Abstract
Purpose of review
This review summarizes new evidence for an intrinsic control system in the aldosterone-sensitive distal nephron in which purinergic signaling regulates sodium transport and governs renal sodium excretion.
Recent findings
Electrophysiological studies identify epithelial Na+ channels (ENaC) as final effectors of purinergic signaling via P2Y2 receptors in the distal nephron. Inhibition of ENaC by autocrine/paracrine purinergic signaling reduces sodium reabsorption allowing an appropriately graded pressure-natriuresis response when delivery of sodium to the distal nephron is high. Disruption of this intrinsic control mechanism decreases sodium excretion and therefore has a pro-hypertensive effect. Because purinergic inhibition of ENaC is tonic yet submaximal, its enhancement increases sodium excretion and therefore has an anti-hypertensive action.
Summary
Purinergic inhibitory regulation of ENaC is a key component of an intrinsic control system that enables the distal nephron to respond appropriately to the delivered load of sodium. This control system is physiologically important and functions in parallel with extrinsic control by the renin-angiotensin-aldosterone system, enabling sodium excretion to keep pace with sodium intake, especially when intake is high, and thereby maintain arterial blood pressure. Disruption of intrinsic control of sodium transport by the distal nephron likely contributes to diseases such as arterial hypertension.
Keywords: P2Y2, ENaC, ATP, hypertension, sodium transport
Introduction
Renal sodium excretion influences arterial blood pressure (ABP) by affecting blood volume and electrolyte composition. Blood pressure is inversely coupled to sodium excretion through negative-feedback regulation by the renin-angiotensin-aldosterone system (RAAS). Sodium excretion is fine-tuned by the adrenal mineralocorticoid aldosterone, which acts at principal cells in the aldosterone-sensitive distal nephron (ASDN) to promote reabsorption of sodium from urine. Activity of epithelial Na+ channels (ENaC), which are localized to the apical membrane of principal cells, is limiting for this sodium reabsorption. As blood pressure and/or effective circulating volume fall, secretion of aldosterone increases and stimulates ENaC in the ASDN to limit sodium excretion in defense of ABP. Mutations that alter the activity or proper regulation of ENaC by the RAAS have been shown to change ABP by affecting renal sodium excretion. Thus, dysregulation of ENaC is a recognized cause of inheritable forms of blood pressure disorders [1-3].
Whereas RAAS regulation of ENaC is a major determinant of renal sodium excretion, this alone does not fully explain the relation between ASDN-dependent sodium excretion, ABP and sodium balance. This is apparent, for example, in the phenomenon of “aldosterone-escape” in which sodium excretion remains robust in the face of a positive sodium balance and elevated levels of circulating mineralocorticoid [4;5]. Moreover, it should be emphasized that although the temporal coupling between changes in ABP and renal sodium excretion is tight, pressure-induced changes in circulating aldosterone is comparatively slow. In addition, ENaC have substantial activity in the ASDN of adrenalectomized animals where mineralocorticoid is completely absent (Stockand JD, personal communication). This suggests that whereas aldosterone is robustly capable of increasing ENaC activity, its absence is less effective at decreasing it.
As posited by Guyton, gain in the relation between changes in ABP and sodium excretion is steep (i.e., infinite) in the presence of a normal-functioning kidney [6]. The question then is what decreases the activity of ENaC to facilitate sodium excretion in states with low RAAS activity, such as high ABP and/or high salt intake? Emerging evidence, as discussed here, suggests that a control mechanism intrinsic to the ASDN tightly couples ENaC activity to the flow of urine or to the load of sodium delivered by more proximal segments of the nephron. This control mechanism functions together with the RAAS to precisely tune the activity of ENaC. It is dependent on nucleotide agonists in urine that signal in a paracrine/autocrine manner via luminal purinergic receptors to reduce sodium reabsorption as urine sodium delivery rises, thereby enabling an appropriately graded pressure-natriuresis response. Disruption of intrinsic control in the ASDN decreases sodium excretion and promotes a rise of ABP. Figure 1A contains an illustration of the components of this intrinsic control system.
