Abstract
The virulence of numerous Gram-negative bacteria is under the control of a quorum sensing process based on synthesis and perception of N-acyl homoserine lactones. Rhodococcus erythropolis, a Gram-positive bacterium, has recently been proposed as a biocontrol agent for plant protection against soft-rot bacteria, including Pectobacterium. Here, we show that the γ-lactone catabolic pathway of R. erythropolis disrupts Pectobacterium communication and prevents plant soft-rot. We report the first characterization and demonstration of N-acyl homoserine lactone quenching in planta. In particular, we describe the transcription of the R. erythropolis lactonase gene, encoding the key enzyme of this pathway, and the subsequent lactone breakdown. The role of this catabolic pathway in biocontrol activity was confirmed by deletion of the lactonase gene from R. erythropolis and also its heterologous expression in Escherichia coli. The γ-lactone catabolic pathway is induced by pathogen communication rather than by pathogen invasion. This is thus a novel and unusual biocontrol pathway, differing from those previously described as protecting plants from phytopathogens. These findings also suggest the existence of an additional pathway contributing to plant protection.
Introduction
Numerous Gram-negative plant-pathogenic bacteria use a communication system based on synthesis and perception of N-acyl-homoserine lactones (NAHSLs); this system involves a quorum sensing (QS) mechanism that controls genes determining pathogenicity and colonization of the host surface [1]. The QS mechanisms of the causative agents of soft-rot, including members of the genera Erwinia now reclassified as Dickeya and Pectobacterium [2]–[4], have been extensively studied. They have also been documented in the economically important phytopathogen Pectobacterium atrosepticum under host influence [5]–[7]. QS triggers an attack by soft-rot bacteria once there is a sufficient number of bacterial cells, and ensures the coordinated production of massive amounts of lytic enzymes to degrade plant tissues and thereby obtain nutrients [8]. This coordinated overproduction of lytic enzymes is suspected to help soft-rot bacteria overwhelm plant defenses [9]. Generating signaling molecules is therefore an important element of the plant-pathogen interaction. Liu and co-authors [10] estimated that transcription of 26% of the genes in P. atrosepticum SCRI1043, including numerous virulence genes, differed between a NAHSL synthase mutant and the wild-type, further implicating QS in pathogenesis. These observations suggest that the means of communication used by pathogenic bacteria are potential targets for the development of a novel biocontrol strategy: rather than directly eradicating the pathogen, interfering with communication may reduce the expression of virulence systems and thus pathogenicity [11]–[13].The potato is one of the world’s major crops [14], [15] and epidemiologic studies report devastating potato diseases suggestive of the emergence of new pectinolytic agents [16], [17]. We are therefore developing a novel biocontrol strategy for the S. tuberosum model based on the selective stimulation of NAHSL-degrading bacteria [18]–[20]. This approach to soft-rot control involves the use of the Gram-positive Rhodococcus erythropolis strain R138 as a biocontrol agent (BCA): it is able both to degrade diverse γ-lactones effectively in vitro [21] and to suppress the maceration of tubers in hydroponic and field culture conditions [22], [23].
However, the mechanism by which this strain controls soft-rot has never been elucidated in planta, although it is suspected that it is linked to NAHSL-breakdown. We recently discovered a lactone assimilation pathway in R. erythropolis: the lactonase QsdA hydrolyzes the lactone bond of a wide range of flavoring compounds containing a γ-butyrolactone ring coupled to an alkyl chain; the open-chain form of these substrates is then degraded by a β- or ω-oxidation step and the products join the β-ketoadipate pathway and the Krebs cycle [21]. Here, we report an analysis of the involvement of this γ-lactone catabolic pathway in the control of tuber soft-rot by R. erythropolis R138. The functions of QsdA, the key enzyme of this catabolic pathway, were investigated by transferring the qsdA gene to a heterologous host (Escherichia coli). Also, we constructed a R. erythropolis R138 qsdA deletion mutant and studied NAHSL breakdown in tubers in its presence. In situ qsdA transcription was followed by confocal laser scanning microscopy (CLSM) using an R. erythropolis strain carrying a plasmid-borne qsdA::gfp transcriptional fusion. The QS process and tuber soft-rot were generated using a virulent P. atrosepticum isolate and were quenched with a recombinant of this strain synthesizing an NAHSL-lactonase and consequently producing only negligible amounts of NAHSLs. Pectobacterium quorum sensing and rhodococcal quorum quenching were successfully characterized in planta. We demonstrate that the γ-lactone catabolic pathway is induced by pathogen communication, and is involved both in NAHSL breakdown and in the control of the disease.
Materials and Methods
Bacterial Strains, Growth Media and Culture Conditions
The characteristics of bacterial strains and plasmids used are presented in Table 1. Agrobacterium tumefaciens NT1 used as a biosensor to detect NAHSL was grown in minimal Agrobacterium Broth (AB) at 25°C [24]. Pectobacterium atrosepticum CFBP 6276 was grown in PGA minimal medium supplemented with 0.4% (w/v) polygalacturonic acid (Sigma-Aldrich, St. Louis) at 25°C as described elsewhere [25] and Escherichia coli DH5α in Luria-Bertani medium (AES Chemunex, Bruz, France) at 37°C. For R. erythropolis strains cultivated at 25°C, the non-selective growth media used were TY to prepare bacterial suspensions for inoculation of potato tubers and LBP for the conjugative transfer of DNA. TY medium contained 0.5% (w/v) tryptone (Difco, Le Pont de Claix, France), 0.2% (w/v) yeast extract (Difco) and 5% (v/v) of a phosphate buffer (6% (w/v) K2HPO4 (Merck, Fontenay-sous-Bois, France) and 4% (w/v) NaH2PO4 (Merck)). LBP medium contained 1% (w/v) Bacto Peptone (Difco), 0.5% (wt/v) yeast extract, and 1% (w/v) NaCl (Sigma-Aldrich). To evaluate R. erythropolis R138 density in potato tubers, the minimal medium described by Barbey et al. [21] was used, with the following modification: gamma-caprolactone (GCL, Sigma-Aldrich), the sole carbon source, was included at 2 g/l. Growth media were supplemented as appropriate with kanamycin at a concentration of 30 µg/ml for E. coli and 200 µg/ml for R. erythropolis, and with 10 µg/ml tetracycline for P. atrosepticum, or 100 µg/ml ampicillin for E. coli, and solidified with agar (15 g/l). All cultures were grown on a rotary shaker (180 rpm). Growth was monitored spectrophotometrically at 580 nm. All cultures were inoculated at an initial OD580 of 0.05.
