Abstract
Background
The meningococcal serogroup A (MenA) polysaccharide conjugate vaccine used in Sub-Saharan Africa does not prevent disease caused by MenW or MenX strains, which also cause epidemics in the region. We investigated the vaccine-potential of native outer membrane vesicles with over-expressed factor H-binding protein (NOMV-fHbp), which targeted antigens in African meningococcal strains, and was combined with a MenA polysaccharide conjugate vaccine.
Methodology/Principal Findings
The NOMV-fHbp vaccine was prepared from a mutant African MenW strain with PorA P1.5,2, attenuated endotoxin (ΔLpxL1), deleted capsular genes, and over-expressed fHbp in variant group 1. The NOMV-fHbp was adsorbed with Al(OH)3 and used to reconstitute a lyophilized MenA conjugate vaccine, which normally is reconstituted with liquid MenC, Y and W conjugates in a meningococcal quadrivalent conjugate vaccine (MCV4-CRM, Novartis). Mice immunized with the NOMV-fHbp vaccine alone developed serum bactericidal (human complement) activity against 13 of 15 African MenA strains tested; 10 of 10 African MenX strains, 7 of 7 African MenW strains, and 6 of 6 genetically diverse MenB strains with fHbp variant group 1 (including 1 strain from The Gambia). The combination NOMV-fHbp/MenA conjugate vaccine elicited high serum bactericidal titers against the two MenA strains tested that were resistant to bactericidal antibodies elicited by the NOMV-fHbp alone; the combination elicited higher titers against the MenA and MenW strains than those elicited by a control MCV4-CRM vaccine (P<0.05); and high titers against MenX and MenB strains. For most strains, the titers elicited by a control NOMV-fHbp knock out vaccine were <1∶10 except when the strain PorA matched the vaccine (titers >1∶000).
Conclusion/Significance
The NOMV-fHbp/MenA conjugate vaccine provided similar or higher coverage against MenA and MenW strains than a quadrivalent meningococcal conjugate vaccine, and extended protection against MenX strains responsible for epidemics in Africa, and MenB strains with fHbp in variant group 1.
Introduction
Devastating epidemics of meningococcal disease have occurred in sub-Saharan Africa for over one hundred years [1]. In 1996, 25,000 deaths were reported, which was the largest meningococcal epidemic on record. Most meningococcal disease in sub-Saharan Africa is caused by serogroup A (MenA) strains. In contrast, in industrialized countries nearly all meningococcal disease is caused by strains producing capsular serogroups B, C or Y [2]–[4]. Until recently, control of meningococcal epidemics in Africa remained an unsolved public health challenge. After more than ten years of work [5]–[7] a MenA polysaccharide-protein conjugate vaccine (MenAfriVac) was introduced [8]–[10]. As of December 3rd 2012, 100 million people in sub-Sahara had received the vaccine (http://www.path.org/news/pr121203-menafrivac.php). Early data indicated that vaccination has been effective in both preventing MenA meningococcal disease [11] and decreasing colonization by MenA strains [12]. Thus, this vaccine has the potential to eliminate MenA epidemics [11]. Widespread vaccination, however, could allow emergence of strains with other serogroups such as X (MenX) or W (MenW), which also cause epidemics in this region [13]–[15]. Although it is not easy to determine whether the vaccination campaign has affected disease caused by strains with non-vaccine serogroups, in Burkina Faso, which was one of the first countries to use the MenA conjugate vaccine, more than 5000 cases and 550 deaths caused by MenW isolates were reported during the first 5 months of 2012 (WHO Surveillance Bulletins, http://www.who.int/csr/don/2012_05_24/en/index.html).
Commercial quadrivalent meningococcal A,C,Y and W conjugate vaccines are available in industrialized countries [16]–[19]. While these vaccines could prevent MenA or MenW disease in sub-Sahara, the vaccines are not affordable since this region is one of the poorest in the world. Also quadrivalent vaccines do not prevent MenX disease, or disease caused by strains with serogroup B (MenB), which are responsible for meningococcal disease in industrialized countries and could arise in Africa in the future.
