Abstract
Background
Variable number of tandem repeats (VNTRs) that are widely distributed in the genome of Yersinia pestis proved to be useful markers for the genotyping and source-tracing of this notorious pathogen. In this study, we probed into the features of VNTRs in the Y. pestis genome and developed a simple hierarchical genotyping system based on optimized VNTR loci.
Methodology/Principal Findings
Capillary electrophoresis was used in this study for multi-locus VNTR analysis (MLVA) in 956 Y. pestis strains. The general features and genetic diversities of 88 VNTR loci in Y. pestis were analyzed with BioNumerics, and a “14+12” loci-based hierarchical genotyping system, which is compatible with single nucleotide polymorphism-based phylogenic analysis, was established.
Conclusions/Significance
Appropriate selection of target loci reduces the impact of homoplasies caused by the rapid mutation rates of VNTR loci. The optimized “14+12” loci are highly discriminative in genotyping and source-tracing Y. pestis for molecular epidemiological or microbial forensic investigations with less time and lower cost. An MLVA genotyping datasets of representative strains will improve future research on the source-tracing and microevolution of Y. pestis.
Introduction
Plague, one of the most devastating infectious diseases in human history, is a reemerging infectious disease causing outbreaks in several areas since the early 1980s. Yersinia pestis, the causative agent of plague, has killed hundreds of millions of people in the three major historical plague pandemics [1], [2]. Y. pestis was listed as one of four Category A selected bacterial agents by the USA Center for Disease Control and Prevention, and it could be potentially used as a war weapon or bioterrorism agent in the future, posing significant threats on the health and safety of human beings [3]. The demand for preparedness for biological terrorism threats and natural plague outbreaks has renewed interest in the detection, identification, and source-tracing of Y. pestis, especially by methods that can determine the origin of the outbreak strain.
Y. pestis evolved from Yersinia pseudotuberculosis no later than 2,600 years ago [4], [5]. The relatively short evolutionary history of Y. pestis accounts for its limited phenotypic and genetic diversity [6], [7]. Traditionally, Y. pestis was classified into three biovars by western scientists [8], according to their ability to reduce nitrate and utilize glycerol: Antiqua (positive for both), Medievalis (negative for nitrate reduction and positive for glycerol utilization), and Orientalis (positive for nitrate reduction and negative for glycerol utilization). Microtus, a new biovar, are usually rhamnose-positive and of low virulence or avirulent for guinea pigs and has been proposed based on whole genome sequencing and genetic analysis [9], [10]. The Mcirotus biovar also includes strains from the FSU, which were called Pestoides by Russian scientists [11]. However, this biovar-based system provide little information for tracing the origin of the organism, and some biovars had been proven genetically heterogeneous [12]. Several methods have been developed for Y. pestis genotyping, including ribotyping, multi-locus variable number of tandem repeats (VNTRs) analysis (MLVA), clustered regularly interspaced short palindromic repeats (CRISPRs), different regions (DFRs), insertion sequence (IS) and single nucleotide polymorphisms (SNPs) [12]–[20].
SNPs can be used as gold markers for genotyping certain bacteria [21]. Utilizing next-generation sequencing technology and phylogenomic analysis, we portrayed the SNP profiles from 133 Y. pestis strains and constructed a full parsimony phylogenetic tree of this species based on 2,298 SNPs [22]. Morelli et al. defined 24 subpopulations in a global collection of 286 Y. pestis strains, by using 933 SNPs that identified from 17 whole genome sequences. [5]. Two sets of VNTR markers which include 25 and 46 loci respectively, were used by Pourcel et al. [4]. The whole genome-wide SNPs provide phylogenetic analysis of extremely high discrimination power, with almost every strain in one individual genotype. However, the time and cost of next-generation sequencing technology make it unfeasible for routine applications in most laboratories.
VNTR usually has higher mutation rate than SNP, and MLVA assay could provide higher discrimination power when multiple loci are used in genotyping, compared to SNP tests including only limited number of pre-identified SNPs [23]. Two sets of VNTR markers which include 25 and 46 loci respectively, were employed by Pourcel et al. [17] and Klevytska et al. [18], [24] to genotype Y. pestis. In addition to Y. pestis, other genetically monomorphic species, including Bacillus anthracis, Acinetobacter baumannii, Mycobacterium tuberculosis, and Francisella tularensis, were analyzed by MLVA to obtain fine-scaled genotyping results [25]–[28]. However, the high mutation rates of VNTR loci also increase the possibility of reversions and convergent mutations, which may lead to homoplasies and bias in phylogenetic reconstruction [21].
In this study, we investigated the polymorphisms and features of 88 VNTRs in Y. pestis, including 24 newly identified ones and 64 previously reported ones, in 97 strains with global diversity. We then developed a simple hierarchical MLVA genotyping scheme based on “14+12” selected loci from the 88 VNTRs, which is consistent with SNP analysis, with significant time- and cost-saving. A total of 956 strains were screened using this scheme to generate a genetic diversity datasets to investigate the source-tracing ability of this method for Y. pestis.
Materials and Methods
Strains and DNA
We selected 79 Chinese Y. pestis strains, 18 publicly available Y. pestis whole genome sequences, and 4 genomes of Y. pseudotuberculosis to evaluate the genome-wide polymorphisms of VNTR loci (Table S1). Additionally, 859 diverse Y. pestis strains, including 842 from 18 plague foci of China, isolated between1943 and 2005, and 17 from Mongolia, were selected and screened using the newly established MLVA genotyping method. Of the 956 Y. pestis strains, 909 were previously reported in DFR genotyping analysis [16]. All the Chinese isolates were collected by the Qinghai Institute for Endemic Diseases Prevention and Control, the Center for Disease Control and Prevention of Xinjiang Uygur Autonomous Region, and the Yunnan Institute for Endemic Disease Control and Prevention. The DNAs of the Mongolian isolates were kindly provided by Dr. Jing Wang from the Institute of Health Quarantine, Chinese Academy of Inspection and Quarantine. The bacteria were cultivated in nutrient agar at 26°C for 48 h, and the genomic DNAs were extracted by conventional sodium dodecyl sulfate lysis and phenol-chloroform extraction.
