Abstract
Tick-borne zoonoses are considered as emerging diseases. Tick repellents represent an effective tool for reducing the risk of tick bite and pathogens transmission. Previous work demonstrated the repellent activity of the phenylpropanoid eugenol against Ixodes ricinus; here we investigate the relationship between molecular structure and repellency in a group of substances related to that compound. We report the biological activity of 18 compounds varying for the presence/number of several moieties, including hydroxyl and methoxy groups and carbon side-chain. Each compound was tested at different doses with a bioassay designed to measure repellency against individual tick nymphs. Both vapor pressure and chemical features of the tested compounds appeared to be related to repellency. In particular, the hydroxyl and methoxy groups as well as the side-chain on the benzene ring seem to play a role. These results are discussed in light of available data on chemical perception in ticks. In the course of the study new repellent compounds were identified; the biological activity of some of them (at least as effective as the “gold standard” repellent DEET) appears to be very promising from a practical point of view.
Introduction
Ticks are obligate blood-feeding ectoparasites of great veterinary and public health importance. Ticks biting humans can cause severe allergic reactions, but the primary concern is their ability to transmit a number of disease-causing agents during the blood meals. For instance, in Europe, Lyme borreliosis and Tick-borne encephalitis, which are transmitted by the tick Ixodes ricinus (L.), are considered emerging diseases [1], [2].
Avoidance of tick-infested areas is the primary personal protection measure to prevent tick bites; however, in order to minimize the risk of tick contact, some simple preventive measures can be taken, such as a sensible behavior or appropriate clothing. Tick repellents and acaricides represent effective tools for reducing the risk of tick bite and pathogens transmission, before and after tick contact, respectively [3]. Tick repellent compounds in different commercial formulations are available and have been demonstrated to reduce the risk of bites when applied to clothing or bare skin (see for example [4]). Since its registration for commercial use, DEET (N,N-Diethyl-3-methyl-benzamide or N,N-Diethyl-meta-toluamide) has become the main active ingredient in most commercial insect- and tick-repellents used on human skin [4]. Although DEET and other common products based on synthetic pyrethroids are very effective and relatively safe repellents (for DEET safety see for example [5]–[7]), some concern exists about possible adverse effects on human health [8], [9] and people frequently perceive synthetic repellents as a potential source of toxicity [10]. Moreover, arthropods show differential responses to these products, indicating the possibility of adaptation and emerging resistance or insensitivity [11]–[14]. For these reasons the development of novel repellents could be of great value and so would be the discovery of new bioactive plant-derived compounds, which are generally more acceptable to people unwilling to use synthetic chemicals [4]. In this regard, a better knowledge about the molecular determinants of the biological activity of such substances may be very important from a practical point of view, in that it may allow a targeted development of new repellents or a faster screening of candidate natural compounds.
Preliminary experiments conducted in our laboratory suggested a biological activity of Ocimum basilicum L. (sweet basil) on I. ricinus [15], integrating previous data about the biological effects of this and related Ocimum species against other arthropods [16]–[20], including other tick species [21], [22]. The compound responsible for the repellency was later identified as eugenol using a bioassay assisted HPLC fractionation protocol followed by GC-MS identification [23]. Eugenol appeared to be as repellent as the reference substance DEET at the doses of 1000 and 100 µg; however, unlike eugenol, DEET was also active at the dose of 10 µg.
Several biological effects of eugenol on invertebrates have already been demonstrated; some examples are: pre- and postsynaptic effects on snail neurons [24]; insecticidal activity [25], [26]; acaricidal activity [27], [28]; repellent activity against insects [29]. Apart from our previous study, comments about a possible activity of eugenol on I. ricinus are also available [30], [31]. However, to our knowledge, no attempts have been made so far to correlate the chemical features of this substance to the repellency against any tick species.
Ecological interactions of ticks are largely mediated by chemical compounds that can affect several crucial stages of the life cycle [32], however the functional basis of their actions are not yet completely understood. In other arthropods a detailed knowledge of the functional basis of chemical mediated communication has been achieved thanks to the fruitful integration of several investigation techniques, including analytical chemistry, neurobiology, molecular biology, and most recent genomic approaches [33]. In the case of ticks, limited genomic data as well as reduced homology with other well studied arthropods has made the task particularly challenging. In fact, what is actually known about tick olfaction is confined to the important morphological and electrophysiological studies on the olfactory sensilla, that are concentrated in the Haller's organ, located on the tarsi of the first pair of legs [32], [34]–[38]. As regards further components of the olfactory system, neither olfactory receptors nor odorant-binding proteins (OBPs) have been described in ticks, while only 1 chemosensory protein (CSP) has been found, so far, in Ixodes scapularis Say [39].
For these reasons, the elucidation of the molecular basis of chemoreception in ticks needs to be pursued from a variety of indirect routes, including studies on the relationship between structure and activity of semiochemicals. Several studies on this relationship have been carried out on other arthropods using different approaches, either applying mathematical models to numerically express the chemical structure of the compounds or simply relating the presence of a given chemical feature to the repellency [40]–[43].
We investigated the molecular determinants of the repellent activity of eugenol and analyzed the factors accounting for such bioactivity. In view of this goal, several substances with similar structure were tested against I. ricinus nymphs and a graphical approach was used to investigate the relationship between structure and bioactivity. The study showed that certain combinations of chemical features are related to repellency; new effective repellent compounds were also identified. Some working hypotheses on the molecular basis of chemoreception in I. ricinus were drawn from the results.
Materials and Methods
Ethics statement
The bioassays were carried out using I. ricinus nymphs collected from May to November by dragging in mixed woodlands and ecotones in Friuli Venezia Giulia (north-eastern Italy). I. ricinus is not an endangered or protected species; therefore, according to local regulations, no specific permissions were required either for collecting the specimens employed in the experimental work or for the field sampling in private properties.
Ticks used in this study
Nymphs were used for this study as this is the most important developmental stage from the epidemiological point of view, being both abundant in the environment and active in human biting and pathogen transmission.
Collected nymphs were kept inside sealed polypropylene tubes, in which a high relative humidity was provided by a damp strip of filter paper, and were maintained at room temperature in the dark until using in the assays. Most ticks were tested within 3 weeks after collection. In any case, only ticks that appeared to be active in the storing tubes were used for the bioassays. Each tick was used only once.