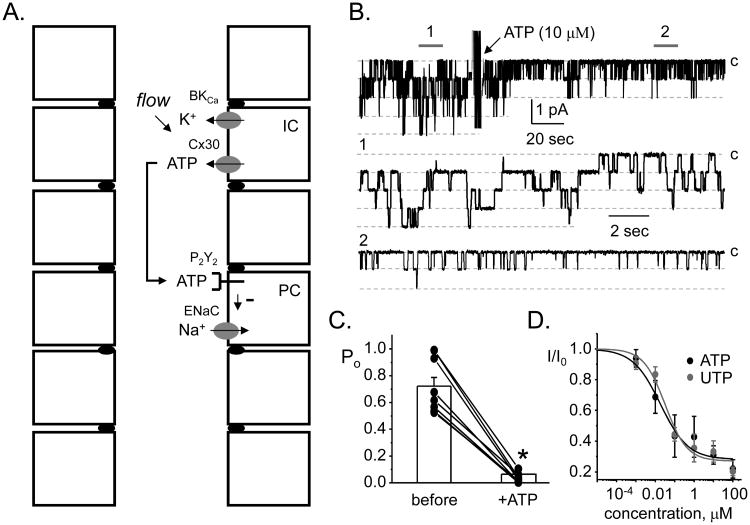
Figure 1. Inhibitory purinergic regulation of ENaC mediates intrinsic control of sodium transport in the ASDN.
A. A schematic of the ASDN showing the components mediating intrinsic control. B. A single channel, cell-attached patch clamp experiment on a principal cell in the isolated, split-open ASDN showing the effects of ATP on ENaC in the apical membrane. In this experiment, inward current is downwards and the closed state is noted with C. Areas below the gray bars, 1 and 2, are shown below on a faster time scale. Data originally presented in [10]. C. Summary of these effects of ATP on ENaC open probability in isolated, split open ASDN. Experiments were similar to that shown in 1B. Data originally presented in [8]. *significant decrease vs. before treatment. D. Dose response curves showing the effects of ATP and UTP on ENaC activity in isolated, split-open ASDN. Experiments similar to that shown in 1B. Data originally presented in [10].
ENaC is the final effector of purinergic signaling in the ASDN
Recent electrophysiological studies identified ENaC as final effectors of inhibitory purinergic signaling in the mammalian ASDN [7-10]. These detailed patch clamp studies were performed on the isolated, split-open mammalian ASDN and are definitive in the sense that they directly quantify effects of purinergic agonism on the open probability of ENaC in paired, single channel experiments. As shown by the results re-presented in figure 1B-D [10], both ATP and UTP at physiological concentrations decrease the activity of ENaC in cell-attached patches in a manner consistent with a receptor mediated mechanism [10-12]. Purinergic agonists decrease activity by affecting gating to reduce open probability [8-10;13]. Actions of purinergic agonists on ENaC are rapid and reversible, consistent with tight spatial and temporal coupling between the receptor and channel.
P2Y2 receptors signal inhibitory purinergic regulation of ENaC in the ASDN
Purinergic agonists target two types of receptors: ionotropic P2X and metabotropic P2Y [12]. The former are ligand-gated, non-selective cation channels, whereas the latter are seven transmembrane-spanning G-protein coupled receptors (GPCR) linked by Gαq/11 to phopholipase C (PLC). Receptor subtype specific agonists and antagonists in patch clamp experiments assaying the activity of ENaC identified apical P2Y2 receptors as responsible for most of the inhibitory purinergic regulation in the isolated, split-open ASDN [8]. Perhaps the most definitive proof for the importance of P2Y2 receptors in mediating inhibitory control is the finding that gene deletion of this receptor abolishes ATP regulation of ENaC [8;9].
Signaling pathway coupling P2Y2 receptors to ENaC
It follows logically from the findings described above that cellular transduction pathways in the ASDN coupling the P2Y2 receptor to ENaC include signaling through Gαq/11 to PLC. Effects of PLC inhibitors on ATP-dependent regulation of ENaC in the ASDN are consistent with this [7;8]. In addition, receptor-independent activation of PLC mimics inhibitory effects on ENaC of ATP-induced activation of P2Y2 receptors. Moreover, the reverse is also true: inhibition of PLC mimics actions of antagonism and knockout of this receptor [8;13].
Neither IP3-dependent calcium signaling nor DAG-dependent PKC activation, which are expected to occur downstream of PLC stimulation, contribute to ATP-dependent regulation of ENaC in the ASDN. Instead, findings point to depletion of apical membrane phosphoinositide 4,5-bisphosphate (PIP2) by PLC-mediated hydrolysis [8;13;14]. Consistent with this mechanism, membrane PIP2 is required for normal ENaC activity with its depletion causing decreased open probability [13;15].