VNTR Loci
Pourcel et al. [17] and Klevytska et al. [18], [24] independently developed two sets of MLVA typing protocols that contained 25 and 46 VNTRs loci, respectively. Given the fact that seven loci were shared by both protocols, 64 previously defined were re-evaluated in combination with the newly selected VNTR loci in this study. Tandem Repeats Finder 4.0 [29] was also used to find tandem repeats (TRs) in the chromosomes of five Y. pestis genomes (CO92, Nepal516, Antiqua, KIM, and 91001) [30]–[33], with alignment parameters of 2, 7, and 7 (match, mismatch, and indel), a minimum alignment score of 80, and a maximum period size of 200. A total of 280 VNTR loci were identified. Twenty-four of these loci that exhibited variations across the five genomes but were missed by previous studies were also selected in the present study for further analysis (Table 1). For consistency with previous nomenclatures, we did not change the names of the reported loci. Accordingly, loci named “M+number” were from Klevytska [18], [24], and those named “yp+number+ms+number” from Pourcel [17]. The newly identified loci in this research were named “N+number” (Table 1).
Table 1. Characteristics of the 88 VNTR loci.
| VNTR ID | In ORF | ML$(bp) | CO92 Position | Alleles | DI | Reference |
| yp0120ms01 | Overlap | 18 | 120612.120755 | 5 | 0.41 | [17] |
| yp0559ms15 | No | 15 | 559948.559977 | 2 | 0.12 | [17] |
| yp0581ms40 | No | 17 | 581354.581472 | 4 | 0.54 | [17] |
| yp0718ms41# | No | 17 | 718786.718904 | 3 | 0.53 | [17] |
| yp1018ms44 | No | 17 | 1018653.1018737 | 3 | 0.08 | [17] |
| yp1118ms69 | No | 16 | 1118998.1119093 | 4 | 0.49 | [17] |
| yp1335ms46 | No | 7 | 1335252.1335286 | 15 | 0.87 | [17] |
| yp1580ms70 | Yes | 9 | 1580109.1580153 | 11 | 0.82 | [17] |
| yp1895ms21 | Yes | 18 | 1895349.1895510 | 3 | 0.08 | [17] |
| yp1925ms71 | No | 14 | 1925713.1925782 | 4 | 0.33 | [17] |
| yp2058ms51 | Yes | 21 | 2058747.2058788 | 3 | 0.18 | [17] |
| yp2769ms06 | Overlap | 60∼ | 2769354.2769833 | 16 | 0.85 | [17] |
| yp3057ms09# | Yes | 18 | 3058032.3058625 | 16 | 0.73 | [17] |
| yp3060ms56# | Overlap | 16 | 3060574.3060653 | 6 | 0.73 | [17] |
| yp3236ms73# | Yes | 18 | 3236823.3236912 | 4 | 0.52 | [17] |
| yp3245ms74 | Yes | 15 | 3245601.3245690 | 4 | 0.52 | [17] |
| yp4042ms35 | Yes | 15 | 4042417.4042491 | 4 | 0.56 | [17] |
| yp4425ms38# | No | 16 | 4425343.4425470 | 7 | 0.68 | [17] |
| M02 | No | 1 | 3122635.3122644 | 5 | 0.36 | [18] |
| M06 | No | 2 | 462711.462722 | 7 | 0.66 | [18] |
| M09 | Yes | 3 | 2871853.2871870 | 7 | 0.22 | [18] |
| M12c | No | 4 | 2325280.2325319 | 14 | 0.88 | [18] |
| M15# | No | 5 | 3696792.3696816 | 7 | 0.61 | [18] |
| M18 | No | 6 | 2771484.2771519 | 12 | 0.86 | [18] |
| M21# | No | 7 | 427497.427531 | 7 | 0.74 | [18] |
| M22* | No | 7 | 1343780.1343870 | 23 | 0.93 | [18] |
| M23* | No | 7 | 3458682.3458730 | 16 | 0.89 | [18] |
| M25* | No | 7 | 2984639.2984792 | 21 | 0.93 | [18] |
| M26 | No | 8 | 3358733.3358748 | 2 | 0.18 | [18] |
| M27 | No | 8 | 3081535.3081686 | 26 | 0.94 | [18] |
| M28* | Overlap | 8 | 1553973.1554028 | 13 | 0.89 | [18] |
| M29* | No | 8 | 2238495.2238558 | 11 | 0.8 | [18] |
| M31 | No | 8 | 2315966.2316029 | 12 | 0.88 | [18] |
| M33* | Yes | 9 | 845399.845632 | 15 | 0.85 | [18] |
| M36 | No | 10 | 2878786.2878805 | 7 | 0.54 | [18] |
| M41 | Yes | 12 | 1896606.1896629 | 2 | 0.02 | [18] |
| M43* | No | 12 | 174513.174572 | 9 | 0.78 | [18] |
| M49 | No | 14 | 3158808.3158849 | 4 | 0.39 | [18] |
| M52 | Yes | 15 | 1198069.1198113 | 2 | 0.18 | [18] |
| M54 | No | 16 | 932708.932755 | 8 | 0.82 | [18] |
| M55 | No | 16 | 1868893.1868924 | 4 | 0.27 | [18] |
| M56 | Overlap | 17 | 2640246.2640279 | 5 | 0.26 | [18] |
| M61# | No | 18 | 2552583.2552672 | 7 | 0.6 | [18] |
| M65 | Overlap | 19 | 2999986.3000042 | 4 | 0.14 | [18] |
| M66 | No | 20 | 3555712.3555791 | 4 | 0.36 | [18] |
| M68 | No | 20 | 150777.150816 | 7 | 0.27 | [18] |
| M69 | Yes | 21 | 772786.772827 | 1 | 0 | [18] |
| M71 | Overlap | 21 | 3768639.3768680 | 4 | 0.12 | [18] |
| M73 | Yes | 30 | 2748784.2748843 | 3 | 0.13 | [18] |
| M74 | Yes | 36 | 3114610.3114681 | 3 | 0.17 | [18] |
| M76 | Overlap | 41 | 4366037.4366118 | 2 | 0.27 | [18] |
| M77 | Overlap | 45 | 54675.54899 | 4 | 0.23 | [18] |
| M79 | No | 8 | 3832848.3832927 | 14 | 0.88 | [18] |
| M37(ms07) | No | 10 | 2916163.2916232 | 7 | 0.56 | [17] & [18] |
| M72(ms54) | No | 22 | 2612799.2612842 | 3 | 0.51 | [17] & [18] |
| M59(ms05) | Overlap | 17 | 1935787.1935939 | 7 | 0.47 | [17] & [18] |
| M58(ms04) # | No | 17 | 1290131.1290266 | 8 | 0.71 | [17] & [18] |
