Abstract
Background
The significance of ezrin immunoexpression and prognosis for osteosarcoma is still controversial. The aim was to provide a meta-analysis for ezrin immunoexpression and prognostic features of osteosarcoma patients.
Methods
A detailed search was made in MEDLINE, EMBASE and the Web of Knowledge for relevant original articles published in English; methodological quality of the included studies was also assessed. Two reviewers extracted data independently. Studies were pooled and summary hazard ratios (HRs) and odds ratio (ORs) with corresponding confidence intervals (CIs) were calculated.
Results
Final analysis of 318 patients from 5 eligible studies was performed. Combined HR of ezrin immunohistochemical staining suggested that positive immunoexpression had an unfavorable impact on osteosarcoma patients' overall survival (n = 223 in 4 studies; HR = 4.79; 95% CI: 1.50–15.30; P = 0.008) but not on event-free survival (n = 202 in 3 studies; HR = 1.59; 95% CI: 0.61–4.15; P = 0. 0.342). Combined OR of ezrin immunohistochemical staining indicated that positive immunoexpression was associated with recurrence (n = 134 in 2 studies; OR = 3.79; 95% CI: 1.49–9.64; P = 0.005) but not with serum ALP level (n = 160 in 2 studies; OR = 2.16; 95% CI: 0.09–52.50; P = 0.637) and histological response to neoadjuvant chemotherapy(n = 260 in 4 studies; OR = 0.87; 95% CI: 0.37–2.03; P = 0.740).
Conclusions
The results of this meta-analysis suggest that ezrin positive immunoexpression confers a higher risk of recurrence and a worse survival in osteosarcoma patients. Large prospective studies are needed to provide solid data to investigate the precise prognostic significance of ezrin.
Introduction
Osteosarcoma is the most common bone cancer in children and young adults [1], [2]. In the past decades, the combination of multi-agent neoadjuvant chemotherapy has improved 5-year survival from 20% to about 70% in patients without metastasis [3], [4]. However, patients that present with metastatic disease or who relapse with disseminated tumors usually have a poor prognosis. To improve patient's prognosis, several attempts have been made to develop methods for predicting metastasis, based on tumor metastasis-associated genes and proteins [3], [5], [6].
Ezrin, also known as cytovillin or villin2, is a member of the ERM (Ezrin-Radixin-Moesin) family, which act as membrane organizers and linkers between plasma membrane and cytoskeleton [7]. It is involved in cell adhesion to the extracellular matrix and in cell–cell interactions, Rho mediated signal transduction pathway and the Akt mediated apoptotic pathway [8], [9]. These findings suggested that it might play a pivotal role in the development of human cancer.
The first research that identified an association between ezrin expression and outcome in osteosarcoma was published in 2004 [10]. In clinical research, a correlation between ezrin expression and malignancy has been reported among various human cancers, such as colorectal cancer, prostate cancer, ovarian cancer and melanoma [11]–[16]. We previously reported that mRNA level of ezrin could be a prognostic factor and a predictor of potential lung metastasis in Chinese osteosarcoma patients [17]. Recently, the discovery of small molecules that prevent osteosarcoma cells metastasis by directly targeting ezrin made it necessary to analyze the predictive value of ezrin in osteosarcoma with lines of published clinical investigations [17]–[23]. However, the prognostic value of ezrin expression in osteosarcoma patient's survival or other clinical features, such as recurrence and histological response to adjuvant chemotherapy, remains controversial. On the one side, several reports revealed that ezrin was a negative prognostic factor for survival for OS patients [17]–[19], [20], [23]; on the other side, several findings demonstrated that ezrin immunoexpression had no value of predicting the prognosis [18], [19].
In this study, we sought to conduct a systematic review and meta-analysis to estimate whether ezrin immunoexpression was associated with outcome of osteosarcoma patient. A pool of studies published between 2007 and 2010 on the association between ezrin immunoexpression and patient's survival, serum alkaline phosphatase (ALP) level, histological response to adjuvant chemotherapy and recurrence in osteosarcoma were analyzed. We found that ezrin mmunoexpression had an unfavorable impact on osteosarcoma patients' overall survival and was associated with recurrence.