Development of polysaccharide-protein conjugate vaccines for prevention of MenB disease has not been possible because the MenB polysaccharide is an autoantigen [20]. Newly discovered protein antigens are under investigation as vaccines that target MenB disease. One of the most promising is factor H-binding protein (fHbp) [21], [22], which is an important virulence determinant [23], [24], and is expressed by nearly all strains [25], [26]. fHbp is contained in two meningococcal B vaccines [27]–[31]. Recently one of these vaccines (Bexsero, Novartis Vaccines, Siena Italy), was approved by the European Commission for immunization of individuals two months of age and older [32]. Second-generation vaccines using chimeric fHbp [33], [34], new adjuvants [35], or native (non-detergent treated) outer membrane vesicle (NOMV) vaccines from mutants with genetically attenuated endotoxin and over-expressed fHbp (NOMV-fHbp) [36], [37], also are in development, and offer the prospect of enhancing immunogenicity and breadth of protection.
More than 600 different fHbp amino acid sequence variants have been identified (http://pubmlst.org/neisseria/fHbp/). Each one is given a unique identifier (ID number). Based on amino acid relatedness, fHbp sequences have been classified into two sub-families (A or B) or three variant groups (v.1, v.2, or v.3) [21], [22]. In general, serum antibodies elicited by fHbp vaccines have bactericidal activity only against strains with fHbp in the same variant group or sub-family as the vaccine [21], [22], [27], [28], [30], [31], [33], [34], [38]–[41].
In a recent study, we immunized mice with a prototype NOMV-fHbp vaccine prepared from a mutant MenB strain over-expressing fHbp ID 1, which is a prevalent variant group 1 sequence among MenB strains in the United States [26]. The animals developed serum bactericidal antibody responses against MenB strains and also against epidemic MenA, W and X strains from sub-Saharan Africa [42]. While these results were promising, neither the PorA variable region (VR) type of the mutant group B strain used to prepare the NOMV vaccine, or the fHbp ID, were present among African meningococcal strains. Also, we had used a plasmid construct to over-express fHbp in the mutant vaccine strain. As a consequence the resulting mutant strain would be too unstable for use in a commercial vaccine production. In the present study we used an engineered promoter containing neisserial porA and nadA promoter sequences, which integrated into the bacterial chromosome and provided stable over-expression of fHbp. Mutant strains with this promoter are potentially suitable for commercial NOMV vaccine production. Further the mutant strain incorporated new knowledge about the advantages of using non-fH binding fHbp mutants as vaccine antigens [43]–[45], which were gained from studies of MenB vaccines, into a single prototype strain designed specifically to target antigens in epidemic meningococcal strains from sub-Sahara Africa. Because our long term goal is a vaccine intended to extend protection elicited by the MenA conjugate vaccine being used in sub-Sahara to cover MenW and X strains, we also investigated the feasibility of combining the NOMV-fHbp vaccine with a MenA polysaccharide-protein conjugate vaccine.
Methods
Objectives
The primary objective was to investigate the immunogenicity in mice of a prototype meningococcal NOMV-fHbp vaccine prepared from a mutant strain from Africa engineered to over-express fHbp when combined with a MenA polysaccharide-protein conjugate vaccine. Our hypothesis was that the combination NOMV-fHbp/MenA conjugate vaccine would elicit similar or higher serum bactericidal antibody responses against epidemic meningococcal MenA, W or X strains than a U.S./European-licensed quadrivalent meningococcal polysaccharide-protein conjugate vaccine.