| M51(ms20) | Yes | 15 | 1814480.1814524 | 4 | 0.51 | [17] & [18] |
| M42(ms45) | No | 12 | 1108967.1109002 | 4 | 0.18 | [17] & [18] |
| M34(ms62)* | Yes | 9 | 4280839.4280892 | 22 | 0.93 | [17] & [18] |
| M19 | No | 6 | 809405.809578 | 28 | 0.89 | [24] |
| M24 | No | 7 | 808089.808130 | 6 | 0.69 | [24] |
| M70 | Yes | 21 | 3793499.3793540 | 2 | 0.02 | [24] |
| M75 | Yes | 18 | 802532.802675 | 7 | 0.62 | [24] |
| N0171 | Overlap | 18 | 172086.172157 | 5 | 0.38 | This study |
| N0320 | Overlap | 8 | 3208218.3208273 | 12 | 0.81 | This study |
| N0322 | No | 7 | 322213.322254 | 12 | 0.86 | This study |
| N0335 | No | 18 | 335291.335362 | 6 | 0.39 | This study |
| N0865# | No | 18 | 865723.865758 | 5 | 0.63 | This study |
| N1093 | Yes | 18 | 1093917.1093970 | 3 | 0.1 | This study |
| N1398 | Overlap | 17 | 1398764.1398814 | 4 | 0.25 | This study |
| N1606* | No | 9 | 1607058.1607093 | 7 | 0.81 | This study |
| N1736 | Yes | 27 | 1736544.1736570 | 2 | 0.39 | This study |
| N2117* | Yes | 9 | 2117885.2117911 | 12 | 0.86 | This study |
| N2486# | No | 17 | 2486193.2486243 | 3 | 0.56 | This study |
| N2577* | No | 7 | 2577991.2578039 | 14 | 0.85 | This study |
| N2896# | No | 16 | 2896594.2896641 | 6 | 0.73 | This study |
| N2976# | No | 18 | 2976893.2976946 | 4 | 0.55 | This study |
| N3322 | No | 19 | 3322024.3322099 | 4 | 0.42 | This study |
| N3624 | Yes | 18 | 3624857.3624928 | 2 | 0.06 | This study |
| N3634 | Overlap | 16 | 3634057.3634120 | 3 | 0.15 | This study |
| N3743 | No | 8 | 3743960.3743999 | 12 | 0.85 | This study |
| N3763 | Yes | 18 | 3763926.3763997 | 4 | 0.33 | This study |
| N3773* | Yes | 9 | 3773759.3773803 | 10 | 0.85 | This study |
| N3779# | No | 17 | 3780032.3780099 | 4 | 0.55 | This study |
| N4268 | No | 7 | 4268345.4268414 | 25 | 0.95 | This study |
| N4287 | Overlap | 19 | 4287530.4287567 | 5 | 0.37 | This study |
| N4556 | Overlap | 19 | 4556736.4556792 | 4 | 0.17 | This study |
14+12 VNTRs used in rapid genotyping system were labeled with # and *, separately.
ML: motif length, DI: Nei’s diversity index.
The primers used in this study, including 64 previously reported ones [17], [18], [24] and 24 for amplifying newly identified VNTRs, are listed in Table S2. All primer sets were labeled with a phosphoramidite fluorescent dye (6-Fam and Hex) on the 5′ terminus.
PCR Amplification
A solution of 10 µL of PCR mixture contained 4 ng of DNA template, 0.5 µM of each primer, 0.4 unit of Taq DNA polymerase, 70 µM of dNTPs, and 10×PCR buffer (170 mM KCl, 35 mM Tris-HCl, 8 mM MgCl2, pH 8.3).The amplification was carried out in a DNA thermocycler (MJ Research PTC-225) with pre-denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 40 s, annealing at 58°C for 40 s, and elongation at 72°C for 1 min. A final 5-min elongation at 72°C was performed after the last cycle to ensure complete amplicon extension. The DNA of strain 91001 was amplified together as reference in each run.
GeneScan Fragment Analysis
The PCR products were diluted five times and mixed with formamide and Rox 500-labeled fragment standards at a ratio of 2∶7:1. Fluorescently labeled amplicons were visualized by capillary electrophoresis on ABI PRISM 310 Genetic Analyzer. Amplicon sizes were estimated with Applied Biosystems GeneScan analysis software, and the sizes of amplicons from strain 91001 were used as references to determine the copy number for each allele as previously described [17].
Clustering Analysis
The copy numbers of all VNTRs were imported into the BioNumerics software package version 5.10 (Applied-Maths, Belgium) as character datasets. Clustering analysis of VNTR results was performed using the categorical multi-state coefficient and the Ward dendrogram. The minimal spanning tree (MSTree) was constructed from a similarity matrix.
Results and Discussion
General Features of VNTR Loci
TR regions are widely distributed in the Y. pestis genome, with 280 alleles of the TRs identified from five published genomes (Figure 1). In addition to the 64 previously defined loci, 24 new ones were selected because of their differences of repeat numbers across the five genomes. The 88 loci mentioned above were further analyzed in this study (Table 1). The motif lengths of these repeats varied from 1 bp to 60 bp, 84.1% ranging from 7 bp to 21 bp. More than half (48/88, 54.5%) of the VNTRs were located in the intergenic regions. Twenty-four (27.3%) VNTRs were located within the ORFs, and their repeat unit lengths were divisible by three, indicating in-frame insertion/deletions in the corresponding proteins (Table 1).
