Abstract
Background
Neuromyelitis optica (NMO) and relapsing-remitting multiple sclerosis (RRMS) are difficult to differentiate solely on clinical grounds. Optical coherence tomography (OCT) studies investigating retinal changes in both diseases focused primarily on the retinal nerve fiber layer (RNFL) while rare data are available on deeper intra-retinal layers.
Objective
To detect different patterns of intra-retinal layer alterations in patients with NMO spectrum disorders (NMOSD) and RRMS with focus on the influence of a previous optic neuritis (ON).
Methods
We applied spectral-domain OCT in eyes of NMOSD patients and compared them to matched RRMS patients and healthy controls (HC). Semi-automatic intra-retinal layer segmentation was used to quantify intra-retinal layer thicknesses. In a subgroup low contrast visual acuity (LCVA) was assessed.
Results
NMOSD-, MS- and HC-groups, each comprising 17 subjects, were included in analysis. RNFL thickness was more severely reduced in NMOSD compared to MS following ON. In MS-ON eyes, RNFL thinning showed a clear temporal preponderance, whereas in NMOSD-ON eyes RNFL was more evenly reduced, resulting in a significantly lower ratio of the nasal versus temporal RNFL thickness. In comparison to HC, ganglion cell layer thickness was stronger reduced in NMOSD-ON than in MS-ON, accompanied by a more severe impairment of LCVA. The inner nuclear layer and the outer retinal layers were thicker in NMOSD-ON patients compared to NMOSD without ON and HC eyes while these differences were primarily driven by microcystic macular edema.
Conclusion
Our study supports previous findings that ON in NMOSD leads to more pronounced retinal thinning and visual function impairment than in RRMS. The different retinal damage patterns in NMOSD versus RRMS support the current notion of distinct pathomechanisms of both conditions. However, OCT is still insufficient to help with the clinically relevant differentiation of both conditions in an individual patient.
Introduction
Neuromyelitis optica (NMO) is a rare autoimmune central nervous system (CNS) condition that predominantly affects the optic nerves and the spinal cord [1], [2]. While NMO had previously been regarded as variant of multiple sclerosis (MS), the recent detection of a highly specific serum biomarker for NMO, antibodies to the most abundant astrocytic CNS water channel aquaporin-4 (AQP4), as well as histopathological data has made clear that NMO is a condition distinct from MS [1], [3]–[7].
An early and accurate diagnosis of NMO and distinction from MS is crucial as prognosis is usually worse than in MS and treatment options for both diseases differ considerably [8]. Although the broad availability of commercial testing for antibodies to AQP4 has facilitated differentiation of NMO from MS, a correct diagnosis remains challenging, in particular in those NMO patients who test negative for AQP4 antibodies. Thus, a considerable number of patients are still misdiagnosed with MS [1], [9].
Optical coherence tomography (OCT) is a non-invasive interferometry technique that has been used to measure retinal nerve fiber layer (RNFL) thickness, total macular volume (TMV) and ganglion cell layer (GCL) thickness in MS and other neurological diseases [10]–[14]. In MS, numerous studies have consistently shown reduced RNFL, TMV, and GCL across disease subtypes and in both eyes with and without prior history of optic neuritis (ON) [14]–[22]. Moreover, measures of retinal neuroaxonal damage were found to correlate with brain atrophy in MS [23]–[27].
Previous OCT studies have shown that ON in NMO causes more severe neuronal damage and greater thinning of the RNFL than ON in MS [28]–[34], which is in line with the clinical experience that visual acuity in NMO is usually more severely affected and visual outcome poorer than in MS [2], [8]. Moreover, and again in contrast to MS, increase in retinal damage as measured by OCT seems to be exclusively linked to clinical ON attacks in NMO while progressive retinal neuroaxonal damage independent of clinically apparent ON as in MS is rare in NMO [35].
In NMO, there are only few studies on retinal OCT data acquired with the high-resolution spectral-domain (SD) technology [36]–[39] most of which did not present data on segmentation of deeper retinal layers. The goal of our study was to analyze OCT measures from all retinal layers in patients with NMO or MS rigorously matched for history of ON and in healthy controls (HC), and to relate these measures to visual functions. We were interested whether the more severe retinal affection in NMO-ON as compared to MS-ON described in earlier OCT studies would also be detectable in deeper retinal layers and whether damage patterns differ between both conditions. If so, OCT could be an additional tool to aid in the clinically challenging differential diagnosis of MS and NMO.
Materials and Methods
Study Participants
Seventeen patients with NMO and NMO spectrum disorder (NMOSD) [5] were prospectively recruited from the outpatient clinics of the NeuroCure Clinical Research Center and of the Department of Neurology, Charité Universitaetsmedizin Berlin. Thirteen patients fulfilled the 2006 diagnostic criteria for NMO, and 4 had NMOSD (3 with longitudinally extensive transverse myelitis (LETM), 1 with recurrent optic neuritis) with AQP4 antibodies [40]. Ninety four percent (16/17) were seropositive for AQP4 antibodies [41]–[43]. HC and relapsing-remitting MS (RRMS) patients according to the 2010 revised McDonald criteria [44] were matched from the center’s research database according to age and gender. NMOSD and MS patients were additionally matched eye-wise for history of ON, determined by medical record review. Exclusion criteria were eye or retina diseases other than ON, refractive error greater than ±6 dpt and acute ON or treatment with corticosteroids within three months prior to OCT examination.
Ethics Statement
The study was approved by the local ethics committee at the Charité - Universitätsmedizin Berlin and was conducted following the Declaration of Helsinki in its currently applicable version, the guidelines of the International Conference on Harmonisation of Good Clinical Practice and the applicable German laws. All participants gave informed written consent.
Optical Coherence Tomography
OCT examination was performed with the Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) without pupil dilatation. RNFL thickness was measured using a 3.4 mm circular scan around the optic nerve head with the device’s standard protocol and segmentation algorithm with activated eye tracker. Ring scan parameters included into analysis were average peripapillary RNFL (pRNFL), quadrant RNFL thicknesses (figure 1A), and nasal to temporal RNFL ratio (N/T ratio). The TMV was assessed by a custom scan that uses 61 vertical B-scans (each with 768 A-Scans, ART = 13 frames) with a scanning angle of 30°×25° focusing on the fovea. All scans passed published quality control criteria [45].