Molecular determinants and mechanisms that enable ENaC to respond to purinergic signaling
Detailed mutagenesis studies of ENaC identified two regions important for regulation by phosphoinositides. These include cytosolic residues in the extreme amino-terminus and those immediately following the second transmembrane domain [15;16]. Both regions are rich in conserved basic residues. Deletion or charge-neutralization of these basic residues disrupts phosphoinositide regulation of channel activity. The activities of several types of ion channels are dependent on membrane PIP2 levels [16-18]. Bona-fide PIP2 binding sites in these channels are rich in conserved basic residues. This suggested that similar sites in ENaC are also phosphoinositide-binding sites. Supporting this conclusion are findings that ENaC does indeed bind phosphoinositides [19;20]. Deletion of basic residues in these putative binding sites disrupts phosphoinositide binding. Residues at the amino-terminus of ENaC appear particularly important for PIP2 regulation. The latter is evident in data showing that deletion and neutralization of basic residues in this region not only disrupts PIP2 regulation but locks the channel in a normal gating state as well [15]. Such an observation is consistent with the amino-terminus of the channel functioning as a negative regulator of open probability when bound membrane PIP2 is absent. Accordingly, PIP2 binding sequesters this negative regulator allowing the channel to gate normally.
Inhibitory purinergic regulation of ENaC is tonic and intrinsic to the distal nephron
Recent evidence indicates that purinergic tone in the ASDN is mediated by locally released nucleotides acting in an autocrine/paracrine capacity. By positioning a biosensor in direct contact with the apical membrane of cells in an isolated, perfused ASDN, Sipos and colleagues [21] demonstrated physiologically important ATP release from intercalated cells in this nephron segment. Inasmuch as these findings indicate that intercalated cells are an important source of nucleotide secretion in the ASDN and that the final effector, ENaC, is localized to principal cells, a paracrine mode of nucleotide action is implicated. The specific modality sensed to trigger inhibitory ATP release, be it flow, pressure or even urinary sodium concentration, though, has yet to be firmly established.
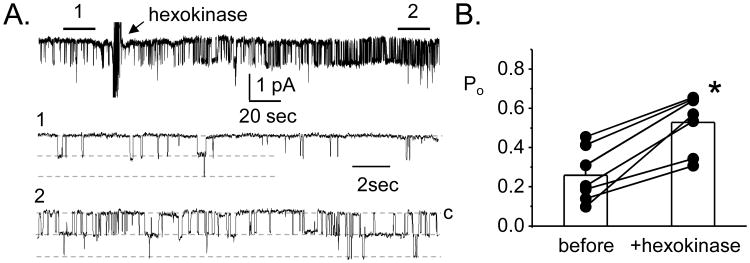
Additional support for local ATP release from the ASDN comes from using the activity of ENaC as a reporter for purinergic signaling. The broad-spectrum purinergic receptor inhibitor suramin and an enzyme capable of consuming endogenous ATP, hexokinase, both rapidly increase the activity of ENaC in isolated, split-open ASDN naive to exogenous purinergic stimulation [8;9;10]. The results re-presented in figure 2 show the effects of hexokinase on ENaC [8]. This tone is absent in P2Y2 receptor knockout mice as demonstrated by ENaC having a higher basal activity in ASDN isolated from these mice and by the lack of response to suramin/hexokinase. Such findings demonstrate that the ASDN in isolation contains all the components of a local purinergic control system governing sodium reabsorption, and that this system has tone. Further support of this conclusion comes from evidence that ATP release and urinary ATP levels are significantly lower and ENaC activity is higher in connexin 30 (Cx30) knockout mice compared to wild-type contorls [21;22]. In the nephron, Cx30 is expressed only in the apical membranes of intercalated cells, and Cx30 hemichannels function as conduits for nucleotide secretion in the ASDN [21;23;24].
Figure 2. There is purinergic tone in the ASDN.
A. A single channel, cell-attached patch clamp experiment showing the effects of hexokinase on ENaC activity in an ASDN naïve to exogenous ATP. In this experiment, inward current is downwards. Areas below the bars, 1 and 2, are shown below on a faster time scale. B. Summary showing the effects of hexokinase on ENaC activity in patch clamp experiments similar to that in 2A. *significant increase vs. before treatment with hexokinase. Data originally presented in [8].