Methods
Search Strategy and Selection Criteria
Medline, PubMed, Embase, and Web of Science were searched (last search was updated on 18th Nov. 2012, using the search terms: ‘ezrin’, ‘cytovillin’, ‘villin2’, ‘vil2’,‘prognosis’ and ‘osteosarcoma’). All searched studies were retrieved, and their bibliographies were checked for other relevant publications. References of selected articles and reviews were also searched manually for additional relevant studies. When more than one of the same patient populations was included in several publications, only the most recent or complete study was used to avoid duplication of information.
Two independent reviewers assessed the eligibility of studies by reviewing titles and abstracts identified by the search. Differences were resolved by discussion. The inclusion criteria required that the identified articles were studies including: (1) ezrin expression evaluated in the primary osteosarcoma tissues; (2) relationship demonstrated between ezrin expression and osteosarcoma clinicopathological parameters or prognosis; (3) ezrin expression examined by immunohistochemistry; (4) articles published as a full paper in English; (5) studies provided sufficient information to estimate hazard ratio (HR) and 95% confidence interval (CI). The exclusion criteria were as follows:(1) duplicate studies on the same patients;(2) studies written in a language other than English;(3) letters, reviews, case reports, conference abstracts, editorials, expert opinion reviews and abstracts.
Data Extraction and Study Assessment
Two investigators extracted data from eligible studies independently, discussed discrepancies, and reached consensus for all items. The following data were collected from each study: first author's name, year of publication, country, total number of patients, age, sex, and immunohistochemical technique, rate of ezrin positive expression. Duplication of data was avoided by matching the author's name and the name of the research centers.
Statistical Analysis
Hazard ratio(HR) and its variance for each individual study were extracted or calculated based on the published researches according to the methods described by Parmar [24]. A HR>1 was considered as a risk factor for worse survival in patient with positive ezrin immunoexpression. This impact of ezrin on survival was considered as statistically significant if the corresponding 95% CI for the summary HR did not overlap 1 unit. Odds ratio (OR) was used to measure the relationship of ezrin immunoexpression and histological response to neoadjuvant chemotherapy, serum ALP level and recurrence. Heterogeneity between the studies was tested using Q-statistics. It was considered statistically significant if P value less than 0.10 and was also quantified using the I 2 metric (I 2<25%, no heterogeneity; I 2 = 25–50%, moderate heterogeneity; and I 2>50%, strong heterogeneity) [25], [26]. If the heterogeneity was existed, we used a random-effects model in place of a fixed-effects model [27].All P values were two sided. Statistical calculations were performed using STATA version 12.0. and Review manager 5.0.
Results
Study Selection and Characteristics
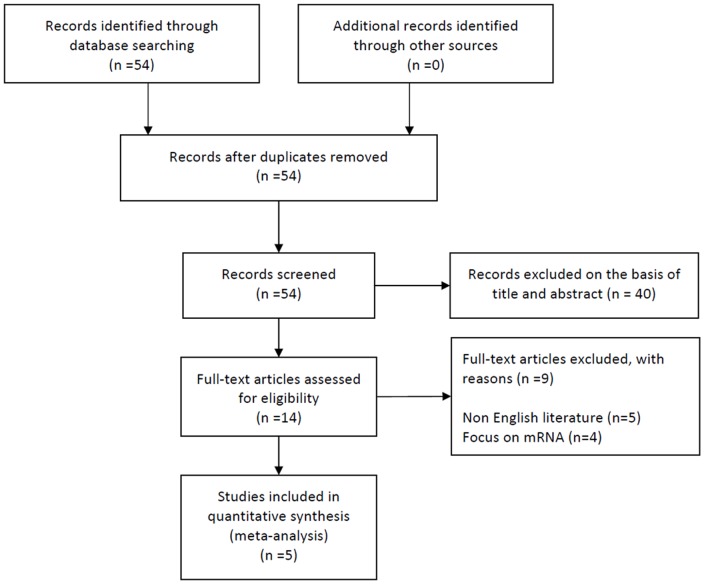
Fifty-five relevant citations were identified for initial review using search strategies as described previously. Of these, fifty were initially excluded after read the titles and abstracts (17 on cell lines; 20 meeting abstracts; 1 review article; 1 on tumor grades; 1 patent; 4 on mRNA expression; 5 published in Chinese) (Figure 1). Ultimately, the systematic literature search yielded a total of 5 studies comprising 318 patients for final analysis [18]–[21], [23]. The characteristics of retained 5 studies are listed in Table 1. These studies that were published from 2007 to 2010 met the inclusion criteria for our meta-analysis.
