Abstract
Background and aims
Reduced vitamin D levels may play a significant role in the development of fractures and musculoskeletal pains reported in patients on aromatase inhibitors (AIs) for breast cancer. In this study, we evaluated the vitamin D status in postmenopausal women with non-metastatic breast cancer who were about to start AI therapy.
Methods
This study was conducted on community dwelling postmenopausal subjects, aged 35–80 years, with early non-metastatic breast cancer (up to stage IIIA), who were about to start therapy using third generation AIs. Symptoms of joint and muscle pains were obtained using a modified Leuven menopausal questionnaire. 25-hydroxyvitamin D [25(OH)D] was evaluated by radioimmunoassy while bone mineral density (BMD) of the lumbar spine and the proximal femur by dual energy X-ray absorptiometry (DXA)
Results
Of the 145 participants (mean age = 60.96 ± 0.88 years), 63/145 (43.5%) had baseline levels of 25(OH)D of < 20 ng/ml (deficient), 50/145 (34.5%) had levels between 20–29 ng/ml (insufficient), and only 32/145 (22%) had > 30 ng/ml (sufficient); thus, 113/145 (78%) had low 25(OH)D levels (i.e. < 30ng/ml). Arthralgias and myalgias were found in 61.3% and 43% of patients, respectively; and of those, 83.3% and 88.1% had 25(OH)D of < 30ng/ml, respectively.
Conclusions
Prevalence of vitamin D deficiency is high in breast cancer women and this may increase the risk of bone loss and fractures in those who are going to start AIs. Moreover, musculoskeletal pains are common in breast cancer women, even before the initiation of AIs and in association with low vitamin D in the majority. Future studies may be needed to establish the contribution of low vitamin D, if any, on the prevalence of musculoskeletal pains in women on AIs.
Keywords: Vitamin D, Osteoporosis, breast cancer, aromatase inhibitors
Introduction
Several factors that predispose patients to suboptimal levels of vitamin D include inadequate sunlight exposure, poor dietary intake, decreased cutaneous synthesis of vitamin D, and conditions or medications that reduce vitamin D absorption or increase vitamin D degradation (1;2). Any illness that is associated with debilitation, either temporarily or permanently, may predispose to low levels of vitamin D perhaps because of limited outdoor activity and poor intake (1;3).
Breast cancer is the most common form of cancer among women in the US (ACS Facts and Figures 2009
(http://www.cancer.org/docroot/STT/content/STT_1x_Cancer_Facts__Figures_2009.asp), and with the improvement in survival with new therapies, there is a growing number of breast cancer survivors who need long-term management of complications resulting from these treatment modalities . Aromatase inhibitors (AIs) are gaining widespread acceptance as a better and safer alternative to tamoxifen in the management of women with estrogen receptor positive breast cancer (ER+) (4;5). There is, however, a concern for their negative side effects on bone health, as they have been reported to increase the incidence of fractures and the number of women developing ostepenia/osteoporosis with therapy (6–10). Furthermore, disturbing musculoskeletal complaints such as joint pains or arthralgias, muscle pains or myalgias and bone pains have been reported to be highly prevalent in patients treated with AIs (6–9) and may result in discontinuation of the therapy in a subset of patients (11).
In addition to a disturbance in calcium and mineral metabolism (12;13), suboptimal vitamin D level has also been reported to be associated with musculoskeletal aches and pains (14;15). Recent reports indicate that 75.6% to 88% of breast cancer survivors have low 25-hydroxyvitamin D [25(OH)D] levels, i.e. < 30 ng/ml (16;17). It is possible that inadequate vitamin D levels may be also highly prevalent among newly diagnosed breast cancer cases primarily due to limited outdoor activity resulting from the surgery and series of chemotherapy and radiation therapy, although they may have normal levels prior to these procedures or at the time of diagnosis. While suggestions on the role of low vitamin D on the etiology of musculoskeletal pains (14) were mostly from studies conducted in otherwise healthy individuals, its contribution on the reported high prevalence of musculoskeletal aches and pains in breast cancer patients on aromatase inhibitor has not been established. The objectives of our study were: firstly, to determine the prevalence of Vitamin D deficiency in patients with newly diagnosed breast cancer who were about to initiate AI therapy; and secondarily, to evaluate the relationship between musculoskeletal complaints and the vitamin D status in patients with newly diagnosed breast cancer who were about to initiate AI therapy.