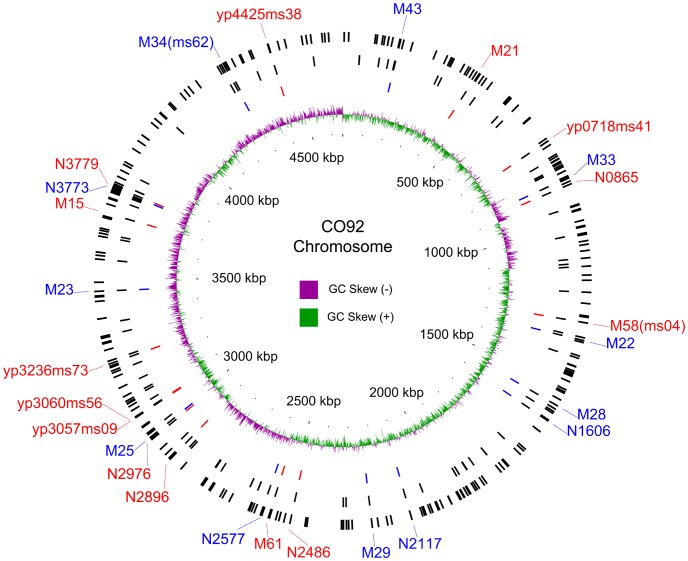
Figure 1. Distribution of the tandem repeats in CO92 genome.
From outer to inner, the bars in circles displayed i) the genomic positions of 280 tandem repeats, ii) position of 88 VNTRs, and iii) position of 14+12 VNTRs used in the hierarchical genotyping system. Red font indicates the 14 VNTRs used in the first step typing, and blue indicates the 12 VNTRs in the second step.
Sixteen VNTRs overlapped with the ORFs, with the start or stop codon of the corresponding ORF located within the repeat regions. Six of these VNTRs overlapped with the start codons (Table 2), the copy number variations of which can cause elongation/shortening or frame shifts and possibly lead to inactivation of the ORFs (Figure S1A). Ten VNTRs overlapped with the stop codons (Table 2). In contrast to that of the VNTRs with start codons, the copy number variation in these VNTRs might have less effect on the encoding of ORFs (Figure S1B).
Table 2. Repeat sequences and features of 16 VNTRs that overlapped with ORFs.
| VNTR ID | Motif sequence of VNTRs (5′-3′)* | Position of overlapping | Gene ID | Production |
| N1398 | AGTACGGAGGTGATAAA | 5′-end | YPO1239 | putative bacteriophage protein |
| M77 | CAACGTTGGCATTGGATATCCAGGTGGGACCGTGGTCCCAATTAC | 5′-end | pCD76 | hypothetical protein |
| M71 | TTTGCTCTATTGATCTTGCGC | 5′-end | YPO3379 | hypothetical protein |
| N3634 | GTTATTTGATAGGACC | 5′-end | YPO3262 | hypothetical protein |
| M56 | TATCCCTATGACGCTAT | 5′-end | YPO2346 | conserved hypothetical protein |
| yp2769ms06 | TTTCTAAGCTGCCTGTGCAGCAGTGAAC+ spacer** | 5′-end | YPO2469 | hypothetical protein |
| yp3060ms56 | AGATTGTATTTGAAAT | 3′-end | YPO2727 | conserved hypothetical protein |
| yp0120ms01 | GCAGTTAACCTTTTCACT | 3′-end | YPO0112 | conserved hypothetical protein |
| N0320 | AGTGACGC | 3′-end | YPO2872 | exodeoxyribonuclease VII large subunit |
| N4287 | CGAGATGTTTGATTAGGTC | 3′-end | YPO3820 | putative P-type cation-translocating membrane ATPase |
| N4556 | GAGTAGTTGTCAAGAGTGC | 3′-end | YPO4039 | xylulose kinase |
| M28 | GTTAATTG | 3′-end | YPO1379 | seryl-tRNA synthetase |
| N0171 | GTTAAGGTTAGCTATGCA | 3′-end | YPO0158 | siroheme synthase |
| M76 | TGTGGTGATCTAAGCGCAACACAGTTAAAAACAGATTTTAA | 3′-end | YPO3891 | hypothetical protein |
| M65 | TAACAAGTTCAATATGTAA | 3′-end | YPO2674 | putative exported protein |
| M59(ms05) | TTAGCCCATATCAGTAA | 3′-end | YPO1697 | putative chaperone protein; K07346 fimbrial chaperone protein |
Bold font indicates start and stop codons.
yp2769ms06 was a CRISPR locus, the motif sequence of which was composed by “direct sequence+spacer.” The start codon of YPO2469 was located within the first spacer of this CRISPR.
Diversity of VNTRs inY. pestis Populations
According to the whole genome-wide SNPs analysis, the population structure of Y. pestis was described as including 5 main branches (Branch 0–4), 9 major populations and 23 sub-populations [4], [5]. We selected 97 strains that belonged to 21 sub-populations representing most of the genetic diversity of Y. pestis (Figure S2 and Table S1), the DNA from another two sub-populations, 3.ANT2 and 4.ANT1, were unavailable in this study for determining polymorphism of 88 VNTRs.