Figure 1. Flow diagram of study selection.
Table 1. Characteristics of eligible studies evaluating ezrin expression and OS or EFS in osteosarcoma patients.
| Author (year) | Patient number (M/F) | Mean age (range) | Metastasis (yes/no) | Neoadjuvant chemotherapy protocol | Country | Antibody source | Dilution | Definition of positive | Ezrin positive (%) (cytoplasm/membrane) |
| Boldrini (2010) | 52(27/25)* | 15.9(7–25)** | 28/24 | BOTG2000/COG9754$ | Brazil | LabVision | N/A | ≥1 cells | 76.5(50/50) |
| Kim (2009) | 70(37/33) | 15.7 (3.8–64.4) | 0/70 | N/A | Korea | NeoMarkers, Fremont, CA. | 1∶300 | >10% cells | 55.7(N/A) |
| Ferrari (2008) | 95(62/33) | 16(4–39) | 0/95 | MTX, ADM, CDP,IFO# | Italy | Neomarkers, Fremont, CA. | 1∶200 | ≥1% cells | 80(49/51) |
| Salas (2007) | 37(23/14) | 15(6.7–54.2) | 2/35 | T10/SFOP OS94 | France | Sigma | 1/250 | ≥1% cells | 62(N/A) |
| Kim (2007) | 64(45/19) | 19.4(4–58)** | 0/64 | Modified T10 | Korea | Santa Cruz,CA | 1∶100 | ≥1 cells | 51.6(N/A) |
N/A: not available;
34 samples were investigated;
average age.
MTX: methotrexate, ADM: doxorubicin, CDP: cisplatin, IFO: ifosfamide;
Brazilian Osteosarcoma Treatment Group 2000 and Children Oncology Group 9754.
The immunohistochemical technique for ezrin expression detection was summarized in Table 1. A HR on event-free survival (EFS) and/or overall survival (OS) could be extracted from these studies, respectively. Most of the survival data were obtained from five eligible studies [18]–[21], [23]. Four of the included studies investigated the association between ezrin immunoexpression and histological response to neoadjuvant chemotherapy response [19]–[21], [23], two studies analyzed the relationship between ezrin immunoexpression and serum ALP level [19], [20].
Methodological quality of the studies
Four included studies were assessed by two authors with high levels of methodological quality (>6 stars) according to Newcastle–Ottawa quality assessment scale [28].
Impact of ezrin immunoexpression on EFS and OS of osteosarcoma patients
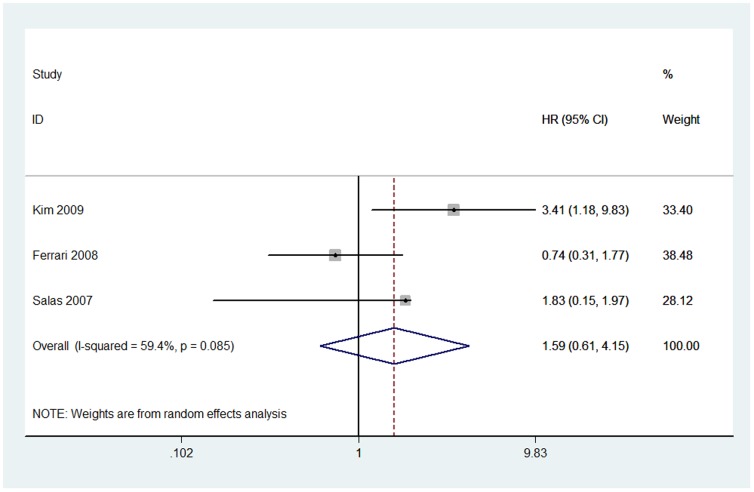
Three studies with a total of 202 osteosarcoma patients dealing with ezrin immunoexpression and EFS were meta-analyzed [19], [20], [23]. Because of heterogeneity (I 2 = 59.4%), a random effect model was adopted in this analysis. The pooled HR was 1.59 (95% CI: 0.61–4.15; Z = 0.95; P = 0.342), illustrating that ezrin immunoexpression was not significantly associated with the EFS of osteosarcoma patients (Figure 2).
