Abstract
Aim
Patients with metastatic osteosarcoma (OS) have a poor outcome with conventional therapies. Zoledronic acid (ZA) is a third-generation bisphosphonate that reduces skeletal-related events in many adult cancers, and preclinical data suggests a possible benefit in OS. This study assessed the maximum tolerated dose (MTD) and feasibility of ZA when combined with chemotherapy in patients with metastatic OS.
Patients and Methods
Patients with a histologic diagnosis of OS were eligible if they were <40 years of age, had initially metastatic disease, and met organ function requirements. Treatment combined surgery and a conventional chemotherapy regimen. ZA was given concurrent with chemotherapy for a total 8 doses over 36 weeks. Three dose levels of ZA were tested: 1.2 mg/m2 [max 2 mg], 2.3 mg/m2 [max 4 mg] and 3.5 mg/m2 [max 6 mg]. The MTD was determined during induction. Six patients were to be treated at each dose level, with an additional 6 patients treated at the MTD to help assess post-induction feasibility.
Results
Twenty-four patients (median age 13.5 years [range, 7-22]; 16 females) were treated. Five patients experienced dose limiting toxicities (DLTs) during induction, including 3 patients treated with 3.5 mg/m2. DLTs included hypophosphatemia, hypokalemia, hyponatremia, mucositis, limb pain and limb edema. There were no reports of excessive renal toxicity or osteonecrosis of the jaw. The MTD was defined as 2.3 mg/m2 (max 4 mg).
Conclusions
ZA can be safely combined with conventional chemotherapy with the MTD of 2.3 mg/m2 (max 4 mg) for patients with metastatic osteosarcoma.
Keywords: Metastatic Osteosarcoma, Zoledronic acid, Chemotherapy
INTRODUCTION
Osteosarcoma is the most common maligna nt bone tumor in children and young adults. Patients with metastatic disease at diagnosis have an estimated five-year event-free survival of less than 20% despite the use of aggressive surgical and medical therapy.1-8 To improve outcomes for these patients, it is imperative that new therapeutic approaches be identified.9
Zoledronic acid (ZA) is a potent third-generation bisphosphonate which targets the microenvironment of bone, improves bone strength and reduces tumor-related pain and skeletal related events in several adult cancers through inhibition of osteoclast activity and bone resorption. Successful studies using ZA together with systemic therapy have led to US Food and Drug Administration approval for use in adults with solid tumors and bone metastases.10-14 Preclinical studies suggest ZA has direct antitumor activity in a variety of tumors, including osteosarcoma. ZA has been shown to inhibit primary tumor growth, reduce lung metastases, and prolong survival in animal models of osteosarcoma.15-18 Bisphosphonates inhibit osteosarcoma cell line in a time- and dose-dependent manner.19, 20 In addition, ZA has been shown to possibly synergize with commonly used chemotherapy agents such as doxorubicin21 and ifosfamide.22
Side effects of bisphosphonates include electrolyte disturbances, flu-like symptoms, nausea, and less commonly, but more seriously, osteonecrosis of the jaw and renal dysfunction.23, 24 Because some of these side effects overlap with those from conventional chemotherapy agents used to treat osteosarcoma, a trial to determine the appropriate ZA dose in combination with these drugs, as well as the safety and feasibility of this approach was performed. An exploratory evaluation of the effect of ZA on event-free survival (EFS), necrosis grading and N-telopeptide levels (amarker of bone turnover) were conducted.
METHODS
Study Aims
The primary aims were: 1) to assess the feasibility and safety of adding ZA to the standard chemotherapy treatment of patients with newly diagnosed metastatic osteosarcoma, and 2) to determine the maximum tolerated dose of ZA when used in combination with other chemotherapy agents used to treat osteosarcoma. Secondary aims were: 1) to assess the histologic response and EFS in patients with metastatic osteosarcoma treated with standard chemotherapy and ZA compared to that of a similar cohort of patients treated on INT-0133 and CCG-7943, and 2) to test whether markers of bone resorption are associated with risk for analytic event in patients with metastatic osteosarcoma.