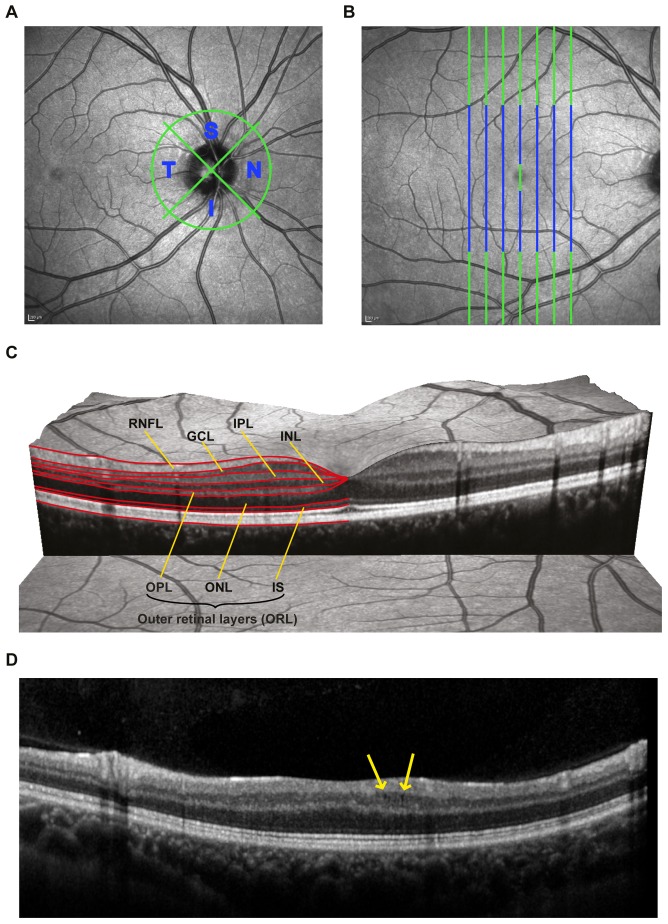
Figure 1. Sample OCT measurement and segmentation.
A) Sample scanning laser ophthalmoscopy image showing the peripapillary ring-scan for retinal nerve fiber layer analysis. Nasal and temporal quadrants were analyzed separately B) Sample scanning laser ophthalmoscopy image showing the B-scans included in the segmentation procedure (green and blue) and the area included into analysis (blue only). C) Sample macular scan showing the segmentation lines and intra-retinal layer layout. Red segmentation lines provided by the software define the macular retinal nerve fiber layer (mRNFL), the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), and inner segments of the photoreceptor layer (IS). OPL, ONL and IS were analyzed combined as outer retinal layers (ORL). D) Sample B-scan of an NMOSD patient with microcystic macular edema (MME).
Intra-retinal Layer Segmentation
Heidelberg Engineering provided a beta software version with a multilayer segmentation algorithm for macular volume scans (Spectralis software version 5.5.0.5, Eye Explorer Software 1.7.0.0). To analyze the inner retinal layers of macular volume scans a subset of B-scans were segmented and manually corrected by an experienced rater in a blinded fashion. The multilayer analysis was performed on the central B-scan through the fovea and on three B-scans each in nasal and temporal direction. Every fourth B-scan from the original volume scan was used resulting in a distance between analyzed B-scans of approximately 500 µm. The mean thickness was calculated for the following retinal layers (figure 1C): macular RNFL (mRNFL), GCL, inner plexiform layer (IPL) and inner nuclear layer (INL). The outer retinal layers (ORL), including the outer plexiform layer (OPL), outer nuclear layer (ONL) and inner photoreceptor layer segments (IS), were analyzed in combination. We analyzed thickness data within a box centered around the fovea and excluding the superior and inferior parts of the scan prone to vessel artifacts as well as the central fovea (Figure 1B). Microcystic macular edema (MME) was defined as presence of cystic lesions located in the inner nuclear layer on at least two adjacent B-scans [46].
Visual Function Testing
High contrast visual acuity (VA) of both eyes was assessed monocularly for both eyes using ETDRS charts integrated in the Optec 6500 P system (Stereo Optical Co, Inc, Illinois, USA) on the same day as OCT examination in all groups. The resulting value was converted into Snellen equivalents. Low contrast visual acuity (LCVA) was assessed using Functional Acuity Contrast Testing with the Optec 6500 P system. Both eyes were tested in monocular mode following the standards published by the American National Standards Institute with best correction under photopic (“day light” with target luminance value of 85 cd/m2) conditions without glare as previously described in detail [47]. Briefly, the linear sine-wave grating charts tested for five spatial frequencies, each with nine levels of contrast. Contrast sensitivity was then recorded as the lowest contrast level achieved by a patient for each spatial frequency. From the five spatial frequency measurements, a function over all measurements was calculated using a least square curve fit and the area under the curve was then established as the area under this function between the lowest and highest spatial frequency, providing a summary expression over all measurements.
Statistical Analysis
Cohort differences were analyzed using Kruskal Wallis test for age of all groups and Mann-Whitney-U tests (MWU) for time since diagnosis and time since last ON of the NMOSD and the MS group. To investigate differences in OCT measures between the different cohorts and between ON and NON eyes, generalized estimation equation models (GEE) with working correlation matrix ‘exchangeable’ were used accounting for within-patient inter-eye dependencies. In all GEE models, diagnosis was used as independent variables and OCT parameters as dependent variables. To account even for potential effects of demographic variables we also corrected for age and gender. The monocular association between GCL and visual function was tested using linear regression models (LR) with GCL as independent and visual function as dependent variable with no further covariates. For subgroup analyses (ON or NON eyes) only the respectively matched HC eyes were included in the analyses, resulting in different, but exactly eye-wise matched cohorts.
All statistical analyses were performed with SPSS 20 (IBM SPSS, NY, USA), all graphical figures were created using R (basic R version 2.15.2 including the ggplot2 package). Statistical significance was achieved at p<0.05. All tests should be understood and interpreted as constituting exploratory data analysis in such way that no previous power calculation or adjustments for multiple testing were made.
Results
The demographic data of the study participants is summarized in table 1. Complete data was available from all patients for OCT and high contrast VA. In addition, LCVA was available from 13 NMOSD patients. To avoid inclusion bias only the LCVA results of the respectively matching 13 MS patients were included. Six NMOSD and 6 MS patients had a history of ON for both eyes, eight patients in each group had a unilateral ON and three patients had LETM without history of ON. There were no significant differences in age between the cohorts (Kruskal Wallis test, p = 0.893), yet disease duration was significantly longer in the MS group compared to the NMOSD group (MWU, p = 0.026). There was no significant difference in time since last ON between MS and NMOSD patients’ eyes with a history of ON (MWU, p = 0.181).