Table 1. Bacterial strains and plasmids.
| Strain or plasmid (synonym) | Relevant characteristic(s) | Source or reference |
| Agrobacterium tumefaciens | ||
| A. tumefaciens NT1 | NT1 derivative of strain C58, carrying pZLR4; Biosensor usedfor NAHSL detection; GmR | [38] |
| Escherichia coli | ||
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 | [49] |
| DH5α | Host for cloning; SupE44 ΔlacU169 (Φ80lacZΔM15) hsdR17recA1 endA1 gyrA96 thi-1 relA1 | Lab collection |
| DH5α(pUC19) (Ec) | Strain DH5α carrying pUC19; ApR | This study |
| DH5α(pUC19-qsdA) (Ec-qsdA) | QsdA-expressing DH5α; ApR | This study |
| Pectobacterium atrosepticum | ||
| CFBP 6276 | Potato soft-rot pathogen; NAHSL producer | [25] |
| 6276(pME6000) (Pa-QS+) | Strain 6276 carrying pME6000, a broad-host-range cloningvector; TcR | [40] |
| 6276(pME6000-aiiA) (Pa-QS–) | Strain 6276 carrying pME6000 containing the aiiA genefrom Bacillus sp. A24 under the constitutive Plac promoter; TcR | [40] |
| Rhodococcus erythropolis | ||
| R138 (BCA) | NAHSL-degrading isolate obtained from hydroponic cultureof potato plants | [50] |
| R138(pEPR1-qsdA::gfp) (BCA-qsdA::gfp) | R138 transformed with pEPR1 qsdA-gfp | This study |
| R138 ΔqsdA (BCA-ΔqsdA) | R138 with a 813 bp fragment deleted from the qsdA gene | This study |
| Plasmids | ||
| pZLR4 | traG::lacZ/traR reporter system | [38] |
| pME6000 | Broad-host-range cloning vector, TcR | [51] |
| pME6000-aiiA (pME6863) | pME6000 carrying the aiiA gene from Bacillus sp. A24 underthe constitutive Plac promoter | [52] |
| pAKE604 | Conjugative suicide vector for qsdA gene deletion; KmR | [26] |
| pAKE604 ΔqsdA | pAKE604 containing the qsdA upstream and downstreamregions; KmR | This study |
| pEPR1 | Shuttle promoter-probe vector carrying the promoterlessgfpuv reporter gene; KmR | [28] |
| pEPR1-qsdA::gfp | pEPR1 with a qsdA::gfp uv transcriptional fusion; KmR | This study |
| pUC19 | Cloning vector for E. coli; ApR | Lab. collection |
| pUC19-qsdA | pUC19 with a 1376 bp PCR fragment containing the qsdAgene; ApR | This study |
KmR, ApR, GmR and TcR indicate resistance to kanamycin, ampicillin, gentamicin and tetracycline, respectively. NAHSL, N-acyl homoserine lactone; CFBP, Collection Française de Bactéries associées aux Plantes, Institut National de la Recherche Agronomique (INRA), Angers, France.
Construction of aqsdA Deletion Mutant of R. erythropolis R138
A markerless qsdA deletion mutant was constructed by using the conjugative suicide vector pAKE604 [26], which cannot replicate in R. erythropolis. The regions upstream and downstream (919 and 780 bp) from qsdA were amplified using standard conditions with Extensor Hi Fidelity polymerase (Thermo scientific, Courtaboeuf, France) and the oligonucleotide pairs qsdA_up_fw/qsdA_up_rv (5′ GTATGTCTAGATGAATCCTGTGTGGTCGTC 3′ with the XbaI site italicized; 5′ TGAACATGCATGACATGCTCGTGCATCAAC 3′ with the NsiI site italicized) and qsdA_do_fw/qsdA_do_rv (5′ CTAGTATGCATCTGCTCGAACGTGGTGTCA 3′ with the NsiI site italicized; 5′ CAGATGAATTCGATAGATCTGCGCTGCG 3′ with the EcoRI site italicized), respectively. These primers were designed from the sequence of the relevant 5 kb of the genome of R. erythropolis R138, kindly provided by D. Faure (CNRS, ISV Gif/Yvette, France). The PCR fragments were inserted into pAKE604 to give pAKE604 ΔqsdA which was transferred into R. erythropolis R138 by biparental mating, as previously described by van der Geize et al. [27] with the following modifications: donor and recipient cells were mixed and spotted onto sterile nitrocellulose filters (GE Healthcare, Orsay, France), which were placed on dry LBP agar plates overnight at 30°C. Then, the mating mixture was suspended in 1 ml LBP liquid medium and 0.1 ml aliquots were spread on LBP agar plates supplemented with kanamycin (200 µg/ml), to select for the presence of the plasmid, and nalidixic acid (30 µg/ml) to kill the E. coli S17-1 donor cells. To test for the excision of the suicide plasmid from the chromosomal DNA, the co-integrate isolate was suspended in 1 ml of sterile saline solution and 0.1 ml aliquots of serial dilutions were plated on LBP agar medium supplemented with 10% sucrose. PCR analysis then DNA sequencing were used to verify the construction of the in-frame qsdA deletion and resulting truncated version of the qsdA gene (156 bp in length instead of 969 bp for the wild-type gene).
Construction of aqsdA::gfp Transcriptional Fusion in R. erythropolis R138 Strain
The promoter region of the qsdA gene was amplified with the Extensor Hi Fidelity polymerase using primers FQDAN (5′ CCAATGCATGGTAGGCATCGGGACATTCT 3′ with NsiI site italicized) and RQDAB (5′ CGCGGATCCCGATCGAACCCCTGACTGT 3′ with BamHI site italicized) and cloned as a 184-bp NsiI-BamHI fragment into the vector pEPR1 [28] upstream from the gfp gene, creating a qsdA::gfp transcriptional fusion. After sequencing the insert, this plasmid-designated pEPR1-qsdA::gfp was introduced in R. erythropolis R138 cells by electroporation as described by Vesely et al. [29]. Briefly, 100 µl of electrocompetent bacterial cells were mixed with 0.5 µg of plasmid DNA and electroshocked at 2.5 kV for 7 ms using a Savant electroporator (Thermo Fisher Scientific). After electroporation, bacterial cells were cultivated in 1 ml of TY broth at 25°C for 5 h with shaking (180 rpm) then plated on TY medium containing kanamycin and incubated for 3 days at 25°C. Among the Kmr clones obtained, one was chosen and checked for the presence of pEPR1qsdA-gfp by PCR analysis.
