Abstract
The recent awareness that eukaryotic cells can be linked and communicate via membranous nanotubes (NTs) has extended previous conceptions of cell-to-cell interaction. Apart from mediating functional connectivity between a broad range of cells, facilitating intercellular transmission of electric signals or various cellular components, there is strong evidence for participation of NTs in pathological processes of particular medical interest. In our recent study, we showed for the first time the existence of nanotubular connections between human primary peritoneal mesothelial cells (HPMCs) and provided insights to their actin/filopodia mediated building mechanism. Furthermore, we showed that tumor necrosis factor (TNF) significantly increased NT formation between HPMCs, pointing to a crucial role of NTs during inflammatory processes. Moreover, our study showed a strong correlation of NT occurrence and cellular cholesterol contents, demonstrating an interdependence of NT mediated cell communication, cytokine action and cholesterol homeostasis. Here, we further provide analysis on NT-formation processes.
Keywords: nanotubes, intercellular communication, tumor necrosis factor, human mesothelial cells, Arp2/3, calcium channels, statins
In the context of inflammatory immune reactions, intercellular communication plays a crucial role. Recent findings demonstrated that cells are able to interact via membranous channels initially termed tunneling nanotubes (TNTs).1 TNTs were initially characterized as thin intercellular membrane channels, tensed between cultivated pheochromocytoma (PC12) cells at their nearest distance and without contact to the substratum, displaying diameters from 50–200 nm and lengths of up to several cell diameters.1 The structures with their remarkable architecture were shown to contain F-actin and/or microtubule backbones and to facilitate the intercellular transmission of various cellular components, including organelles as well as plasma membrane constituents or the transfer of electric signals.2,3 Although few publications have demonstrated the existence of NTs in vivo,4 their occurrence, architecture and function in the body is still a matter of considerable debate and may substantially vary in accordance to the respective species, tissue, developmental stage, age, genetic background and pathophysiological variations. However, there is culminating evidence for a participation of NTs in several pathological processes of substantial medical interest. NTs were proposed to be involved in the intercellular spread of prion proteins,5,6 and viral proteins, e.g., during HIV infections,7-9 the transfer of drug resistance between cancer cells10 or the transfer of Aβ peptides in the context of Alzheimer disease.11
In the immune system, human peritoneal mesothelial cells (HPMCs), the resident cells of the peritoneal cavity, bear an effective antigen-presenting function for T cells and thereby play a relevant role during the immune response in the peritoneal cavity e.g., during peritonitis.12,13 Facing this background, we have shown that NTs are formed in the human peritoneum and that their occurrence correlates with defined pathophysiological conditions.14 Since in vivo analyses of NTs in humans are unfeasible, we developed HPMC primary cultures from omentum obtained during abdominal surgery or from effluents of overnight bags from patients undergoing peritoneal dialysis (PD) treatment.14 We could demonstrate that primary HPMCs establish discrete NT connections de novo and provide support for a filopodia/actin mediated formation process.14 Moreover, we showed that the number of NTs is significantly increased upon stimulation of cells with TNF pointing to an important participation of NTs during inflammatory reactions.14 Our study additionally revealed a strong correlation of NT occurrence with cellular cholesterol contents. We could show that incubation of cells with the statin simvastatin led to a significant increase in the formation of NTs.14 To address the question whether this observation was a mere in vitro phenomenon, we isolated HPMCs from omentum of a patient undergoing statin treatment and assessed NT-numbers. The results showed that the numbers of NTs being built between these cells were comparably high as the numbers found for cells stimulated with simvastatin.14 This observation shows that statins strongly influence NT-formation processes at least in this particular type of cell with potential impact for clinical treatments. The underlying molecular mechanisms however remain elusive at this stage and are subject of future research in the field.
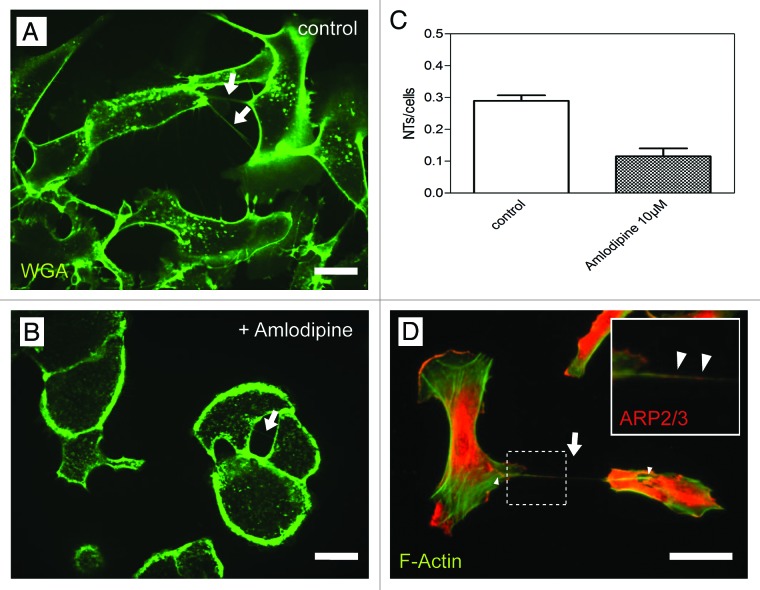
In further experiments we investigated a possible involvement of calcium channels during NT-formation. In this context studies from Beum et al. highlighted the occurrence of thin structures, so called streamers, during complement-mediated cytolysis of B cells only in presence of Ca2+.15 Salter et al. reported that Ca2+ fluxes in dendritic cells promote cell morphology changes which coincide with membrane spreading and lamellipodia extension.16 This led us to test whether the blocking of calcium channels in HPMCs with the drug amlodipine has an impact on NT-formation. Our experiments revealed that incubation of the cells with amlodipine resulted in significant lower NT-numbers as compared with the control experiment (Fig. 1 A-C). This clear decrease in NT-numbers argues for an important involvement of calcium channels in NT-formation processes, although the precise mechanism remains elusive.