Materials and Methods
Study Population
This study is conducted on community dwelling postmenopausal subjects with non-metastatic breast cancer recruited from the St. Louis metropolitan area and neighboring counties. These subjects were mostly recruited by referrals from oncologists, and from flyers and letters. This study was in accordance with the guidelines in the Declaration of Helsinki for the appropriate treatment of human subjects. The protocol was approved by the Washington University School of Medicine review board, and written informed consent was obtained from each participant.
The subjects were postmenopausal women participating in a study on bone loss with aromatase inhibitor therapy, aged 36–80 years old, with early stage estrogen receptor positive breast cancer (up to Stage 111A), who were about to start therapy using third generation AIs (anastrozole, letrozole or exemestane). At the time of participation, all of the participants had undergone surgery, and were finished or about to finish chemotherapy and or radiation therapy. Subjects who were taking medications affecting bone metabolism such as estrogen, bisphosphonate (alendronate, risedronate, ibandronate, and zoledronic acid), selective estrogen receptor modulators (raloxifene, tamoxifen), GnRH analogs, glucocorticoids of at least 5 mg a day of prednisone or equivalent for ≥ 1 month, or phenytoin; and those having diseases or conditions which would alter bone metabolism including hyperthyroidism, osteomalacia, chronic liver disease, renal failure, hypercortisolism, malabsorption and alcoholism were excluded from the study. Current tobacco users were excluded, whereas past smokers who stopped smoking for 6 months were allowed into the study.
Clinical Data
Symptoms of joint and muscle pains were obtained using a menopausal questionnaire, i.e. the modified Leuven questionnaire, which details the different symptoms related to the musculoskeletal, vasomotor, psychiatric, and genitourinary systems (18). Severity of each menopausal symptom is graded from 1 to 5 with 1 being no symptom and five having the worst or intolerable symptoms. Data on estrogen exposure were assessed through a number of variables as age at menarche, average number of periods per year during the reproductive years, number of years of birth control pill use, total number of pregnancies, number of pregnancies to term, months of lactation, age at menopause and years since menopause (YSM). A family history of osteoporosis was coded as positive in the presence of a first degree relative diagnosed with osteoporosis, kyphosis, and fragility fractures in the absence of secondary causes.
The use of calcium and vitamin D supplements was obtained through a questionnaire given at study entry and expressed as average daily intake in mg/day and International Units (IU)/ day, respectively. Alcohol intake was expressed as the average number of alcoholic drink-equivalents consumed over a 1-wk period. A can of beer (336 mL), a glass of wine (112 mL), and 28 mL of a heavy alcoholic beverage (i.e. > 40% proof alcohol) were considered one drink-equivalent. Previous smoking was expressed in pack-years and was estimated as the number of 20-cigarette packs smoked per day multiplied by the number of years of smoking. Physical activity was expressed as a numerical score and was defined as sedentary (score of 1: sitting or lying most of the day), moderately active (score of 2: being on one’s feet more than half of a day), and very active (score of 3: engaging in regular physical exercise) (19). Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2). The waist to hip ratio was calculated as the ratio between waist circumference, taken at the umbilical level, and the hip circumference, measured 6 in.(15.24 cm) below the anterior superior iliac spine.
Biochemical Data
Serum for 25(OH)D measurement was obtained at study entry and was performed by radioimmunoassay using DiaSorin Kits through the Barnes-Jewish Hospital Laboratory (DiaSorin Inc, Stillwater, MN).