Descriptions of Procedures
Selection of fHbp sequence variant and vaccine strain
We previously identified four prevalent fHbp amino acid sequence variants among a collection of 106 MenA, W and X meningococcal isolates from 17 countries in Africa [42] (see also Figure S1). These four fHbp sequence variants (including related variants, each differing from the respective prevalent variant by 1 amino acid) were present in 81% of the isolates. With the exception of fHbp ID 22/23 in variant group 2 (which was in 58% of MenW isolates), the three other prevalent fHbp sequence variants were in variant group 1∶100% of MenA isolates, 95% of MenX isolates, and 34% of MenW isolates. For over-expression of fHbp, we selected fHbp ID 9 as potentially the most cross-reactive protein among the prevalent variant group 1 proteins [42]. The amino acid sequence of fHbp ID 9 was 96 and 94 percent identical to ID 4/5 or 74, respectively, and was predicted to elicit protective anti-fHbp antibodies against MenA, W and X strains with fHbp in variant group 1. For coverage against MenW strains with fHbp variant 2, we selected an invasive MenW strain (Sudan 1/06), to prepare the mutant vaccine strain. The PorA (P1.5,2) of this strain was identical to that of all epidemic ST-11 MenW strains tested from sub-Saharan Africa with fHbp in variant groups 1, 2, or 3. This particular PorA subtype also was related to P1.5–1,2–36, and P1.5–1,2–2, which are found in clonal complex (CC) 175 MenW strains that cause disease in the sub-Sahara but are less common than CC11 MenW strains. The PorA P1.2,5 in vaccine also was antigenically related to PorA 5-1,10-1, which are present in recent CC181 MenX strains [42], [46]. Thus, the PorA from the vaccine strain was predicted to elicit broadly protective anti-PorA antibodies against the majority of MenW isolates and, possibly, against some MenX strains from the sub-Saharan region irrespective of the fHbp sequence variant.
Stable over-expression of fHbp ID 9
Strain Sudan 1/06 is naturally a low-to-medium expresser of fHbp ID 9 [42]. For over-expression of fHbp we replaced the native fHbp promoter with an engineered hybrid nadA-porA promoter (Figure 1, Panel A). In the engineered promoter, designated EH, the sequence between −10 and −35 in the PorA promoter that contained the poly G tract, which promotes slip-strand miss-pairing, was replaced with the sequence (ATATGCCTCCTTTCATA) from the neisserial nadA promoter to stabilize expression of the downstream gene (fHbp, nmb1870, Figure 1, Panel B).
Figure 1. Schematic representation of the genetic strategy to over-express fHbp ID 9 R41S.
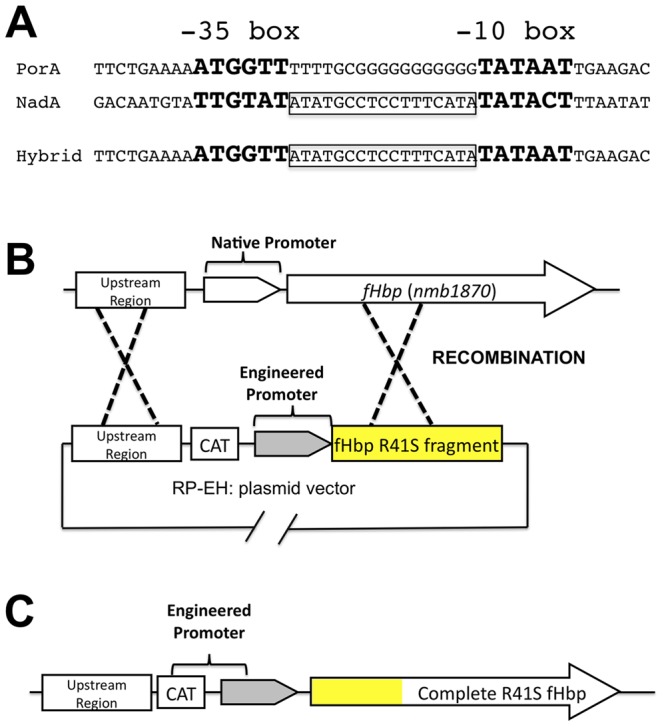
Panel A: Sequences of natural porA and nadA promoters and the engineered (Hybrid) promoter where the sequence between the −10 and −35 boxes of the porA promoter containing the poly G tract was replaced with the sequence from the Neisserial nadA promoter to eliminate slip-strand miss-pairing. Although not shown, in the hybrid promoter the porA promoter sequence (−164 to −35 and −10 to +57) was left intact. Panels B-C: Strategy for replacement of the native fHbp promoter by the engineered promoter sequence, and introduction of the R41S mutation in fHbp to decrease fH binding (see text for additional details). Panel B shows the genomic environment of the fHbp gene (nmb1870) and the plasmid RP-EH used. CAT, represents the chloramphenicol resistance cassette used for selection of the recombinants. The yellow R41S box represents the first 255 base pairs of the fHbp ID 9 gene containing the mutation that leads to the R41S change in the mature mutant ID 9 fHbp. Panel C represents the organization of the region after the recombination.