Bioassay
To test the effect of different stimuli on the behavior of ticks, a simple lab bioassay was used that was described previously [15], [23]. Briefly, a circular arena was obtained placing upside down a 6 cm diameter glass Petri dish. Two concentric circles were drawn on the inner surface of the Petri dish, having 1 cm radius (start line or line A) and 2 cm radius (finish line or line B). The treatment was applied with a pipette outside the finish line on the outer surface of the Petri dish in 100 µl volume of solvent (acetone). After the complete evaporation of the solvent, the arena was placed on a wet piece of filter paper inside a larger Petri dish (Figure 1); in this way a positive humidity gradient was created encouraging the centrifugal movement of the tick. A single nymph was placed with a fine paint brush in the center of the arena and carefully observed throughout the experiment; the time spent to go from line A over line B was recorded.
Figure 1. Glass arena used in the bioassays.
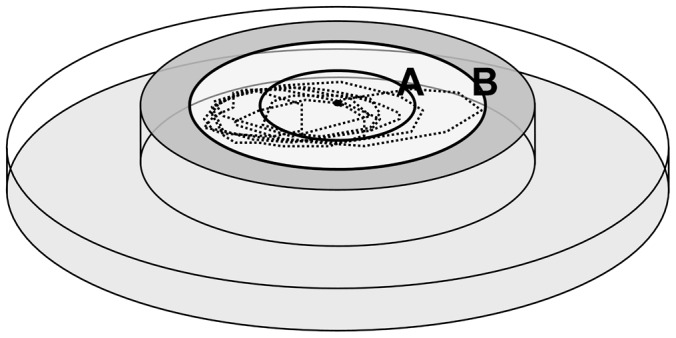
A 6 cm diameter glass Petri dish was placed upside down in a larger Petri dish containing a wet piece of filter paper (light grey). Two concentric circles were drawn on the inner surface of the Petri dish, having 1 cm radius (start line or line A) and 2 cm radius (finish line or line B). The treatment was applied with a pipette outside the finish line (dark grey) on the outer surface of the Petri dish in 100 µl volume of solvent. After the complete evaporation of the solvent, a single nymph was placed with a fine paint brush in the center of the arena and the time spent to go from line A over line B was recorded. The dashed line represents the track captured from the video of a single tick moving on an arena treated with a repellent substance (in this case 100 µg of eugenol).
Under these conditions, in presence of non-repellent stimuli the nymph simply goes straight from the center of the arena to the edge of it, whereas if a repellent substance is used, the tick walks towards the finish line, turning before reaching the treated area.
If the start line was not crossed before 45 seconds, the nymph was discarded; if after 3.5 minutes the tick did not cross the finish line, 210 seconds was recorded as the finish time.
Tested substances
Several compounds differing from eugenol for 1 or more molecular features were tested. In particular, since eugenol consists of a hydroxyl group (–OH), a methoxy group (–O–CH3) and an allyl side-chain (–CH2–CH = CH2) on a benzene ring, commercially available substances in which the hydroxyl and methoxy groups were maintained, eliminated or replaced with a methoxy or a hydroxyl group respectively were tested as well as compounds with an additional methoxy group. The allyl side-chain was maintained, eliminated or replaced with a methyl, ethyl or vinyl side-chain. Tested substances are reported in Table 1, along with eugenol. Pure compounds were purchased from Sigma, Aldrich, Sigma-Aldrich and SAFC.
Table 1. List of the substances considered in this study.
| Abbr | CAS N° | Substance name (and synonyms) | Differences from eugenol |
| EUG | 97-53-0 | 4-Allyl-2-methoxyphenol (4-Allylguaiacol; Eugenol) | - |
| SYR | 91-10-1 | 2,6-Dimethoxyphenol (Syringol) | no side-chain + extra methoxy group |
| ADP | 6627-88-9 | 4-Allyl-2,6-dimethoxyphenol (2,6-Dimethoxy-4-allylphenol) | extra methoxy group |
| CAT | 120-80-9 | 1,2-Benzenediol (Catechol; 1,2-Dihydroxybenzene) | no side-chain + hydroxyl instead of methoxy group |
| MCA | 452-86-8 | 4-Methyl-1,2-benzenediol (4-Methylcatechol) | methyl side-chain + hydroxyl instead of methoxy group |
| ECA | 1124-39-6 | 4-Ethyl-1,2-benzenediol (4-Ethylcatechol) | ethyl side-chain + hydroxyl instead of methoxy group |
| DMB | 91-16-7 | 1,2-Dimethoxybenzene (Veratrol) | no side-chain + methoxy instead of hydroxyl group |
| DMT | 494-99-5 | 1,2-Dimethoxy-4-methyl-benzene (3,4-Dimethoxytoluene; 4-Methylveratrol) | methyl side-chain + methoxy instead of hydroxyl group |
| DMS | 6380-23-0 | 1,2-Dimethoxy-4-vinyl-benzene (3,4-Dimethoxystyrene; 4-Vinylveratrole) | vinyl side-chain + methoxy instead of hydroxyl group |
| ADB | 93-15-2 | 4-Allyl-1,2-dimethoxybenzene (Methyleugenol) | methoxy instead of hydroxyl group |
| GUA | 90-05-1 | 2-Methoxyphenol (2-Hydroxyanisole; Guaiacol) | no side-chain |
| MMP | 93-51-6 | 2-Methoxy-4-methylphenol (Creosol) | methyl side-chain |
| EGU | 2785-89-9 | 4-Ethyl-2-methoxy-phenol (4-Ethylguaiacol) | ethyl side-chain |
| MVP | 7786-61-0 | 2-Methoxy-4-vinylphenol (4-Vinyl-guaiacol) | vinyl side-chain |
| MAN | 100-84-5 | 1-Methoxy-3-methyl-benzene (3-Methylanisole) | methyl side-chain + no hydroxyl group |
| EAN | 10568-38-4 | 1-Ethyl-3-methoxy-benzene (3-Ethylanisole) | ethyl side-chain + no hydroxyl group |
| VAN | 626-20-0 | 1-Methoxy-3-vinyl-benzene (3-Vinylanisole) | vinyl side-chain + no hydroxyl group |
| EST | 140-67-0 | 1-Allyl-4-methoxybenzene (4-Allylanisole; Estragole) | no methoxy group + methoxy instead of hydroxyl group |
Experimental protocol
Compounds listed in Table 1 were tested at different doses. The dose was calculated according to the substance purity as from the details provided by the supplier. For each dose, 10 bioassays against the stimulus to be tested and 10 bioassays against the negative control (in this case the arenas were treated with 100 µl of the solvent alone) were conducted. Each bioassay was run with a different tick in a different arena.