Bone mineral density (BMD)
Bone mineral density (BMD) of the lumbar spine and the proximal femur were measured at the time of entry into the study by dual energy X-ray absorptiometry (DXA) using Hologic QDR 4500 (Hologic Inc., Waltham Ma, U.S.A.). In the Bone Health Program at Washington University where all the bone density tests were performed, the coefficient of variability for this technique using QDR 4500 densitometer in our center is 1.09% for the lumbar spine and 1.2% for the total femur (20).
Statistical analysis
Results were expressed as means ± SE. Group comparisons were done by analysis of variance (ANOVA) for continuous variables and by chi-square analysis for categorical variables. The data were managed using Excel 2003 (Microsoft Corp., Redmond, WA) and were analyzed using Statgraphic Plus 5.0 (Manugistic, Inc., Rockville, MD)
Results
A total of 145 postmenopausal women (113 Caucasians, 29 African-Americans and 3 Asian) with breast cancer participated in the study. Levels of 25(OH)D were divided into 3 groups: < 20 ng/ml as group 1 (deficient), 20–29 ng/ml as group 2 (insufficient) and ≥ 30ng/ml as group 3 (sufficient) (normal level: 30 to 100 ng/ml). Based on this criteria (Figure 1), 63/145 (43.5%) of the subjects fell under group 1, 50/145 (34.5%) under group 2, and 32/145 (22%) under group 3. Thus, 78% (113/145) of the breast cancer subjects were below the vitamin D level considered to be optimal. Eighty four of the 145 participants (57.9%) were taking vitamin D supplements either from a multivitamin or from a vitamin D pill. Under the different vitamin D groups, 28/63 (44.4%) in group 1, 32/50 (64%) in group 2, and 24/32 (75%) in group 3 were on some form of vitamin D supplements at the time of presentation.
Figure 1.
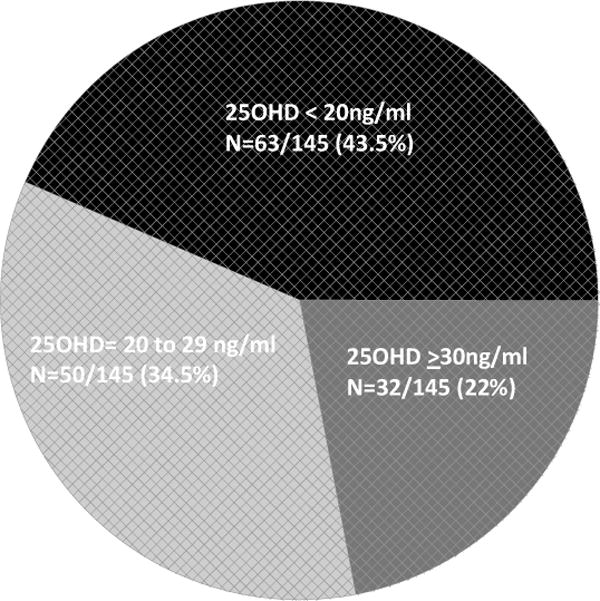
Vitamin D distribution among newly diagnosed women with breast cancer
Table 1 shows the clinical features of the study population divided according to vitamin D levels. As expected, the mean intake of vitamin D supplements is lower in vitamin D deficient and insufficient groups in comparison to the optimal vitamin D group, though the difference was not statistically significant. The average total calcium intake from both dietary and supplements was also significantly higher in the normal vitamin D group (group3) in comparison to low vitamin D groups (1 and 2) (p = 0.02). The activity score was borderline high in the normal vitamin D group relative to the low vitamin D groups (p = 0.05), while the body mass index (BMI) was significantly lower among patients with normal vitamin D relative to those in the low vitamin D groups (p = 0.01). There were no significant differences in the clinical features such as age, YSM, alcohol intake, smoking history and family history of osteoporosis among the different vitamin D groups (Table 1).
Table 1.