In previous studies, fHbp vaccines with an R41S mutation had decreased fH binding and showed increased immunogenicity in human fH transgenic mice than control fHbp vaccines that bound human fH [43], [44]. In the present study, we introduced the R41S mutation into a fragment containing the first 255 base pairs of the fHbp gene, which is a highly conserved region [38], [47] among the variant group 1 fHbp sequences and was used as the 3′ recombination arm. The engineered EH promoter and the plasmid vector, which contains the promoter and the 255 base pairs of the fHbp gene including the mutation encoding for R41S, are illustrated in Figure 1, Panels B and C, respectively. Using this promoter we generated a mutant of strain Sudan 1/06, designated OE fHbp (OE, over-expressed), and used flow cytometry with anti-fHbp mAb JAR 5 to assess the relative amounts of fHbp on the bacterial surface of live bacteria [48]. The mutant strain with over-expressed fHbp R41S had ∼10-fold increased fHbp on its surface compared with the parent wildtype strain (Figure 2).
Figure 2. Surface-accessible fHbp on live bacteria of the mutant vaccine strain Sudan 1/06 with over-expressed fHbp as measured by flow cytometry.
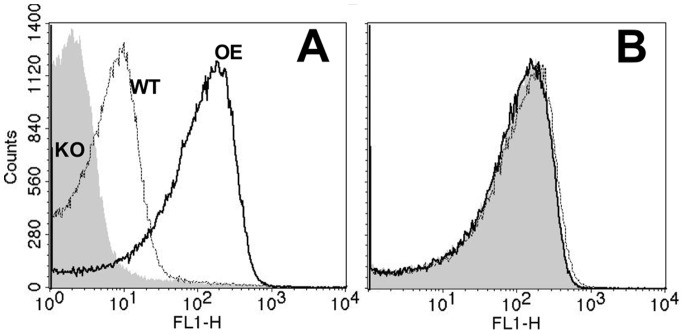
A. Binding of anti-fHbp mAb, JAR 5. WT, parental strain with wildtype fHbp expression; KO, mutant with fHbp gene inactivated; OE, strain with the engineered promoter and mutant gene encoding fHbp ID 9 with the R41S amino acid substitution. Panel B: Detection of MenW using anticapsular mAb JW-W1 as a control.
For the final vaccine strain we inserted a second copy of the engineered promoter, the R41S fHbp gene, a terminator, and a different antibiotic marker, neomycin phosphotransferase, that confers kanamycin resistance, into the lpxL1 locus to generate a mutant with two copies of the R41S fHbp and penta-acylated LOS instead of hexa-acylated LOS in the parental strain [49]. Penta-acylated LOS has 50- to 100-fold diminished endotoxin activity compared to that of wildtype hexa-acylated LOS [49]–[51] (See Discussion section). The second copy of the engineered promoter/fHbp gene also increased fHbp levels in the NOMV by ∼25% over those achieved by a single copy in the native fHbp locus (data not shown). Finally, to create a capsular null mutant, we deleted a chromosomal segment (cssA-cssEw) by recombining a cassette that conferred erythromycin resistance to enable selection of the recombinants. This mutation eliminated the MenW polysaccharide in the NOMV vaccine, avoided the potential problem of induction of anti-capsular antibody hyporesponsiveness [52], [53], and helped ensure production of a consistent NOMV vaccine.