All the substances were tested at the doses of 100 and 1000 µg, corresponding to 6.37 and 63.65 µg/cm2 respectively. If a substance was found repellent at 100 µg, it was also tested at 10 µg and if repellent at 10 µg, it was tested at 1 µg.
Bioassays were all run at room temperature under daylight conditions. Temperature was monitored at the beginning and at the end of each experimental session; observed temperature variations were from 0.1 to 1.1°C. The minimum and maximum temperatures recorded in the whole period in which the bioassays were carried out were 21.1 and 28.7°C respectively; however, room temperature did not seem to affect the bioassay results.
Statistical analysis of the results
Data from the bioassays were not normally distributed, therefore the median was used as a central tendency estimator and non parametric methods were used for hypothesis testing. In order to check for possible significant differences between treatments and negative controls, the time spent by ticks to move from line A over line B was compared using the nonparametric Mann-Whitney U test.
To represent the repellency of compounds, we used the ratio obtained dividing the median of time before crossing the finish line recorded in the bioassays against the stimulus by the median recorded in the bioassays against the negative control. Correlation between substances' repellency and vapor pressure was tested using Spearman's rank correlation coefficient, assigning a rank from 0 to 3 to the substances according to their repellency as follows: rank 3: compounds that are repellent at 10 µg (low dose); rank 2: repellent at 100 µg (medium dose); rank 1: repellent only at 1000 µg (high dose); rank 0: not repellent at any dose.
Results
Repellency of pure compounds
The bioactivity of the pure compounds tested in this study is reported in Table 2. Out of all the tested substances, 2 (MAN, EAN) were not repellent at all, 3 (GUA, DMB, EST) proved to be repellent only at the highest dose of 1000 µg, while 6 (MMP, EGU, SYR, DMT, DMS, VAN) were repellent also at the dose of 100 µg, this medium dose being the minimum at which eugenol was repellent in the previous study [23]. Five compounds (MVP, ADP, ADB, CAT, MCA) were repellent at the dose of 10 µg, that is the minimum dose at which DEET proved to be repellent with this bioassay [23]; remarkably, a substance (ECA) demonstrated a statistically significant difference between the negative control and the treatment even at the lowest dose of 1 µg.
Table 2. Bioactivity of pure compounds at different doses.
| Stimulus | Dose (µg) | Treatment median (s) | Negative control median (s) | P | Treatment/negative control |
| EUG 1 | 1 | 17.5 | 24.0 | n.s. | 0.7 |
| EUG 1 | 10 | 22.5 | 14.0 | n.s. | 1.6 |
| EUG 1 | 100 | 128.0 | 9.5 | <0.01 | 13.5 |
| EUG 1 | 1000 | 192.5 | 12.0 | <0.01 | 16.0 |
| EUG 2 | 10 | 7.5 | 14.0 | n.s. | 0.5 |
| EUG 2 | 100 | 182.0 | 9.5 | <0.01 | 19.2 |
| EUG 2 | 1000 | 193.5 | 12.0 | <0.01 | 16.1 |
| EUG 3 | 10 | 18.0 | 14.0 | n.s. | 1.3 |
| EUG 3 | 100 | 80.0 | 9.5 | <0.01 | 8.4 |
| EUG 3 | 1000 | 187.0 | 12.0 | <0.01 | 15.6 |
| SYR | 10 | 20.0 | 18.5 | n.s. | 1.1 |
| SYR | 100 | 184.0 | 18.5 | <0.01 | 9.9 |
| SYR | 1000 | 195.5 | 18.0 | <0.01 | 10.9 |
| ADP | 1 | 12.5 | 12.0 | n.s. | 1.0 |
| ADP | 10 | 166.0 | 16.5 | <0.01 | 10.1 |
| ADP | 100 | 192.0 | 19.5 | <0.01 | 9.8 |
| ADP | 1000 | 197.5 | 16.5 | <0.01 | 12.0 |
| CAT | 1 | 12.0 | 12.0 | n.s. | 1.0 |
| CAT | 10 | 53.5 | 12.0 | <0.01 | 4.5 |
| CAT | 100 | 193.0 | 12.0 | <0.01 | 16.1 |
| CAT | 1000 | 193.5 | 9.5 | <0.01 | 20.4 |