Heterologous Expression of QsdA inE. coli
The qsdA gene including its promoter region was amplified using primers QsdAF (5′TAATAAGAATTCTGACATGTCGAGTGGTTCCT 3′ with EcoRI site italicized) and QsdAR (5′TAATAATCTAGAGCTGACAGTCCTGTCGAAGT 3′ with XbaI site italicized) and the amplified fragment was inserted into pUC19 to give pUC19-qsdA; this was introduced into E. coli DH5α by transformation using the standard heat-shock method (42°C for 45 s). The appropriate insert was confirmed by sequencing.
Biocontrol Assays on Potato Tubers
Overnight cultures of P. atrosepticum CFBP 6276, R. erythropolis R138, E. coli DH5α and their derivative strains (see Table 1) were washed in 0.9% NaCl. The surface of Solanum tuberosum cv. Allians tubers were sterilized by incubation in a 1% (v/v) bleach solution for 10 min followed by rinsing with distilled water. They were inoculated by injection into the intramedulla (to a depth of 1 cm) with 10 µl of cell suspension containing the following bacterial combinations: [107 CFU of P. atrosepticum 6276/6276(pME6000)/6276(pME6000-aiiA) with 4×107 CFU of R. erythropolis R138/R138 ΔqsdA/R138(pEPR1-qsdA::gfp)] or [107 CFU of P. atrosepticum 6276 with 2×107 CFU of E. coli DH5α(pUC19)/DH5α(pUC19-qsdA)]. For controls, the suspensions of R. erythropolis R138 and/or P. atrosepticum 6276 were replaced with 0.9% NaCl. The inoculated tubers were incubated in a Minitron incubator (Infors, Massy, France) at 25°C with a relative humidity of 80±2%. One, two, three and seven days after infection, eight to 18 tubers for each condition were sectioned across the middle, and photographed. These experiments were conducted three times at different times of the year. The Mann and Whitney test was used to assess differences in maceration symptoms between groups (α = 0.05).
Analysis of Bacterial Populations Collected from Inoculated Potato Tubers
A 1 cm-diameter cookie cutter was used to collect standardized tuber samples from both parts of each sectioned tuber. Each sample was homogenized and 500 mg aliquots of the tuber homogenate were suspended in 5 ml of sterile saline solution. The suspensions were serially diluted, and plated on PGA minimal medium, selective minimal medium containing GCL, TY medium supplemented with 200 µg/ml kanamycin, and LB supplemented with 100 µg/ml ampicillin to count P. atrosepticum 6276, R. erythropolis R138, R. erythropolis R138(pEPR1-qsdA::gfp), and E. coli DH5α, respectively. Three independent experiments were performed and for each experiment, three independent samples were analyzed for each condition.
Extraction and Quantification of NAHSLs from Potato Tubers
For each condition, NAHSL was extracted from three samples of three different potato tubers. Reported values are means ± SD of three independent experiments. Samples of 500 mg of tuber (fresh weight) were suspended in 2 ml of sterile saline solution and vortexed vigorously for 1 min. One ml of this suspension was then mixed with an equal volume of dichloromethane (Fisher scientific, Illkirch, France) and centrifuged at 3,000 g for 10 min. The organic phase was collected and the aqueous phase was again extracted with dichloromethane as described above. The two resulting organic phases were combined, dried over anhydrous magnesium sulfate (Sigma-Aldrich), evaporated to dryness, and stored at −20°C until analysis. These dried extracts were analyzed by HPLC-MS, as previously described [30], [31]. NAHSL was also assayed using the A. tumefaciens biosensor NT1 and thin-layer chromatography silicate plates (C18-reverse phase, Whatman) according to Shaw et al. [32]. Eleven NAHSL standards were used, six N-acyl- (from butanoyl- to tetradecanoyl-) and five N-3-oxo- (from 3-oxo-hexanoyl- to 3-oxo-tetradecanoyl-) -L-HSL. The synthetic standards and stock solutions were prepared in HPLC-grade ethyl acetate (Fisher Scientific, France) and stored at −20°C.
Sampling of Potato Tubers for Confocal Laser Scanning Microscopy (CLSM)
Bacterial smears were made from inoculated potato tuber tissue and fixed by heating on glass slides. Slides were examined by inverted CLSM (LSM 710, Carl Zeiss MicroImaging, Le Pecq, France). To excite the GFP in bacterial cells, a 488 nm laser with 509 nm emission filters was used. Confocal images were acquired with Zen 2009® software (Carl Zeiss MicroImaging) using the same gains and offset parameters for all images. Three bacterial smears from three different tubers were analyzed for each condition.
Results
Tuber Soft-rot Associated withP. atrosepticum is Triggered by N-3-oxo-octanoyl-L-HSL-based QS
Two methods were tested for evaluating NAHSL production: high-performance liquid chromatography coupled to mass spectrometry (HPLC-MS) as described in Latour et al. [30] and thin-layer chromatography (TLC) with the biosensor Agrobacterium tumefaciens NT1 for detection [32]. TLC gave better results with all inoculated tubers, probably because it has a lower limit of NAHSL detection (0.5 fmol for N-3-oxo-octanoyl-L-HSL) [32]. This high sensitivity is a consequence of the signaling molecule produced by P. atrosepticum being the same as that used naturally by the biosensor A. tumefaciens and therefore being best recognized by this bacterium.
In vitro characterization of NAHSL production both by TLC and HPLC-MS showed that most P. atrosepticum strains, including the P. atrosepticum strain 6276 used in this study, produce N-3-oxo-octanoyl-L-HSL when grown in minimal medium with polygalacturonic acid [18]. Potato tubers were inoculated with P. atrosepticum 6276 and NAHSL was subsequently extracted. TLC identified the extracted NAHSL as N-3-oxo-octanoyl-L-HSL, the same as the NAHSLs produced by P. atrosepticum in a synthetic medium inducing synthesis of virulence factors [7], [18] and the signaling molecule used by this pathogen in the host plant. No NAHSLs were detected in tubers inoculated with P. atrosepticum 6276(pME6000-aiiA) expressing the Bacillus AiiA lactonase [33]. Hereafter, for simplicity, this NAHSL auto-quencher strain is referred to as Pa-QS–.
P. atrosepticum 6276 strains carrying or not carrying the empty vector pME6000 (Pa-QS+) were used both as typical NAHSL producers and virulent potato pathogens. These two strains produced similar amounts of NAHSL in the potato tubers (10 to 950 ng/g of tuber, 1 to 7 days after inoculation). Pa-QS+ induced tissue maceration, and the diameter of the lesion increased with time; the non-NAHSL producing derivative strain, Pa-QS– did not induce tissue maceration (Fig. 1A). This confirms that QS regulation is required for the virulence of P. atrosepticum. No maceration was detected in potato tubers inoculated only with R. erythropolis BCA, a bacterium that does not express the pectinolytic enzymes produced by soft-rot bacteria (Fig. 2A).