Figure 1. Influence of Amlodipine and recruitment of Arp2/3 during NT-formation. (A, B) High resolution 3D live-cell fluorescence images of NTs (arrows) connecting primary mesothelial cells one hour after plating on a collagen I coated glass cover slide in absence (A) and presence (B) of the calcium channel blocker Amlodipine. Cell plasma membranes were stained with WGA Alexa Fluor® 488. (C) Quantitative analysis of the NTs/cells ratio in Amlodipine treated cells compared with untreated cells. (D) Co-immunostaining of F-actin and Arp2/3 showing Arp2/3 (arrowheads in the magnified insert) being present at the NT-basis (arrowheads) and within the NT (arrow) tensed between two individual mesothelial cells. Scale bars: 20 µm.
Based on our recent observation that the formation of NTs between HPMCs is actin and respectively filopodia based, we further analyzed the NT-formation process in greater detail to unravel components of the underlying molecular machinery. In this context, we focused on two actin related proteins - Arp2 and Arp3 - which are responsible for the generation of branched networks of actin filaments and which are localized to filopodia e.g., during cell spreading.17 To assess their involvement during NT-formation, we stained HPMCs connected via NTs for F-actin and the Arp2/3 complex subunit ARP2 (Fig. One D). Respective experiments show that Arp2/3 is located at the bases (Fig. One D, arrowheads) as well as inside NTs along with F-actin (Fig. One D and Insert, arrows).
Whether these or related proteins and the underlying molecular mechanisms are indeed key players during NT-formation – and thus promising targets for novel therapeutic treatments in view of severe diseases like cancer, viral infections etc. – will now be subject of thorough future investigations.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/22686
References
- 1.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 2.Hurtig J, Chiu DT, Onfelt B. Intercellular nanotubes: insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:260–76. doi: 10.1002/wnan.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci U S A. 2010;107:17194–9. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT): A potential mechanism for intercellular HIV trafficking. Commun Integr Biol. 2009;2:243–4. doi: 10.4161/cib.2.3.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gousset K, Zurzolo C. Tunnelling nanotubes: a highway for prion spreading? Prion. 2009;3:94–8. doi: 10.4161/pri.3.2.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunning CJ, Reyes JF, Steiner JA, Brundin P. Can Parkinson’s disease pathology be propagated from one neuron to another? Prog Neurobiol. 2012;97:205–19. doi: 10.1016/j.pneurobio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–8. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol. 2011;6:658–75. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowinski S, Alakoskela JM, Jolly C, Davis DM. Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1. Methods. 2011;53:27–33. doi: 10.1016/j.ymeth.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Pasquier J, Galas L, Boulangé-Lecomte C, Rioult D, Bultelle F, Magal P, et al. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J Biol Chem. 2012;287:7374–87. doi: 10.1074/jbc.M111.312157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Cui J, Sun X, Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18:732–42. doi: 10.1038/cdd.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausmann MJ, Rogachev B, Weiler M, Chaimovitz C, Douvdevani A. Accessory role of human peritoneal mesothelial cells in antigen presentation and T-cell growth. Kidney Int. 2000;57:476–86. doi: 10.1046/j.1523-1755.2000.00867.x. [DOI] [PubMed] [Google Scholar]
- 13.Valle MT, Degl’Innocenti ML, Bertelli R, Facchetti P, Perfumo F, Fenoglio D, et al. Antigen-presenting function of human peritoneum mesothelial cells. Clin Exp Immunol. 1995;101:172–6. doi: 10.1111/j.1365-2249.1995.tb02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranzinger J, Rustom A, Abel M, Leyh J, Kihm L, Witkowski M, et al. Nanotube action between human mesothelial cells reveals novel aspects of inflammatory responses. PLoS One. 2011;6:e29537. doi: 10.1371/journal.pone.0029537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beum PV, Lindorfer MA, Peek EM, Stukenberg PT, de Weers M, Beurskens FJ, et al. Penetration of antibody-opsonized cells by the membrane attack complex of complement promotes Ca(2+) influx and induces streamers. Eur J Immunol. 2011;41:2436–46. doi: 10.1002/eji.201041204. [DOI] [PubMed] [Google Scholar]
- 16.Salter RD, Watkins SC. Dendritic cell altered states: what role for calcium? Immunol Rev. 2009;231:278–88. doi: 10.1111/j.1600-065X.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnston SA, Bramble JP, Yeung CL, Mendes PM, Machesky LM. Arp2/3 complex activity in filopodia of spreading cells. BMC Cell Biol. 2008;9:65. doi: 10.1186/1471-2121-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]