Abstract
Most land plants are able to form symbiotic associations with fungi, and in many cases these associations are necessary for plant and fungal survival. These plant/fungal associations are formed with mycorrhizal (arbuscular mycorrhizal or ectomycorrhizal) or endophytic fungi, fungi from distinct phylogenetic lineages. While it has been shown that mycorrhizal fungi are able to transfer nutrients to plant roots in exchange for carbon, endophytes have been thought as asymptomatic colonizers. Recently, however, it has been shown that some insect pathogenic endophytic fungi are able to transfer insect derived nitrogen to plant roots, likely in exchange for plant sugars. Here we explore potential convergent evolutionary strategies for nutrient transfer between insect pathogenic endophytes and mycorrhizal fungus.
Keywords: convergent evolution, endophyte, fungus, insect pathogen, plant symbiosis
More than 90% of all land plant species engage in symbiotic relationships with fungi and are dependent upon these interactions for survival. These relationships with plants are formed with mycorrhizal (endo- and/or ectomycorrhizal fungi) or endophytic fungi. Endophytic fungi live within plant tissues; this includes root as well as foliar inhabitants, of which many fungi are host and tissue specific.1 Generally, endophytic fungi may be defined as asymptomatic plant colonizers, and an endophyte may not remain within the plant tissue throughout its life. Functional endomycorrhizal fungi, however, are explicitly excluded as “endophytes,” as they are specialized for nutrient transfer from sources outside of the root2 and, more specifically, are phylogenetically distinct from most groups of endophytes.3,4 The endophytic arbuscular mycorrhizal fungi (AM) are placed in a separate monophyletic phylum, the Glomeromycota.5 The term endophyte is thus defined by location and does not address the nature of the relationship with the plant or its phylogenetic status. Therefore, AM fungi are a specialized group of endophytic fungi with a unique phylogenetic status. The ectomycorrhizal fungal species, on the other hand, are among the Basidiomycota as well as the Ascomycota. Here, the definition includes that ectomycorrhizal fungi produce a hyphal sheath, or mantle, covering the root tip and a hartig net of hyphae surrounding the plant cells within the root cortex.6 Endophytic associations differ from mycorrhizas primarily by the absence of a localized interface of specialized hyphae and the absence of synchronized plant-fungus development.7 Brundett7 also suggested that in endophytic fungal associations there is little nutrient transfer to plants. However, several non-mycorrhizal endophytic fungi can provide nutrients to plants.8
Here we explore recent findings that endophytic fungi can also specialize in nutrient transfer from outside of the root, specifically, from soil-borne insects that they can infect.8 These endophytic, insect pathogenic fungi (EIPF) are phylogenetically distinct from AM fungi. In this minireview we suggest convergent evolutionary strategies for nutrient transfer to plants with some species of EIPF, AM fungi as well as ectomycorrhizal fungi.
Nutrient Transfer in AM and Endophytic Fungi
AM fungi are well known as mediators of nutrient transfer to plants. The majority of vascular plants, (80–90%)9 are able to form associations with AM fungi, and these associations have been described, in part, by nutrient exchange. Initial studies on nutrient transfer focused on the ability of AM fungi to provide roots with phosphate and it was found that the arbuscule of these fungi, specifically the peri-arbuscular membrane, contained phosphate transporters.10 Providing the fungus with increased levels of plant-derived carbon resulted in increased transference of phosphate.11 This suggests a coordinated barter of nutrients between plant and fungus. Some fungi can also provide nitrogen to plants.10,12 Glomus species have the ability to transfer nutrients, including nitrogen to the roots of leguminous plants such as Medicago.10,13 Ceratobasidium cornigerum has also been shown to provide orchids (Goodyera repens) with nitrogen in exchange for carbon.14
Some fungal endophytes can affect plant growth and plant responses to pathogens, herbivores and environmental change; others produce useful or novel secondary metabolites. Root endophytes colonize healthy plant roots, however, one of the defining characteristics has been that endophytes do not have nutrient transfer interfaces commonly observed in AM fungi.15,16 This definition is not without exception. An increase in nutrient content and growth was observed for Carex sp, Pinus contorta and Vulpia ciliata when inoculated with dark septate endophytic fungi.17-19
The dark septate endophytic fungus Heteroconium chaetospira forms a functional symbiosis with Brassica campestris (Chinese cabbage) where the fungus transfers nitrogen to, and receives carbon from, the plant.20 The Brassicaceae do not usually form mycorrhizal associations so this association with H. chaetospira, as well as others,8 suggests that endophytes can also transfer nutrients to plants. Endophytic associations can also result in more efficient nutrient acquisition since root associated fungal hyphae are able to obtain soil nutrients from areas too small for plant roots to penetrate.21
Recently, some insect pathogenic fungi have been shown to form endophytic asociations with plant roots.22-24 In particular, one EIPF, Metarhizium robertsii, can transfer insect-derived nitrogen to plants.8 M. robertsii infected a soil insect after which the fungal mycelia colonized the host plant (switchgrass and haricot bean) where nitrogen transfer was detected.8 The ectomycorrhizal fungus, Laccaria bicolor was also able to transfer insect derived nitrogen from springtails to the roots of eastern white pine trees.25 However, L. bicolor is not known as an insect pathogen and it is possible that L. bicolor transferred nitrogen to plants from insect cadavers. The ability of EIPF to transfer insect derived nitrogen to plant roots indicates a fundamental shift in the way these fungi are viewed within the ecosystem as they represent the ability of the plant to regain nitrogen previously lost by insect herbivory.