Investigation of the relation between BKCa channels and ATP release in the ASDN provides additional support for the importance of intercalated cells as a source of urinary ATP [25]. BKCa channels are localized principally to the apical membrane of intercalated cells in the ASDN. BKCa channels respond to mechanical stimulation, such as shear-stress resulting from changes in flow, and are known to mediate flow-dependent K+ secretion in the ASDN [26-28]. SiRNA knockdown of a functionally important subunit of the BKCa channel, the BK-β4 subunit, decreases ATP release and K+ secretion in immortalized intercalated cells held in culture [25]. The connexin inhibitor, carbenoxolone, has a similar effect on ATP release in these cultured cells. Moreover, BK-β4 knockout mice with high rates of distal urine flow have significantly less ATP in their urine compared to wild-type animals under identical conditions. The activity of ENaC in isolated, split-open ASDN from these knockout animals is also elevated consistent with decreased tonic regulation by inhibitory purinergic signaling (Stockand JD, personal communication). Such findings suggest coupled electrochemical efflux between K+ and ATP as part of the mechanism for shear-stress-induced ATP release in intercalated cells.
Changes in urinary nucleotide levels reflect ABP/sodium balance
Increases in the rate of flow of the perfusate stimulate ATP secretion from intercalated cells in the isolated, perfused ASDN [21]. Moreover, connexin hemichannels, which mediate at least part of this nucleotide secretion are sensitive to mechanical stimulation [21;24]. These findings establish a cause and effect relation between factors influencing urine flow with nucleotide secretion at the ASDN. A positive correlation between dietary sodium and urinary nucleotide concentration extends this relation to the systemic level [9;10;25]. Thus, nucleotide secretion at the ASDN ultimately reflects ABP and/or sodium balance. Consistent with this, increased dietary sodium fails to increase urinary ATP levels in Cx30 knockout mice [10;22]. Moreover, Cx30 knockout mice on a high sodium diet excrete less sodium: have a lower fractional excretion of sodium (FeNa) compared to wild-type animals treated in an identical manner [22]. A decrease in FeNa, by definition, points to impaired tubule function likely reflecting inappropriate sodium retention by the ASDN. Supporting this is the finding that Cx30 knockout animals maintained on a high sodium diet have elevated ABP i.e., salt-sensitive hypertension, that is ameliorated by treatment with amiloride [21]. Amiloride blocks ENaC to increase renal sodium excretion. Cx30 knockout mice also have an impaired pressure-natriuresis response, excreting less sodium and urine volume at high ABP compared to wild-type animals [21]. This impaired pressure-natriuresis response reflects inappropriate sodium retention by the ASDN providing a mechanism for the observed salt-sensitive increase of ABP.
Urinary nucleotide levels link the activity of ENaC in the ASDN to changes in ABP
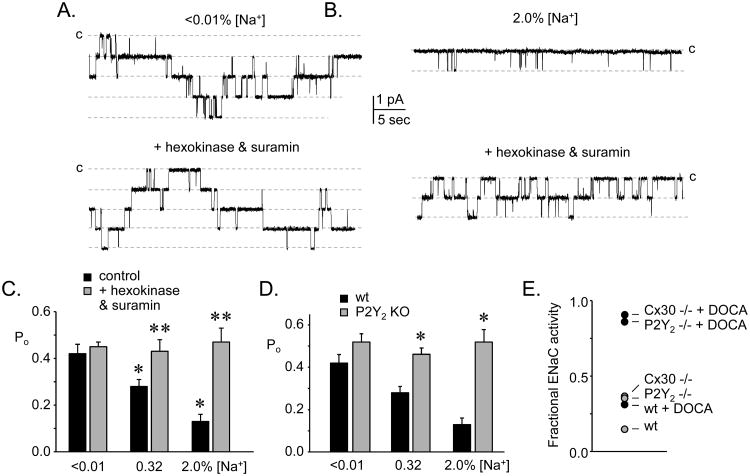
As shown by results represented in figure 3, the activity of ENaC is inversely related to dietary sodium intake [9;29]. Clearly this reflects regulation, in part, by RAAS particularly when sodium intake is low and RAAS activity is high. The capacity of ENaC to respond to a change in sodium intake, as reflecting feedback regulation, can be quantified by dividing the activity of channels in the isolated ASDN of animals maintained on a high sodium diet by that of channels from animals maintained on a sodium free regimen [10;22]; see figure 3E). As this fractional ENaC activity approaches zero, capacity to excrete sodium rises. Conversely, as fractional ENaC activity approaches unity, sodium excretion capacity falls. In a corresponding fashion, ABP is expected to be normal in the first instance and elevated in the latter, particularly in the presence of increased sodium intake.
Figure 3. Purinergic inhibition of ENaC allows this channel to respond appropriately to sodium balance.