Figure 2. Forest plot showing the association between ezrin expression and event-free survival (EFS) of osteosarcoma.
The summary HR and 95% CIs were shown (according to the random-effects estimations).
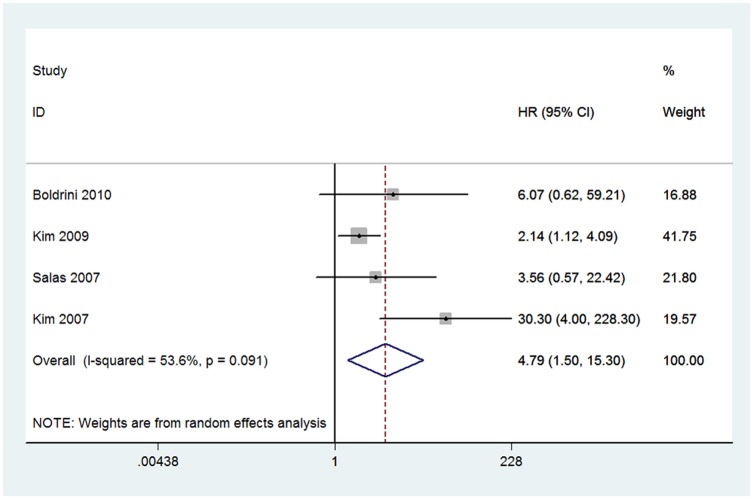
Four studies including 223 patients reported the correlation between ezrin immunoexpression and OS were also meta-analyzed [18], [20], [21], [23]. Due to heterogeneity (I 2 = 53.6%), a random effect model was selected. The combined HR was 4.79 (95% CI: 1.50–15.30; Z = 2.64; P = 0.008), which demonstrated that ezrin immunoexpression was significantly associated with the poor OS of osteosarcoma patients (Figure 3).
Figure 3. Forest plot showing the association between ezrin expression and overall survival (OS) of osteosarcoma.
The summary HR and 95% CIs were shown (according to the random-effects estimations).
Ezrin immunoexpression and histological response to neoadjuvant chemotherapy
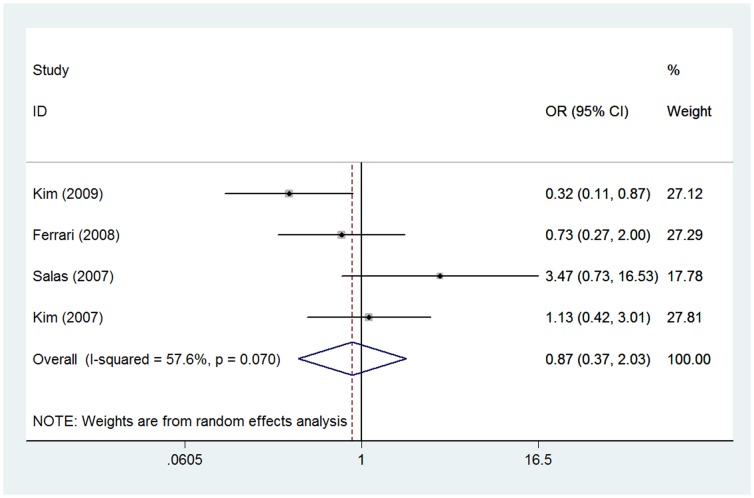
Four of these included studies with 260 patients investigated the association between ezrin immunoexpression and histological response to neoadjuvant chemotherapy response [19]–[21], [23]. A random effect model was selected for analyze due to heterogeneity (I 2 = 57.6%). The combined OR was 0.87 (95% CI: 0.37–2.03; Z = 0.33; P = 0.740), suggesting that ezrin immunoexpression was not associated with histological response of osteosarcoma patients (Figure 4).