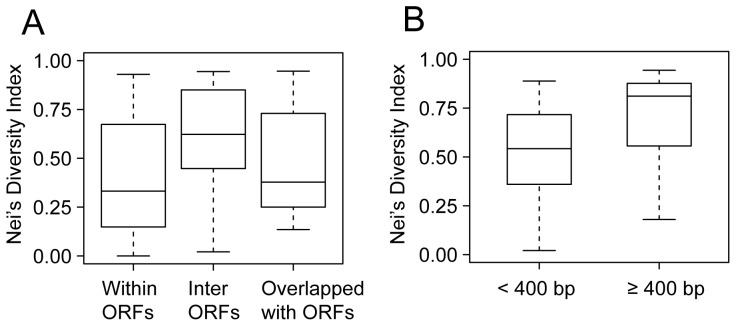
It was presumed that although VNTRs had high mutation rates [23], purifying selection would have purged the deleterious mutations, which resulted in VNTRs of different diversity according to their functional importance. To quantify the genetic diversity of VNTRs, we calculated the Nei’s diversity index [DI = 1−∑(allele frequency)2] of 97 strains for each VNTR locus. The VNTRs in Y. pestis exhibited high variability, with the DIs ranging from 0 to 0.95. M69, identified by Klevytska et al., revealed no diversity in our samples, with a DI of zero (Table 1). As expected, the VNTRs in the intergenic regions showed a significantly higher DI than those within ORFs (Figure 2A, Mann–Whitney U test, P = 0.0018). Interestingly, for the 48 VNTRs located between ORFs, the DIs were largely different according to their relative positions to the neighboring genes. The VNTRs located less than 400 bp from the 5′ end of the genes, the copy number variations of which were supposed to affect the binding of transcription factors to the promoter region, showed a lower DI (Figure 2B, Mann–Whitney U test, P = 0.0286). Thus, the polymorphisms of VNTRs in such genomic regions possibly influenced gene transcription regulation, thereby experiencing stronger purifying selection.
Figure 2. Nei’s DI across VNTRs in different genomic positions.
A. DIs across three groups of 88 VNTRs according to their relative position to ORFs. B. DIs of 48 VNTRs located between ORFs. The two groups were defined by their distance to the 5′-end of the downstream ORF. Box plot indicates median (horizontal line), interquartile range (box), and minimum and maximum values (whiskers).
As mentioned above, six VNTRs overlapped with the 5′ end of the ORFs, and their variations were supposed to result in length variation or frame shift mutations of the corresponding genes (Table 2). Therefore, except for that of yp2769ms06, a CRISPR locus rather than a real VNTR, the DIs of the five other VNTRs were also constrained by purifying selection, presenting low values of 0.12 to 0.26 (Table 1).
The 10 VNTRs that overlapped with the 3′ end of the ORFs were supposed to have relatively higher DIs because they received less selection pressure. However, the loci among them still exhibited very low DIs. For example, the DI of locus M65, overlapping with an ORF that encoded putative exported protein, was 0.14. Another locus, N4556, which overlapped with a xylulose kinase encoding gene, revealed a DI value of 0.17. The low DIs suggest that such VNTR variations might also affect gene function and was therefore constrained by selection pressures. One possible explanation was that the motifs in these loci were not 100% identical, and the copy number variations would lead to frame shift mutations. Although the DIs implied a possible relationship between the polymorphism of VNTRs and the function of the associated genes, further investigation needs to be performed to provide more robust evidences on such correlations.
Loci Selection for Hierarchical Genotyping
Using all 88 VNTRs in genotyping Y. pestis could generate extremely high resolution, which distinguished all 97 strains into unique genotypes for each (Figure 3A). However, using too many loci would largely increase operation time and workload, which will not satisfy the need for rapid genotyping and source-tracing when facing plague natural outbreak or bioterrorism attack. Therefore, we optimized the combination of VNTRs (Figure S3), which used a small portion of loci to reduce the time and costs, without significantly decreasing the discrimination power or distorting the accuracy of the genotyping results (Figure S2).
Figure 3. Clustering analysis of 97Y. pestis and four Y. pseudotuberculosis strains based on different sets of VNTRs.
A. MSTree based on 88 loci, B. MSTree based on 39 loci. SNP-based populations were labeled with different colors.
Firstly, we removed the VNTRs in the known accessory genome to avoid the bias caused by possible missing data in genotyping work. Twenty-three large genomic regions (termed as Different regions, DFRs) were previously reported missing in certain Y. pestis strains (i.e., located in accessory genome) [16], [34], [35], and six of the 88 VNTRs were located or overlapped with these DFRs. The absence of genomic regions led to failed amplifications of alleles in some strains, such as M75. Seventeen strains (17.5%) obtained no amplicon for this locus, which resulted in a large amount of missing data. Therefore, these six VNTRs were excluded from the genotyping scheme.
Secondly, we removed yp2769ms06, which was actually a CRISPR rather than a VNTR locus. Although CRISPR is an alternative genotyping marker for bacteria [13], [36], [37], we still excluded it from the MLVA typing system for two reasons: a). The CRISPR had different variation patterns and more complicated mutation mechanisms compared with normal VNTRs. Therefore, the cluster analysis that combined the variations generated from the two types of repeat regions could lead to bias; b). Yp2769ms06, defined as “YPa” in previous researches [13], [14], has approximately 14 repeat units, corresponding to ca. 1 kbp of amplicons for this locus. According to our observation, the large size and specific arrangement of the nucleotide sequence of the YPa would cause difficult in amplification and copy number determination.
Thirdly, as the loci with low DI values contributed relatively less to the discrimination power of the whole scheme, we removed 42 VNTRs with DI less than 0.5. Based on the remaining 39 VNTRs, we built an MSTree for the 97 Y. pestis strains using four Y. pseudotuberculosis strains as the outgroup (Figure 3B). Although 49 VNTRs were removed, the remaining ones provided similar discrimination and could still distinguish all strains into unique genotypes for each. However, the phylogenetic relationship across the strains revealed by 39-loci MLVA deviated slightly from the population structure defined by SNP analysis (Figure S2) [4], [5]. For example, 0.PE2 in Branch 0 was clustered with 2.ANT in Branch 2, and the 1.ANT of Branch 1 was grouped with strains of Branch 0. The high mutation rate of VNTRs increases the rate of homoplasies in phylogenetic reconstruction and confounds the phylogenetic relationship across the strains [6]. Considering that all 39 VNTRs, which were supposed to possess higher mutation rates and homoplasy rates [23], revealed higher diversities, the confound phylogenetic relationships across these strains based on those loci are understandable.