Single channel current traces of ENaC in cell attached patches on ASDN isolated from mice maintained on a sodium free (A) and a high sodium diet (B) without (top) and with (bottom) addition of suramin plus hexokinase to the bathing solution. Inward current is downward with closed state noted with C. *significant decrease compared to sodium free (<0.01%) condition; **significant increase compared to control. Data originally presented in [9]. C. Summary graph showing the effects on ENaC activity of suramin plus hexokinase (gray bars) as compared to basal activity (black bars) in ASDN from mice fed a sodium free, regular sodium and high sodium diet. *significant increase compared to wild-type under identical feeding conditions. Data originally presented in [9]. D. Summary graph comparing ENaC activity in ASDN from wild-type (black bars) versus P2Y2 receptor knockout (gray bars) mice maintained with sodium free, regular sodium and high sodium diets. Data originally presented in [9]. E. Summary graph showing fractional ENaC activity (activity with high sodium feeding / activity with sodium free feeding) in wild-type (wt) and P2Y2 receptor and Cx30 knockout mice in the absence (gray circles) and presence (black circles) supplementing with DOCA. Activity calculated as fNPo where f is the frequency of observing at least one active ENaC in a patch, N is the mean number of ENaC per patch that contained active channels, and Po is open probability. Data originally presented in [10] and [22].
Activity of ENaC in P2Y2 receptor knockout mice, as noted above, is elevated compared to wild-type animals, and blockade of P2Y2 receptors and metabolism of ATP have no effect on the activity of channels in ASDN harvested from knockout mice in contrast to their effects in wild-type controls [8-10]; see also figure 3). The explanation for this is that ENaC in P2Y2 receptor knockout animals is incapable of sensing or responding to ATP. Bypassing the receptor by directly stimulating PLC, though, does decrease ENaC activity in P2Y2 receptor knockout mice [8]. Fractional ENaC activity in the P2Y2 receptor knockout mouse is elevated compared to that in wild-type mice and is nearing equal to that in wild-type mice where RAAS signaling has been saturated by treatment with DOCA [10;22]; see also figure 3E). With respect to ENaC, this demonstrates that loss of purinergic regulation is functionally equivalent to saturated regulation by RAAS. ENaC activity also is elevated in Cx30 mice and does not respond to suramin or hexokinase [22]. This is because the capacity of the ASDN to secrete ATP is compromised in this latter case such that ENaC cannot respond to factors that influence ATP levels or signaling since the nucleotide is simply not present. Fractional ENaC activity in Cx30 knockout animals is also elevated compared to wild-type animals and is similar to that in P2Y2 receptor knockout animals and wild-type animals treated with DOCA. However, ENaC in both Cx30 and P2Y2 receptor knockout animals retain some of their ability to respond to changes in dietary sodium and aldosterone, albeit with greater activity across all dietary sodium regimens, particularly with high sodium feeding, compared to wild-type animals [9;10;22].
A major effect of aldosterone on ENaC in both wild-type and knockout animals is to increase channel number, whereas knockout of P2Y2 receptor and Cx30 increases only channel open probability [9;10;22]. This difference in final effect likely explains how intrinsic regulation is able to function in parallel with regulation by the RAAS (discussed below). Within this framework of parallel extrinsic (i.e., RAAS) and intrinsic (i.e., purinergic) regulation, the increased activity and compromised capacity of ENaC to respond to changes in sodium intake (shown as elevated fractional ENaC activity) in knockout animals almost certainly reflects disruption of regulation by ATP signaling. Indeed, the latter is consistent with evidence for increased activity of ENaC in wild-type but not knockout animals during interdiction of purinergic regulation by suramin or hexokinase [9;10;22]. Linking sodium intake-dependent ATP secretion in the ASDN to control of ENaC activity is the finding that the fold increase of ENaC activity in response to blockade of purinergic signaling is greater in wild-type animals fed a high sodium diet compared to animals fed a low sodium diet in which there is no observed response [9;10;22]; see figure 3C). Thus, fractional ENaC activity is elevated in knockout animals because they are incapable of responding to changes in urinary nucleotide levels, as the latter reflect ABP/sodium intake. However, ENaC in these knockout animals retain their full capacity to respond to RAAS signaling which also reflects ABP/sodium intake. This is consistent with intrinsic regulation and extrinsic regulation by RAAS operating in parallel, though often asynchronously, to control ENaC function, with stimulation of the former lowering activity and the latter increasing activity to provide a complete continuum of control. Consistent with this, saturating aldosterone levels in Cx30 and P2Y2 receptor knockout mice markedly increase fractional ENaC activity pushing it towards unity indicating the almost complete loss of the ability of ENaC to respond to feedback regulation [10;22]. As expected, this treatment in knockout mice also compromises sodium excretion and markedly increases ABP in a sodium dependent manner compared to untreated knockout animals and wild-type animals treated with DOCA [9;10;21;22;30].