Table 1. Demographic and clinical overview.
| NMOSD | RRMS | HC | ||
| Subjects | n | 17 | 17 | 17 |
| Gender | Female | 16 | 16 | 16 |
| Male | 1 | 1 | 1 | |
| Age [years] | mean ± SD | 40.8±12.3 | 41.2±12.7 | 41.4±12.4 |
| Min–Max | 19–63 | 20–64 | 21–66 | |
| Time since diagnosis [months] | Mean ± SD | 44.3±38.2 | 93.7±65.8 | n/a |
| Min–Max | 3–129 | 3–240 | n/a | |
| AQP4-Ig positive | n | 16 | n/a | n/a |
| Eyes | n | 34 | 34 | 34 |
| Eyes with a history of ON | n | 20 | 20 | n/a |
| Time since last ON [months] | Mean ± SD | 45±41 | 65±46 | n/a |
| Min–Max | 3–130 | 3–160 | n/a |
Abbreviations: NMOSD: neuromyelitis optica spectrum disorders; RRMS: relapsing-remitting multiple sclerosis; HC: healthy controls; AQP4-Ig: aquaporin 4 antibodies; ON: optic neuritis, n/a = not applicable (the resp. data did not apply to this group).
OCT Measures in NMOSD and MS Eyes with ON
mRNFL, GCL and IPL were significantly reduced in NMOSD-ON eyes compared to HC (table 2). On the contrary, INL thickness and the outer retinal layers were significantly increased in NMOSD-ON eyes when compared to HC eyes. NMOSD-ON eyes also showed a significantly more pronounced mRNFL, GCL and IPL thinning than MS-ON eyes while slight differences in INL and the outer retinal layers were not significant (sample cases in figure 2).
Table 2. Retinal morphology in eyes after ON.
| NMOSD-ON | MS-ON (vs. NMOSD-ON) | Matched HC (vs. NMOSD-ON) | ||||||||
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | B (SE) | p | Mean ± SD | Min–Max | B (SE) | p | |
| Average pRNFL (µm) | 58.5±21.2 | 28.0–93.4 | 85.3±13.3 | 62.3–105.2 | −26.7 (5.3) | <0.001 | 100.1±10.8 | 80.7–122.3 | 41.5 (5.1) | <0.001 |
| inferior pRNFL (µm) | 79.18±29.3 | 36.1–134.9 | 115.4–17.9 | 81.1–144.9 | −35.9 (7.1) | <0.001 | 131.0±14.9 | 97.4–155.0 | −51.6 (7.1) | <0.001 |
| superior pRNFL (µm) | 73.6±26.1 | 25.2–124.9 | 105.7±18.4 | 71.8–138.0 | −31.9 (7.1) | <0.001 | 120.6±16.0 | 94.0–157.1 | −46.7 (7.2) | <0.001 |
| nasal pRNFL (µm) | 38.6±19.5 | 11.3–87.8 | 67.7±15.1 | 37.8–89.0 | −29.1 (5.4) | <0.001 | 76.5±19.7 | 35.5–105.0 | −37.8 (5.9) | <0.001 |
| temporal pRNFL (µm) | 42.4.±16.6 | 17.9–78.7 | 52.5±14.7 | 35.1–89.8 | −9.9 (4.6) | 0.031 | 72.2±10.4 | 53.2–95.6 | −29.7 (4.3) | <0.001 |
| TMV (mm3) | 7.96±0.47 | 7.04–8.67 | 8.31±0.48 | 7.44–9.02 | −0.34 (0.14) | 0.019 | 8.52±0.53 | 7.75–9.38 | −0.56 (0.15) | <0.001 |
| mRNFL (µm) | 25.6±6.2 | 13.2–35.5 | 29.4±4.0 | 22.8–37.5 | −3.7 (1.5) | 0.015 | 33.4±2.3 | 29.5–36.4 | −7.8 (1.4) | <0.001 |
| GCL (µm) | 30±8.5 | 9.1–46.3 | 36.1±5.8 | 27.1–44.4 | −6.1 (2.2) | 0.007 | 45.5±5.1 | 37.8–56.3 | −15.4 (2.1) | <0.001 |
| IPL (µm) | 26.0–7.0 | 16.4–38.2 | 33.1±7.0 | 22.5–44.8 | −7.0 (2.1) | <0.001 | 35.9±5.2 | 27.6–45.5 | −9.8 (1.9) | <0.001 |
| INL (µm) | 41.5±5.3 | 34.5–54.1 | 39.3±3.1 | 33.4–44.6 | 0.075 | 38.9±3.1 | 33.2–45.9 | 2.6 (1.3) | 0.045 | |
| Outer layers (µm) | 114.6±6.6 | 96.7–128.5 | 113.5±9.6 | 102.0–134.8 | 0.683 | 110.3±6.94 | 99.2–122.9 | 4.2 (2.0) | 0.035 | |
Results of retinal OCT outcomes of NMOSD and MS patients’ eyes after optic neuritis and healthy controls; GEE results showing the differences between NMOSD-ON and MS-ON and NMOSD-ON to healthy controls.
Abbreviations: NMOSD: neuromyelitis optica spectrum disorder; MS: multiple sclerosis; NON: eyes without history of optic neuritis; HC: healthy controls; OCT: optical coherence tomography; p/mRNFL: peripapillary and macular retinal nerve fiber layer; TMV: total macular volume; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; SD: standard deviation; Min: minimum; Max: maximum; B: coefficient estimate from generalized estimating equation models (GEE), SE: standard error from GEE coefficient estimates.
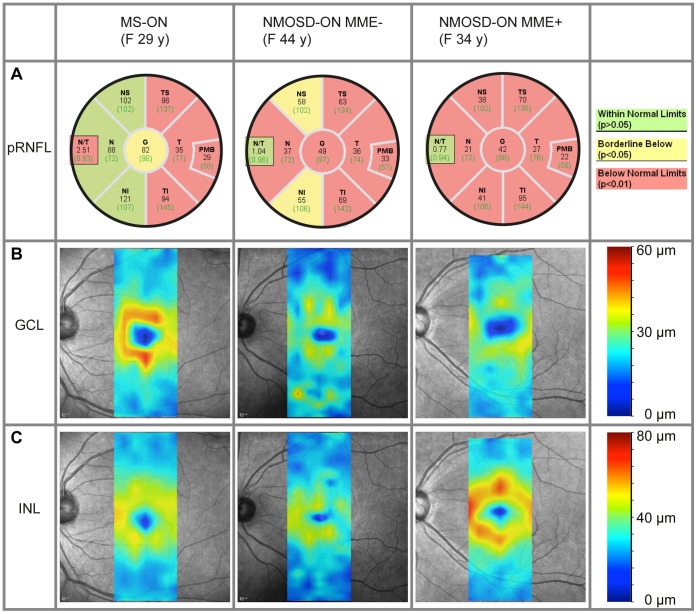
Figure 2. Sample patient data from NMOSD and MS eyes.
