTPase from flies to humans suggests conservation of an important physiological function(s). A series of studies using both expression of active and dominant-negative Rit mutants and RNAi-mediated gene silencing approaches had identified putative roles for Rit in the control of signaling pathways involved in neuronal morphogenesis,6,7 differentiation8-10 and cell survival.8,11 However, whether any of these functions constitute an essential cellular role for Rit remained uncharacterized.
To critically assess the physiological function(s) of Rit, we begin by examining the impact of D-Ric GTPase loss in Drosophila. While vertebrates express 35 members of the Ras branch of the small GTPase superfamily, Drosophila express only 14 orthologs, including RIC, which shares an effector domain and > 65% amino acid identity with the vertebrate Rit and Rin GTPases.12,13 D-Ric null flies were viable, fertile and displayed no obvious developmental defects. Because earlier studies had identified a role for Rit signaling in cell survival,8 the susceptibility of D-Ric mutants to stress was examined. Adult flies displayed a reduced resistance to numerous environmental stresses, when compared with wild-type controls, indicating that RIC-dependent survival signaling cannot be compensated for by any other Drosophila GTPases. Moreover, the amplitude and duration of p38 MAPK signaling was blunted in null flies in response to heat shock, supporting a role for RIC-p38 signaling in recovery from thermal stress. To extend the analysis to vertebrates we generated a Rit1 knockout mouse.14 Rit null animals were born at the expected Mendelian ratio and displayed no gross morphological or anatomical abnormalities, suggesting that as seen with RIC deletion, Rit function is not essential for cellular proliferation or embryonic development.
To evaluate the contribution of Rit to cell survival, primary mouse embryonic fibroblasts (MEFs) were cultured from wild-type and Rit null littermates. Surprisingly, Rit−/− MEFs displayed a selective vulnerability to reactive oxygen species (ROS). Analysis of cellular kinase cascades found that hydrogen peroxide treatment resulted in the activation of p38, ERK and ERK5 in wild-type MEFs. In contrast, the activation of p38, and ERK were reduced in Rit−/− MEFs, suggesting that Rit-dependent regulation of one or more of these pathways might contribute to survival in response to oxidative injury. Inhibition of p38, but not ERK signaling, was shown to disrupt Rit-dependent survival signaling in both primary MEFs,14 but also in a variety of cultured cell lines.11 These results motivated studies to explore the requirement for Rit in stress-mediated p38 activation. Silencing of Rit (using either RNAi-mediated knockout or Rit−/− MEFs) dramatically suppressed p38 activation downstream of a variety of stresses, and in a number of mammalian cell lines, supporting a general role for Rit in coupling cellular stress to a p38-dependent survival signaling.
The identification of p38 as a key element in Rit-dependent survival signaling was unexpected as p38 activation is commonly associated with the induction of cell death.3 Further analysis found that, in parallel with inhibition of stimulus-mediated p38 activation, Rit silencing inhibited stress-induced Akt activation, mirroring the results from ROS stimulated Rit−/− MEFs.14 Recent work has identified a pro-survival cascade in which p38 promotes Akt activation within a novel HSP27 scaffolded complex.15-18 Within the complex, p38 activates MAPK activated protein kinase 2 (MAPKAPK2 or MK2), resulting in the phosphorylation of HSP27 and Akt, leading to cellular responses including the inhibition of apoptosis (Fig. 1).19 Expression of activated Rit in PC6 cells was shown to stimulate MK2 activity and increase levels of phosphorylated HSP27 in a p38-dependent fashion, while silencing endogenous Rit inhibited stress-mediated MK2 and HSP27 phosphorylation. Consistent with a role for Rit in the regulation of this cascade, MK2, HSP27 and Akt phosphorylation were decreased in Rit−/− MEFs following ROS exposure, when compared with similarly treated wild-type MEFs. Akt signaling is known to regulate the balance between cell survival and apoptosis.20 Phosphorylation of Bad of S136, a pro-apoptotic member of the Bcl-2 family, results in its sequestration in the cytosol, promoting cell survival.21,22 Consistent with a role for Akt signaling in MEF survival following oxidative stress, in Rit null cells, Bad S136 phosphorylation was significantly reduced.