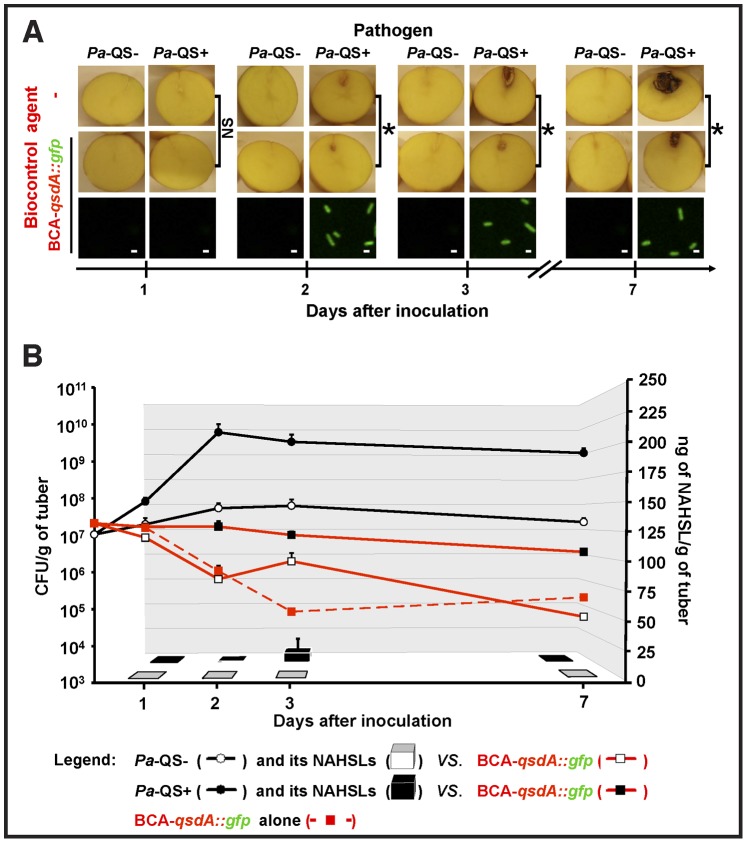
Figure 1. Induction ofqsdA gene transcription, NAHSL-breakdown and biocontrol activity of R. erythropolis in potato tubers.
(A) qsdA gene transcription and biocontrol activity of the R. erythropolis BCA-qsdA::gfp against P. atrosepticum 6276 defective (Pa-QS–) or not (Pa-QS+) for NAHSL production were analyzed at 1, 2, 3 and 7 days after inoculation of S. tuberosum var. Allians tubers. For the controls, one of the two strains was replaced in the inoculum with a 0.9% NaCl solution. Asterisks indicate significantly less severe maceration symptoms in the presence of the BCA-qsdA::gfp, as assessed with the Mann and Whitney test (α = 0.05). The fluorescence of the BCA-qsdA::gfp was analyzed by confocal laser scanning microscopy. (B) The numbers of P. atrosepticum (black lines) and R. erythropolis (red lines) bacteria per unit weight (CFU/g fresh weight of potato tubers), and NAHSL concentration (ng/g of potato tubers; black and white bars) were determined for each condition in potato tubers. For lines and bars, each value is the mean of three replicates with the standard deviation indicated. NS, non-significant; NAHSL, N-acyl homoserine lactone.
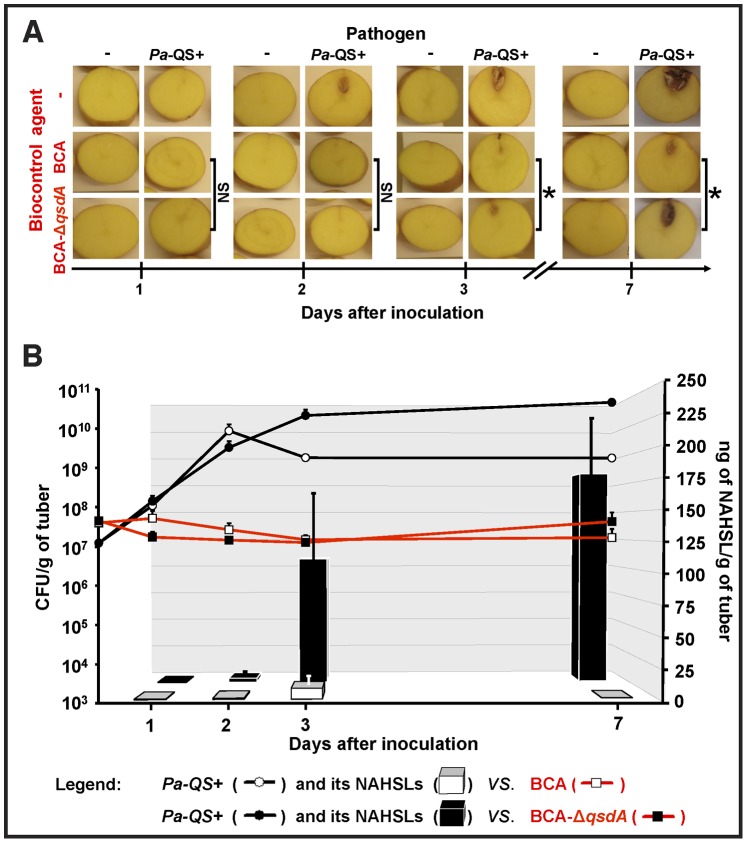
Figure 2. NAHSL-breakdown and biocontrol activity of theR.erythropolis qsdA deletion mutant in potato tubers.
(A) The R. erythropolis R138 wild-type (BCA) and the R. erythropolis R138 ΔqsdA (BCA-ΔqsdA) strains were compared for biocontrol activity against P. atrosepticum 6276 (Pa-QS+) 1, 2, 3 and 7 days after inoculation of potato tubers. For the controls, one or both strains were replaced in the inoculum with a 0.9% NaCl solution. Significant differences (Mann and Whitney test; α = 0.05) in maceration symptoms between infected tubers inoculated with the BCA or the BCA-ΔqsdA are indicated with an asterisk. (B) Population dynamics of P. atrosepticum and R. erythropolis bacteria (CFU/g fresh weight of potato tubers; black and red lines respectively), and NAHSL concentrations (ng/g of potato tubers; black and white bars) were determined for each condition in potato tubers. For lines and bars, each value is the mean of three replicates with the standard deviation indicated. NS, non-significant; NAHSL, N-acyl homoserine lactone.
qsdA Gene Transcription in R. erythropolis is Induced in Potato Tuber by P. atrosepticum NAHSLs
To analyze the qsdA gene transcription during biocontrol assays on potato tubers, the R. erythropolis R138 strain harboring a transcriptional qsdA::gfp fusion (BCA-qsdA::gfp) was generated (see the Materials and Methods). The extent of maceration was lower in tubers co-inoculated with Pa-QS+ and BCA-qsdA::gfp than in tubers inoculated with Pa-QS+ alone 2 to 7 days post-inoculation (p<0.05) (Fig. 1A). This indicates that the biocontrol capacity of R. erythropolis R138 had not been altered by transformation with the recombinant plasmid carrying the qsdA::gfp fusion.
