Abstract
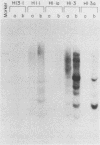
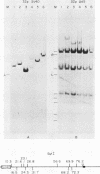
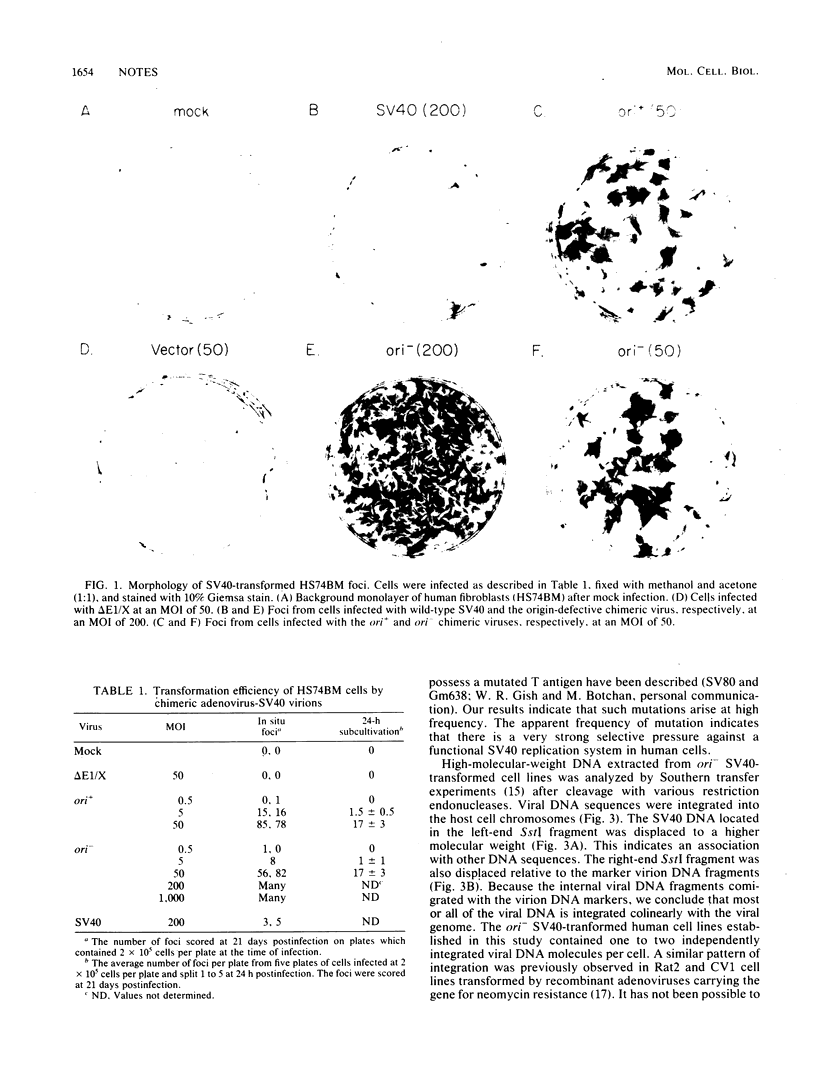
The origin-defective simian virus 40 (SV40) mutant 6-1 has been useful in transforming human cells (Small et al., Nature [London] 296:671-672, 1982; Nagata et al., Nature [London] 306:597-599, 1983). However, the low efficiency of transformation achieved by DNA transfection is a major drawback of the system. To increase the efficiency of SV40-induced transformation of human fibroblasts, we used recombinant adenovirus-SV40 virions which contain a complete SV40 early region including either a wild-type or defective (6-1) origin of replication. The SV40 DNA was cloned into the adenovirus vector in place of early region 1. Cell lines transformed by viruses containing a functional origin of replication produced free SV40 DNA. These cell lines were subcloned, and some of the subclones lost the ability to produce free viral DNA. Subclones that failed to produce free viral DNA were found to possess a mutated T antigen. Cell lines transformed by viruses containing origin-defective SV40 mutants did not produce any free DNA. Because of the high efficiency of transformation, we suggest that the origin-defective chimeric virus is a convenient system for establishing SV40-transformed cell lines from any human cell type that is susceptible to infection by adenovirus type 5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Van Doren K. Palindromic adenovirus type 5-simian virus 40 hybrid. J Virol. 1983 Jan;45(1):91–103. doi: 10.1128/jvi.45.1.91-103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Transformation of human skeletal muscle cells by simian virus 40. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6581–6585. doi: 10.1073/pnas.80.21.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Diamond B., Bloom B. R. The generation of human monocyte/macrophage cell lines. Nature. 1983 Dec 8;306(5943):597–599. doi: 10.1038/306597a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Slater M. L., Dermody J. J., Mandel M. Replication of simian virus 40 DNA in normal human fibroblasts and in fibroblasts from xeroderma pigmentosum. J Virol. 1981 Aug;39(2):481–489. doi: 10.1128/jvi.39.2.481-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Owens R. B., Hiller A. J., Nelson-Rees W. A., Johnston J. O. The biology of human cells in tissue culture. I. Characterization of cells derived from osteogenic sarcomas. Int J Cancer. 1976 Feb 15;17(2):219–234. doi: 10.1002/ijc.2910170211. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Doren K., Hanahan D., Gluzman Y. Infection of eucaryotic cells by helper-independent recombinant adenoviruses: early region 1 is not obligatory for integration of viral DNA. J Virol. 1984 May;50(2):606–614. doi: 10.1128/jvi.50.2.606-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Jha K. K., Mulder C., Basilico C., Ozer H. L. Human fibroblasts transformed by the early region of SV40 DNA: analysis of "free" viral DNA sequences. Virology. 1980 Jul 30;104(2):439–453. doi: 10.1016/0042-6822(80)90346-3. [DOI] [PubMed] [Google Scholar]