As described below, the resultant capsular null mutant vaccine strain with attenuated endotoxin and over-expressed R41S fHbp was used to produce the NOMV-fHbp vaccine. We created a second mutant strain with the same modifications and antibiotic resistance genes but lacking the fHbp gene (NOMV-fHbp KO), which was used to produce a control NOMV vaccine without fHbp (NOMV-KO). The results of characterization of the different NOMV vaccines are provided in the Results section.
Preparation of NOMV vaccines
Bacteria were grown at 37°C with shaking at 300 rpm for 10 hours in a 600 mL culture in Frantz media to early stationary phase (OD600 nm 3.8 to 4.2 after 10 hours of growth). Phenol 0.5% (w/v) was added to inactivate the bacteria. After overnight incubation at 4°C, the culture was centrifuged for 40 minutes at 4000 x g and the supernatant was filtered through a 0.45 µM Millipore filter unit (PES, 500ml, 90 mm diameter, Thermo Fisher Scientific, MA, US) to remove remaining bacteria. The supernatant was concentrated to ∼100 ml in an ultrafiltration cell (Millipore Model 8400, Millipore Ultrafiltration Membranes, PLHK, 76 mm diameter, 100,000 NMWL, Millipore, MA, US), and centrifuged at 100,000 g for 2 hours (41,100 rpm, Ti60 rotor, Beckman ultracentrifuge). The NOMV blebs were re-suspended in 3% sucrose and 0.2 M glycine, pH 8.0, and stored at –20°C.
NOMV characterization
Major proteins in the NOMV vaccines were separated by SDS PAGE and visualized with Commassie Blue staining (see Figure S2). The concentration of fHbp in the NOMV was measured by a quantitative Western blot, which had a linear range between 0.1 to 2 mg/ml [38]. The anti-fHbp mAb used was JAR 5 [54]. The presence of fHbp, PorA, MenW capsule, and residual fH-binding in the NOMV were measured by ELISA, which was performed as previously described [43].
Mouse immunization
CD-1 female mice were obtained from Charles River (Wilmington, MA, US). Groups of five-week-old mice (12 mice per group) were immunized with NOMV-fHbp or NOMV-KO vaccines (2.5 µg of total protein). As an additional control, we immunized mice with a quadrivalent meningococcal conjugate vaccine (Menveo, Novartis), which was designated “meningococcal conjugate vaccine 4-CRM,” or MCV4-CRM. Each human dose of MCV4-CRM contains 10 µg of MenA oligosaccharide and 5 µg each of oligosaccharides of MenC, Y and W conjugated with CRM197 protein. The commercial vaccine is supplied in two vials, one containing lyophilized MenA conjugate, which is reconstituted immediately before use with liquid MenC, Y and W conjugate vaccines in the second vial. The vaccine for humans does not use an aluminum adjuvant. For the mouse immunogenicity study, the vaccine was adsorbed with 0.6 mg of aluminum hydroxide (2% Alhydrogel, Brenntag Biosector), and we used one-fifth of the human dose (i.e., for mice, 1 µg of each of the MenC, Y and W oligosaccharide conjugates and 2 µg of the MenA conjugate). The NOMV vaccines were also adsorbed with aluminum hydroxide (0.6 mg per dose). For the combination vaccine, we reconstituted 2 µg of lyophilized MenA conjugate vaccine with the liquid suspension of NOMV-fHbp vaccine (2.5 µg of protein) that had been adsorbed with aluminum hydroxide. Three injections were given, each separated by 3 weeks, and terminal blood samples were obtained three weeks after the last dose.
Measurement of serum antibody responses by ELISA
Serum IgG anti-fHbp Ab responses were measured by ELISA, which was performed as described previously [45]. Serum anticapsular antibody responses were measured using MenA polysaccharide coupled to adipic acid dihydrazide [55]. For coating the plates we used 5 µg/mL of the derivatized polysaccharide.