Figure 4. Forest plot showing the association between ezrin expression and histological response to neoadjuvant chemotherapy in osteosarcoma.
The summary OR and 95% CIs were shown (according to the random-effects estimations).
Ezrin immunoexpression and serum ALP level
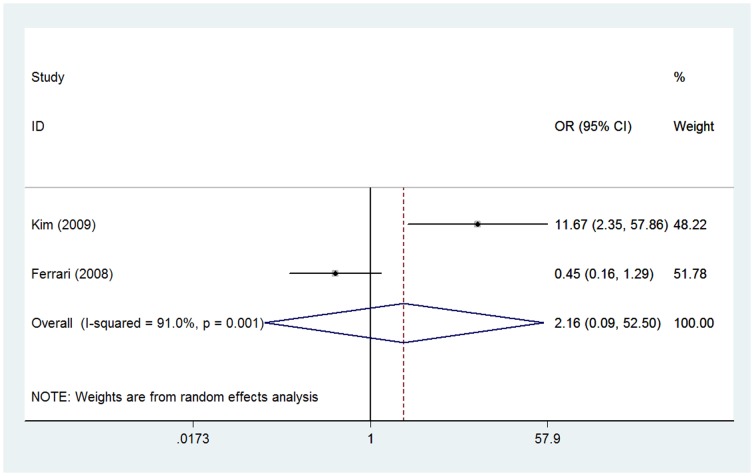
Two of these included studies with 160 patients investigated the association between ezrin immunoexpression and serum ALP level [19], [20]. Because of heterogeneity (I 2 = 91.0%), a random effect model was adopted in this analysis. The combined OR was 2.16 (95% CI: 0.09–52.50; Z = 0.47; P = 0.637), suggesting that ezrin immunoexpression was not correlated to serum ALP level (Figure 5).
Figure 5. Forest plot showing the association between ezrin expression and serum ALP level in osteosarcoma.
The summary OR and 95% CIs were shown (according to the random-effects estimations).
Ezrin immunoexpression and recurrence
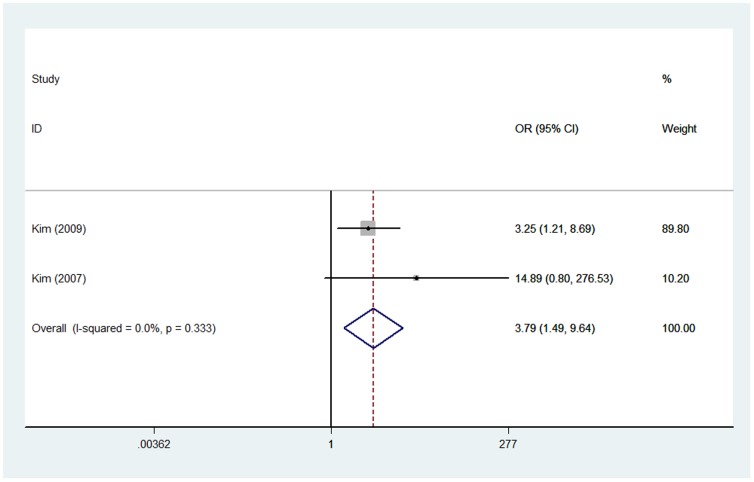
Two of these included studies with 134 patients investigated the relationship between ezrin immunoexpression and local recurrence [20], [21]. A fixed effect model was selected for analyze since no heterogeneity was detected (I 2 = 0.0%). The combined OR was 3.79 (95% CI: 1.49–9.64; Z = 2.80; P = 0.005), indicating that ezrin immunoexpression was significantly related to local recurrence (Figure 6).
Figure 6. Forest plot showing the association between ezrin expression and recurrence in osteosarcoma.
The summary OR and 95% CIs were shown (according to the fixed-effects estimations).