A) Peripapillary retinal nerve fiber layer (pRNFL) thickness data (in µm) for average RNFL (G) and sectors (nasal-superior quadrant (NS), temporal-superior (TS), temporal, temporal-inferior (TI), nasal-inferior (NI) and nasal (N)) for a multiple sclerosis (MS) patient’s eye with a previous optic neuritis (ON) (left), a neuromyelitis optica spectrum disorder (NMOSD) patient’s eye with a previous ON without microcystic macular edema (MME) (center), and an NMOSD patient’s eye with previous ON and MME (right). Background colors describe the comparison to a healthy reference group from the device’s database. B) and C) Thickness maps of the retinal ganglion cell layer (GCL, B) and inner nuclear layer (INL, C) respective to the patients’ data from A).
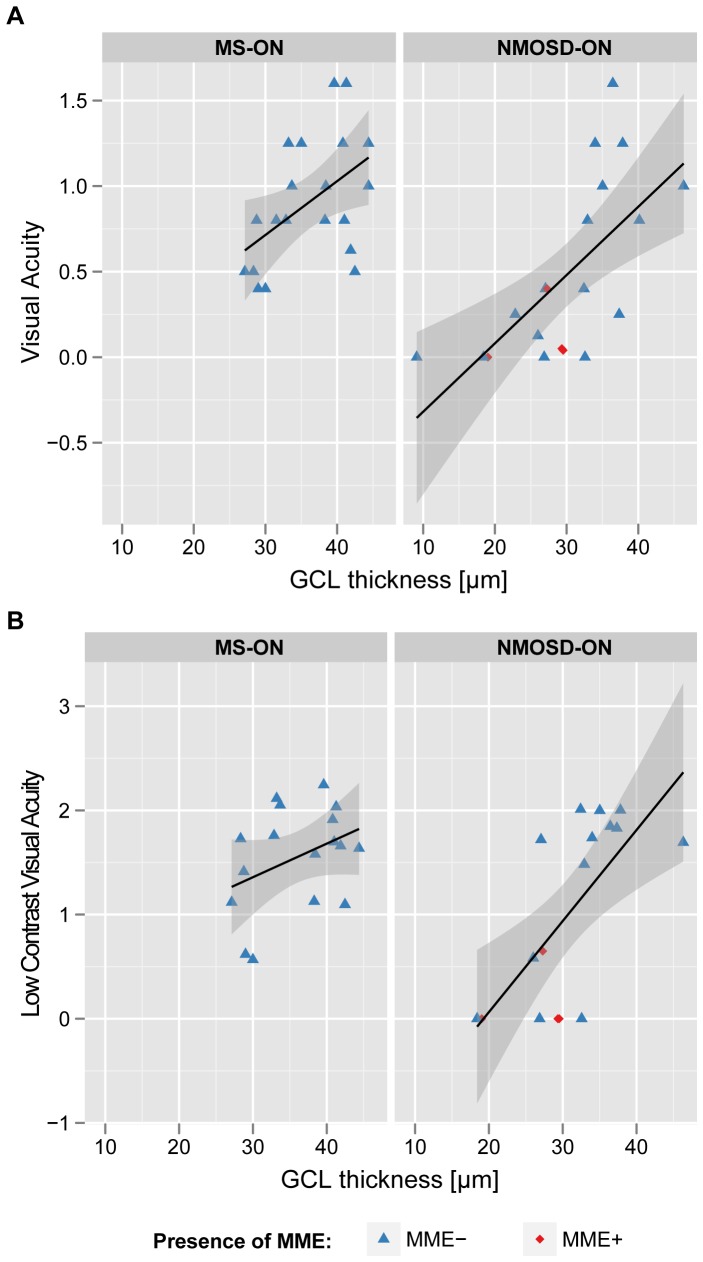
ON in NMOSD eyes resulted in stronger visual function impairment than ON in MS eyes: High contrast VA was significantly lower with NMOSD-ON eyes (Mean ± SD: 0.48±0.51) compared to MS-ON eyes (0.91±0.37, GEE: B = −0.4, SE = 0.1, p = 0.002). Likewise, low contrast LCVA was reduced with NMOSD-ON eyes (1.03±0.89) when compared to MS-ON eyes (1.55±0.50, GEE: B = −0.5, SE = 0.2, p = 0.024). GCL thickness was related to VA in NMOSD-ON eyes (LR: R2 = 0.441, p = 0.001) better than in MS-ON eyes (LR: R2 = 0.242, p = 0.028) (figure 3A). The same held true for LCVA in NMOSD-ON (LR: R2 = 0.467, p = 0.002) versus MS-ON eyes (LR: R2 = 0.143, p = 0.135) (figure 3B).
Figure 3. Correlation between visual function and retinal morphology.
Scatterplots illustrating relations of ganglion cell thickness of neuromyelitis optica spectrum disorder (NMOSD) and multiple sclerosis (MS) patients’ eyes with a previous optic neuritis to A) high contrast visual acuity (determined by ETDRS charts) and B) low contrast visual acuity determined by functional acuity contrast testing.
Different Affection of Quadrant pRNFL in NMOSD and MS after ON
pRNFL thickness was significantly decreased in eyes from patients with either of the two disorders compared to HC, with NMOSD-ON eyes being significantly more affected than MS-ON eyes in all quadrants (table 2). As it is a long-standing clinical experience that optic disc pallor and optic atrophy in MS-ON eyes may exhibit a temporal preponderance and as also histopathological and OCT works have shown particular damage to temporal axons [48]–[50], we were curious whether a predilection of the temporal quadrant of pRNFL would also be detectable in NMOSD. While pRNFL was indeed primarily reduced in the temporal quadrant in MS-ON eyes, pRNFL in NMOSD-ON eyes was more broadly reduced with thinning involving other quadrants. We used the ratio of nasal versus temporal RNFL thickness (N/T ratio) for comparing quadrant-wise thinning in the groups NMOSD-ON and MS-ON. N/T ratio was significantly lower in NMOSD-ON than in MS-ON (0.93±0.35 vs. 1.36±0.43; GEE: B = −0.38, SE = 0.13, p = 0.004), meaning that in relation to the temporal quadrant, the nasal quadrant was much stronger affected in NMOSD than in MS. These results are emphasized by the representative pRNFL quadrant thickness values in figure 2.