Clinical features of the study population according to vitamin D levels
| Group I < 20 | Group II 20–29 | Group III > 30 | * P | |
|---|---|---|---|---|
| Mean 25(OH)D (ng/ml) | 13.4 ± 0.5 | 24.2 ± 0.6 | 36.5 ± 0.7 | < 0.001 |
|
| ||||
| Total number of subjects | 63 | 50 | 32 | |
| Caucasians | 41 | 43 | 29 | |
| African-Americans | 19 | 7 | 3 | |
| Asian | 3 | 0 | 0 | |
|
| ||||
| Age (years) | 59.7 ± 1.2 | 61.8 ± 1.3 | 61.9 ± 1.7 | 0.42 |
|
| ||||
| YSM | 14.5 ± 1.7 | 14.9 ± 1.9 | 13.2 ± 2.4 | 0.85 |
|
| ||||
| BMI (kg/m2) | 31.4 ± 0.8 | 30.2 ± 0.9 | 27.4 ± 1 | 0.02 |
|
| ||||
| Waist to hip ratio | 0.87 ± 0.009 | 0.86 ± 0.009 | 0.83 ± 0.01 | 0.04 |
|
| ||||
| History of past smoking | ||||
| Percent past smokers | 42% | 26.5% | 40.6% | 0.2 |
| Total past smoking (pack-years) | 10.7 ± 2 | 6.7 ± 2 | 9.9 ± 2.6 | 0.38 |
|
| ||||
| Positive family history of osteoporosis | 26.6% | 44% | 31.2% | 0.15 |
|
| ||||
| Activity score | 1.66 ± 0.09 | 1.76 ± 0.1 | 2.0 ± 0.13 | 0.05 |
|
| ||||
| Vitamin D intake from supplements (IU/day) | 201 ± 120 | 390 ± 142 | 575 ± 170 | 0.19 |
|
| ||||
| Average daily calcium intake (mg/day) | 493 ± 78 | 691 ± 89 | 855 ± 112 | 0.02 |
|
| ||||
| Spine BMD | 1.05 ± 0.01 | 1.06 ± 0.02 | 1.03 ± 0.02 | 0.6 |
|
| ||||
| Total Hip BMD | 0.96 ± 0.01 | 0.96 ± 0.01 | 0.94 ± 0.02 | 0.7 |
|
| ||||
| Femoral Neck BMD | 0.80 ± 0.01 | 0.80 ± 0.01 | 0.78 ± 0.02 | 0.6 |
|
| ||||
| Trochanteric BMD | 0.72 ± 0.01 | 0.71 ± 0.01 | 0.70 ± 0.02 | 0.8 |
|
| ||||
| Intertrochanteric BMD | 1.13 ± 0.02 | 1.16 ± 0.01 | 1.12 ± 0.02 | 0.3 |
P by ANOVA for continuous variables and χ2 analysis.
Analysis according to the time of presentation showed no significant seasonal variation in 25(OH)D levels i.e. warmer season (i.e. April to September = 23.4 ± 1.2 ng/ml), compared to those who came during the cold season (i.e. October to March = 21.3 ± 1.2 ng/ml) p = 0.2 (Table 2). Compared to Caucasians, African-Americans had significantly lower 25(OH)D levels (16.8 ± 1.7 vs. 23.8 ± 0.9, p = < 0.001) (Table 2). Since there were only 3 Asian participants, these subjects were not included in the analysis for racial difference in vitamin D level. Dividing the BMD of the participants according to vitamin D levels showed no significant differences in BMD among the different vitamin D groups in any of the skeletal sites examined.
Table 2.
Vitamin D levels (ng/ml) according to the time of presentation and racial background
| Time of presentation | April to September | 23.4 ± 1.2 | P = 0.2 |
|---|---|---|---|
| October to March | 21.3 ± 1.2 | ||
| Race | Caucasians | 23.8 ± 0.9 | P < 0.009 |
| African-Americans | 16.8 ± 1.7 |
Analysis of complaints related to the musculoskeletal system in the 137 women who completed the menopausal questionnaire showed that 84 (61.3%) and 59 (43%) had mild to severe arthralgias and myalgias, respectively, at baseline prior to initiation of AIs. Fifty-four of these patients (39.4%) had both symptoms concurrently. As shown in figure 2, serum 25(OH)D levels were low in 70 of the 84 (83.3%) (p = 0.03) patients with arthralgias, and in 52 of the 59 (88%) (p = 0.008) subjects with myalgias. Out of 54 subjects who complained of both arthralgias and myalgias, 48 (88.8%) (p = 0.009) had serum 25(OH)D of less than 30 ng/ml.