Convergent Evolution in Nutrient Transfer in AM and Endophytic Fungi
The earliest fossil evidence of mycorrhizal associations with plant roots is ca. 460 million years ago.26 The early evolutionary origin of these mutually beneficial partnerships suggests that fungal/root associations, specifically mycorrhizae, may have benefited plants as well as fungi and has significantly affected the global distribution of flora.27 These associations are not trivial since these symbioses enhance the overall fitness of the plant.27 However, not all root-associated fungi are mycorrhizae, and despite the fact that mycorrhizal fungi have been ubiquitously noted, other root associated fungi (i.e., endophytes) have been discovered. These non-Glomeromycota fungi are most likely not the product of continued co-evolution with plants followed by fungal divergences, but have separately undergone convergent evolution as plant root associates. That is, beneficial plant/fungal associations have evolved separately several times over the past 400 my.
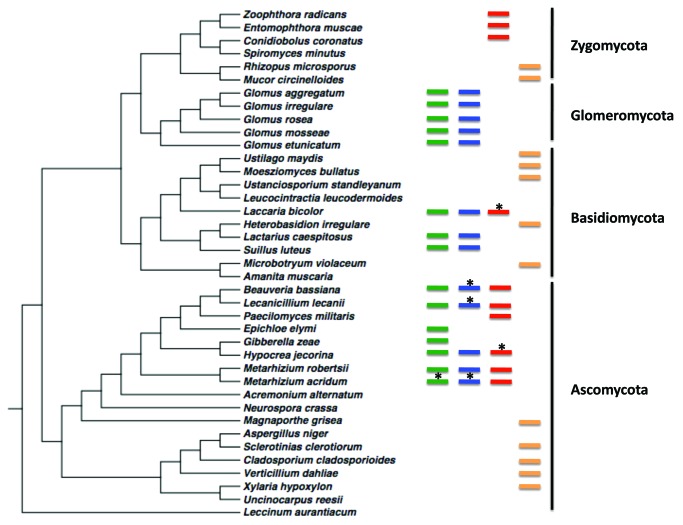
A fungal phylogeny constructed in Figure 1 shows the phylogenetic position of the Glomeromycota in relation to EIPF and ectomycorrhizal fungi. The EIPF are phylogenetically distant from the Glomeromycota but are also involved in nutrient transfer to plants. Endophytic fungi may have multiple ecological niches since they are not restricted to the host plant. Metarhizium is taxonomically related to other endophytic fungi, notably Claviceps (Fig. 1.). We suggest that these fungi have undergone convergent evolution as plant symbionts. However, Metarhizium is also a generalist insect pathogen that can infect over 200 insect species.28 Metarhizium is a plant symbiont due to phylogenetic heritage (i.e., that they are closely related to other endophytes), and it has also evolved mechanisms as a generalist insect pathogen. This bifunctional lifestyle as a plant endophyte and an insect pathogen may have allowed Metarhizium to provide plants with insect-derived nitrogen. Approximately, 10% of insect biomass is nitrogen.29 One of the driving forces behind this mutualism is most likely the barter for plant carbon. In the soil, carbon can be a limiting resource for various fungal species.30 A number of saprotrophs obtain carbon from decaying plant matter, but this method of acquisition is inefficient for uncompetitive fungi.30 Several root associated fungal species are able to utilize plant-derived carbon,14,31-33 which could lead to a nitrogen-carbon barter. EIPF may have been drawn to plant roots as a niche initially due to the sugars present within root exudates.