To further analyze the potential value for differential diagnosis we calculated ROC curves. Here, pRNFL showed an AUC of 0.835 (corresponding to 60% sensitivity at 90% specificity to identify an NMOSD related optic neuritis by this measure) and N/T ratio an AUC of 0.775 (corresponding to 50% sensitivity at 90% specificity). These values should be interpreted with caution due to the low number of cases and the exploratory nature of the analysis and are only given for comparison of our results to other studies.
To investigate whether the N/T ration may be NMOSD specific or whether a severe ON might lead in general to a broader loss of pRNFL, we selected 20 additional eyes with a history of ON from 17 RRMS patients presenting with very thin pRNFL values from our OCT database. In order to analyze MS patients with the most severe pRNFL reduction, we simply chose those 17 RRMS patients in our database with the lowest pRNFL values without any further criteria or a specific cutoff. Despite these eyes (59.2±6.0 µm) showing a comparable pRNFL thickness with NMOSD-ON eyes (58.5±21.2 µm, p = 0.984), the N/T ratio in these eyes (1.47±0.42) was in the range of the originally matched MS-ON eyes (GEE: p = 0.361) and statistically highly significantly different from NMOSD-ON eyes (GEE: B = −0.53, SE = 0.13, p<0.001), further supporting the evidence for a different damage pattern in NMOSD compared to MS.
None of the MS-ON eyes showed a pRNFL below 46.6 µm whereas 9 NMOSD-ON eyes (45%) had a pRNFL below this value. No MS-ON eyes showed an N/T ratio below 0.61, while 5 NMOSD-ON eyes (25%) were below this limit. Four NMOSD-ON eyes (20%) showed both pRNFL and N/T ratio below these cut-off values.
Role of Microcystic Macular Edema (MME)
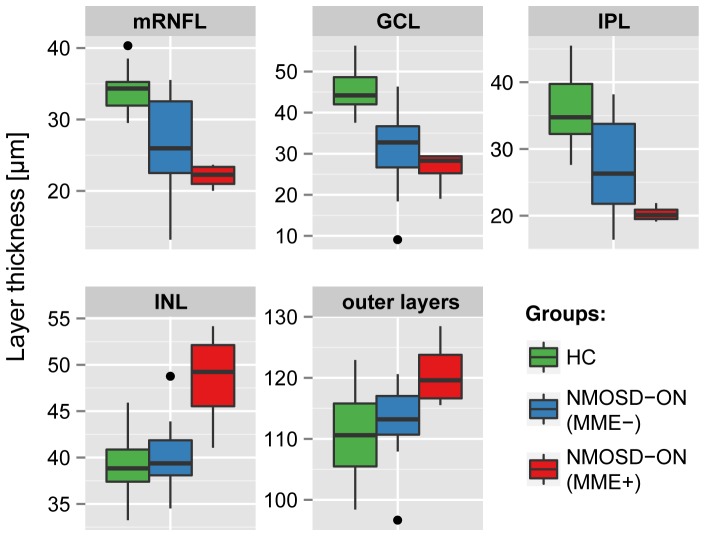
Four eyes from three NMOSD patients (75%) were affected by MME (figure 1D). All patients with MME reported a previous ON in the respective eye. In contrast, no MS patient or HC was affected by MME. Eyes with MME (MME+) in NMOSD patients showed severely reduced RNFL, GCL, and IPL, but increased INL and outer layers in comparison to NMOSD-ON eyes without MME (MME-) (figure 4, MME+ in red).
Figure 4. Intra-retinal layer thickness in NMOSD-ON eyes with and without microcystic macular edema.
Layer thicknesses for the macular retinal nerve fiber layer (mRNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL) and the combined outer retinal layers for NMOSD-ON eyes with (MME+, in red) and without (MME-, in blue) microcystic macular edema and healthy controls eyes (HC, in green). Outer retinal layers include outer plexiform layer, outer nuclear layer and inner photoreceptor layer segments.
All MME+ eyes performed very poorly in high and low visual acuity testing with three eyes being legally blind. VA was significantly lower in MME+ eyes (Mean ± SD 0.12±0.19) than in MME- eyes (0.57±0.53, GEE: B = 0.609, SE = 0.123, p<0.001) and LCVA was reduced in MME+ eyes (0.16±0.33) when compared to MME- eyes (1.30±0.83, GEE: B = 1.237, SE = 0.163, p<0.001).
To test whether the above reported INL and outer layer thickening in NMOSD-ON eyes over HC eyes was a result of MME, we performed all corresponding group comparisons also under exclusion of MME affected eyes and their respective controls. Comparing these cohorts, no significant increase of INL (p = 0.363) and outer layers (p = 0.336) was detected in NMOSD eyes anymore. pRNFL (B = −38.6, SE = 5.8) and the inner retinal layers mRNFL (B = −6.9; SE = 1.7), GCL (B = −15.2, SE = 2.6) and IPL (B = −8.7, SE = 2.0) were still significantly decreased in comparison to HC eyes (all p<0.001). These layers were also reduced in NMOSD-ON eyes compared to MS-ON eyes (mRNFL: B = - 3.7, SE = 1.5, p = 0.015; GCL: B = −6.1, SE = 2.2, p = 0.007; IPL: B = −7.0, SE = 2.1, p = 0.001).
NMOSD vs. MS Eyes without Previous Optic Neuritis
Finally, we investigated whether NMOSD patient’s eyes without any history of ON showed retinal changes in comparison to healthy controls. Detailed results comparing retinal measures of NMOSD-NON with eyes from HC are given in table 3. In summary, NMOSD-NON eyes did not differ significantly from HC eyes in any of the OCT layer measures. For comparison with MS, we additionally compared MS-NON eyes with HC. MS-NON eyes showed reduced thickness in macular RNFL and GCL and a slight non-significant thickening of INL and outer layers.