| MCA | 1 | 13.0 | 15.0 | n.s. | 0.9 |
| MCA | 10 | 195.5 | 15.0 | <0.01 | 13.0 |
| MCA | 100 | 190.5 | 15.0 | <0.01 | 12.7 |
| MCA | 1000 | 192.0 | 15.0 | <0.01 | 12.8 |
| ECA | 1 | 16.5 | 9.0 | <0.05 | 1.8 |
| ECA | 10 | 185.5 | 9.0 | <0.01 | 20.6 |
| ECA | 100 | 192.5 | 9.0 | <0.01 | 21.4 |
| ECA | 1000 | 196.5 | 9.0 | <0.01 | 21.8 |
| DMB | 10 | 12.5 | 9.5 | n.s. | 1.3 |
| DMB | 100 | 12.5 | 9.5 | n.s. | 1.3 |
| DMB | 1000 | 196.0 | 9.5 | <0.01 | 20.6 |
| DMT | 10 | 7.0 | 8.5 | n.s. | 0.8 |
| DMT | 100 | 106.0 | 8.5 | <0.01 | 12.5 |
| DMT | 1000 | 199.5 | 8.5 | <0.01 | 23.5 |
| DMS | 10 | 11.5 | 10.0 | n.s. | 1.2 |
| DMS | 100 | 188.5 | 12.5 | <0.01 | 15.1 |
| DMS | 1000 | 200.0 | 10.0 | <0.01 | 20.0 |
| ADB | 10 | 28.5 | 10.0 | <0.05 | 2.9 |
| ADB | 100 | 184.5 | 16.5 | <0.01 | 11.2 |
| ADB | 1000 | 194.0 | 10.0 | <0.01 | 19.4 |
| GUA | 100 | 13.0 | 13.0 | n.s. | 1.0 |
| GUA | 1000 | 51.5 | 12.5 | <0.01 | 4.1 |
| MMP | 10 | 10.5 | 12.0 | n.s. | 0.9 |
| MMP | 100 | 63.0 | 8.5 | <0.01 | 7.4 |
| MMP | 1000 | 200.5 | 10.0 | <0.01 | 20.1 |
| EGU | 10 | 11.5 | 10.0 | n.s. | 1.2 |
| EGU | 100 | 184.0 | 8.5 | <0.01 | 21.6 |
| EGU | 1000 | 181.5 | 14.0 | <0.01 | 13.0 |
| MVP | 10 | 22.0 | 10.0 | <0.05 | 2.2 |
| MVP | 100 | 148.0 | 12.5 | <0.01 | 11.8 |
| MVP | 1000 | 191.5 | 10.0 | <0.01 | 19.2 |
| MAN | 100 | 17.0 | 24.5 | n.s. | 0.7 |
| MAN | 1000 | 8.5 | 14.0 | n.s. | 0.6 |
| EAN | 100 | 11.0 | 14.0 | n.s. | 0.8 |
| EAN | 1000 | 22.5 | 14.0 | n.s. | 1.6 |
| VAN | 100 | 16.5 | 8.5 | <0.05 | 1.9 |
| VAN | 1000 | 182.5 | 10.0 | <0.01 | 18.3 |
| EST | 100 | 14.0 | 9.0 | n.s. | 1.6 |
| EST | 1000 | 181.5 | 12.5 | <0.05 | 14.5 |
“P” indicates the statistical significance of the difference between treatment and negative control (n.s. = no statistically significant difference). “Treatment/negative control” represents the ratio between the treatment and negative control medians. The results of three independent replicates of eugenol from [23] are reported as a reference (EUG 1, EUG 2, EUG 3).
To focus the attention only on the most active compounds and simplify further analysis, we considered as repellent, at a certain dose, only substances showing a ratio between the treatment and the negative control median of about 10 or higher (Table 2). According to this rule, 3 compounds were repellent at the dose of 10 µg (ADP, MCA, ECA), 7 at 100 µg (EGU, MVP, SYR, DMT, DMS, ADB, CAT; eugenol would fall into this class), 4 only at the dose of 1000 µg (MMP, DMB, VAN, EST) and 3 were not repellent at all (GUA, MAN, EAN). In the rest of the article, this classification will be used.
Correlation between repellency and vapor pressure
Spearman's rank coefficient highlighted a statistically significant correlation between repellency and vapor pressure of the tested substances (rS = −0.8993; P<0.001); in particular, it appeared that the lower the vapor pressure, the higher the repellency (Figure 2).
Figure 2. Repellency and vapor pressure (listed in descending order) of each tested compound.
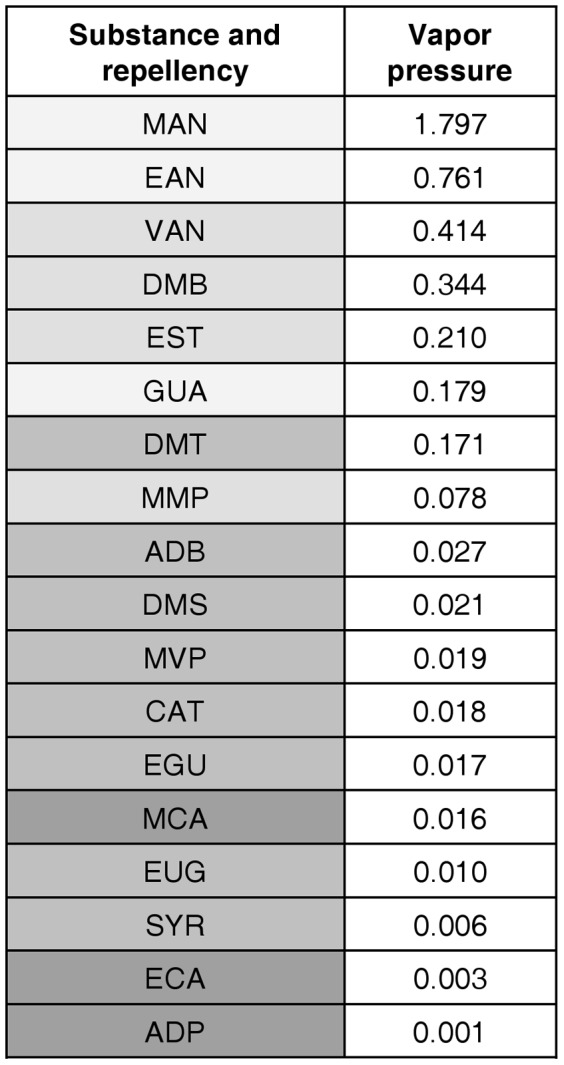
Grey tones indicate the repellency of each molecule: dark grey represents molecules that are repellent at the lowest dose (10 µg); medium grey, molecules repellent at the medium dose (100 µg); light grey, molecules repellent only at the highest dose (1000 µg); very light grey, molecules which are not repellent even at the highest dose. Predicted vapor pressure values (mmHg) at 25°C are from: ACD/Labs Predicted Properties (In: ChemSpider website. URL: <http://www.chemspider.com>, accessed 21Jan 2010).