CLSM of bacterial smears from inoculated tubers was used to assess GFP-associated fluorescence. No such fluorescence was detected in potato tubers inoculated with the BCA-qsdA::gfp strain alone (data not shown) or in combination with Pa-QS– (Fig. 1A). GFP-expressing bacteria were only detected between 2 and 7 days after co-inoculation with BCA-qsdA::gfp and Pa-QS+ (Fig. 1A), suggesting that the transcription of the qsdA gene is only induced if NAHSLs are produced. The population dynamics of the pathogen and of the BCA-qsdA::gfp strains were examined and inoculated tubers were assayed for NAHSLs. The density of the Pa-QS+ population in the presence of R. erythropolis increased from 107 CFU/g to 6×109 CFU/g 2 days post-inoculation (Fig. 1B). The population of Pa-QS– increased more slowly (from 107 to 5×107 CFU/g 3 days post-inoculation). In both cases, the populations remained relatively stable thereafter. The population of R. erythropolis remained constant until day 3 in the presence of Pa-QS+ and then decreased from 107 to 3×106 CFU/g. The concentration of NAHSL decreased 30 to 7,000 fold between days 1 to 7 in tubers co-inoculated with Pa-QS+ and BCA-qsdA::gfp strains, whereas there was no such decrease in tubers inoculated only with Pa-QS+ (data not shown). In contrast, in the presence of Pa-QS–, the population of R. erythropolis decreased more strongly with a similar fitness to that observed with the population inoculated alone (Fig. 1B). This suggests that the growth of BCA-qsdA::gfp is promoted by the assimilation of NAHSLs and/or tuber cell lysates associated with the presence of Pa-QS+. These various results clearly indicate that BCA-qsdA::gfp degrades NAHSL.
QsdA Involvement in Quorum Quenching and Control of Tuber Soft-rot due toP. atrosepticum
To investigate the role of the γ-lactone catabolic pathway in the control of tuber soft-rot due to P. atrosepticum, a qsdA deletion mutant and a heterologous QsdA expression system were constructed (see Materials and Methods). The presence of a single copy of the qsdA gene in the genome of R. erythropolis R138 was verified by Southern hybridization using a qsdA probe (data not shown). The R. erythropolis R138 ΔqsdA (BCA-ΔqsdA) strain was compared to the R. erythropolis parental strain for its biocontrol ability against P. atrosepticum and for NAHSL-degrading activity. Tubers were co-inoculated with P. atrosepticum Pa-QS+ and each of the two R. erythropolis strains: significant differences in tissue maceration were observed from the third day (p<0.05) (Fig. 2A). The density of P. atrosepticum increased from 107 to 9×109 CFU/g by day 2 in the presence of the BCA strain and from 107 to 2×1010 CFU/g by day 3 in the presence of the BCA-ΔqsdA strain (Fig 2B). The population of P. atrosepticum in the presence of the BCA strain decreased to 2×109 CFU/g on day 3 and remained constant thereafter. In the presence of the BCA-ΔqsdA strain, the population of P. atrosepticum increased twofold after day 3. The density of R. erythropolis (both BCA and BCA-ΔqsdA) stayed relatively constant over the duration of the experiment at about 4×107 CFU/g. NAHSL titers in tubers co-inoculated with the BCA-ΔqsdA strain were approximately 10 and 1,400 fold higher than in tubers co-inoculated with the parental strain on 3 and 7 days post-inoculation, respectively (Fig. 2B). This clearly implicates QsdA in NAHSL inactivation in potato tubers.
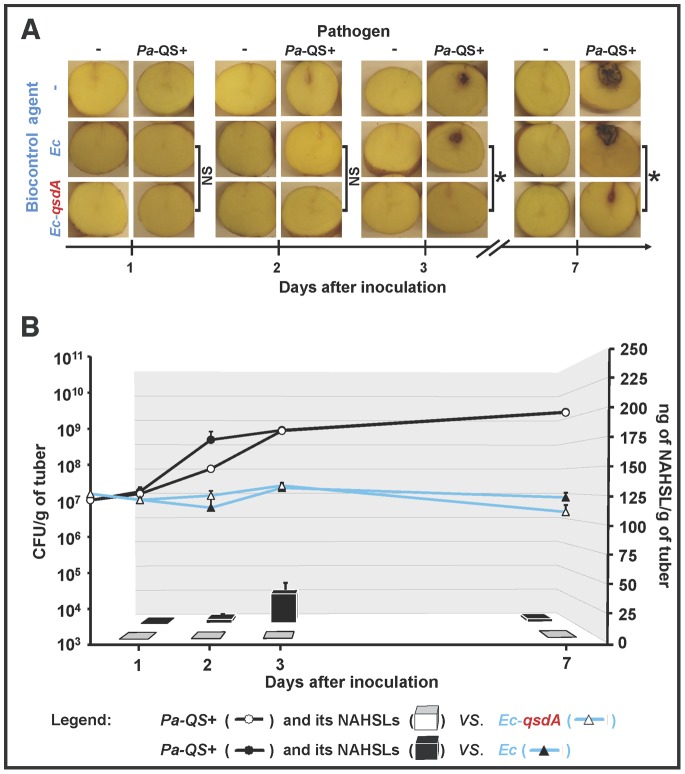
The qsdA gene was introduced into E. coli, a bacterium unable to degrade NAHSLs and the heterologous expression of QsdA was verified. The QsdA-expressing strain E. coli DH5α(pUC19-qsdA) (hereafter called Ec-qsdA) and the control E. coli DH5α strain carrying the empty pUC19 vector (Ec) were compared for biocontrol activity against P. atrosepticum and their ability to degrade NAHSL molecules. Potato tubers inoculated with the P. atrosepticum strain alone or in combination with Ec exhibited similar symptom severity (Fig. 3A); co-inoculation of tubers with the pathogen and Ec-qsdA resulted in a significantly less tissue maceration 3 and 7 days post-inoculation. The density of P. atrosepticum stayed constant for 24 h post-inoculation but then increased from 107 to 108 or 5×108 CFU/g on day 2 in the presence of Ec-qsdA or Ec, respectively (Fig. 3B). A similar growth was noted from day 3 post-inoculation. The population density of P. atrosepticum increased to 2×109 CFU/g on day 7 days whether co-inoculated with Ec or Ec-qsdA, whereas the E. coli population density (Ec or Ec-qsdA) remained constant over the seven-day duration of the experiment at about 107 CFU/g. NAHSL was assayed in each condition: the amounts of these molecules in tubers co-inoculated with Ec-qsdA were approximately 20 to 100 fold lower than in tubers co-inoculated with Ec (Fig. 3B), confirming the in planta QS quenching ability of QsdA.