Figure 1. Phylogeny of a subsample of fungal species representing four fungal taxa with examples of plant symbionts (endomycorrhizal, ectomycorrhizal and endophytes), the ability of these symbionts to transfer nutrients to plants, fungi that are insect pathogens and those that are plant pathogens. The tree shown was created using ITS1 5.8s rRNA and ITS2 sequences. Green bars indicate plant symbionts, Blue bars indicate that nutrient transfer has been shown between the fungal symbiont and the plant. Red bars indicate insect pathogens. Yellow bars indicate plant pathogens. Asterisks above bars indicate potential, but yet untested, capacity of the fungus. For strain numbers, sequences and accession numbers, contact corresponding author.
However, not all endophytes behave in the same manner with respect to nutrient acquisition. Generally, endophytic fungi can be placed into two groups, clavicipitaceous and non-clavicipitaceous.34 EIPF are clavicipitaceous fungi and are able to acquire nutrients from the insects they infect and can then transfer those nutrients to host plants.8 Non-insect pathogens that are plant symbionts, however, are generally decomposers of decaying plant materials found in soil; through this decomposition they are able to acquire nutrients necessary for potential exchange with plant roots.35 The fungal phylogeny shown in Figure 1 indicates that the divergence between Ascomycete EIPF and Glomeromycota occurred early in fungal evolutionary history. The recent discovery that several ascomycetous insect pathogens are also able to form plant root associations suggests a possible convergence in plant symbiosis and nutrient exchange strategies between these fungi and the Glomeromycota as well as some of the ectomycorrhizal fungi. More specialized insect pathogens are found in the Zygomycetes, specifically in the Entomophthorales. Most of these fungi are fastidious and obligate insect pathogens36 and have not yet been reported as plant associates (Fig. 1).
The ability of AM fungi, EIPF and some species of ectomycorrhizal fungi, three phylogenetically disparate fungal groups, to act as mediators of plant nutrient transfer suggests convergent evolution as root colonizers and conduits of nutrient transfer. The challenge is to identify convergent evolutionary strategies at the molecular level in order to identify fungal genes that mediate root colonization as well as nutrient transfer. By using well-known mycorrhizal species as genetic models for EIPF, gene homologs involved in plant/fungus communication and/or nutrient transfer may be identified. In Glomus and Laccaria species there is an upregulation of ammonium transporters during plant root association.10,37 These fungi are from distinct fungal lineages and this finding indicates they have developed similar methods for nitrogen transfer to plant roots. It has also been shown that in phylogenetically disparate fungal plant symbionts there is an increase in simple sugar transporters during root colonization that coincides with an increase in monosaccharide transporters in plant roots.38
With respect to phosphate translocation, in some Glomus species there was an increase in the expression of phosphate transporters in the extraradical and intraradical mycelia during root association;39 this increase in expression has also been observed in Hebeloma cylindrosporum during root symbiosis.40 This upregulation of phosphate transporters in fungal symbionts during root colonization corresponds to an increase in plant root phosphate transporters.41 Similar expression patterns have also been seen in root-associated fungus with respect to sulfate transporters.42 It is likely that numerous fungal species have developed transporters and evolved similar nutrient exchange programs in order to barter for the acquisition of carbon from plants. The next step is to elucidate the molecular mechanisms by which plants sense the acquisition of fungal-derived nutrients and allow a symbiotic association that draws carbon from the plant.