Correspondence between repellency and structure
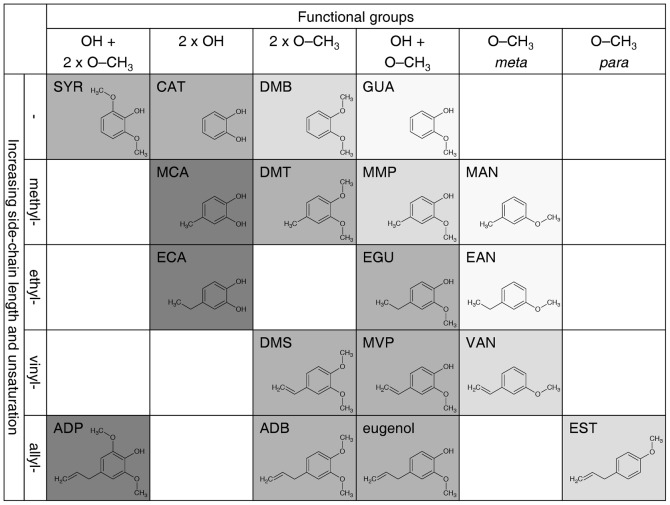
To highlight possible correspondences between repellency and molecular structure we used a 2-dimensional graph, where compounds are ordered according to the functional groups attached to the benzene ring and the length and saturation of the carbon side-chain; in the graph, repellency is rendered with increasing tones of a color so that darker areas mark combinations of chemical features that appear to have a stronger influence on repellency (Figure 3).
Figure 3. Graphical classification of the compounds tested in this study and repellency.
Columns contain molecules with the same combination of functional groups (hydroxyl –OH and methoxy –O–CH3 groups), but with a different side-chain (no side-chain or methyl, ethyl, vinyl, allyl side-chains) on the benzene ring; rows contain molecules that have the same side-chain but different functional groups. Grey tones indicate the repellency of each molecule: dark grey represents molecules repellent at the lowest dose (10 µg); medium grey, molecules repellent at the medium dose (100 µg); light grey, molecules repellent only at the highest dose (1000 µg); very light grey, molecules which are not repellent even at the highest dose.
The compound classification in this artificial space pointed out the role of oxygen; in fact, it appeared that both the hydroxyl and methoxy groups were, at least partly, involved in repellency, since compounds having either 2 or 3 hydroxyl or methoxy groups (in various combinations) were more repellent than those carrying only 1 methoxy group. Moreover, compounds with 2 hydroxyl groups or with 1 hydroxyl and 2 methoxy groups showed the highest repellency.
The carbon side-chain also seemed to play a role: in fact, the longer the chain, the higher the repellency.
Discussion
Many of the compounds tested in this study, which are all similar, to some extent, to the repellent eugenol, appeared to be active in the bioassay.
In principle, in the bioassay used here, contact between the tick's sensory apparatus and the treated area of the arena is possible. However, according to our observations, this happened only in a very few cases, strongly suggesting that olfaction rather than tasting is involved in the observed biological activity of tested compounds.
In general, the mode of action of repellents on blood feeding arthropods is still somehow controversial. Several different hypotheses have been postulated so far that could all be valid under different circumstances. For example, in the case of the widely used DEET, some Authors proposed a mechanism implying an interference with the recognition of attractive odors (host or food odors), rather than a real repellent activity of the substance itself [44], [45]. Similarly, Pellegrino et al. [12] considered DEET as a modulator of the general olfactory receptor activity, capable of disrupting the insect odor code. In contrast, other Authors suggested an effective avoidance-behavior induced by the recognition of the molecule by some specific arthropod olfactory receptors, irrespective of other stimuli (see [13], [14], [46] for examples regarding insects; [47] for ticks). Both hypotheses are probably valid under different conditions, as suggested by the results in [48], [49], where repellent molecules demonstrate, in different circumstances, either inhibitory or excitatory activities on olfactory receptors, by reducing agonist-evoked currents or eliciting currents in the absence of agonists, respectively. However, the avoidance behavior of the repellent treated area observed with this bioassay (see, for example, the track in Figure 1) is consistent with an effective repellency of the tested substances. Moreover, possible attractive olfactory stimuli coming from the observer should not play a major role in this case, since the observer always wore gloves and stayed at a distance during the bioassays; as a matter of fact, ticks moving in the arena did not show any preferential direction regardless of the observer's position.
Anyway, the relationship between the characteristics of a molecule and its biological activity against a certain arthropod species is not yet clearly understood, despite several attempts to correlate physical, structural and chemical properties of a molecule with its repellency have been made (see for example [40]–[42] and citations therein). Nevertheless, Paluch et al. [41] found that vapor pressure and electronic and electrotopological descriptors are important components in models describing the repellency of sesquiterpenes against mosquitoes; moreover, Davis ([50], cited in [51]) found that both oxygen functional groups and vapor pressure are important factors related to insect repellent activity.
As regards vapor pressure, we found that the lower the vapor pressure, the higher the repellency (Figure 2). Also the highly repellent substance DEET presents a vapor pressure of 0.001 mmHg at 25°C, that is comparable with that of the most repellent compounds tested here. As stated in a similar case study [43], the low or nil bioactivity of compounds with higher vapor pressure could not be a direct consequence of a lack of intrinsic repellency since it may result from a quick disappearance of the substances from the arena. However, this does not seem the case of our study, given the relatively low vapor pressure of all compounds tested here, so that the experimenter could perceive their odor coming from the arena throughout the assay and for a long time after the end of it, confirming the persistency of the substances. Like in [41], the observed negative correlation between vapor pressure and bioactivity could also be due to the bioassay setup, involving a static air design and a small space in relation to the size of the assayed animal. In fact, under these conditions, a lower vapor pressure allows the maintenance of a well-defined gradient between the treated and untreated areas in the bioassay environment. Conversely, large air flow-through systems could lead to a positive correlation between repellency and vapor pressure, since, in that case, the higher the volatility of the compound, the higher the amount that could possibly reach the arthropod's sensilla from the distance. In any case, the significant correlation between repellency and vapor pressure does not necessarily imply that the vapor pressure itself directly influences repellency, since vapor pressure is related to the molecular structure of the compound, which could be the major driver of repellency.
As for the chemical structure, the role of different moieties in the repellent properties of insect repellents has been investigated by several Authors (see for example [41], [42] and citations therein). In these studies, amides, imides, phenols, alcohols, hydroxy ethers, glycols and hydroxy esters were all shown to be involved in the repellent properties of the molecules. Moreover, within specific classes of molecules, repellency seemed to increase from acetate to the corresponding alcohol to the corresponding ester, and unsaturated alcohols were better repellents compared with saturated ones (effects of unsaturation are considered also in [43]). In general, several studies emphasized the importance of the oxygen (in particular of the hydroxyl –OH groups), that was confirmed here.