Serum bactericidal antibody activity
Serum bactericidal titers were measured as previously described using early log-phase bacteria grown for approximately 2 h in Mueller-Hinton broth (BD Biosciences, Franklin Lakes, NJ, US) supplemented with 0.25% glucose (w/v) and 0.02 mM cytidine 5′-monophospho-N-acetylneuraminic acid (CMP-NANA, Sigma-Aldrich, St, Louis, MO, US). To insure that all of the test strains expressed sialylated LOS, we added CMP-NANA to the culture media since some N. meninigitidis strains (particularly serogroup A) require exogenous CMP-NANA for sialylation of LOS while others have mechanism(s) for endogenous sialylation [56], [57]. Strains with sialylated LOS are more resistant to bactericidal activity than strains with no or partial sialylated LOS [58]. Therefore, the titers reported are conservative estimates of protective activity. The complement source was IgG-depleted human serum, which was prepared as previously described [38]. We tested serum bactericidal activity against 38 strains (15 MenA, 6 MenB, 7 MenW and 10 MenX). Source, phenotypic and genetic characterization on the strains [42], [59]–[61] are summarized in Table 1.
Table 1. Summary of meningococcal strains used to test serum bactericidal activity.
| Strain Name | Secondary Strain Designation | Capsular Group | Country | Year | fHbp Protein ID* | 1/Serum Bactericidal GMT† | Clonal Complex, (ST) | PorA VR Type | Reference | |
| NOMV-KO | NOMV-fHbp | |||||||||
| Senegal1/99 | A1 | A | Senegal | 1999 | 5 | <10 | 281 | 5 (5) | P1.20,9 | [59] |
| BuFa6/07 | A2 | A | Burkina Faso | 2007 | 5 | 16 | 108 | 5 (2859) | P1.20,9 | [59] |
| E23/03 | A3 | A | Ethiopia | 2003 | 5 | <10 | <10 | 5 (7) | P1.20,9 | [59] |
| Z1275 | A4 | A | Niger | 1963 | 4 | <10 | 100 | 1 (1) | P1.5–2,10 | [60] |
| E2/88 | A5 | A | Ethiopia | 2003 | 5 | <10 | 346 | 5 (7) | P1.20,9 | [59] |
| Z2491 | A6 | A | Gambia | 1983 | 5 | <10 | 6861 | 4 (4) | P1.7,13–1 | [61] |
| Niger1/95 | A7 | A | Niger | 1995 | 5 | 31 | 463 | 5 (5) | P1.20,9 | [59] |
| Niga3/07 | A8 | A | Nigeria | 2007 | 5 | <10 | 178 | 5 (7) | P1.20,9 | [59] |
| C50/01 | A9 | A | Congo | 2001 | 5 | <10 | <10 | 5 (7) | P1.20,9 | [42] |
| LNP20868 | A10 | A | Burkina Faso | 2003 | 5 | <10 | 306 | 5 (2859) | P1.20,9 | [59] |
| LNP20790 | A11 | A | Burkina Faso | 2003 | 5 | <10 | 71 | 5 (2859) | P1.20,9 | [59] |
| Nigeria4/03 | A12 | A | Nigeria | 2003 | 5 | 632 | 1643 | 5 (7) | P1.20,9 | [42] |
| Sudan11/07 | A13 | A | Sudan | 2007 | 5 | <10 | 109 | 5 (7) | P1.20,9 | [42] |
| BuFa20030378 | A14 | A | Burkina Faso | 2007 | 5 | <10 | 160 | 5 (6035) | P1.20,9 | [42] |
| Chad5/07 | A15 | A | Chad | 2007 | 5 | <10 | 183 | 5 (7) | P1.20,9 | [42] |