Figure 2.

Percentage of breast cancer women with complaints of arthralgias and myalgias or both who have low vs. normal 25-hydroxyvitamin D levels
Discussion
Our study showed that a majority of newly diagnosed breast cancer patients had low vitamin D at baseline prior to initiating AI therapy, with nearly 44% who can be considered as vitamin D deficient (less than 20 ng/ml). As expected, the mean vitamin D level was lower for African-Americans compared to Caucasians. Our data showed that patients with higher vitamin D levels (group 3) had lower BMI and higher activity score. We also found that joint and muscle pains were common among women with breast cancer at baseline prior to initiation of aromatase inhibitors, and majority of women with these complaints had suboptimal levels of vitamin D at presentation. Considering that these patients were going to initiate AI treatment, our findings are clinically relevant, given the increased incidence of bone loss and fractures, and the high prevalence of athralgias and myalgias in patients on AIs.
Serum PTH begins to rise when vitamin D levels fall below 30–32 ng/ml (75–80 nmol/L) (12;13) resulting in osteoclast activation leading to bone loss. Inadequate vitamin D levels in patients with breast cancer may, therefore, pose as additional risk for bone loss when superimposed on aromatase inhibitor therapy. An increase in the incidence of fractures, and the diagnosis of osteoporosis have been reported in large clinical trials involving third generation aromatase inhibitors (6–10). In these trials, however, the vitamin D status and the calcium intake of the participants were mostly unknown, and these patients were also not given supplements of calcium and vitamin D. In a smaller study by Geisler et.al. (17), 88% of the subjects who were randomized to either exemestane or placebo, had 25(OH)D levels that was less than 30ng/ml. This study also showed a significant reduction in femoral neck bone density at the end of 2 years. Since the low vitamin D levels in these patients were uncorrected throughout the study, it remains unknown if correction of low vitamin D levels would be able to prevent or reduce bone loss in these patients.
The current clinical guidelines from the American Society of Clinical Oncology recommend supplementation with calcium and vitamin D and BMD measurements in women on AIs at baseline and annually thereafter (21). However, routine vitamin D measurements to assess vitamin D status are not part of the current guidelines in the management of bone health among breast cancer patients especially those who are about to initiate AIs. On the other hand, it should be also noted that reformulation of the radioimmunoassay and drifts in the assay performance (method bias and imprecision) have raised important issues on the consistency of vitamin D measurement across laboratories in the last years, as reported by an NIH advisory in 2009 (http://ods.od.nih.gov/News/NCHS_Vitamin_D_Data_Advisory.aspx).
Recent recommendations from the National Osteoporosis Foundation suggest a vitamin D intake of 800 to 1000 IU daily ((http://www.nof.org/professionals/Clinicians_Guide.htm, an intake even smaller to that proposed by NIH of 2000 UI in post menopausal women (http://ods.od.nih.gov/factsheets/vitamind.asp#h8). These doses are higher than previous recommendations to reflect the recent recognition of the widespread nature of inadequate vitamin D levels in the general population. Heaney and colleagues estimated that the body requires 3000–5000 IU of vitamin D daily to maintain vitamin D requirements during the winter months along with cutaneously synthesized accumulation from sunlight exposure during the preceding summer months (22). Given the co-morbid condition which may predispose to inadequate vitamin D levels, women with newly-diagnosed breast cancer, especially those who will initiate AIs, may benefit from higher doses of vitamin D (23).