Most arbuscular mycorrhizal fungi are obligate biotrophs43 and are unable to grow in pure culture and transformation systems are not available for many of these fungi. This provides an opportunity for Metarhizium to act as a potential model to test the role of nutrient transporters and plant permissiveness in these endophytes since gene knockout systems,44 as well as the fungal genome,45 are readily available.46
Conclusions
Traditional research on insect pathogenic fungi has generally been focused on fungal virulence toward insect hosts and therefore largely ignored other aspects of their soil ecology. The discovery that some insect pathogenic fungi are able to form endophytic associations with the capacity of nutrient transfer strongly indicates convergence in nutrient transfer strategies between a number of fungal lineages, most likely as a method to obtain carbon from plant roots. Subsequent findings that EIPF are capable of nutrient transfer to plant roots fundamentally changes the way these fungi must be viewed in soil habitats. These fungi are not solely insect pathogens and should be viewed as an integral part of plant health and as an important part of the soil nutrient cycle on which plants rely in order to acquire limiting soil nutrients.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/22321
References
- 1.Carroll G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbionts. Ecology. 1988;69:2–9. doi: 10.2307/1943154. [DOI] [Google Scholar]
- 2.Brundrett MC. Co-evolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–9. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- 4.Porras-Alfaro A, Herrera J, Sinsabaugh RL, Odenbach KJ, Lowrey T, Natvig DO. Novel root fungal consortium associated with a dominant desert grass. Appl Environ Microbiol. 2008;74:2805–13. doi: 10.1128/AEM.02769-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shubler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res. 2001;105:1413–21. doi: 10.1017/S0953756201005196. [DOI] [Google Scholar]
- 6.Carlile MJ, Watkinson SC, Gooday GW. Parasites and mutualistic symbionts. In: The Fungi. Second Edition. Burlington, MA: Academic Press, 2001; 363-460. [Google Scholar]
- 7.Brundrett MC. Diversity and classification of mycorrhizal associations. Biol Rev Camb Philos Soc. 2004;79:473–95. doi: 10.1017/S1464793103006316. [DOI] [PubMed] [Google Scholar]
- 8.Behie SW, Zelisko PM, Bidochka MJ. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science. 2012;336:1576–7. doi: 10.1126/science.1222289. [DOI] [PubMed] [Google Scholar]
- 9.Malloch DW, Pirozynski KA, Raven PH. Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants (A Review) Proc Natl Acad Sci USA. 1980;77:2113–8. doi: 10.1073/pnas.77.4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaude N, Bortfeld S, Duensing N, Lohse M, Krajinski F. Arbuscule-containing and non-colonized cortical cells of mycorrhizal roots undergo extensive and specific reprogramming during arbuscular mycorrhizal development. Plant J. 2012;69:510–28. doi: 10.1111/j.1365-313X.2011.04810.x. [DOI] [PubMed] [Google Scholar]
- 11.Bücking H, Shachar-Hill Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005;165:899–911. doi: 10.1111/j.1469-8137.2004.01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–23. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 13.Cox G, Tinker PB. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. New Phytol. 1976;77:371–8. doi: 10.1111/j.1469-8137.1976.tb01526.x. [DOI] [Google Scholar]
- 14.Cameron DD, Leake JR, Read DJ. Mutualistic mycorrhiza in orchids: evidence from plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol. 2006;171:405–16. doi: 10.1111/j.1469-8137.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 15.Brundrett MC. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In: Schulz B, Boyle C, Seiber TN eds. Microbial Root Endophyts. Vol 9, Soil Biol. Berlin: Springer-Verlag, 2006: 281-98. [Google Scholar]
- 16.Schulz B, Boyle C. What are endophytes? Schulz B, Boyle C, Seiber TN eds. Microbial Root Endophytes. Vol. 9, Soil Biol. Berlin: Springer-Verlag, 2006; 1-13. [Google Scholar]
- 17.Haselwandter K, Read RJ. The significance of root-fungus association in two Carex species of high-alpine plant communities. Oecologia. 1982;53:352–4. doi: 10.1007/BF00389012. [DOI] [PubMed] [Google Scholar]
- 18.Jumpponen A, Mattson KG, Trappe JM. Mycorrhizal functioning of Phialocephala fortinii with Pinus contorta on glacier forefront soil: interactions with soil nitrogen and organic matter. Mycorrhiza. 1998;7:261–5. doi: 10.1007/s005720050190. [DOI] [PubMed] [Google Scholar]
- 19.Newsham KK. Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpia ciliata ssp. ambigua. New Phytol. 1999;144:517–24. doi: 10.1046/j.1469-8137.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Usuki F, Narisawa K. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia. 2007;99:175–84. doi: 10.3852/mycologia.99.2.175. [DOI] [PubMed] [Google Scholar]