Evidence that purinergic and RAAS regulation operate in parallel to fine-tune ENaC activity in the ASDN
Perhaps the strongest evidence to date that intrinsic control and regulation by RAAS function in parallel to modulate ENaC activity comes from studies investigating the role of purinergic signaling in aldosterone-escape [10;22]. This is when sodium excretion is elevated appropriately in the presence of a sodium load despite high levels of aldosterone [4;5]. Wild-type animals display aldosterone-escape, in part, because ENaC activity cannot be saturated by RAAS signaling alone in the presence of a positive sodium balance [9;10]. Under these conditions, intrinsic control is providing a counterbalance to the inappropriate, considering the context of sodium balance, actions of RAAS on ENaC. This is protective. Knockout of the P2Y2 receptor or Cx30 compromises this protection [10;22]. In the presence of a saturating level of aldosterone, sodium excretion and ENaC activity are lower and higher, respectively, in knockout mice compared to wild-type animals under a variety of sodium intake regimens. As noted above, fractional ENaC activity approaches 1.0 in knockout mice treated with aldosterone but is only ∼0.25 in wild-type animals. Moreover, knockout but not wild-type animals treated with aldosterone and consuming a high sodium diet have elevated ABP [9;10;21;22;30]. Thus, intrinsic regulation of ENaC by purinergic signaling is necessary for aldosterone-escape and loss of this regulation disturbs the normal regulation of ABP.
Physiological consequences of purinergic regulation of ENaC
The above discussion begins to reveal the physiological importance of intrinsic control of renal sodium excretion as mediated by paracrine regulation of ENaC in the ASDN. This system enables the distal nephron to respond appropriately to upstream input and to thereby maintain ABP within a normal range by increasing renal sodium and volume excretion. Restating this, intrinsic control functioning in parallel with RAAS enables appropriate compensatory pressure-natriuresis at elevated ABP. An example of how this intrinsic control system impacts systemic regulation of ABP is the recent finding that specific P2Y2 receptor agonism dose-dependently decreases ABP in wild-type but not P2Y2 receptor knockout mice [31]. In parallel, P2Y2 receptor agonism dose-dependently increased urinary sodium excretion in wild-type but not knockout animals by increasing FeNa and fractional excretion of fluid without affecting glomerular filtration rate. Similar to Cx30 knockout mice, P2Y2 receptor knockout mice have an impaired pressure-natriuresis response [30-32]. Again, this provides the mechanism for hypertension in these latter animals, particularly in the presence of a high sodium diet.
Pathophysiological consequences of disrupting purinergic regulation of ENaC
Knockout mice lacking the P2Y2 receptor have increased ABP in the presence of low RAAS activity [30]. This increase of ABP is associated with decreased plasma K+ levels and impaired renal sodium excretion. Because potassium secretion counterbalances sodium reabsorption in the mammalian ASDN and because RAAS is normally suppressed in the face of elevated ABP, the findings above indicate that the ASDN is dysfunctional in P2Y2 knockout mice and that sodium reabsorption is improperly elevated due to inappropriately activated ENaC even in the presence of suppressed RAAS. The renal and ABP phenotype of P2Y2 receptor knockout mice are consistent with those of transgenic and knock-in mice with hyperactive ENaC [33-35] and humans with Liddle's syndrome: a form of hypertension resulting from gain-of-function mutations in ENaC [1;36]. Amiloride also ameliorates the renal and ABP phenotypes in these latter cases. In fact, all of the above represent variations of end-organ resistance to the normal feedback signals that target ENaC to influence ABP. P2Y2 receptor knockout mice simply represent end-organ resistance to intrinsic regulation as mediated by paracrine actions of ATP.
Renal dysfunction and salt-sensitive hypertension of Cx30 knockout mice similarly represent improper regulation of ENaC by disrupted intrinsic control [21;22]. There are several related forms of inheritable hyper- and hypo-tension associated with abnormal renal sodium handling in the ASDN, to include apparent mineralocorticoid excess, glucocorticoid remediable aldosteronism, Geller syndrome and pseudohypoaldosteronism type I [1-3]. In contrast to P2Y2 receptor and Cx30 knockout animals, these represent variations of dysfunctional feedback control of ENaC by RAAS signaling or surrogates of this system. This predicts that the pathophysiological consequences of disrupting intrinsic control should mirror, in a general sense, improper regulation by RAAS. Current findings agree with this.