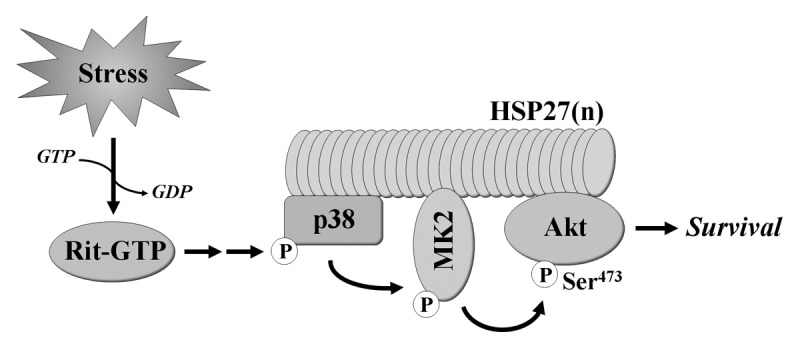
Figure 1. Rit signaling confers resistance to cellular stress. New evidence indicates that Rit plays a key role in stress-mediated cell survival by directing p38 MAPK toward the activation of MK2 and Akt within a HSP27 scaffolded signaling complex.
Selective association of Ras and Rho family GTPases with scaffolded MAPK cascades is one mechanism known to confer specificity to MAPK signaling.23 Consistent with a unique role for Rit in channeling ROS-mediated p38 activation to the MK2/HSP27/Akt pathway, using either dominant-negative mutants or RNAi silencing approaches to disrupt MK2 or HSP27, had no effect on Akt activation downstream of the closely related H-Ras and Rap1A GTPases. Moreover, co-immunoprecipitation studies found that p38, MK2, HSP27 and Akt were capable of associating with Rit, either individually or indirectly as part of a larger complex, but with the exception of p38, these proteins failed to associate with H-Ras. Together, these data support a model in which Rit signaling regulates a conserved survival function that involves Rit association with and regulation of a p38-MK2-HSP27-Akt signaling complex (Fig. 1).
The conservation of the Rit-p38 cascade from Drosophila to man highlights its importance to cell survival and potentially to the regulation of additional p38-mediated biological processes.3 These include the regulation of pro-survival transcriptional cascades,24-26 control of cell cycle progression27 and the lifespan.28 In future it will be important to determine whether Rit might modulate these, or other p38-dependent processes. In addition, while p38 inhibitors are under development for the treatment of inflammatory and autoimmune disease, cancer and cardiovascular disorders, toxicity of these agents has been an issue.29 Given the chronic nature of these disorders, and the complex regulatory role played by p38 signaling, greater insight into molecular mechanisms governing p38-mediated survival signaling may aid in the development of new therapies which limit adverse clinical outcomes.
In conclusion, the finding that Rit functions as a critical regulator of an evolutionarily conserved, p38-dependent survival signaling cascade, by directing p38 activity toward the activation of Akt within a HSP27 scaffolded complex, represents an important step forward in our understanding of Rit function and provides insight into a molecular mechanism coupling p38 activity to cell survival.
Acknowledgments
This work was supported by Public Health Service grant NS045103 from the National Institute of Neurological Disorders and Stroke (D.A.A.) and by NIH Grant Number P20GM103486 from the National Institute of General Medical Sciences, its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/22297
References
- 1.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 2.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 3.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 4.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 5.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–36. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andres DA, Shi GX, Bruun D, Barnhart C, Lein PJ. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J Neurochem. 2008;107:1436–47. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer ML, Shao H, Andres DA. Induction of neurite extension and survival in pheochromocytoma cells by the Rit GTPase. J Biol Chem. 2002;277:20160–8. doi: 10.1074/jbc.M201092200. [DOI] [PubMed] [Google Scholar]
- 9.Shi GX, Andres DA. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol Cell Biol. 2005;25:830–46. doi: 10.1128/MCB.25.2.830-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi GX, Rehmann H, Andres DA. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–47. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi GX, Jin L, Andres DA. A rit GTPase-p38 mitogen-activated protein kinase survival pathway confers resistance to cellular stress. Mol Cell Biol. 2011;31:1938–48. doi: 10.1128/MCB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CH, Della NG, Chew CE, Zack DJ. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J Neurosci. 1996;16:6784–94. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wes PD, Yu M, Montell C. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 1996;15:5839–48. [PMC free article] [PubMed] [Google Scholar]
- 14.Cai W, Rudolph JL, Harrison SM, Jin L, Frantz AL, Harrison DA, et al. An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Mol Biol Cell. 2011;22:3231–41. doi: 10.1091/mbc.E11-05-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, et al. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–23. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 16.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–35. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 17.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 18.Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H, Shen X. MAPK-activated protein kinase-2 (MK2)-mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27. J Biol Chem. 2006;281:37215–26. doi: 10.1074/jbc.M603622200. [DOI] [PubMed] [Google Scholar]
- 19.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 21.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 22.del Peso L, González-García M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 23.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 24.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–90. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 25.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–70. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–32. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 29.Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5:929–39. doi: 10.2174/1568026054985911. [DOI] [PubMed] [Google Scholar]