Patients
Enrollment was restricted to patients 40 years old or younger with newly diagnosed, biopsy-proven, high-grade metastatic osteosarcoma. Patients were classified as having pulmonary metastases if they had biopsy proven lung metastasis or if they had 3 or more lesions, each ≥ 5 mm in maximum diameter, or a single lesion ≥ 1 cm. Patients were classified as having bone metastases if they had confirmation of bone scintigraphy or plain radiograph abnormalities either by MRI scan or biopsy or both. Eligibility requirements included adequate renal function as defined as a normal serum creatinine level or creatinine clearance ≥70ml/min/1.73 m2; adequate liver function with bilirubin ≤ 1.5 × normal and AST or ALT ≤ 2.5 × normal; adequate cardiac function with fractional shortening on echocardiogram ≥ 29% or ejection fraction by radionuclide angiography ≥ 50%; and adequate bone marrow function with peripheral absolute neutrophil count (ANC) ≥ 1000/μL, platelet count ≥ 100,000/μL (transfusion independent) and hemoglobin ≥ 10g/dL (with or without RBC transfusions). All patients and/or their parents or legal guardians must have given informed consent, and all institutional and federal requirements for human studies must have been met.
Therapy
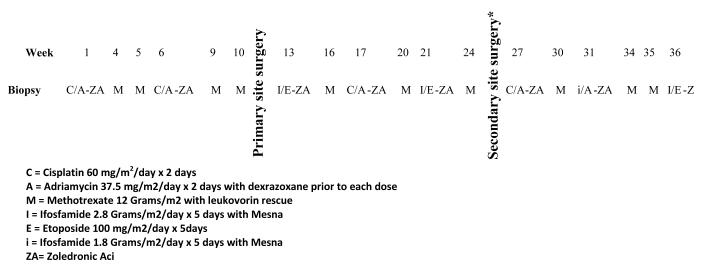
All patients received ZA in combination with conventional chemotherapy. The schema for therapy is depicted in Figure 1. ZA dose was assigned at study entry. The dose escalation plan involved 4 levels of ZA and cohorts of 6 patients per level, expanding to 12 patients at the MTD to better define short- and long-term toxicities of the therapy. The first cohort of patients received ZA at dose of 2.3 mg/m2 (max 4 mg) with plans to increase the dose to 3.5 mg/m2 (max 6 mg,) and then 4.6 mg/m2 (max 8 mg) if tolerated. Enrollment to a particular dose level was suspended after the sixth patient in the cohort was enrolled. During the times of suspension for safety analysis, up to 6 patients could be enrolled at the lower dose of 1.2 mg/m2 (max 2 mg). All patients were given age-appropriate supplementation of calcium and vitamin D.
Figure 1.
Treatment Schema for AOST06P1.
Toxicity
A dose level was considered tolerable if at most one patient experienced dose-limiting toxicity (DLT) during Induction (Weeks 1 through 12). If 2 or more patients at a particular dose level experienced DLT, the level was considered not tolerable and there was no further escalation. The MTD was defined as the highest dose at which no more than one patient experienced dose-limiting toxicity (DLT). Adverse events were graded using the NCI Common Toxicity Criteria (version 3.0). Hematological toxicity was considered dose-limiting if either ANC < 1000/μL or platelet count < 100,000/μL delayed scheduled surgery on the primary site by more than 2 weeks. Non-hematological DLT was defined as any grade 3 or 4 non-hematologic toxicity thought to be possibly, probably or definitely related to ZA with the exception of: grade 3 nausea and vomiting controlled with adequate antiemetic prophylaxis, grade 3 transaminase (AST/ALT) elevation that occurred during the evaluation period but resolved to ≤ grade 2 before the planned dose of therapy after definitive surgery, grade 3 fever or infection, grade 3 or 4 hypocalcemia, grade 3 mucositis, grade 3 fatigue that returned to ≤ grade 2 before the planned dose of therapy after definitive surgery, or grade 3 decreased joint range of motion or joint effusion related to the primary tumor. Each adverse event was defined as either unlikely or unrelated to ZA or possibly, probably or definitely related to ZA. Attribution to ZA was assigned by the primary oncologist at the treating institution.