Table 3. Retinal morphology in eyes without previous ON.
| Matched HC | NMOSD-NON (vs. HC) | MS-NON (vs. HC) | |||||||
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | p | Mean ± SD | Min–Max | B (SE) | p | |
| Average pRNFL (µm) | 97.0±12.1 | 80.4–120.8 | 99.5±7.5 | 88.9–113.7 | 0.5 | 92.5±8.9 | 71.5–100.9 | 0.69 | |
| TMV (mm3) | 8.52±0.53 | 7.75–9.38 | 8.45±0.34 | 8.02–9.16 | 0.355 | 8.59±0.28 | 8.21–9.13 | 0.086 | |
| mRNFL (µm) | 34.4±2.9 | 26.6–40.3 | 34.8±2.0 | 32.4–38.4 | 0.61 | 31.7±2.9 | 27.0–39.4 | −2.4 (1.1) | 0.025 |
| GCL (µm) | 44.9±4.2 | 37.5–52.8 | 43.3±2.0 | 36.6–50.5 | 0.293 | 41.3±5.3 | 31.5–49.0 | −3.6 (1.7) | 0.039 |
| IPL (µm) | 35.8±3.8 | 30.4–44.3 | 35.3±4.4 | 26.9–40.9 | 0.69 | 34.4±3.5 | 29.0–39.0 | 0.266 | |
| INL (µm) | 39.1±2.4 | 33.8–43.8 | 38.4±3.4 | 32.3–46.7 | 0.507 | 40.5±2.2 | 36.3–43.7 | 0.095 | |
| Outer layers (µm) | 110.5±6.5 | 98.4–122.9 | 109.3±5.4 | 94.7–116.3 | 0.52 | 113.3±5.4 | 103.1–123.9 | 0.201 | |
Results of retinal OCT outcomes of NMOSD and MS patients’ eyes without history of optic neuritis and healthy controls; GEE results showing the differences between NMOSD-NON and MS-NON to healthy controls. Outer retinal layers include outer plexiform layer, outer nuclear layer and inner photoreceptor layer segments.
Abbreviations: NMOSD: neuromyelitis optica spectrum disorder; MS: multiple sclerosis; NON: eyes without history of optic neuritis; HC: healthy controls; OCT: optical coherence tomography; p/mRNFL: peripapillary and macular retinal nerve fiber layer; TMV: total macular volume; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; SD: standard deviation; Min: minimum; Max: maximum; B: coefficient estimate from generalized estimating equation models (GEE), SE: standard error from GEE coefficient estimates.
Discussion
In this study we used SD-OCT with intra-retinal segmentation to investigate retinal layer changes in 17 NMOSD patients in comparison to matched RRMS patients and HC. In line with clinical data and previous OCT studies [32], [36], [37] we show that neuroaxonal retinal damage displayed by thinning of the peripapillary and macular RNFL and of the GCL is more severe in ON eyes from patients with NMOSD than in ON eyes from patients with MS. This is paralleled by poorer visual functions as assessed by high and low contrast visual acuity. Interestingly, the association of structural retinal damage and impairment of visual function was more apparent in NMOSD-ON eyes than in MS-ON eyes: GCL thickness was a much stronger predictor of both ETDRS visual acuity and LCVA measured by Functional Acuity Contrast Testing in NMOSD than in MS. Half of NMOSD-ON eyes had pRNFL values below 46.6 µm versus none of the MS-ON eyes, and the mean pRNFL difference between both groups was 27 µm. This difference is in striking accordance with values reported by two previous seminal studies in NMO using the older time domain OCT technology [29], [30]. The stronger association between morphology and visual function in NMOSD-ON eyes that tend to have lower axonal and neuronal OCT measures may suggest that below a certain threshold of neuroaxonal loss retinal neurons and axons are no longer able to sufficiently maintain visual function. Other studies in patients with ON or NMO further underscore the assumption of a threshold RNFL thickness, below which visual acuity becomes very poor [30], [51], [52]. This has important implications as early and effective therapeutic interventions following ON should aim to prevent substantial retinal damage and thus poor visual function which negatively influences the patient’s quality of life [53], [54]. Several recent studies have indeed shown that therapeutic interventions with corticosteroids, plasma exchange or erythropoietin after an ON attack may help preserve retinal axons and this effect can be monitored by OCT [28], [55], [56]. This further supports the argument of some authors that strict immunosuppression is mandatory in NMO [57]. Importantly, non-affected eyes in NMOSD had normal visual function and preserved retinal layers.
Our results support previous findings on a distinct distribution of RNFL thinning across quadrants in NMOSD-ON eyes versus MS-ON eyes. Consistent with Naismith et al. [29] and Monteiro et al. [32], the temporal preponderance of RNFL damage typical for MS was not detectable in NMOSD eyes. Here, other quadrants were as well severely affected, resulting in a significantly lower nasal to temporal pRNFL ratio in NMOSD versus MS. However, given the limited sample size in our and the two previous cohorts and the substantial overlap of data between MS and NMOSD, the N/T ratio is currently of limited value to differentiate between a NMOSD-ON and a MS-ON eye on an individual level. The pathophysiological aspects, however, deserve discussion. The temporal quadrant of the RNFL contains the papillo-macular bundle, which is built by parvocellular axons that consist of smaller, thinly myelinated fibers with rapid firing rates. Interestingly, several diseases besides MS show a predominant impairment of these parvocellular axons such as Leber’s hereditary optic neuropathy, OPA1 related dominant optic nerve atrophy, and spinocerebellar ataxia type 1 [58]. All these diseases share a presumed insult to mitochondria as one key event in the disease process. Owing to their small volume and fast firing rates, parvocellular axons may be more vulnerable to energy depletion resulting from impaired mitochondrial function [59].
Support for this assumption stems from an autopsy study by Evangelou et al. who demonstrated a selective vulnerability to injury of parvocellular axons and neurons in the anterior optic pathway in MS [60]. In contrast, the more even distribution of retinal damage in NMOSD-ON eyes as compared to MS-ON may indicate that damage to parvocellular axons is not a key feature in NMO but other mechanisms are more important.
In line with some previous reports [30], [33], NMOSD-NON eyes exhibited no apparent retinal damage as all OCT measures were not different from controls. This is concordant with the notion that retinal damage in NMO is linked to clinically manifest ON attacks and does not occur progressively or as a consequence of subclinical optic neuropathy as is the case in MS. Accordingly, a secondary progressive course has been rarely described in NMO [35]. In contrast to our and other groups Syc et al. and Sotirchos et al. reported GCL plus IPL thinning also in NMOSD eyes without history of ON [36], [39]. As the authors acknowledge, subclinical disease activity in NMOSD is not a widely appreciated phenomenon. These discrepant findings from studies using high resolution SD-OCT with intra-retinal segmentation can currently not be resolved; the use of two different OCT devices and segmentation techniques and the relatively low number of NMOSD-NON eyes in both studies with an inherent risk for statistical errors may have played a role. However, the topic deserves to be addressed in larger studies, as the finding of subclinical optic neuropathy in NMO would question our current pathophysiological understanding of NMO.