Figure 3. NAHSL-breakdown and biocontrol activity of the QsdA-expressingE. coli strain in potato tubers.
(A) E. coli DH5α(pUC19) (Ec) and E. coli DH5α(pUC19-qsdA) (Ec-qsdA) were compared for biocontrol activity against P. atrosepticum 6276 (Pa-QS+) 1, 2, 3 and 7 days after inoculation of potato tubers. For the controls, one or both strains were replaced in the inoculum with a 0.9% NaCl solution. Significant differences (Mann and Whitney test; α = 0.05) in maceration symptoms between infected tubers inoculated with the Ec or the Ec-qsdA strains are indicated with an asterisk. (B) Population dynamics of P. atrosepticum and E. coli bacteria (CFU/g fresh weight of potato tubers; black and blue lines respectively), and NAHSL concentration (ng/g of potato tubers; black and white bars), were determined for each condition in potato tubers. For lines and bars, each value is the mean of three replicates with the standard deviation indicated. NS, non-significant; NAHSL, N-acyl homoserine lactone.
Discussion
Rhodococcal cells have a large number of metabolic pathways and express numerous bioconversion and degradation activities. This has led to the use of these bacteria for the catabolism of recalcitrant molecules [34]–[36]. R. erythropolis both expresses an effective NAHSL-degrading activity and shows biocontrol ability to protect potato tubers; it is therefore an attractive candidate BCA against soft-rot bacteria by quorum quenching based-biocontrol [19], [37]. This strategy could be applied to the protection of numerous horticultural and vegetable crops and to the control of other plant pathogens that use N-3-oxo-octanoyl-L-HSL (for example Agrobacterium spp.) or γ-butyrolactones (Streptomyces spp.), as signaling molecules [38], [39].
The molecular mechanisms involved in this protection have not previously been identified in planta. Although the beneficial action of R. erythropolis has been evidenced in plant models, the activity responsible for the protection has mostly been studied using bacterial cells extracted from the host, then grown on synthetic media [13], [14], [19]. Recently, we discovered a catabolic pathway in R. erythropolis involved in the assimilation of various γ-lactones composed of a five-member ring linked to an aliphatic chain [21]. Here, we report an analysis of the involvement of this metabolic pathway in the biocontrol activity of R. erythropolis in potato tubers. We focused on the key marker of this catabolic route, the lactonase QsdA. QsdA catalyzes the ring-opening of γ-lactones, a selective and limiting operation involved in the first step of the pathway. Downstream from the qsdA gene in the R. erythropolis genome there are contiguous sequences encoding proteins strongly suspected to belong to the same pathway. For example, fadD, a gene under the same promoter as qsdA, codes for the long-chain fatty acid-CoA ligase FadD, which activates, by CoA thioester linkage, the aliphatic acids resulting from QsdA activity [21]. We first examined the transcriptional activity of the qsdA gene, in the form of a qsdA::gfp transcriptional fusion, in R. erythropolis co-inoculated with the pathogen into tubers. CLSM analysis revealed that GFP-expressing bacteria were first detectable 2 days after inoculation; consistent with this, NAHSL assays indicated that the qsdA gene was transcribed only in the presence of a sufficient concentration of NAHSL. No fluorescence was observed in the presence of the P. atrosepticum strain defective for NAHSL production. This threshold concentration of NAHSLs was reached when the P. atrosepticum population density reached a certain level, corresponding to the ‘quorum’ population density (after 48 h in our experimental model). This point in the potato infection marks the transition between the multiplication phase characterized by a rapid increase in the pathogenic bacterial density and the invasive phase typified by a stable population density and the massive production of lytic enzymes mediated by QS [40]. The absence of the characteristic multiplication and soft-rot phases in tubers inoculated with Pa-QS– was due to the absence of NAHSLs (or the production of amounts below the detection limits of the quantification methods). Indeed, NAHSL signals are essential for regulating the production of lytic enzymes, which release nutrients for the pathogen [8], [10]. Only the presence of Pa-QS+ promoted a sustainable survival of the populations of R. erythropolis in tubers over the seven-day duration of the experiment, enabling them to exert their biocontrol effect. An E. coli strain heterologously expressing QsdA was able to protect tubers against the pathogen with the same effectiveness as the wild-type R. erythropolis strain. This clearly confirms the role of QsdA in the protection of potato tubers against P. atrosepticum. Moreover, tissue maceration was significantly greater from the third day post-inoculation to the end of the experiment when the BCA-ΔqsdA mutant strain rather than the QsdA-expressing parental strain was used for co-infections. This difference was associated with a 10 fold greater P. atrosepticum population in the presence of the BCA-ΔqsdA strain and to higher NAHSL levels.
The deletion of the qsdA gene did not completely abolish the biocontrol activity of R. erythropolis R138. There are at least two possible explanations. Rhodococci produces multiple homologs of catabolic enzymes, enhancing metabolic versatility [35], [36]. Therefore, there may be one or more alternative enzymes for lactone catabolism working in addition to QsdA. The second possibility is that the inactivation of this pathway may provoke a switch in metabolism and the initiation of an alternative metabolic pathway taking over the function of the first pathway. This possibility deserves careful consideration, given that 3-oxo substituted-HSL molecules have been demonstrated to be bactericidal in Gram-positive bacteria [41]. Indeed, Gram-positive bacteria may have developed NAHSL-degrading enzymes to protect themselves against the antibacterial activity of NAHSLs and to enhance their survival in the natural environment [41], [42]. Were this the case, bacteria may possess alternative metabolic pathways for NAHSL inactivation to compensate for the inactivity of one of them. These alternative metabolic routes may involve a lactonase other than QsdA, an acylase which liberates a free homoserine lactone and a fatty acid, or an oxido-reductase whose activity results in silencing the QS-regulated processes; the degradation products of such activities cannot act as signal molecules [33]. Note that traces of these three activities have been detected in vitro in other R. erythropolis strains [43]–[45].