| H44/76 | B1 | B | Norway | 1976 | 1 | <10 | 736 | 32 (32) | P1.7,16 | [42] |
| SK140 | B2 | B | US | 2005 | 4 | <10 | 1151 | 41/44 (5097) | P1.7–2,4 | [42] |
| MD01327 | B3 | B | US | 1999 | 4 | <10 | 893 | 41/44 (2973) | P1.18,25 | [42] |
| NZ98/254 | B4 | B | New Zealand | 1998 | 14 | <10 | 368 | 41/44 (42) | P1.7–2,4 | [42] |
| M01573 | B5 | B | US | NA | 55 | <10 | 125 | 41/44 (44) | P1.7–1,1 | [22] |
| 675 | B6 | B | The Gambia | 1995 | 347 | <10 | 3065 | 41/44 (8233) | P1.7–1,1 | [42] |
| ††Su1/06 | W1 | W | Sudan | 2006 | 9 | 23280 | 25411 | 11 (11) | P1.5,2 | [59] |
| BuFa2-03 | W2 | W | Burkina Faso | 2003 | 23** | 2014 | 1243 | 11 (11) | P1.5,2 | [59] |
| Nigeria1/04 | W3 | W | Nigeria | 2004 | 22** | 87 | 34 | 175 (2881) | P1.5–1,2–36 | [42] |
| C6/01 | W4 | W | Congo | 2001 | 350 | 221 | 1753 | 175 (175) | P1.5–1,2–2 | [42] |
| BF 17/01 | W5 | W | Burkina Faso | 2001 | 9 | 1374 | 1294 | 11 (11) | P1.5,2 | [42] |
| Uganda12/06 | W6 | W | Uganda | 2006 | 349 | 1533 | 781 | 11 (11) | P1.5,2 | [42] |
| Mali1/06 | W7 | W | Mali | 2006 | 23** | 1309 | 753 | 11 (11) | P1.5,2 | [42] |
| BF 12/03 | X1 | X | Burkina Faso | 2003 | 73 | 38 | 1627 | 181 (751) | P1.5–1,10–1 | [42] |
| Kenya1/06 | X2 | X | Kenya | 2006 | 74 | <10 | 310 | UA (5403) | P1.19,26 | [42] |
| Uganda 23/06 | X3 | X | Uganda | 2006 | 74 | <10 | 277 | UA (5403) | P1.19,26 | [42] |
| Uganda 3/06 | X4 | X | Uganda | 2006 | 74 | <10 | 112 | UA (5403) | P1.19,26 | [42] |
| Uganda14/06 | X5 | X | Uganda | 2006 | 74 | <10 | 118 | UA (5403) | P1.19,26–4 | [42] |
| BF 5/97 | X6 | X | Burkina Faso | 1997 | 73 | <10 | 239 | 181 (751) | P1.5–1,10–1 | [42] |
| BuFa2-97 | X7 | X | Burkina Faso | 1997 | 73 | <10 | 446 | 181 (751) | P1.5–1,10–1 | [59] |
| BuFa7/07 | X8 | X | Burkina Faso | 2007 | 74 | 93 | 279 | 181 (181) | P1.5–1,10–1 | [59] |
| HF24 | X9 | X | S. Africa | 1970s | 4 | 21 | 160 | 181 (3687) | P1.7,9 | [42] |
| HF78 | X10 | X | S. Africa | 1970s | 4 | 11 | 359 | 181 (3687) | P1.7,9 | [42] |
As designated on the public website at http://pubmlst.org/neisseria/fHbp/. **fHbp sequence variant in group 2 (sub-family A); all other fHbp sequence variants are in group 1 (sub-family B) as designated on the public website.
For strains A01–A05, W01–W04, X01–X03, and B01–B05, the reciprocal GMT was calculated from titers of three serum pools (four animal each). These are shown graphically along with GMT of negative control mice immunized with aluminum hydroxide alone in Figure 5. For the remaining strains shown in Table 1, the reciprocal GMT was calculated from titers of two serum pools.
Strain used for construction of the vaccine strain.
In this publication we made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford [93], [94].