Assessment of Publication Bias
Because the number of study included in our meta-analysis was comparatively few, we did not draw funnel plot to demonstrate publication bias.
Discussion
This meta-analysis showed that ezrin immunoexpression had an unfavorable impact on overall survival and was associated with recurrence in patients with osteosarcoma. No significant predictive associations were found between ezrin expression and EFS, histological response to neoadjuvant chemotherapy and ALP level.
However, these conclusions should be deciphered with several issues that could not be overcame: First, the results of ezrin immunoexpression were less powerful because only 5 studies were included for analysis with a relatively small sample size of 318 patients; second, as a result of small sample size, stratified analysis of different race was not performed. Because of the varied incidence by race at young population [29], we could not exclude race as a factor that might have influence on outcome of osteosarcoma patients.
In terms of survival, cautions should be paid to interpret the results of different significance of EFS and OS. First, four studies dealing with OS were included, whereas only three reports focus on EFS were eligible, and the recruiting patients were 233 and 202 respectively, which were relatively small sample size compare with other cancer studies. Second, there was 13.4% (30/223) of patients with metastasis were recruited in OS analysis, but it's only about 1% (2/202) of counterpart in EFS analysis. Since ezrin was demonstrated to be the key role of metastasis, it's obviously that different proportion of patients with potential to experience metastasis would make outcome different. These differences could lead to inhomogeneity of analysis.
It's reasonable to expect that recurrence rate accompanies with short EFS. However, the significant association between ezrin and recurrence did not result in a correlation between ezrin and EFS. This could be explained by different studies were included in recurrence and EFS (Figure 1) analysis (Figure 5).
Although ezrin was a key component factor for metastasis, it also played an important role in multidrug resistance and cell survival [30], [31]. Unfortunately, this meta-analysis showed no remarkable correlations between ezrin expression and histological response to neoadjuvant chemotherapy and ALP level despite the combined OR for ALP as high as 2.16. These negative results might due to that only two eligible studies with heterogeneity were analyzed. Therefore, more precise estimates of these relationships would be possible if more researches were included.
Meanwhile, some limitations in this meta-analysis should be noticed: First, marked heterogeneity of included studies existed in analysis except recurrence analysis. The heterogeneity was probably due to following reasons: primary antibodies from different companies, different cut-off points for positive staining, different immunoreactivity pattern (membrane/cytoplasm), baseline characteristics of patients (tumor stage, country) and the duration of follow-up. The variables mentioned above may make the pooled results less reliable. Second, there might be some biases if studies other than English were excluded. Third, the relatively small sample sizes in the included studies result in that even very powerful prognostic factors may not have become significant. Fourth, HRs were calculated from data or extrapolated from survival curves in the eligible studies, the HR information obtained by statistical software unavoidably developed a decrease of reliability.
Conclusion
This meta-analysis suggests that ezrin was seems to be associated with a worse prognosis for OS and recurrence of patients with osteosarcoma with available evidence. However, more patients should be enrolled to improve statistical power and avoid clinical heterogeneity; moreover, large prospective studies are still needed to provide solid data to investigate the precise prognostic significance of ezrin.
Acknowledgments
We thank Dr Ren Shengxiang from Shanghai Pulmonary Hospital at Tongji University, for his personal advice for this paper.