Some indication of a possible relationship between unsaturation and repellency was noted in the present study, by comparing, for example, the biological activity of EAN (saturated ethyl side-chain) and VAN (unsaturated vinyl side-chain). A similar relationship was reported also by Moore [52, cited in 42], who observed that unsaturated alcohols are more repellent than saturated ones. However, the case of EGU (saturated ethyl side-chain) and MVP (unsaturated vinyl side-chain), showing a similar repellency despite the presence of a double bond in the latter, suggests caution in drawing any definitive conclusions on this subject. In general, the importance of unsaturation can be confirmed, but the direction of this effect does not always seem clear, as also noted, for example, in [43], where an unsaturation in a certain position could increase or decrease the repellency depending on the molecules considered.
In any case, the data presented in this study confirm the strong influence of the molecular structure on the bioactivity of repellent compounds in ticks as already observed in other arthropods. In theory, this may be related to the variable volatility of different compounds, so that, for example, the presence of oxygen would not be important per se but for the effect that an oxygen atom can have on the vapor pressure of the compound. However, a vast body of evidence about the importance of steric and chemical interactions between signals and receptors in chemosensory systems points towards this direction.
In general, odorants are recognized by several olfactory receptors with distinct affinities and each receptor has a distinct odor-response profile [53]–[59]. Studies on the vertebrate olfactory system have demonstrated the existence of receptors activated by eugenol and structurally related substances. For example, the rat vanilloid receptor 1 is activated by eugenol and capsaicin (a molecule sharing the methoxyphenol moiety with eugenol) [60], and the so called “eugenol mouse olfactory receptor” (mOR-EG) recognizes eugenol and similar substances (EGU and MMP in the present work) [55]. It was also demonstrated that, despite mOR-EG has some tolerance for certain substitutions in the target molecule, it is however highly sensitive for other structural changes [55]. Noteworthy, in the same study, mOR-EG did not respond to guaiacol (GUA), a substance that did not show any repellency in the present study. Insects have also got olfactory receptors that respond to eugenol or structurally related compounds: for example, AgOr65 (in the mosquito Anopheles gambiae Giles) is responsive to eugenol and similar substances [54], [57], [61]; likewise, Or59a (in the Drosophila fly larvae) responds to methyleugenol (ADB in the present study) and to the similar anisole (methoxybenzene) and methylphenol [56]; methyleugenol (ADB) has also been shown to upregulate the odorant receptor co-receptor Orco in the Bactrocera dorsalis (Hendel) fly [62]. Unfortunately, only few studies have been carried out so far on tick olfactory receptors [32], [34]–[38]. For example, Leonovich [34] demonstrated the presence of phenol and lactone olfactory receptors in the distal sensilla of the I. ricinus Haller's organ. Altogether, the available evidences suggest that at least 1 receptor with high affinity for substances with molecular structure similar to that of eugenol could be present in ticks as well. In this case, such a receptor (or receptors) could well be involved in the recognition of the substances considered in this study.
In addition to olfactory receptors, odorant-binding proteins (OBPs) and chemosensory proteins (CSPs) are involved in the insect olfactory system activity. In general, each OBP can bind several ligands with different affinities, depending on the cavity volume and conformation and the residues of the binding site [63]–[66]; for example, eugenol proved to be the best ligand assayed for AmelOBP14, an Apis mellifera L. OBP [63], [65]. Unfortunately, the scarcity of data about the proteins involved in odorants recognition in ticks, strongly limits the potential of studies relating the chemical structure of ligands to the binding affinity of OBPs in these arthropods. In fact, in an exhaustive comparative genomic analysis of the odorant gene families in 20 arthropod species, only 1 CSP gene and no OBP and olfactory receptor genes (or even pseudogenes) were found in the whole I. scapularis genome (the only tick genome completely sequenced so far) [39].
Regardless the mechanisms accounting for the repellency of eugenol and related compounds against ticks, the question about the ultimate causes of such repellency remains open. In this respect, the acaricidal effects of some of these compounds (that could result, for example, from primordial arms races between herbivores and plants [67]) on other tick species may provide a possible clue [27].
In conclusion, despite the incomplete knowledge of tick's chemosensory system prevented a more detailed investigation, some molecular features influencing the bioactivity of tick repellents were identified in this study; this could allow, in the future, an easier comprehension of the underlying mechanisms of repellency. Under a practical point of view, the remarkable biological activity of some of the compounds tested here appears to be very promising even though further studies, investigating toxicology, persistence and efficacy under field conditions, will be necessary to confirm their potential as novel ingredients for tick repellents.