Fourthly, for further reducing the number of loci and acquiring a genotyping result as consistent as possible with phylogeny based on SNPs, we investigated the phylogenies constructed by all of the possible combination of the subsets from these 39 VNTRs. Unfortunately, no VNTR combination could perfectly reconstruct the population structure defined by SNPs. A 14-VNTR scheme produced a phylogeny that mostly approximated the SNP-based analysis, in which all strains were clustered into the major populations correctly (Figure 4). However, the discrimination power of 14 VNTRs was not high enough to resolve the strains into subpopulation level. Therefore, in addition to these 14 VNTR loci, we used another 12 VNTRs from the previous 39 ones to further distinguish the strains of major populations into different sub-populations. As shown in Figure 4, with the loci M23, M43, and N2577, the major population 0.ANT could be divided into sub-population 0.ANT1, 0.ANT2, and 0.ANT3. Five additional loci M33, M25, M34, N3773, and M22 could resolve the 1.IN into 1.IN1, 2, and 3. 1.ORI could be divided into sub-population 1.ORI1, 2, and 3 by adding four loci M29, M28, N1606, and N2117. M25 could classify 2.MED into 2.MED1, 2, and 3 and 2.ANT into 2.ANT1, 2, and 3.
Figure 4. Genotyping results of 97Y. pestis and four Y. pseudotuberculosis strains based on 14+12 VNTRs.
The trees were built by Ward. The twelve VNTRs used for defining the sub-populations were indicated on the arrows.
Finally, a hierarchical genotyping scheme was established using “14+12” VNTR loci, which could define the Y. pestis strains into sub-populations. Briefly, a 14-VNTR locus analysis designates isolates into major populations, and then additional one to five loci could classify them into sub-populations. Notably, if only a limited number of strains need to be genotyped, it would be more convenient to determine the polymorphism of all 26 VNTRs simultaneously before performing a hierarchical analysis.
Establishment of Extensive MLVA Datasets for Source-tracing Plague Outbreaks
The accuracy of source-tracing would depend not only on the robust typing method but also on extensive datasets including samples with as much diversity as possible. Accordingly, we determined the profiles of “14+12” VNTR loci in additional 859 Y. pestis strains. Combining these with the MLVA results of 97 strains used for scheme development (Table S1), the datasets including genotyping information on 956 Y. pestis strains was obtained.
As most of these strains had detailed information on isolation time and location, these data consisted of valuable resources for tracing the source of plague outbreaks. Based on the primary 14 VNTRs, we reconstructed the phylogeny of these strains. The geographical clustering patterns of the MLVA genotypes, with the strains isolated from the same or neighboring region, were grouped together in a manner consistent with the SNP analysis (Figure S4) [4]. Then, with the secondary 12 loci, we separately constructed phylogenetic trees of the strains of five major populations to validate the discrimination power of these loci. A total of 139 strains, including 25 pre-defined 2.MED ones, were clustered based on 14 loci and M25 (Figure S5). All strains were group into three major branches with 2.MED3, 2.MED2, and 2.MED1. A total of 137 strains, including nine pre-defined 2.ANT ones, were clustered based on the same set of VNTRs used in 2.MED (Figure S6). Except for one 2.ANT2 strain 351001 grouped with three 2.ANT1 ones, the other two sub-populations in 2.ANT were clustered with their relatives. Sixteen pre-defined 0.ANT1, 2, and 3 strains clustered with their close-related ones, respectively, with 14 primary VNTRs and M23, M43, as well as N2577 (Figure S7). For population 1.IN, 207 isolates were divided into three clusters, 1.IN1, 2 and 3, with their respective closely related ones together based on 14 loci and M34, M33, M25, M22, as well as N3773 (Figure S8).
Biovar Orientalis is a relatively young group, and fewer variations were accumulated in their genomes. Thus, strains of the 1.ORI population did not perfectly cluster into 1.ORI1, 2, and 3 sub-populations (Figure S9). For example, strain IP 275 (pre-defined as 1.ORI3) grouped with a strain of 1.ORI2, and another strain of 1.ORI3, MG05-1020, grouped with four 1.ORI1 strains. As the copy number data of IP275 and MG05-1020 were deciphered from their draft genome sequences, the distorted clustering results were possibly caused by sequencing or assembly errors.
Although certain strains from several sub-populations were not clustered perfectly by the hierarchical scheme of “14+12” VNTR loci, this scheme would provide a high-resolution population structure of Y. pestis, which is compatible with SNP-based phylogeny.
Conclusions
In this study, we investigated the features of 88 diversified VNTRs in Y. pestis and developed a simple genotyping scheme based on the selected “14+12” VNTRs. This hierarchical genotyping method not only provides enough discrimination power but also reduces time and cost substantially. This scheme also reduces the possible distortion of homoplasies caused by rapid mutations of VNTR loci. Meanwhile, an MLVA genotyping datasets including 956 strains was obtained for future source-tracing attempts. We noticed that Microtus strains showed heterogeneous typing results by this scheme. Other MLVA assays also failed to reconstruct reliable phylogenetic topology for the ancient Microtus (Pestoides) strains [19], [38]. This is might because of the fact that, some VNTR loci mutated so fast that they got saturated during the evolution and led to homoplasies in different lineages [23]. An alternative typing scheme will be a hierarchical assay consisting of canonical SNP analysis to determine the major phylogenetic groups, and subsequent specific MLVA to determine detailed position in the terminals of the phylogenetic tree, as what had been achieved in Bacillus anthracis [39].
The large amount of genetic diversity information provided by this study could also benefit the microevolution research on Y. pestis. However, most strains in this datasets were from China, and MLVA information on more Y. pestis strains from other countries by international collaborations is necessary to enrich and validate this datasets for global microbial forensics investigation of this pathogen [29].
Supporting Information
The possible influence on gene coding by variations of VNTRs that overlapped with ORFs. The ORF was indicated by grey, and motifs of VNTR were colored by blue. A. The motifs that contained start codon. The insertion or deletion of motif would possibly result in length variation of the ORF. B. The motifs that contained end codon. The variation of copy number wouldn’t change the coding of ORF.