The feasibility of administering ZA and ifosfamide was assessed in patients who received the combination of ifosfamide and ZA followed by methotrexate (2 weeks later), because this potentially nephrotoxic combination had not been previously investigated. The incidence of grade 4 renal toxicity associated with long-term administration of ZA was investigated. All patients who completed planned therapy or who had grade 4 renal toxicity after planned week 13 therapy that required a dose reduction of ZA were considered for this analysis. Grade 3 or higher renal toxicity was considered a contraindication for post-induction administration of ZA.
Outcome Analysis
Event-free survival (EFS) was defined as the time from enrollment until disease progression, death, or last patient contact, whichever came first. A patient who experienced disease progression or death was considered to have an analytic event; otherwise the patient was censored at last contact. Survival (S) was defined as the time from enrollment until death or last contact. Death, regardless of cause, was considered an event. Otherwise, the patient was censored at the date of last contact. EFS and S were estimated using the method of Kaplan and Meier.25 Disease outcome was compared with that reported for newly-diagnosed patients with metastatic disease enrolled on COG studies CCG-7943 26 and INT-0133 27 using the log-rank test (p ≤ 0.025). Histopathologic analysis of resected primary tumor (% tumor necrosis) was required on all patients at end of Induction for assessment of response to chemotherapy. The necrosis grading was quantified by maximum necrosis grading according to the system of Huvos. Greater than 90% necrosis was defined as a good response.
Bone Resorption Biological Assay
Participation in bone resorption studies was optional. Urine was to be collected at baseline prior to treatment, at the end of Induction, and at the end of therapy. All urine samples were processed and frozen until batch analysis. Urine samples were thawed at 4°C overnight and a commercially available immunoassay kit (Osteomark® NTx Urine [Wampole Laboratories, Princeton, NJ]) was used to measure urine NTx excretion in duplicate (coefficient of variation ≤ 10%). To normalize for fluctuations in renal clearance, urine NTx excretion was expressed in relation to urine creatinine concentrations (Parameter™ Creatinine [R&D Systems, Minneapolis, MN]) and reported in nanomolar bone collagen equivalents (BCE) per millimolar urine creatinine (nM BCE/mM creatinine).
RESULTS
Patient Population
AOST06P1 was opened in August 2008 and the last patient was enrolled in August 2010. All patients enrolled were eligible and are included in this report. Data current to December 2011 were used in this analysis. Twenty-four (24) patients with metastatic osteosarcoma were enrolled on AOST06P1. The characteristics of these 24 patients are summarized in Table 1. The primary disease sites were femur (15 patients), tibia (5 patients), humerus (2 patients), fibula (1 patient) and pelvis (1 patient). Twenty-one patients (87.5%) had lung involvement and 10 (42%) had metastatic disease involving the bones.
Table 1.
Patient Characteristics
| Characteristics | Number |
Percent
(%) |
|---|---|---|
| Age at enrollment (yrs) | ||
| 0-9 | 4 | 17.7 |
| 10-17 | 14 | 58.3 |
| 18+ | 6 | 25.0 |
| Sex | ||
| Male | 8 | 33.3 |
| Female | 16 | 66.7 |
| Race | ||
| White | 13 | 54.2 |
| Black | 7 | 29.2 |
| American Indian, Aleutian, or Eskimo | 1 | 4.2 |
| Other | 3 | 12.4 |
| Ethnicity | ||
| Non-Hispanic | 20 | 83.3 |
| Hispanic | 3 | 12.5 |
| Unknown | 1 | 4.2 |
| Primary tumor site | ||
| Humerus | 2 | 8.3 |
| Femur | 15 | 62.5 |
| Tibia | 5 | 20.8 |
| Fibula | 1 | 4.2 |
| Acetabulum | 1 | 4.2 |
| Metastatic tumor site(s) | ||
| One lung only | 5 | 20.8 |
| Both lungs only | 7 | 29.2 |
| Bone * | 10 | 41.7 |
| Other * | 2 | 8.3 |
*****Eight patients have metastases in bone and lung (s); two patients have just bone metastases.
One patient has metastases in lymph nodes and both lungs; another patient has just lymph node metastases.