Funding Statement
This work was supported by National Natural Scientific Foundation of China (NSFC) (No: 81102038, 81172105, and 81172548). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Picci P (2007) Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyers PA, Gorlick R (1997) Osteosarcoma. Pediatr Clin North Am 44: 973–989. [DOI] [PubMed] [Google Scholar]
- 3. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, et al. (2006) Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 106: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 4. Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, et al. (1986) The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 314: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 5. Clark JC, Dass CR, Choong PF (2008) A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol 134: 281–297. [DOI] [PubMed] [Google Scholar]
- 6. Bakhshi S, Radhakrishnan V (2010) Prognostic markers in osteosarcoma. Expert Rev Anticancer Ther 10: 271–287. [DOI] [PubMed] [Google Scholar]
- 7. Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. [DOI] [PubMed] [Google Scholar]
- 8. Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M (2003) Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell 14: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krishnan K, Bruce B, Hewitt S, Thomas D, Khanna C, et al. (2006) Ezrin mediates growth and survival in Ewing's sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin Exp Metastasis 23: 227–236. [DOI] [PubMed] [Google Scholar]
- 10. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, et al. (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature medicine 10: 182–186. [DOI] [PubMed] [Google Scholar]
- 11. Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M (2005) The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res 7: R365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elzagheid A, Korkeila E, Bendardaf R, Buhmeida A, Heikkila S, et al. (2008) Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum Pathol 39: 1737–1743. [DOI] [PubMed] [Google Scholar]
- 13. Geiger KD, Stoldt P, Schlote W, Derouiche A (2000) Ezrin immunoreactivity is associated with increasing malignancy of astrocytic tumors but is absent in oligodendrogliomas. Am J Pathol 157: 1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makitie T, Carpen O, Vaheri A, Kivela T (2001) Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci 42: 2442–2449. [PubMed] [Google Scholar]
- 15. Moilanen J, Lassus H, Leminen A, Vaheri A, Butzow R, et al. (2003) Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol 90: 273–281. [DOI] [PubMed] [Google Scholar]
- 16. Weng WH, Ahlen J, Astrom K, Lui WO, Larsson C (2005) Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res 11: 6198–6204. [DOI] [PubMed] [Google Scholar]
- 17. Xu-Dong S, Zan S, Shui-er Z, Li-na T, Wen-xi Y, et al. (2009) Expression of Ezrin correlates with lung metastasis in Chinese patients with osteosarcoma. Clinical and Investigative Medicine Médecine Clinique Et Experimentale 32: E180–188. [DOI] [PubMed] [Google Scholar]
- 18. Boldrini E, Peres SV, Morini S, de Camargo B (2010) Immunoexpression of Ezrin and CD44 in patients with osteosarcoma. Journal of pediatric hematology/oncology : official journal of the American Society of Pediatric Hematology/Oncology 32: e213–217. [DOI] [PubMed] [Google Scholar]
- 19. Ferrari S, Zanella L, Alberghini M, Palmerini E, Staals E, et al. (2008) Prognostic significance of immunohistochemical expression of ezrin in non-metastatic high-grade osteosarcoma. Pediatric blood & cancer 50: 752–756. [DOI] [PubMed] [Google Scholar]
- 20. Kim C, Shin E, Hong S, Chon HJ, Kim HR, et al. (2009) Clinical value of ezrin expression in primary osteosarcoma. Cancer research and treatment : official journal of Korean Cancer Association 41: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim MS, Song WS, Cho WH, Lee SY, Jeon DG (2007) Ezrin expression predicts survival in stage IIB osteosarcomas. Clin Orthop Relat Res 459: 229–236. [DOI] [PubMed] [Google Scholar]
- 22. Park HR, Jung WW, Bacchini P, Bertoni F, Kim YW, et al. (2006) Ezrin in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Pathology, research and practice 202: 509–515. [DOI] [PubMed] [Google Scholar]
- 23. Salas S, Bartoli C, Deville JL, Gaudart J, Fina F, et al. (2007) Ezrin and alpha-smooth muscle actin are immunohistochemical prognostic markers in conventional osteosarcomas. Virchows Archiv : an international journal of pathology 451: 999–1007. [DOI] [PubMed] [Google Scholar]
- 24. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 25. DerSimonian R (1996) Meta-analysis in the design and monitoring of clinical trials. Stat Med 15: 1237–1248 discussion 1249–1252. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, et al. (2009) More than numbers: the power of graphs in meta-analysis. Am J Epidemiol 169: 249–255. [DOI] [PubMed] [Google Scholar]
- 28. Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 29. Mirabello L, Troisi RJ, Savage SA (2009) Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 115: 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brambilla D, Zamboni S, Federici C, Lugini L, Lozupone F, et al. (2012) P-glycoprotein binds to ezrin at amino acid residues 149–242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int J Cancer 130: 2824–2834. [DOI] [PubMed] [Google Scholar]
- 31. Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, et al. (2004) The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem 279: 26280–26286. [DOI] [PubMed] [Google Scholar]