(PDF)
SNP-defined phylogenetic relationship of 97 Y. pestis strains. The tree was plotted according to previously reported phylogenies [4], [5]. Each circle represents one sub-population, and the size indicates the number of strains. The major populations are indicated by colored circles. The strains from 3.ANT2 and 4.ANT1 were unavailable, and the branches leading to these two populations are indicated by dot lines.
(PDF)
Procedure of locus selection for rapid genotyping system.
(PDF)
Dendrogram of 956 strains based on 14 primary VNTR loci.
(PDF)
Dendrogram of Y. pestis strains clustered with 2.MED population based on 14+1 VNTR loci. A total of 139 strains were analyzed according to the profiles of 14 primary VNTRs and of the locus M25.
(PDF)
Dendrogram of Y. pestis strains clustered with 2.ANT population based on 14+1 VNTR loci. A total of 137 strains were analyzed according to the profiles of 14 primary VNTRs and the locus M25.
(PDF)
Dendrogram of Y. pestis strains clustered with 0.ANT population based on 14+3 VNTR loci. A total of 186 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M23, N2577, and M43
(PDF)
Dendrogram of Y. pestis strains clustered with 1.IN population based on 14+5 VNTR loci. A total of 207 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M34, N3773, M33, M25, and M22.
(PDF)
Dendrogram of Y. pestis strains clustered with 1.ORI population based on 14+4 VNTR loci. A total of 196 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M29, M28, N1606, and N2117.
(PDF)
Information and repeat numbers in 14+12 loci of 97 representative Y. pestis and 4 Y. pseudotuberculosis strains
(XLSX)
Primers of 88 VNTR loci used in this study. The locus name was described as “locus name_unit length_amplicon size_repeat numbers” in CO92 genome
(XLSX)
Acknowledgments
We thank Drs. Axel Cloeckaert, Mike Seifert and reviewers for their advices and comments on the final draft of this report. We thank all survey staffs and participants.
Funding Statement
This work was funded by the State Key Development Program for Basic Research of China (2009CB522601), the National Key Program for Infectious Diseases of China (2012ZX10004215), the Industry Research Special Foundation of China Ministry of Health (201202021), and the National Natural Science Foundation of China (31071111 and 31000015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Prentice MB, Rahalison L (2007) Plague. Lancet 369: 1196–1207. [DOI] [PubMed] [Google Scholar]
- 2. Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, et al. (2008) Plague: past, present, and future. PLoS Med 5: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broussard LA (2001) Biological agents: weapons of warfare and bioterrorism. Mol Diagn 6: 323–333. [DOI] [PubMed] [Google Scholar]
- 4. Cui Y, Yu C, Yan Y, Li D, Li Y, et al. (2013) Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis . Proc Natl Acad Sci U S A 110: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morelli G, Song Y, Mazzoni CJ, Eppinger M, Roumagnac P, et al. (2010) Yersinia pestis genome sequencing identifies patterns of global phylogenetic diversity. Nat Genet 42: 1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Achtman M (2008) Evolution, population structure, and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol 62: 53–70. [DOI] [PubMed] [Google Scholar]
- 7. Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, et al. (1999) Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis . Proc Natl Acad Sci U S A 96: 14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perry RD, Fetherston JD (1997) Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev 10: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou D, Tong Z, Song Y, Han Y, Pei D, et al. (2004) Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. Journal of Bacteriology 186: 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song Y, Tong Z, Wang J, Wang L, Guo Z, et al. (2004) Complete genome sequence of Yersinia pestis strain 91001, an isolate avirulent to humans. DNA Research 11: 179–197. [DOI] [PubMed] [Google Scholar]
- 11. Anisimov AP, Lindler LE, Pier GB (2004) Intraspecific diversity of Yersinia pestis . Clin Microbiol Rev 17: 434–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achtman M, Morelli G, Zhu P, Wirth T, Diehl I, et al. (2004) Microevolution and history of the plague bacillus, Yersinia pestis . Proc Natl Acad Sci U S A 101: 17837–17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui Y, Li Y, Gorge O, Platonov ME, Yan Y, et al. (2008) Insight into microevolution of Yersinia pestis by clustered regularly interspaced short palindromic repeats. PLoS One 3: e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vergnaud G, Li Y, Gorge O, Cui Y, Song Y, et al. (2007) Analysis of the three Yersinia pestis CRISPR loci provides new tools for phylogenetic studies and possibly for the investigation of ancient DNA. Adv Exp Med Biol 603: 327–338. [DOI] [PubMed] [Google Scholar]
- 15. Pourcel C, Salvignol G, Vergnaud G (2005) CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151: 653–663. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Dai E, Cui Y, Li M, Zhang Y, et al. (2008) Different region analysis for genotyping Yersinia pestis isolates from China. PLoS One 3: e2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pourcel C, Andre-Mazeaud F, Neubauer H, Ramisse F, Vergnaud G (2004) Tandem repeats analysis for the high resolution phylogenetic analysis of Yersinia pestis . BMC Microbiol 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klevytska AM, Price LB, Schupp JM, Worsham PL, Wong J, et al. (2001) Identification and characterization of variable-number tandem repeats in the Yersinia pestis genome. J Clin Microbiol 39: 3179–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Cui Y, Hauck Y, Platonov ME, Dai E, et al. (2009) Genotyping and phylogenetic analysis of Yersinia pestis by MLVA: insights into the worldwide expansion of Central Asia plague foci. PLoS One 4: e6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guiyoule A, Grimont F, Iteman I, Grimont PA, Lefevre M, et al. (1994) Plague pandemics investigated by ribotyping of Yersinia pestis strains. J Clin Microbiol 32: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Comas I, Homolka S, Niemann S, Gagneux S (2009) Genotyping of genetically monomorphic bacteria: DNA sequencing in Mycobacterium tuberculosis highlights the limitations of current methodologies. PLoS One 4: e7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achtman M (2004) Population structure of pathogenic bacteria revisited. Int J Med Microbiol 294: 67–73. [DOI] [PubMed] [Google Scholar]