Toxicity
The most common toxicity during induction attributed to ZA was reversible hypocalcemia. DLTs observed during the dose escalation part of the trial are listed in Table 2. There was one DLT at dose level 1 (1. 2mg/m2), one DLT at dose level 2 (2.3 mg/m2) and three DLTs at dose level 3 (3.5 mg/m2). Hypophosphatemia was the most commonly reported DLT (n= 5) and several patients had multiple defined DLTs.
Table 2.
Dose-Limiting Toxicities Observed During Induction
| Dose Level | Number of Patients Enrolled |
Number of Patients with Dose-Limiting Toxicity |
Dose-limiting Toxicities Observed |
|---|---|---|---|
| 1.2mg/m2 (Dose Escalation) |
6 | 1 | Grade 3 hypokalemia; grade 3 edema |
| 2.3mg/m2 (Dose Escalation) |
6 | 1 | Grade 3 hyopkalemia |
| 3.5 mg/ m2 (Dose Escalation) |
6 | 3 | Grade 3 hypophosphatemia (2 patients); hyponatremia (1 patient) |
| 2.3mg/m2 (After the MTD was Established) |
6 | 2 | Grade 3 hypophosphatemia; Grade 3 dehydration |
An additional 6 patients were enrolled at the defined MTD, a dose of 2.3mg/m2 [max 4mg], to investigate whether there are other less frequent toxicities and to assess post-induction toxicity and feasibility. Two of these six patients exhibited DLTs during induction. In total, of the 12 patients treated at 2.3 mg/m2, 3 had protocol defined DLTs, including two asymptomatic electrolyte disturbances and one grade 3 dehydration. Of the 12 patients treated at the MTD, five (5) received post induction therapy were evaluable for post-induction feasibility analysis. Of the seven removed from therapy at the end of induction, five progressed, one stopped because of dose-limiting toxicity and one refused further therapy (See Supplemental Table 1). One of the five patients exhibited secondary limiting toxicity in the post induction therapy. There were no reports of grade 3 or higher renal toxicity and no reports of osteonecrosis of the jaw. All reported grade 3 or higher toxicities by dose level and phase of therapy are included in Supplemental Table 2.
Outcome
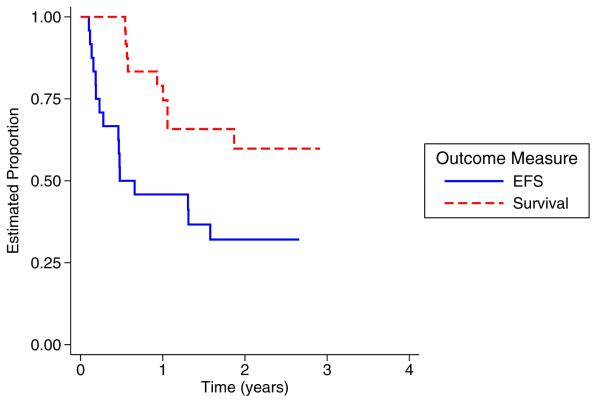
At the time of analysis for this study, 16 patients had experienced a relapse and nine (9) died subsequent to the relapse. Overall EFS and S for the 24 patients were 32% (95% confidence interval 15-51%) and 60% (95% confidence interval 36%-77%), respectively, at 2 years (Figure 2). According to the criteria above, EFS and S outcomes on AOST06P1 were not significantly different than that of INT-0133 (p = 0.079) or CCG-7943 (p = 0.16). The median time of followup for those who had not relapsed at the analytic time was 24 months (range 10 – 31 months). Of the 15 patients who underwent definitive surgery, 5 tumors exhibited a good necrosis grading (> 90% necrosis). Specifically, favorable histologic response was seen in 0 of 5 patients at dose level 1, 3 of 6 at dose level 2 and 2 of 4 at dose level 3.
Figure 2.
Kaplan-Meier overall survival (blue) and event free survival (dashed red) curves.