- 21.Majdi H, Damm E, Nylund J. Longevity of mycorrhizal roots depends on branching order and nutrient availability. New Phytol. 2001;150:195–202. doi: 10.1046/j.1469-8137.2001.00065.x. [DOI] [Google Scholar]
- 22.Sasan RK, Bidochka MJ. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am J Bot. 2012;99:101–7. doi: 10.3732/ajb.1100136. [DOI] [PubMed] [Google Scholar]
- 23.Akello J, Dubois T, Gold CS, Coyne D, Nakavuma J, Paparu P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.) J Invertebr Pathol. 2007;96:34–42. doi: 10.1016/j.jip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Posada F, Vega FE. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao) Mycologia. 2005;97:1195–200. doi: 10.3852/mycologia.97.6.1195. [DOI] [PubMed] [Google Scholar]
- 25.Klironomos JN, Hart MM. Food-web dynamics. Animal nitrogen swap for plant carbon. Nature. 2001;410:651–2. doi: 10.1038/35070643. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson DM. Mycorrhizal evolution. Trends Ecol Evol. 2001;16:64–5. doi: 10.1016/S0169-5347(00)02071-1. [DOI] [PubMed] [Google Scholar]
- 27.Cairney JW. Evolution of mycorrhiza systems. Naturwissenschaften. 2000;87:467–75. doi: 10.1007/s001140050762. [DOI] [PubMed] [Google Scholar]
- 28.St. Leger RJ. Biology and mechanisms of insect-cuticle invasion by deuteromycetous fungal pathogens. In N.C. Beckage, S.N. Thompson and B.A. Federici (ed) Parasites and pathogens of insects, 2. New York, N.Y.: Academic Press 1993; 221-9. [Google Scholar]
- 29.Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, Woods HA, et al. Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat. 2002;160:784–802. doi: 10.1086/343879. [DOI] [PubMed] [Google Scholar]
- 30.Tiunov AV, Scheu S. Carbon availability controls the growth of detritivores (Lumbricidae) and their effect on nitrogen mineralization. Oecologia. 2004;138:83–90. doi: 10.1007/s00442-003-1391-4. [DOI] [PubMed] [Google Scholar]
- 31.Gavito ME, Olsson PA. Allocation of plant carbon to foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiol Ecol. 2003;45:181–7. doi: 10.1016/S0168-6496(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 32.Hawkes CV, Hartley IP, Ineson P, Fitter AH. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob Change Biol. 2008;14:1181–90. doi: 10.1111/j.1365-2486.2007.01535.x. [DOI] [Google Scholar]
- 33.Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, et al. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003;131:1496–507. doi: 10.1104/pp.102.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez RJ, White JF, Jr., Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–30. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 35.Dighton J. Acquisition of nutrients from organic resources by mycorrhizal autotrophic plants. Experientia. 1991;47:362–9. doi: 10.1007/BF01972078. [DOI] [Google Scholar]
- 36.Bidochka MJ, Kamp AM, Amrithra De Croos JN. Insect pathogenic fungi: from genes to populations. Fungal Pathology. Dordrecht, Netherlands: Kluwer Academic Publishing, 2000; 171-95. [Google Scholar]
- 37.Lucic E, Fourrey C, Kohler A, Martin F, Chalot M, Brun-Jacob A. A gene repertoire for nitrogen transporters in Laccaria bicolor. New Phytol. 2008;180:343–64. doi: 10.1111/j.1469-8137.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- 38.Harrison MJ. A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J. 1996;9:491–503. doi: 10.1046/j.1365-313X.1996.09040491.x. [DOI] [PubMed] [Google Scholar]
- 39.Benedetto A, Magurno F, Bonfante P, Lanfranco L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza. 2005;15:620–7. doi: 10.1007/s00572-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 40.Tatry MV, El Kassis E, Lambilliotte R, Corratgé C, van Aarle I, Amenc LK, et al. Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J. 2009;57:1092–102. doi: 10.1111/j.1365-313X.2008.03749.x. [DOI] [PubMed] [Google Scholar]
- 41.Karandashov V, Bucher M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005;10:22–9. doi: 10.1016/j.tplants.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Allen JW, Shachar-Hill Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2009;149:549–60. doi: 10.1104/pp.108.129866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azcon-Aguilar C, Bago B, Barea JM. In: Varma A, Hock B eds. Mycorrhiza: Structure, function, molecular biology and biotechnology. Second Edition. Berlin: Springer-Verlag, 1999:391-407. [Google Scholar]
- 44.Staats CC, Junges A, Fitarelli M, Furlaneto MC, Vainstein MH, Schrank A. Gene inactivation mediated by Agrobacterium tumefaciens in the filamentous fungi Metarhizium anisopliae. Appl Microbiol Biotechnol. 2007;76:945–50. doi: 10.1007/s00253-007-1043-4. [DOI] [PubMed] [Google Scholar]
- 45.Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, Shang Y, et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011;7:e1001264. doi: 10.1371/journal.pgen.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang W, Pei Y, Bidochka MJ. Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can J Microbiol. 2006;52:623–6. doi: 10.1139/w06-014. [DOI] [PubMed] [Google Scholar]