Funding Statement
The work was financed by the Università degli Studi di Udine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Randolph SE (2006) EDEN - emerging diseases in a changing European environment: tick-borne diseases. Int J Med Microbiol 296S1: 84–86. [Google Scholar]
- 2. Vorou RM, Papavassiliou VG, Tsiodras S (2007) Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol Infect 135: 1231–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piesman J, Eisen L (2008) Prevention of tick-borne diseases. Annu Rev Entomol 53: 323–343. [DOI] [PubMed] [Google Scholar]
- 4. Bissinger BW, Roe RM (2010) Tick repellents: Past, present, and future. Pest Biochem Physiol 96: 63–79. [Google Scholar]
- 5. Koren G, Matsui D, Bailey B (2003) DEET-based insect repellents: safety implications for children and pregnant and lactating women. Can Med Assoc J 169: 209–212. [PMC free article] [PubMed] [Google Scholar]
- 6. Schoenig GP, Osimitz TG, Gabriel KL, Hartnagel R, Gill MW, et al. (1999) Evaluation of the chronic toxicity and oncogenicity of N,N-diethyl-m-toluamide (DEET). Toxicol Sci 47: 99–109. [DOI] [PubMed] [Google Scholar]
- 7. Sudakin DL, Trevathan WR (2003) DEET: a review and update of safety and risk in the general population. J Toxicol Clin Toxicol 41: 831–839. [DOI] [PubMed] [Google Scholar]
- 8. Abdel-Rahman A, Shetty AK, Abou-Donia MB (2001) Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Exp Neurol 172: 153–171. [DOI] [PubMed] [Google Scholar]
- 9. Corbel V, Stankiewicz M, Pennetier C, Fournier D, Stojan J, et al. (2009) Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biol 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrington JE (2004) Risk perceptions regarding ticks and Lyme disease: a national survey. Am J Prev Med 26: 135–140. [DOI] [PubMed] [Google Scholar]
- 11. Klun JA, Strickman D, Rowton E, Williams J, Kramer M, et al. (2004) Comparative resistance of Anopheles albimanus and Aedes aegypti to N,N-diethyl-3-methylbenzamide (Deet) and 2-methylpiperidinyl-3-cyclohexen-1-carboxamide (AI3-37220) in laboratory human-volunteer repellent assays. J Med Entomol 41: 418–422. [DOI] [PubMed] [Google Scholar]
- 12. Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB (2011) A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478: 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sfara V, Mougabure-Cueto G, Zerba EN, Alzogaray RA (2011) Adaptation of the repellency response to DEET in Rhodnius prolixus . J Insect Physiol 57: 1431–1436. [DOI] [PubMed] [Google Scholar]
- 14. Stanczyk NM, Brookfield JFY, Ignell R, Logan JG, Field LM (2010) Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc Natl Acad Sci U S A 107: 8575–8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nazzi F, Milani N, Saccon I (2010) A bioassay to assess the activity of repellent substances on Ixodes ricinus nymphs. In: Trends in Acarology: Proceedings of the 12th International Congress. Sabelis MW, Bruin J, editors. Dordrecht, Netherlands: Springer Science+Business Media B.V; 517–519. [Google Scholar]
- 16. Erler F, Ulug I, Yalcinkaya B (2006) Repellent activity of five essential oils against Culex pipiens . Fitoterapia 77: 491–494. [DOI] [PubMed] [Google Scholar]
- 17. Gbolade AA, Oyedele AO, Sosan MB, Adewoyin FB, Soyelu OL (2000) Mosquito repellent activities of essential oils from two Nigerian Ocimum species. J Trop Med Plants 1: 146–148. [Google Scholar]
- 18. Murugan K, Murugan P, Noortheen A (2007) Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta: Diptera: Culicidae). Bioresour Technol 98: 198–201. [DOI] [PubMed] [Google Scholar]
- 19. Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS (2005) Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus . Bioresour Technol 96: 1749–1757. [DOI] [PubMed] [Google Scholar]
- 20. Seyoum A, Pålsson K, Kung'a S, Kabiru EW, Lwande W, et al. (2002) Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: ethnobotanical studies and application by thermal expulsion and direct burning. Trans R Soc Trop Med Hyg 96: 225–231. [DOI] [PubMed] [Google Scholar]
- 21. Dobrotvorsky AK, Tkachev AV, Demenkova LI (1989) Study of the repellent activity of some plants extracts against Ixodes persulcatus. Izvestiya Sibirskogo Otdeleniya Akademii Nauk SSSR. Seriya Biologicheskikh Nauk 2: 62–66 (in Russian).. [Google Scholar]
- 22. Mwangi EN, Hassanali A, Essuman S, Myandat E, Moreka L, et al. (1995) Repellent and acaricidal properties of Ocimum suave against Rhipicephalus appendiculatus ticks. Exp Appl Acarol 19: 11–18. [DOI] [PubMed] [Google Scholar]
- 23. Del Fabbro S, Nazzi F (2008) Repellent effect of sweet basil compounds on Ixodes ricinus ticks. Exp Appl Acarol 45: 219–228. [DOI] [PubMed] [Google Scholar]
- 24. Szabadics J, Erdélyi L (2000) Pre- and postsynaptic effects of eugenol and related compounds on Helix pomatia L. neurons. Acta Biol Hung 51: 265–273. [PubMed] [Google Scholar]
- 25. Waliwitiya R, Kennedy CJ, Lowenberger CA (2009) Larvicidal and oviposition-altering activity of monoterpenoids, trans-anithole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag Sci 65: 241–248. [DOI] [PubMed] [Google Scholar]
- 26. Yang Y-C, Lee S-H, Lee W-J, Choi D-H, Ahn Y-J (2003) Ovicidal and adulticidal effects of Eugenia caryophyllata bud and leaf oil compounds on Pediculus capitis . J Agric Food Chem 51: 4884–4888. [DOI] [PubMed] [Google Scholar]
- 27. Brown HA, Minott DA, Ingram CW, Williams LAD (1998) Biological activities of the extracts and constituents of pimento, Pimenta dioica L. against the southern cattle tick, Boophilus microplus . Insect Sci Appl 18: 9–16. [Google Scholar]
- 28. Kim E-H, Kim H-K, Ahn Y-J (2003) Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J Agric Food Chem 51: 885–889. [DOI] [PubMed] [Google Scholar]
- 29. Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19: 603–608. [Google Scholar]
- 30. Thorsell W, Mikiver A, Tunón H (2006) Repelling properties of some plant materials on the tick Ixodes ricinus L. Phytomedicine 13: 132–134. [DOI] [PubMed] [Google Scholar]
- 31. Tunón H, Thorsell W, Mikiver A, Malander I (2006) Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum . Fitoterapia 77: 257–261. [DOI] [PubMed] [Google Scholar]
- 32.Allan SA (2010) Chemical ecology of tick-host interactions. In: Takken W, Knols BJG, editors. Ecology and Control of Vector-borne Disease Volume 2.Olfaction in Vector-host Interactions. Wageningen, Netherlands: Wageningen Academic Publishers. 327–348.