Bone Resorption Markers
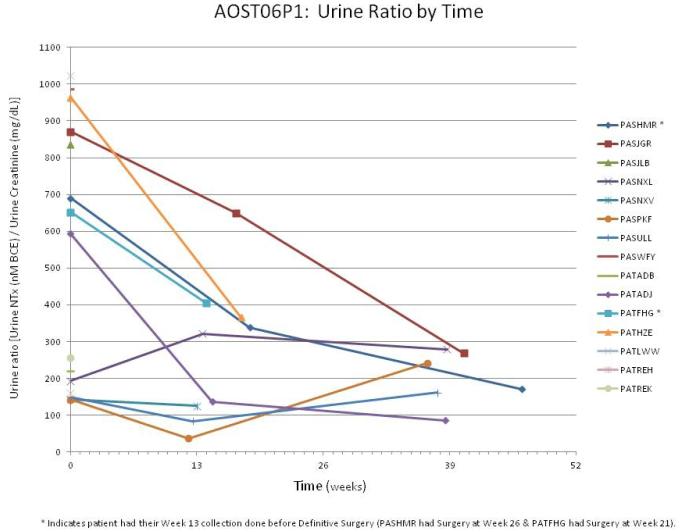
Thirty-one urine samples were obtained from 16 patients: 15 at baseline (before initiation of therapy), 10 collected during therapy, and 6 at the end of therapy. Of the 10 patients who had urine collected during therapy, the samples were obtained at median of 13.9 weeks from enrollment (12.3 to 18.6 weeks) and 8 of the 10 were collected after surgery for the primary tumor. Figure 3 shows the results of the N-telopeptide levels as adjusted for creatinine clearance for each patient by time. The median N-telopeptide urine ratio level decreased from a baseline of 593 nM BCE/mM creatinine (range, 142-1022 nM BCE/mM creatinine) to median level of 229 nM BCE/mM creatinine during therapy (range, 37-650 nM BCE/mM creatinine). Six samples were obtained at the end of therapy with a median level of 206 nM BCE/mM creatinine (range, 86-279 nM BCE/mM creatinine).
Figure 3.
Urine N-telopeptide to creatinine ratio plots by time for each subject submitting samples. Coded patient identifier is listed on the right side of the graph.
DISCUSSION
This multi-institutional study demonstrated that the addition of ZA to 5-drug chemotherapy is safe and feasible for children and young adults with metastatic osteosarcoma. Using this chemotherapy backbone, the MTD of ZA was 2.3 mg/m2 (max 4 mg), which is similar to the dose used in adults in combination with chemotherapy.28 Monitoring and replacement of electrolytes, particularly phosphorus and calcium, is necessary. Since the onset of hypocalcemia varies from a few days after the first treatment to several months after repeated infusions, it would be prudent to recommend extended monitoring and supplementation in future studies. This dose is lower than the MTD of 4 mg/m2 identified in a pediatric Phase I trial of ZA for relapsed neuroblastoma, although the chemotherapy backbone used in that study (oral cyclophosphamide) is much less intensive than employed in the current trial.29 Dosing decisions regarding ZA in future pediatric studies should take into account the therapeutic context in which it is being administered.
Despite the use of 5 conventional chemotherapy agents, outcomes remain unsatisfactory for patients with metastatic osteosarcoma. Targeted agents may have the potential for improving outcome without substantially increasing toxicity. ZA is attractive because of its proven tolerance in adults with metastatic solid tumors, and the preclinical activity demonstrated in various osteosarcoma models. Bisphosphonates may exert antineoplastic properties through inhibition of farnesyl-pyrophosphate synthase and disruption of the mevalonate biosynthetic pathway.30-32 Bisphosphonates may also influence the bone microenvironment and affect metastatic potential. Additionally, the reduced bone resportion with bisphosphonates could improve the integrity of endoprosthetic devices used in reconstructive surgeries.33 Genetic screening of osteosarcomas suggests that several genes involved with osteoclastogenesis or bone resorption are differentially expressed in chemotherapy-resistant pediatric osteosarcoma samples, thereby emphasizing the potential relevance of this therapeutic target.34
Due to potential toxicity overlap, it was essential to determine if ZA could be safely combined with a standard backbone of osteosarcoma treatment. Of particular interest was whether ZA would further exacerbate renal injury or serious electrolyte imbalances which may be associated with cisplatin and ifosfamide use. For example, up to 10% of adults with cancer who receive ZA experience some renal toxicity.24 Although a prior report in osteosarcoma patients suggested that concurrent use of pamidronate with osteosarcoma chemotherapy is safe and feasible, ZA is a far more potent bisphosphonate. 35 In our study, we found that hypocalcemia and hypophosphatemia were the most common side effects, but were generally manageable. These findings were consistent with other reported experiences with ZA in pediatric cancer patients.29, 36 None of the patients enrolled on this study experienced serious (grade 3 or higher) renal dysfunction or osteonecrosis of the jaw; however, risk of these serious complications will still require close monitoring in any future study. Additionally, there is a general lack of knowledge about long-term side effects of ZA use in children, but pre-clinical data suggest that it can affect bone growth and dental development.37, 38