- 23. Vogler AJ, Keys CE, Allender C, Bailey I, Girard J, et al. (2007) Mutations, mutation rates, and evolution at the hypervariable VNTR loci of Yersinia pestis . Mutat Res 616: 145–158. [DOI] [PubMed] [Google Scholar]
- 24. Girard JM, Wagner DM, Vogler AJ, Keys C, Allender CJ, et al. (2004) Differential plague-transmission dynamics determine Yersinia pestis population genetic structure on local, regional, and global scales. Proc Natl Acad Sci U S A 101: 8408–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vogler AJ, Birdsell D, Wagner DM, Keim P (2009) An optimized, multiplexed multi-locus variable-number tandem repeat analysis system for genotyping Francisella tularensis . Lett Appl Microbiol 48: 140–144. [DOI] [PubMed] [Google Scholar]
- 26. Hill V, Zozio T, Sadikalay S, Viegas S, Streit E, et al. (2012) MLVA based classification of Mycobacterium tuberculosis complex lineages for a robust phylogeographic snapshot of its worldwide molecular diversity. PLoS One 7: e41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hauck Y, Soler C, Jault P, Merens A, Gerome P, et al. (2012) Diversity of Acinetobacter baumannii in four French military hospitals, as assessed by multiple locus variable number of tandem repeats analysis. PLoS One 7: e44597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciammaruconi A (2012) Microchip Capillary electrophoresis of multi-locus VNTR analysis for genotyping of Bacillus anthracis and Yersinia pestis in microbial forensic cases. Methods Mol Biol 830: 381–390. [DOI] [PubMed] [Google Scholar]
- 29. Benson G (1999) Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res 27: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song Y, Tong Z, Wang J, Wang L, Guo Z, et al. (2004) Complete genome sequence of Yersinia pestis strain 91001, an isolate avirulent to humans. DNA Res 11: 179–197. [DOI] [PubMed] [Google Scholar]
- 31. Deng W, Burland V, Plunkett G 3rd, Boutin A, Mayhew GF, et al (2002) Genome sequence of Yersinia pestis KIM. J Bacteriol 184: 4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, et al. (2001) Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413: 523–527. [DOI] [PubMed] [Google Scholar]
- 33. Chain PS, Hu P, Malfatti SA, Radnedge L, Larimer F, et al. (2006) Complete genome sequence of Yersinia pestis strains Antiqua and Nepal516: evidence of gene reduction in an emerging pathogen. J Bacteriol 188: 4453–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou D, Han Y, Song Y, Tong Z, Wang J, et al. (2004) DNA microarray analysis of genome dynamics in Yersinia pestis: insights into bacterial genome microevolution and niche adaptation. J Bacteriol 186: 5138–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai E, Tong Z, Wang X, Li M, Cui B, et al. (2005) Identification of different regions among strains of Yersinia pestis by suppression subtractive hybridization. Res Microbiol 156: 785–789. [DOI] [PubMed] [Google Scholar]
- 36.Grissa I, Bouchon P, Pourcel C, Vergnaud G (2007) On-line resources for bacterial micro-evolution studies using MLVA or CRISPR typing. Biochimie doi:10.1016/j.biochi.2007.07.014 [DOI] [PubMed]
- 37. Stragier P, Ablordey A, Meyers WM, Portaels F (2005) Genotyping Mycobacterium ulcerans and Mycobacterium marinum by using mycobacterial interspersed repetitive units. J Bacteriol 187: 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riehm JM, Vergnaud G, Kiefer D, Damdindorj T, Dashdavaa O, et al. (2012) Yersinia pestis lineages in Mongolia. PLoS One 7: e30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simonson TS, Okinaka RT, Wang B, Easterday WR, Huynh L, et al. (2009) Bacillus anthracis in China and its relationship to worldwide lineages. BMC Microbiology 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The possible influence on gene coding by variations of VNTRs that overlapped with ORFs. The ORF was indicated by grey, and motifs of VNTR were colored by blue. A. The motifs that contained start codon. The insertion or deletion of motif would possibly result in length variation of the ORF. B. The motifs that contained end codon. The variation of copy number wouldn’t change the coding of ORF.
(PDF)
SNP-defined phylogenetic relationship of 97 Y. pestis strains. The tree was plotted according to previously reported phylogenies [4], [5]. Each circle represents one sub-population, and the size indicates the number of strains. The major populations are indicated by colored circles. The strains from 3.ANT2 and 4.ANT1 were unavailable, and the branches leading to these two populations are indicated by dot lines.
(PDF)
Procedure of locus selection for rapid genotyping system.
(PDF)
Dendrogram of 956 strains based on 14 primary VNTR loci.
(PDF)
Dendrogram of Y. pestis strains clustered with 2.MED population based on 14+1 VNTR loci. A total of 139 strains were analyzed according to the profiles of 14 primary VNTRs and of the locus M25.
(PDF)
Dendrogram of Y. pestis strains clustered with 2.ANT population based on 14+1 VNTR loci. A total of 137 strains were analyzed according to the profiles of 14 primary VNTRs and the locus M25.
(PDF)
Dendrogram of Y. pestis strains clustered with 0.ANT population based on 14+3 VNTR loci. A total of 186 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M23, N2577, and M43
(PDF)
Dendrogram of Y. pestis strains clustered with 1.IN population based on 14+5 VNTR loci. A total of 207 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M34, N3773, M33, M25, and M22.
(PDF)
Dendrogram of Y. pestis strains clustered with 1.ORI population based on 14+4 VNTR loci. A total of 196 strains were analyzed according to the profiles of 14 primary VNTRs and the loci M29, M28, N1606, and N2117.
(PDF)
Information and repeat numbers in 14+12 loci of 97 representative Y. pestis and 4 Y. pseudotuberculosis strains
(XLSX)
Primers of 88 VNTR loci used in this study. The locus name was described as “locus name_unit length_amplicon size_repeat numbers” in CO92 genome
(XLSX)