Besides bone loss and fractures, musculoskeletal aches and pains (arthralgias and myalgias) have been reported as common complaints in patients taking AIs. The 5-year results of the ATAC (Anastrozole Alone or in Combination with Tamoxifen versus Tamoxifen Alone) trial showed that 35.6% of women receiving anastrozole complained of arthralgias relative to women randomized to tamoxifen (8). Similarly for letrozole and exemestane, 21.3% and 5.4% of women, respectively, randomized to the active drugs also reported arthralgias compared to placebo (11.3%, 3.8% respectively) (6;9). Although there is no evidence that these complaints are related to the use of AIs, they may in some patients, lead to discontinuation of the drug (11). Our study showed that a significant proportion of breast cancer women complained of arthralgias and myalgias even before taking AIs. These same bone and muscle pains are commonly described in patients with vitamin D deficiency and are relieved by vitamin D replacement (15;24;25). Interestingly, from the pool of breast cancer women with these complaints in our study, a significant majority had 25(OH)D levels less than 30 ng/ml. Nevertheless, any direct evidence that low vitamin D is a major factor on the genesis of these symptoms in women on AIs, thus far, remains lacking.
The etiology of the musculoskeletal aches and pains associated with AIs remains unclear, but two factors may work in the pathogenesis of these symptoms, namely profound hypoestrogenemia and low vitamin D. Observations of an increase in the incidence of musculoskeletal pains at the time of menopause relieved by hormone therapy suggests the importance of estrogen status in the etiology of these complaints (26). Likewise, the bone and muscle pains that are commonly described in patients with vitamin D deficiency that are relieved by vitamin D replacement (15;24;25), also imply a causal relationship between low vitamin D and these complaints. Considering the high prevalence of low vitamin D in our women who were about to initiate AI therapy, it is likely that suboptimal levels of vitamin D may contribute to the increase in the incidence and possibly the severity of musculoskeletal symptoms experienced by profoundly estrogen deficient AI treated women.
Our results also showed that the mean vitamin D level for African-Americans was significantly lower than those of Caucasians (27;28). This is not surprising considering the well-known racial differences in the cutaneous synthesis of vitamin D (29–31). The clinical implication of this racial difference in vitamin D levels on bone health among African-Americans on AIs, however, remains undetermined. Consistent with prior reports (32;33), our results suggest an inverse relationship between BMI and circulating vitamin D levels, and that women with low vitamin D are heavier compared to those with normal vitamin D. Several potential explanations have been put forth to explain this observation, one of them is that fat soluble-vitamin D3 is sequestered in the large adipose compartment, resulting in low levels in the serum (32). Our results also showed no significant seasonal variation in vitamin D levels. This is not surprising given the fact that our patients had newly diagnosed breast cancer, and may have limited outdoor activity from the treatment protocol that they have to undergo.
Our study has limitations. Firstly, the limited sample size may not be reflective of the true prevalence of low vitamin D levels in women with breast cancer, especially newly-diagnosed subjects, whose outdoor activity may be poor from courses of radiation and chemotherapy. Nevertheless, our prevalence was concordant with the recently reported findings in a bigger study, conducted on breast cancer survivors (16). Since all our measurements are done at the time of study entry, we have no data on the vitamin D levels in our patients before or at the time of breast cancer diagnosis, thus, it is also possible that they may have longstanding vitamin D deficiency/insufficiency which may account for their symptoms. Alternatively, since we have no information on the prevalence of arthralgias and myalgias in our subjects before the diagnosis of breast cancer, it is also possible that these complaints may be chronic and not related to their vitamin D status nor their current state of health. Nonetheless, these complaints appear to be highly prevalent in our patients even before the initiation of AIs and challenge the notion that they are AI-related. Lastly, we have not measured PTH levels which are expected to increase in states of low vitamin D, and may potentially play a part in bone loss experienced by patients on AIs.