A recent study evaluating the combination of chemotherapy and pamidronate (a less potent bisphosphonate) for patients with osteosarcoma demonstrated a promising 5-year EFS and S of 45% and 62%, respectively, for 11 patients with metastatic disease.35 The outcome of patients on the current study (AOST06P1) was not statistically different when compared with CCG-7943 and INT-0331 trials. However, each of these studies had different eligibility criterion and the patterns of initial sites of metastatic disease were different from AOST06P1. Additionally, the small number of patients enrolled in this Phase I study limits any such historical comparison. A randomized clinical trial evaluating the addition of ZA to chemotherapy in patients with localized osteosarcoma is being conducted in France, and the results of that study may better define the activity of adjuvant ZA.
Biochemical markers of bone turnover, such as N-terminal cross-linking telopeptide of type I collagen provide real-time information on the rate and extent of skeletal turnover which serves as a surrogate of bone pathology. Several studies in other adult cancers have shown bone turnover markers can indicate extent of disease and osteolytic activity, and can provide prognostic information.39-41 N-telopeptide markers tend to decrease in patients with osteosarcoma undergoing therapy, but the relative proportion attributed to ZA administration is unknown. Whether this marker that can be used to monitor disease response or assess prognosis requires additional investigation.
In conclusion, ZA can be safely added to the backbone of chemotherapy used to treat metastatic osteosarcoma. Future studies will hopefully define the potential benefit of ZA in patients with osteosarcoma.
Supplementary Material
Acknowledgements
Dr. Matthew Krasin for his expert input regarding radiation oncology, Kristy Lee Powell for her expert input regarding nursing issues and Andrew Ostrenga for his expert input regarding pharmacy issues. Additionally, Uhma Ganesan and Possi Gontijo were instrumental in providing administrative support as the Protocol Coordinator.
Financial Support: This work was supported in part by the COG NIH grant, Swim Across America, Mt Zion Foundation and Campini Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voute PA, Souhami RL, Nooij M, et al. A phase II study of cisplatin, ifosfamide and doxorubicin in operable primary, axial skeletal and metastatic osteosarcoma. European Osteosarcoma Intergroup (EOI). Ann Oncol. 1999;10(10):1211–8. doi: 10.1023/a:1008361612767. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Papadopolous N, Raymond AK, et al. A phase II study of cisplatin, doxorubicin, and ifosfamide with peripheral blood stem cell support in patients with skeletal osteosarcoma and variant bone tumors with a poor prognosis. Cancer. 2004;101(1):156–63. doi: 10.1002/cncr.20317. [DOI] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–8. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11(3):449–53. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 5.Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19(1):171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 6.Harris MB, Gieser P, Goorin AM, et al. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16(11):3641–8. doi: 10.1200/JCO.1998.16.11.3641. [DOI] [PubMed] [Google Scholar]
- 7.Goorin AM, Harris MB, Bernstein M, et al. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol. 2002;20(2):426–33. doi: 10.1200/JCO.2002.20.2.426. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Briccoli A, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: treatment with cisplatin, adriamycin and high dose of methotrexate and ifosfamide. Oncol Rep. 2000;7(2):339–46. doi: 10.3892/or.7.2.339. [DOI] [PubMed] [Google Scholar]
- 9.Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9(15):5442–53. [PubMed] [Google Scholar]
- 10.Hatoum HT, Lin SJ, Smith MR, Barghout V, Lipton A. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer. 2008;113(6):1438–45. doi: 10.1002/cncr.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98(5):962–9. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 12.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–44. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 13.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100(12):2613–21. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 14.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 15.Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol Cancer Ther. 2007;6(12 Pt 1):3263–70. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- 16.Labrinidis A, Hay S, Liapis V, Findlay DM, Evdokiou A. Zoledronic acid protects against osteosarcoma-induced bone destruction but lacks efficacy against pulmonary metastases in a syngeneic rat model. Int J Cancer. 2010;127(2):345–54. doi: 10.1002/ijc.25051. [DOI] [PubMed] [Google Scholar]