Conclusion
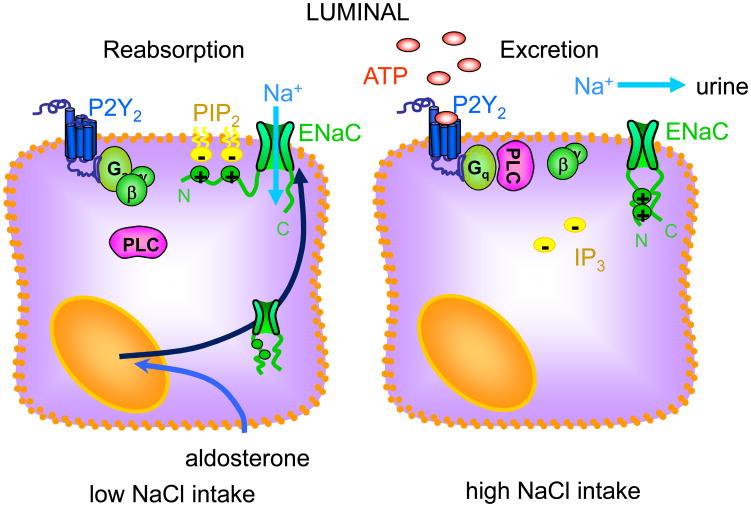
Regulation of ENaC in the ASDN by an intrinsic control system that relies on purinergic signaling, as illustrated in figure 4, enables the kidney to match sodium excretion to sodium balance to maintain ABP in a normal range. This intrinsic control system functions in parallel with RAAS. Compromise of intrinsic control, similar to RAAS, affects ABP through dysregulation of renal sodium excretion.
Figure 4. Intrinsic control of ENaC by inhibitory purinergic signaling facilitates sodium excrtion.
Cellular model showing the protein players and signals modulating intrinsic control of ENaC in the ASDN.
Key points.
The ASDN contains an intrinsic control system that allows renal sodium excretion to be matched to systemic sodium balance to maintain ABP within a normal range.
Intrinsic control is mediated by paracrine signaling via P2Y2 receptors on principal cells.
ENaC are final effectors of inhibitory purinergic signaling in the ASDN.
Loss of intrinsic control decreases sodium excretion and increases ABP.
Renal and ABP phenotypes resulting from dysfunction of intrinsic control are expected to mirror those of aberrant RAAS signaling and ENaC activity.
Acknowledgments
The authors have no conflict of interest to report. Research in the Stockand laboratory is supported by NIH grants R01 DK59594, R01 DK087460 and R01 DK070571 and the AHA grant EIA 0640054N. Research in the Vallon laboratory is supported by R01 HL094728, R01 DK28602, P2 GM066232 and P30 DK079337, and AHA Grant in Aid GRNT344038. Research in the Toney laboratory is supported by R01 HL102310 and P01 HL088052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Schild L. The epithelial sodium channel and the control of sodium balance. Biochim Biophys Acta. 2010;1802:1159–1165. doi: 10.1016/j.bbadis.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Bonny O, Hummler E. Dysfunction of epithelial sodium transport: From human to mouse. Kidney Int. 2000;57:1313–1318. doi: 10.1046/j.1523-1755.2000.00968.x. [DOI] [PubMed] [Google Scholar]
- 4.Knox FG, Burnett JC, Jr, Kohan DE, et al. Escape from the sodium-retaining effects of mineralocorticoids. Kidney Int. 1980;17:263–276. doi: 10.1038/ki.1980.32. [DOI] [PubMed] [Google Scholar]
- 5.Hall JE, Granger JP, Smith MJ, Jr, Premen AJ. Role of renal hemodynamics and arterial pressure in aldosterone “escape”. Hypertension. 1984;6:I183–I192. doi: 10.1161/01.hyp.6.2_pt_2.i183. [DOI] [PubMed] [Google Scholar]
- 6.Guyton AC. The surprising kidney-fluid mechanism for pressure control - its infinite gain! Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 7.Wildman SS, Marks J, Turner CM, et al. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol. 2008;19:731–742. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pochynyuk O, Bugaj V, Rieg T, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Pochynyuk O, Rieg T, Bugaj V, et al. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. 2010;24:2056–2065. doi: 10.1096/fj.09-151506. This article is the first report of regulation of ENaC by inhibitory purinergic tone in the isolated, split-open ASDN. Results in this article begin to demonstrate that loss of this regulation impairs sodium excretion and elevates blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **10.Stockand JD, Mironova E, Bugaj V, et al. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol. 2010;21:1903–1911. doi: 10.1681/ASN.2010040377. This article establishes that loss of purinergic regulation of ENaC in P2Y2 receptor knockout animals abolishes aldosterone-escape. Such findings demonstrate the physiological relevance of purinergic inhibition of ENaC and identify how this intrinsic control system functions in parallel with RAAS to fine-tune ENaC activity and plasma sodium balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Vallon V, Rieg T. Regulation of Renal NaCl and Water Transport by the ATP/UTP/P2Y2 Receptor System. Am J Physiol. 2011 doi: 10.1152/ajprenal.00236.2011. This is an in depth review of purinergic signaling and how such signaling influences salt and water transport in the kidney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol. 2008;294:F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 13.