In summary, vitamin D deficiency/insufficiency is highly prevalent among patients with breast cancer and may contribute to the bone loss and the higher incidence of fractures in patients on AI therapy. It is perhaps advisable to screen and treat suboptimal vitamin D levels particularly in women with breast cancer undergoing this form of hormonal therapy. Our results demonstrate that a significant percentage of women with breast cancer complain of arthralgias and myalgias. Although these symptoms are commonly reported as adverse events in patients on AIs, our results indicate that they are present even before the initiation of these drugs. Our findings also indicate that a significant majority of women with these complaints have suboptimal levels of vitamin D at baseline. While as of yet, there are no data to support the concept that symptoms of arthralgias and myalgias in women taking AIs may also be due to low vitamin D levels, studies looking at the effect of vitamin D replacement in women with musculoskeletal complaints on aromatase inhibitors are ongoing and would perhaps shed more light on the role of suboptimal vitamin D levels on these complaints (34). However, in the opinion of the authors, a randomized placebo-controlled study to determine if vitamin D, using the recently proposed dose by the NIH (or even higher), initiated at the start of aromatase inhibitors would prevent the musculoskeletal symptoms in patients on AIs would be more informative. Given the complexity of vitamin D metabolism, a cross-over study design while controlling for sunlight exposure, dietary intake, latitude, race and season would more appropriate for a meaningful interpretation of the results. Moreover, it also important to know from future studies if reaching a 25(OH)D level of least 30 ng/ml will be enough to alleviate these symptoms or a higher target level will be necessary. Recognizing that severe athralgias and myalgias may result in discontinuation of AIs, establishing a link between these symptoms and vitamin D status may help in the therapeutic approach of these complaints. Finally, routinely checking for these complaints prior to, and after initiation of AIs will help argue against the notion that these symptoms are AI-related and potentially encourage adherence to AIs in the majority of patients.
Acknowledgments
We would like to thank Gerard Moskowitz, PhD for his valuable help in the organization of the data presented and in editing this manuscript.
This work has been supported by NIH grants R03 AR049401 (RAV), and K12 HD01459 (Building Interdisciplinary Research Careers in Women’s Health) and 5R21AR053196-01 (RAV).
Footnotes
Presented in part at the 29th Annual Meeting of the American Society for Bone and Mineral Research, September 16–19, 2007, Honolulu, Hawaii
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res. 2005;20(8):1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Perez EA. Safety of aromatase inhibitors in the adjuvant setting. Breast Cancer Res Treat. 2007;105(Suppl 1):75–89. doi: 10.1007/s10549-007-9704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18(Suppl 8):viii26–viii35. doi: 10.1093/annonc/mdm263. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 7.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 8.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 10.Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7(8):633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 11.Donnellan PP, Douglas SL, Cameron DA, Leonard RC. Aromatase inhibitors and arthralgia. J Clin Oncol. 2001;19(10):2767. [PubMed] [Google Scholar]
- 12.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, wson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 13.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency: what a pain it is. Mayo Clin Proc. 2003;78(12):1457–1459. doi: 10.4065/78.12.1457. [DOI] [PubMed] [Google Scholar]
- 15.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 16.Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88(1):133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisler J, Lonning PE, Krag LE, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer. 2006;42(17):2968–2975. doi: 10.1016/j.ejca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Morales L, Neven P, Timmerman D, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer Drugs. 2004;15(8):753–760. doi: 10.1097/00001813-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 19.rmamento-Villareal R, Villareal DT, Avioli LV, Civitelli R. Estrogen status and heredity are major determinants of premenopausal bone mass. J Clin Invest. 1992;90(6):2464–2471. doi: 10.1172/JCI116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20(2):232–239. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 23.Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223–234. doi: 10.1016/j.breast.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Gloth FM, III, Lindsay JM, Zelesnick LB, Greenough WB., III Can vitamin D deficiency produce an unusual pain syndrome? Arch Intern Med. 1991;151(8):1662–1664. [PubMed] [Google Scholar]
- 25.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 26.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105(5 Pt 1):1063–1073. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 27.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19(5):527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 28.Perry HM, III, Horowitz M, Morley JE, et al. Aging and bone metabolism in African American and Caucasian women. J Clin Endocrinol Metab. 1996;81(3):1108–1117. doi: 10.1210/jcem.81.3.8772584. [DOI] [PubMed] [Google Scholar]
- 29.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 31.Harris SS, wson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 32.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilarrasa N, Maravall J, Estepa A, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30(8):653–658. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]