- 17.Labrinidis A, Hay S, Liapis V, Ponomarev V, Findlay DM, Evdokiou A. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clin Cancer Res. 2009;15(10):3451–61. doi: 10.1158/1078-0432.CCR-08-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104(11):2522–9. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 19.Sonnemann J, Eckervogt V, Truckenbrod B, Boos J, Winkelmann W, van Valen F. The bisphosphonate pamidronate is a potent inhibitor of human osteosarcoma cell growth in vitro. Anticancer Drugs. 2001;12(5):459–65. doi: 10.1097/00001813-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM. Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone. 2003;33(2):216–28. doi: 10.1016/s8756-3282(03)00223-0. [DOI] [PubMed] [Google Scholar]
- 21.Horie N, Murata H, Kimura S, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96(2):255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymann D, Ory B, Blanchard F, et al. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37(1):74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–7. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 24.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74(11):1385–93. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 25.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Second ed John Wiley and Sons, Inc; New York: 2002. [Google Scholar]
- 26.Seibel NL, Krailo M, Chen Z, et al. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: children’s Cancer Group (CCG) 7943. Cancer. 2007;109(8):1646–53. doi: 10.1002/cncr.22553. [DOI] [PubMed] [Google Scholar]
- 27.Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2009;115(22):5339–48. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berenson JR. Zoledronic acid in cancer patients with bone metastases: results of Phase I and II trials. Semin Oncol. 2001;28(2 Suppl 6):25–34. doi: 10.1016/s0093-7754(01)90262-3. [DOI] [PubMed] [Google Scholar]
- 29.Russell HV, Groshen SG, Ara T, et al. A phase I study of zoledronic acid and low-dose cyclophosphamide in recurrent/refractory neuroblastoma: a new approaches to neuroblastoma therapy (NANT) study. Pediatr Blood Cancer. 2011;57(2):275–82. doi: 10.1002/pbc.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9(Suppl 4):3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 31.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9(7):2394–9. [PubMed] [Google Scholar]
- 33.Wilkinson JM, Eagleton AC, Stockley I, Peel NF, Hamer AJ, Eastell R. Effect of pamidronate on bone turnover and implant migration after total hip arthroplasty: a randomized trial. J Orthop Res. 2005;23(1):1–8. doi: 10.1016/j.orthres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Mintz MB, Sowers R, Brown KM, et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res. 2005;65(5):1748–54. doi: 10.1158/0008-5472.CAN-04-2463. [DOI] [PubMed] [Google Scholar]
- 35.Meyers PA, Healey JH, Chou AJ, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011;117(8):1736–44. doi: 10.1002/cncr.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.August KJ, Dalton A, Katzenstein HM, et al. The use of zoledronic acid in pediatric cancer patients. Pediatr Blood Cancer. 2011;56(4):610–4. doi: 10.1002/pbc.22681. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia S, Dumoucel S, Chesneau J, et al. Impact of oncopediatric dosing regimen of zoledronic acid on bone growth: preclinical studies and case report of an osteosarcoma pediatric patient. J Bone Miner Res. 2011;26(10):2439–51. doi: 10.1002/jbmr.453. [DOI] [PubMed] [Google Scholar]
- 38.Hiraga T, Ninomiya T, Hosoya A, Nakamura H. Administration of the bisphosphonate zoledronic acid during tooth development inhibits tooth eruption and formation and induces dental abnormalities in rats. Calcif Tissue Int. 2010;86(6):502–10. doi: 10.1007/s00223-010-9366-z. [DOI] [PubMed] [Google Scholar]
- 39.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23(22):4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 40.Hirsh V, Major PP, Lipton A, et al. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol. 2008;3(3):228–36. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 41.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113(1):193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.