In conclusion, this work documents the nature of the signaling molecules used by P. atrosepticum in planta and their role in virulence. Previous in vitro assays show the intracellular production of catabolites (N-acyl homoserine) in R. erythropolis from NAHSLs by opening the lactone ring by QsdA, and also that other enzymes are required for the complete assimilation of lactones [21], [45]. Our work provides evidence for the involvement of this γ-lactone catabolic pathway in the breakdown of extracellular NAHSL and thus in the biocontrol activity of R. erythropolis. An important point for biocontrol applications is that this pathway is induced not by the invasion step or the presence of the pathogen, but by its QS-based communication. It would be valuable to characterize the mechanisms involved in signal transduction in the BCA, from NAHSL detection to the transcription of corresponding catabolic operons. The γ-lactone pathway of R. erythropolis is an example of a catabolic pathway in which the broad spectrum of the substrates allows it to contribute to bacterial nutrition [21], detoxification [41], [42] and the control of communication. Our experiments suggest that this pathway may well not be the only one involved in the control of soft-rot. These biocontrol pathways can be added to the list of those already described to protect plants from soft-rot bacteria, such as 2,4 diacetylphloroglucinol synthesis in P. fluorescens F113 [46] and the antagonism and/or the induction of systemic resistance by Serratia plymuthica A30 [47], [48]. More generally, the γ-lactone pathway differs from many of these other biocontrol mechanisms in that it is based on a catabolic principle; it is thus unlike antibiosis, iron competition or plant-induced systemic resistance based on the synthesis of secondary metabolic compounds.
Acknowledgments
We are grateful to Dr Jan Nešvera (Laboratory of Molecular Genetics of Bacteria, Institute of Microbiology, Academy of Sciences of the Czech Republic) for the gift of the plasmid pEPR1, to Prof. C. Thomas (School of biosciences, University of Birmingham, UK) for the gift of the plasmid pAKE604, and to Prof. D. Haas (Université de Lausanne, Switzerland) for the gift of the plasmids pME6000 and pME6863. We also thank Olivier Maillot for technical assistance, Christine Farmer and Alex Edelman for linguistic support.
Works are related to COST 631 action -“Understanding and Modeling Plant-Soil Interactions in the Rhizosphere Environment”.
Funding Statement
This research was supported by grants from the Région Haute-Normandie & Ministère délégué à l’Enseignement Supérieur et à la Recherche, GRR VASI (ex-VATA) & FEDER (European Union). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. von Bodman SB, Bauer WD, Coplin DL (2003) Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol 41: 455–482. [DOI] [PubMed] [Google Scholar]
- 2. Barnard AM, Salmond GP (2007) Quorum sensing in Erwinia species. Anal Bioanal Chem 387: 415–423. [DOI] [PubMed] [Google Scholar]
- 3. Charkowski A, Blanco C, Condemine G, Expert D, Franza T, et al. (2012) The role of secretion systems and small molecules in soft-rot enterobacteriaceae pathogenicity. Annu Rev Phytopathol 50: 425–449. [DOI] [PubMed] [Google Scholar]
- 4. Põllumaa L, Alamae T, Maë A (2012) Quorum sensing and expression of virulence in pectobacteria . Sensors 12: 3327–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M (2007) Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum . Proteomics 7: 3527–3537. [DOI] [PubMed] [Google Scholar]
- 6.Monson R, Burr T, Liu H, Hedley P, Toth I, et al. (2012) Identification of genes in the VirR regulon of Pectobacterium atrosepticum and characterization of their roles in quorum sensing-dependent virulence. Environ Microbiol doi:–10.111/j.1462–2920.2012.02822.x [DOI] [PubMed]
- 7.Tarasova N, Gorshkov V, Petrova O, Gogolev Y (2013) Potato signal molecules that activate pectate lyase synthesis in Pectobacterium atrosepticum SCRI1043. World J Microbiol Biotechnol DOI –––10.1007/s11274–013–1281–9 [DOI] [PubMed]
- 8. Toth IK, Birch PRJ (2005) Rotting softly and stealthily. Curr Opin Plant Biol 8: 424–429. [DOI] [PubMed] [Google Scholar]
- 9. Maë A, Montesano M, Koiv V, Palva ET (2001) Transgenic plants producing the bacterial pheromone N-acyl-homoserine lactone exhibit enhanced resistance to the bacterial phytopathogen Erwinia carotovora . Mol Plant Microbe Interact 14: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 10. Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, et al. (2008) Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum . PLoS Pathog 4: e1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bais HP (2012) Shoot the messages not the messengers. Plant Soil 358: 7–10. [Google Scholar]
- 12. Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, et al. (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411: 813–17. [DOI] [PubMed] [Google Scholar]
- 13. Faure D, Dessaux Y (2007) Quorum sensing as a target for developing control strategies for the plant pathogen Pectobacterium . Eur J Plant Pathol 119: 353–365. [Google Scholar]
- 14. Diallo S, Crepin A, Barbey C, Orange N, Burini J-F, et al. (2011) Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol Ecol 75: 351–364. [DOI] [PubMed] [Google Scholar]
- 15.Food and Agriculture Organization (FAO) (2009) International year of the potato 2008, New light on a hidden treasure. Food and Agriculture Organization of the United Nations (ed), Rome. 144 p.