- 33. de Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. J Chem Ecol 34: 882–897. [DOI] [PubMed] [Google Scholar]
- 34. Leonovich SA (2004) Phenol and lactone receptors in the distal sensilla of the Haller's organ in Ixodes ricinus ticks and their possible role in host perception. Exp Appl Acarol 32: 89–102. [DOI] [PubMed] [Google Scholar]
- 35. Soares SF, Borges LMF (2012) Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. Acta Trop 124: 192–198. [DOI] [PubMed] [Google Scholar]
- 36. Donzé G, McMahon C, Guerin PM (2004) Rumen metabolites serve ticks to exploit large mammals. J Exp Biol 207: 4283–4289. [DOI] [PubMed] [Google Scholar]
- 37. Grenacher S, Kröber T, Guerin PM, Vlimant M (2001) Behavioural and chemoreceptor cell responses of the tick, Ixodes ricinus, to its own faeces and faecal constituents. Exp Appl Acarol 25: 641–660. [DOI] [PubMed] [Google Scholar]
- 38. Steullet P, Guerin PM (1994) Identification of vertebrate volatiles stimulating olfactory receptors on tarsus I of the tick Amblyomma variegatum Fabricius (Ixodidae). J Comp Physiol A 174: 27–38. [DOI] [PubMed] [Google Scholar]
- 39. Vieira FG, Rozas J (2011) Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol 3: 476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. García-Domenech R, Aguilera J, Moncef AE, Pocovi S, Gálvez J (2009) Application of molecular topology to the prediction of mosquito repellents of a group of terpenoid compounds. Mol Divers 14: 321–329. [DOI] [PubMed] [Google Scholar]
- 41. Paluch G, Grodnitzky J, Bartholomay L, Coats J (2009) Quantitative structure-activity relationship of botanical sesquiterpenes: spatial and contact repellency to the yellow fever mosquito, Aedes aegypti . J Agric Food Chem 57: 7618–7625. [DOI] [PubMed] [Google Scholar]
- 42. Paluch G, Bartholomay L, Coats J (2010) Mosquito repellents: a review of chemical structure diversity and olfaction. Pest Manag Sci 66: 925–935. [DOI] [PubMed] [Google Scholar]
- 43. Snyder JC, Antonious GF, Thacker R (2011) A sensitive bioassay for spider mite (Tetranychus urticae) repellency: a double bond makes a difference. Exp Appl Acarol 55: 215–224. [DOI] [PubMed] [Google Scholar]
- 44. Ditzen M, Pellegrino M, Vosshall LB (2008) Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319: 1838–1842. [DOI] [PubMed] [Google Scholar]
- 45. Dogan EB, Ayres JW, Rossignol PA (1999) Behavioural mode of action of deet: inhibition of lactic acid attraction. Med Vet Entomol 13: 97–100. [DOI] [PubMed] [Google Scholar]
- 46. Syed Z, Leal WS (2008) Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci U S A 105: 13598–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carroll JF, Klun JA, Debboun M (2005) Repellency of deet and SS220 applied to skin involves olfactory sensing by two species of ticks. Med Vet Entomol 19: 101–106. [DOI] [PubMed] [Google Scholar]
- 48. Bohbot JD, Dickens JC (2010) Insect repellents: modulators of mosquito odorant receptor activity. PLoS One 5: e12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bohbot JD, Fu L, Le TC, Chauhan KR, Cantrell CL, et al. (2011) Multiple activities of insect repellents on odorant receptors in mosquitoes. Med Vet Entomol 25: 436–444. [DOI] [PubMed] [Google Scholar]
- 50. Davis EE (1985) Insect repellents: concepts of their mode of action relative to potential sensory mechanisms in mosquitoes (Diptera: Culicidae). J Med Entomol 22: 237–243. [DOI] [PubMed] [Google Scholar]
- 51. Peterson C, Coats J (2001) Insect repellents - past, present and future. Pest Outlook 12: 154–158. [Google Scholar]
- 52. Moore W (1934) Esters as repellents. J New York Entomol Soc 42: 185–192. [Google Scholar]
- 53. Bohbot JD, Dickens JC (2012) Selectivity of odorant receptors in insects. Front Cell Neurosci 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carey AF, Wang G, Su C-Y, Zwiebel LJ, Carlson JR (2010) Odorant reception in the malaria mosquito Anopheles gambiae . Nature 464: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, et al. (2001) Molecular bases of odor discrimination: reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci 21: 6018–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kreher SA, Kwon JY, Carlson JR (2005) The molecular basis of odor coding in the Drosophila larva. Neuron 46: 445–456. [DOI] [PubMed] [Google Scholar]
- 57. Wang G, Carey AF, Carlson JR, Zwiebel LJ (2010) Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae . Proc Natl Acad Sci U S A 107: 4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gaillard I, Rouquier S, Giorgi D (2004) Olfactory receptors. Cell Mol Life Sci 61: 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benton R (2009) Molecular basis of odor detection in insects. Ann N Y Acad Sci 1170: 478–481. [DOI] [PubMed] [Google Scholar]
- 60. Yang BH, Piao ZG, Kim Y-B, Lee C-H, Lee JK, et al. (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82: 781–785. [DOI] [PubMed] [Google Scholar]
- 61. Pask GM, Jones PL, Rützler M, Rinker DC, Zwiebel LJ (2011) Heteromeric anopheline odorant receptors exhibit distinct channel properties. PLoS One 6: e28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zheng W, Zhu C, Peng T, Zhang H (2012) Odorant receptor co-receptor Orco is upregulated by methyl eugenol in male Bactrocera dorsalis (Diptera: Tephritidae). J Insect Physiol 58: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 63. Iovinella I, Dani FR, Niccolini A, Sagona S, Michelucci E, et al. (2011) Differential expression of odorant-binding proteins in the mandibular glands of the honey bee according to caste and age. J Proteome Res 10: 3439–3449. [DOI] [PubMed] [Google Scholar]
- 64. Lagarde A, Spinelli S, Tegoni M, He X, Field L, et al. (2011) The Crystal Structure of Odorant Binding Protein 7 from Anopheles gambiae Exhibits an Outstanding Adaptability of Its Binding Site. J Mol Biol 414: 401–412. [DOI] [PubMed] [Google Scholar]
- 65. Spinelli S, Lagarde A, Iovinella I, Legrand P, Tegoni M, et al. (2012) Crystal structure of Apis mellifera OBP14, a C-minus odorant-binding protein, and its complexes with odorant molecules. Insect Biochem Mol Biol 42: 41–50. [DOI] [PubMed] [Google Scholar]
- 66. Tsitsanou KE, Thireou T, Drakou CE, Koussis K, Keramioti MV, et al. (2012) Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents. Cell Mol Life Sci 69: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iason GR, Dicke M, Hartley SE (2012) The Ecology of Plant Secondary Metabolites: From Genes to Global Processes. Cambridge University Press. 352.