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol. 2008;294:F38–F46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 14.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens. 2008;17:533–540. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pochynyuk O, Tong Q, Medina J, et al. Molecular Determinants of PI(4,5)P2 and PI(3,4,5)P3. Regulation of the Epithelial Na+ Channel. J Gen Physiol. 2007;130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pochynyuk O, Tong Q, Staruschenko A, Ma HP, et al. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol. 2006;290:F949–F957. doi: 10.1152/ajprenal.00386.2005. [DOI] [PubMed] [Google Scholar]
- 17.Rosenhouse-Dantsker A, Logothetis DE. Molecular characteristics of phosphoinositide binding. Pflugers Arch. 2007;455:45–53. doi: 10.1007/s00424-007-0291-6. [DOI] [PubMed] [Google Scholar]
- 18.Logothetis DE, Petrou VI, Adney SK, Mahajan R. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 2010;460:321–341. doi: 10.1007/s00424-010-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol. 2007;580:365–372. doi: 10.1113/jphysiol.2006.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pochynyuk O, Staruschenko A, Tong Q, et al. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 21.Sipos A, Vargas SL, Toma I, Hanner F, et al. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Mironova E, Peti-Peterdi J, Bugaj V, et al. Diminished paracrine regulation of the epithelial Na+channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286:1054–1060. doi: 10.1074/jbc.M110.176552. This article demonstrates that ENaC is inappropriately hyper-active in Cx30 knockout mice, and that renal function is impaired in these mice. Moreover, the findings in this study demonstrate that ATP secretion through Cx30 is required for aldosterone-escape. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol. 2005;289:F1304–F1312. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 24.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol. 2010;298:R1143–R1155. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC. Coupled ATP and potassium efflux from intercalated cells. Am J Physiol. 2011;300:F1319–F1326. doi: 10.1152/ajprenal.00112.2011. This article provides support for the idea that coupled electrodiffusion of ATP with K+ underlies flow sensitive nucleotide release from intercalated cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieg T, Vallon V, Sausbier M, Sausbier U, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Wei Y, Sun P, et al. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct: role of protein kinases A and C. Am J Physiol. 2009;297:F904–F915. doi: 10.1152/ajprenal.90685.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Morimoto T, Woda C, et al. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol. 2007;293:F227–F235. doi: 10.1152/ajprenal.00057.2007. [DOI] [PubMed] [Google Scholar]
- 29.Vallon V, Hummler E, Rieg T, et al. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol. 2009;20:721–729. doi: 10.1681/ASN.2008040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieg T, Bundey RA, Chen Y, et al. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- **31.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am J Physiol. 2011 doi: 10.1152/ajpregu.00148.2011. These studies demonstrate that specific agonism of P2Y2 receptors increases renal sodium excretion and decreases ABP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol. 2011;300:F657–F668. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahlmann A, Pradervand S, Hummler E, et al. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle's syndrome. Am J Physiol. 2003;285:F310–F318. doi: 10.1152/ajprenal.00016.2003. [DOI] [PubMed] [Google Scholar]
- 34.Pradervand S, Vandewalle A, Bens M, et al. Dysfunction of the epithelial sodium channel expressed in the kidney of a mouse model for liddle syndrome. J Am Soc Nephrology. 2003;14:2219–2228. doi: 10.1097/01.asn.0000080204.65527.e6. [DOI] [PubMed] [Google Scholar]
- 35.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 36.Hansson JH, Nelson-Williams C, Suzuki H, et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11:76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]