Of note are our findings of MME in four NMOSD-ON eyes. MME in these few eyes was responsible for the apparent thickening of the INL in NMOSD-ON eyes versus HC that was no longer present when we excluded these MME eyes from group comparisons. MME has recently been described by Gelfand and colleagues in 15 of 318 MS patients [46]. MME was associated with disease severity, reduced visual acuity and RNFL thinning. Moreover, MME was predominantly located in the INL and was more prevalent in eyes with prior symptomatic ON. However, the question as to whether MME is specific for MS or may also occur in other conditions with optic nerve involvement remains to be answered [61], [62].
In line with this, both Sotirchos et al. and Gelfand et al. [38], [39] reported MME exclusively in NMO eyes affected by ON as was the case in our study. Moreover, all three works consistently found that MME NMO eyes exhibited more severe structural retinal damage and more profoundly impaired visual function than non MME NMO eyes. In this regard, the recent proposal by Balk and colleagues that MME may be linked to Mueller cell pathology is intriguing and warrants further investigation [61]. In NMO one could hypothesize targeting of Mueller cells which are AQP4-containing retinal astrocytes of the retina by AQP4 autoantibodies. In AQP4 knock-out mice, electroretinograms have suggested that lack of AQP4 mildly impairs retinal function, presumably by altered Mueller cell fluid balance [63]. However, it remains to be investigated if AQP4 antibodies that have been shown to be pathogenic in NMO [64], may have access to the retina via a leaky blood-retina barrier and thus can target retinal Mueller cells. This could be another cause of retinal damage beyond a retrograde degeneration of ganglion cells following an attack to axons in the optic nerve [36].
In light of recent studies that have shown a detrimental effect of some MS disease-modifying drugs in NMO [65]–[73], the possibility of using OCT for a more accurate diagnosis of NMO and for a correct differential diagnosis versus MS would be desirable, especially in AQP4 antibody negative patients. However, despite some distinct features of retinal damage we and others have identified by OCT group comparisons, the discriminatory capacity of OCT for a reliable differential diagnosis of the individual patient is still insufficient to be used in clinical routine. Moreover, it is a limitation of our and most previous studies that the sample size of the NMO cohorts were relatively small owing to the rarity of NMO, thus results are prone to statistical errors. Further technical advances in OCT technology with respect to data acquisition and post-processing will hopefully improve the utility of the technique for clinically routine in the near future.
Acknowledgments
We thank Cynthia Kraut for excellent technical support.
Funding Statement
This work was supported by the German Research Council (DFG Exc 257 to FP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, et al. (2012) Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 3. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, et al. (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 4. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6: 805–815. [DOI] [PubMed] [Google Scholar]
- 6. Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, et al. (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125: 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarius S, Paul F, Franciotta D, Waters P, Zipp F, et al. (2008) Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol 4: 202–214. [DOI] [PubMed] [Google Scholar]
- 8. Wingerchuk DM, Weinshenker BG (2003) Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology 60: 848–853. [DOI] [PubMed] [Google Scholar]
- 9. Mealy MA, Wingerchuk DM, Greenberg BM, Levy M (2012) Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol 69: 1176–1180. [DOI] [PubMed] [Google Scholar]
- 10. Bock M, Brandt AU, Dorr J, Pfueller CF, Ohlraun S, et al. (2010) Time domain and spectral domain optical coherence tomography in multiple sclerosis: a comparative cross-sectional study. Multiple Sclerosis 16: 893–896. [DOI] [PubMed] [Google Scholar]
- 11. Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, et al. (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9: 921–932. [DOI] [PubMed] [Google Scholar]
- 12. Noval S, Contreras I, Muñoz S, Oreja-Guevara C, Manzano B, et al. (2011) Optical coherence tomography in multiple sclerosis and neuromyelitis optica: an update. Mult Scler Int 2011: 472790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costello F (2011) Evaluating the use of optical coherence tomography in optic neuritis. Mult Scler Int 2011: 148394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, et al. (2012) Retinal Damage in Multiple Sclerosis Disease Subtypes Measured by High-Resolution Optical Coherence Tomography. Multiple Sclerosis International 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman EM, Cutter G, et al. (2007) Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 69: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 16. Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, et al. (2011) Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 134: 518–533. [DOI] [PubMed] [Google Scholar]
- 17. Burkholder BM, Osborne B, Loguidice MJ, Bisker E, Frohman TC, et al. (2009) Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Archives of Neurology 66: 1366–1372. [DOI] [PubMed] [Google Scholar]
- 18. Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, et al. (2007) Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 68: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 19. Toledo J, Sepulcre J, Salinas-Alaman A, García-Layana A, Murie-Fernandez M, et al. (2008) Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler 14: 906–912. [DOI] [PubMed] [Google Scholar]
- 20. Albrecht P, Ringelstein M, Müller AK, Keser N, Dietlein T, et al. (2012) Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler 18: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 21.Brandt AU, Oberwahrenbrock T, Ringelstein M, Young KL, Tiede M, et al.. (2011) Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 134: e193; author reply e194. [DOI] [PubMed] [Google Scholar]
- 22.Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, et al.. (2013) Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler: in press, doi:10.1177/1352458513489757 [DOI] [PubMed]
- 23. Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, et al. (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 24. Siger M, Dziegielewski K, Jasek L, Bieniek M, Nicpan A, et al. (2008) Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol 255: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 25. Dörr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, et al. (2011) Association of Retinal and Macular Damage with Brain Atrophy in Multiple Sclerosis. PLoS ONE 6: e18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zimmermann H, Freing A, Kaufhold F, Gaede G, Bohn E, et al. (2012) Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler 19: 443–450. [DOI] [PubMed] [Google Scholar]
- 27.Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, et al.. (2012) Relationships Between Retinal Axonal and Neuronal Measures and Global Central Nervous System Pathology in Multiple Sclerosis. Arch Neurol: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merle H, Olindo S, Donnio A, Richer R, Smadja D, et al. (2008) Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci 49: 4412–4417. [DOI] [PubMed] [Google Scholar]
- 29. Naismith RT, Tutlam NT, Xu J, Klawiter EC, Shepherd J, et al. (2009) Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology 72: 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman EM, et al. (2009) Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 73: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green AJ, Cree BAC (2009) Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatr 80: 1002–1005. [DOI] [PubMed] [Google Scholar]