- 16. Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM (2011) Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol 60: 999–1013. [Google Scholar]
- 17. Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Helias V, et al. (2011) Dickeya species: an emerging problem for potato production in Europe. Plant Pathol 60: 385–399. [Google Scholar]
- 18. Crépin A, Barbey C, Beury-Cirou A, Helias V, Taupin L, et al. (2012) Quorum sensing signaling molecules produced by reference and emerging soft-rot bacteria (Dickeya and Pectobacterium spp.). PLoS ONE 7: e35176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crépin A, Barbey C, Cirou A, Tannières M, Orange N, et al. (2012) Biological control of pathogen communication in the rhizosphere: a novel approach applied to potato soft rot due to Pectobacterium atrosepticum . Plant Soil 358: 27–37. [Google Scholar]
- 20. Crépin A, Beury-Cirou A, Barbey C, Farmer C, Helias V, et al. (2012) N-acyl homoserine lactones in diverse Pectobacterium and Dickeya plant pathogens: diversity, abundance, and involvement in virulence. Sensors 12: 3484–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbey C, Crépin A, Cirou A, Budin-Verneuil A, Orange N, et al. (2012) Catabolic pathway of gamma-caprolactone in the biocontrol agent Rhodococcus erythropolis . J Proteome Res 11: 206–216. [DOI] [PubMed] [Google Scholar]
- 22. Cirou A, Mondy S, An S, Charrier A, Sarrazin A, et al. (2012) Efficient biostimulation of the native and introduced quorum-quenching Rhodococcus erythropolis is revealed by a combination of analytical chemistry, microbiology and pyrosequencing. Appl Environ Microbiol 78: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cirou A, Raffoux A, Diallo S, Latour X, Dessaux Y, et al. (2011) Gamma-caprolactone stimulates growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum . Res Microbiol 162: 945–950. [DOI] [PubMed] [Google Scholar]
- 24. Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, et al. (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA 71: 3672–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smadja B, Latour X, Trigui S, Burini J-F, Chevalier S, et al. (2004) Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can J Microbiol 50: 19–27. [DOI] [PubMed] [Google Scholar]
- 26. El-Sayed AK, Hothersall J, Thomas CM (2001) Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology 147: 2127–2139. [DOI] [PubMed] [Google Scholar]
- 27. van der Geize R, Hessels GI, van Gerwen R, van der Meijden P, Dijkhuizen L (2001) Unmarked gene deletion mutagenesis of kstD, encoding 3-ketosteroid Delta1-dehydrogenase, in Rhodococcus erythropolis SQ1 using sacB as counter-selectable marker. FEMS Microbiol Lett 205: 197–202. [DOI] [PubMed] [Google Scholar]
- 28. Knoppova M, Phensaijai M, Vesely M, Zemanova M, Nesvera J, et al. (2007) Plasmid vectors for testing in vivo promoter activities in Corynebacterium glutamicum and Rhodococcus erythropolis . Curr Microbiol 55: 234–239. [DOI] [PubMed] [Google Scholar]
- 29. Vesely M, Patek M, Nesvera J, Cejkova A, Masak J, et al. (2003) Host-vector system for phenol-degrading Rhodococcus erythropolis based on Corynebacterium plasmids. Appl Microbiol Biotechnol 61: 523–527. [DOI] [PubMed] [Google Scholar]
- 30. Latour X, Diallo S, Chevalier S, Morin D, Smadja B, et al. (2007) Thermoregulation of N-acyl homoserine lactone-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum . Appl Environ Microbiol 73: 4078–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morin D, Grasland B, Vallée-Réhel K, Dufau C, Haras D (2003) On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acyl homoserine lactones, quorum-sensing signal molecules, in the presence of biological matrices. J Chromatogr A 1002: 79–92. [DOI] [PubMed] [Google Scholar]
- 32. Shaw PD, Ping G, Daly SL, Cha C, Cronan JE Jr, et al. (1997) Detecting and characterization N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA 94: 6036–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong YH, Zhang LH (2005) Quorum sensing and quorum-quenching enzymes. J Microbiol 43: 101–109. [PubMed] [Google Scholar]
- 34. de Carvalho CC, da Fonseca MM (2005) The remarkable Rhodococcus erythropolis . Appl Microbiol Biotechnol 67: 715–726. [DOI] [PubMed] [Google Scholar]
- 35. Larkin MJ, Kulakov LA, Allen CCR (2005) Biodegradation and Rhodococcus - masters of catabolic versatility. Curr Opin Microbiol 16: 282–290. [DOI] [PubMed] [Google Scholar]
- 36. van der Geize R, Dijkhuizen L (2004) Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr Opin Microbiol 7: 255–261. [DOI] [PubMed] [Google Scholar]
- 37. Jafra S, Przysowa J, Czajkowski R, Michta A, Garbeva P, et al. (2006) Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can J Microbiol 52: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 38. Cha C, Gao P, Chen YC, Shaw PD, Farrand SK (1998) Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant Microbe Interact 11: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 39. Nishida H, Ohnishi Y, Beppu T, Horinouchi S (2007) Evolution of γ-butyrolactone synthases and receptors in Streptomyces . Environ Microbiol 9: 1986–1994. [DOI] [PubMed] [Google Scholar]
- 40. Smadja B, Latour X, Faure D, Chevalier S, Dessaux Y, et al. (2004) Involvement of N-acylhomoserine lactones throughout plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum). Mol Plant Microbe Interact 17: 1269–1278. [DOI] [PubMed] [Google Scholar]
- 41. Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, et al. (2005) Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci USA 102: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roche DM, Byers JT, Smith DS, Glansdorp FG, Spring DR, et al. (2004) Communications blackout? Do N-acylhomoserine-lactone-degrading enzymes have any role in quorum sensing? Microbiology 150: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 43. Park SY, Hwang BJ, Shin MH, Kim JA, Kim HK, et al. (2006) N-acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol Lett 261: 102–108. [DOI] [PubMed] [Google Scholar]
- 44. Uroz S, Chhabra SR, Camara M, Williams P, Oger P, et al. (2005) N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 151: 3313–3322. [DOI] [PubMed] [Google Scholar]
- 45. Uroz S, Oger PM, Chapelle E, Adeline MT, Faure D, et al. (2008) A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl Environ Microbiol 74: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN, et al. (1997) Ecological interaction of a biocontrol Pseudomonas fluorescens strain producing 2,4-diacetylphloroglucinol with the soft rot potato pathogen Erwinia carotovora subsp. atroseptica . FEMS Microbiol Ecol 23: 95–106. [Google Scholar]
- 47. Czajkowski R, de Boer WJ, van Veen JA, van der Wolf JM (2011) Characterization of bacterial isolates from rotting potato tuber tissue showing antagonism to Dickeya sp. biovar 3 in potato in vitro and in planta . Plant Pathol 61: 169–182. [Google Scholar]
- 48. Czajkowski R, de Boer WJ, van Veen, JA, van der Wolf JM (2011) Studies on the interaction between the biocontrol agent, Serratia plymuthica A30, and blackleg-causing Dickeya sp. (biovar 3) in potato (Solanum tuberosum). Plant Pathol 61: 677–688. [Google Scholar]
- 49. Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat Biotechnol 1: 784–791. [Google Scholar]
- 50. Cirou A, Diallo S, Kurt C, Latour X, Faure D (2007) Growth promotion of quorum-quenching bacteria in the rhizosphere of Solanum tuberosum . Environ Microbiol 9: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 51. Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, et al. (1998) Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco necrosis virus. Phytopathology 88: 687–694. [DOI] [PubMed] [Google Scholar]
- 52. Reimmann C, Ginet N, Michel L, Keel C, Michaix P, et al. (2002) Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148: 923–932. [DOI] [PubMed] [Google Scholar]