- 32. Monteiro MLR, Fernandes DB, Apóstolos-Pereira SL, Callegaro D (2012) Quantification of Retinal Neural Loss in Patients with Neuromyelitis Optica and Multiple Sclerosis with or without Optic Neuritis Using Fourier-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci 53: 3959–3966. [DOI] [PubMed] [Google Scholar]
- 33. De Seze J, Blanc F, Jeanjean L, Zéphir H, Labauge P, et al. (2008) Optical coherence tomography in neuromyelitis optica. Arch Neurol 65: 920–923. [DOI] [PubMed] [Google Scholar]
- 34.Bouyon M, Collongues N, Zéphir H, Ballonzoli L, Jeanjean L, et al.. (2013) Longitudinal follow-up of vision in a neuromyelitis optica cohort. Mult Scler, 2013. doi:10.1177/1352458513476562 [DOI] [PubMed]
- 35. Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG (2007) A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 68: 603–605. [DOI] [PubMed] [Google Scholar]
- 36. Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, et al. (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes DB, Raza AS, Nogueira RGF, Wang D, Callegaro D, et al.. (2012) Evaluation of Inner Retinal Layers in Patients with Multiple Sclerosis or Neuromyelitis Optica Using Optical Coherence Tomography. Ophthalmology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelfand JM CB (2013) MIcrocystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol: 1–5. [DOI] [PubMed] [Google Scholar]
- 39. Sotirchos ES, Saidha S, Byraiah G, Mealy MA, Ibrahim MA, et al. (2013) In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 80: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 41. Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, et al. (2007) Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med 4: e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jarius S, Probst C, Borowski K, Franciotta D, Wildemann B, et al. (2010) Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci 291: 52–56. [DOI] [PubMed] [Google Scholar]
- 43. Kalluri SR, Illes Z, Srivastava R, Cree B, Menge T, et al. (2010) Quantification and functional characterization of antibodies to native aquaporin 4 in neuromyelitis optica. Arch Neurol 67: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 44. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tewarie P, Balk L, Costello F, Green A, Martin R, et al. (2012) The OSCAR-IB Consensus Criteria for Retinal OCT Quality Assessment. PLoS ONE 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ (2012) Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 135: 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bock M, Brandt AU, Kuchenbecker J, Dörr J, Pfueller CF, et al. (2012) Impairment of contrast visual acuity as a functional correlate of retinal nerve fibre layer thinning and total macular volume reduction in multiple sclerosis. Br J Ophthalmol 96: 62–67. [DOI] [PubMed] [Google Scholar]
- 48. Frisén L, Hoyt WF (1974) Insidious atrophy of retinal nerve fibers in multiple sclerosis. Funduscopic identification in patients with and without visual complaints. Arch Ophthalmol 92: 91–97. [DOI] [PubMed] [Google Scholar]
- 49. Kerrison JB, Flynn T, Green WR (1994) Retinal pathologic changes in multiple sclerosis. Retina 14: 445–451. [DOI] [PubMed] [Google Scholar]
- 50. Bock M, Brandt AU, Dörr J, Kraft H, Weinges-Evers N, et al. (2010) Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg 112: 647–652. [DOI] [PubMed] [Google Scholar]
- 51. Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, et al. (2008) Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Multiple Sclerosis 14: 893–905. [DOI] [PubMed] [Google Scholar]
- 52. Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, et al. (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58: 383–391. [DOI] [PubMed] [Google Scholar]
- 53. Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, et al. (2009) Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatr 80: 767–772. [DOI] [PubMed] [Google Scholar]
- 54. Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, et al. (2012) Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 119: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakamura M, Nakazawa T, Doi H, Hariya T, Omodaka K, et al. (2010) Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol 248: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 56. Sühs K-W, Hein K, Sättler MB, Görlitz A, Ciupka C, et al. (2012) A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol 72: 199–210. [DOI] [PubMed] [Google Scholar]
- 57. Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, et al. (2010) EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 17: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 58. Stricker S, Oberwahrenbrock T, Zimmermann H, Schroeter J, Endres M, et al. (2011) Temporal Retinal Nerve Fiber Loss in Patients with Spinocerebellar Ataxia Type 1. PLoS ONE 6: e23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Horssen J, Witte ME, Ciccarelli O (2012) The role of mitochondria in axonal degeneration and tissue repair in MS. Mult Scler 18: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 60. Evangelou N, Konz D, Esiri MM, Smith S, Palace J, et al. (2001) Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 124: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 61. Balk LJ, Killestein J, Polman CH, Uitdehaag BMJ, Petzold A (2012) Microcystic macular oedema confirmed, but not specific for multiple sclerosis. Brain 135: e226–e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abegg M, Zinkernagel M, Wolf S (2012) Microcystic macular degeneration from optic neuropathy. Brain 135: e225–e225. [DOI] [PubMed] [Google Scholar]
- 63. Li J, Patil RV, Verkman AS (2002) Mildly abnormal retinal function in transgenic mice without Müller cell aquaporin-4 water channels. Invest Ophthalmol Vis Sci 43: 573–579. [PubMed] [Google Scholar]
- 64. Jarius S, Wildemann B (2010) AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 6: 383–392. [DOI] [PubMed] [Google Scholar]
- 65. Kleiter I, Hellwig K, Berthele A, Kümpfel T, Linker RA, et al. (2012) Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 69: 239–245. [DOI] [PubMed] [Google Scholar]
- 66. Jacob A, Hutchinson M, Elsone L, Kelly S, Ali R, et al. (2012) Does natalizumab therapy worsen neuromyelitis optica? Neurology 79: 1065–1066. [DOI] [PubMed] [Google Scholar]
- 67.Bomprezzi R, Powers JM, Shimizu J, Tsuji S, Weinshenker BG, et al.. (2011) IFNβ-1b may severely exacerbate Japanese opticspinal MS in neuromyelitis optica spectrum: Japanese optic-spinal MS: is it MS or neuromyelitis optica and does the answer dictate treatment? Neurology 77: 195; discussion 195–196. [DOI] [PubMed] [Google Scholar]
- 68. Barnett MH, Prineas JW, Buckland ME, Parratt JDE, Pollard JD (2012) Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler 18: 108–112. [DOI] [PubMed] [Google Scholar]
- 69.Jarernsook B, Siritho S, Prayoonwiwat N (2012) Efficacy and safety of beta-interferon in Thai patients with demyelinating diseases. Mult Scler. [DOI] [PubMed] [Google Scholar]
- 70. Kim S-H, Kim W, Li XF, Jung I-J, Kim HJ (2012) Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler 18: 1480–1483. [DOI] [PubMed] [Google Scholar]
- 71. Papeix C, Vidal J-S, de Seze J, Pierrot-Deseilligny C, Tourbah A, et al. (2007) Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 13: 256–259. [DOI] [PubMed] [Google Scholar]
- 72. Shimizu J, Hatanaka Y, Hasegawa M, Iwata A, Sugimoto I, et al. (2010) IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 75: 1423–1427. [DOI] [PubMed] [Google Scholar]
- 73. Min J-H, Kim BJ, Lee KH (2012) Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 18: 113–115. [DOI] [PubMed] [Google Scholar]