Statistical analyses
Antibody concentrations and titers were transformed (Log10). After confirming the transformed data were normally distributed, we calculated the geometric mean antibody titers. In this calculation, titers below the limit of the assay were assigned a value half of the lower tested dilution. Two-tailed Student’s t tests were used to compare the geometric mean antibody titers between two independent groups of mice. All statistical tests were two-tailed; probability values of less than or equal to 0.05 were considered statistically significant.
Ethics statement
All animal work was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the U.S. National Institutes of Health. The protocol was approved by the Children’s Hospital & Research Center at Oakland Institutional Animal Care and Use Committee (NIH assurance number A3631–01). We used approved operating protocols for vaccine injections, blood collection and euthanasia, which were performed under inhalation isoflurane anesthesia to provide minimal distress to the animals. The mice were monitored by the investigators and the animal caretakers in the Animal Facility, and were provided enrichment in the form of cotton fiber pads. Terminal blood collections were done at the earliest times possible while allowing for maximal immune responses. We used the smallest numbers of animals possible to obtain statistically meaningful data, and we did not duplicate experiments unnecessarily. The clinical isolates used to measure serum bactericidal activity were from collections described in previous studies [41], [53]. The isolates were coded and identified based only on year of isolation and country of origin. Permission to use these isolates was obtained from the Children’s Hospital Oakland Institutional Review Board (IRB). The human complement source for measuring serum bactericidal activity was serum from an adult who participated in a protocol that was approved by the IRB. Written informed consent was obtained from the subject.
Results
Characterization of the NOMV Vaccines
By SDS-PAGE, the expected major proteins in the NOMV were observed (Figure S2). Compared with the NOMV-fHbp vaccine, the NOMV-KO vaccine appeared to have slightly more protein in bands resolving around 38 and 32 kDa, which were consistent with PorB and reduction modifiable protein (Rmp), respectively. The fHbp is known to localize in the same portion of the gel as OpA/OpC proteins [62], and could not be clearly visualized. By Western blot, the amount of fHbp in the NOMV-fHbp vaccine was approximately 12-fold higher than in the control NOMV vaccine prepared from the wild-type strain (Figure 3), which was consistent with the 10-fold increase in live cells estimated by flow cytometry. Interestingly, we also detected a band of approximately 60 kDa in the NOMV-fHbp vaccine, which was consistent with the expected size of a fHbp dimer.
Figure 3. Detection of fHbp in NOMV vaccines by Western blot using anti-fHbp mAb JAR 5.
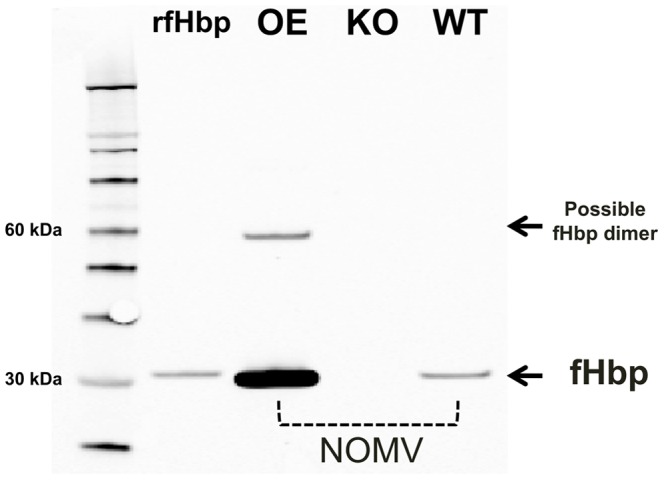
rfHbp, recombinant fHbp ID 9. OE, NOMV-fHbp vaccine with over-expressed R41S mutant fHbp prepared from the mutant vaccine strain with Δlpxl1 and Δcapsule; KO, NOMV prepared from a triple knockout (ΔfHbp, Δlpxl1, and Δcapsule); WT, NOMV vaccine prepared from the wildtype strain. A total of 0.25 µg of NOMV was loaded in each lane.
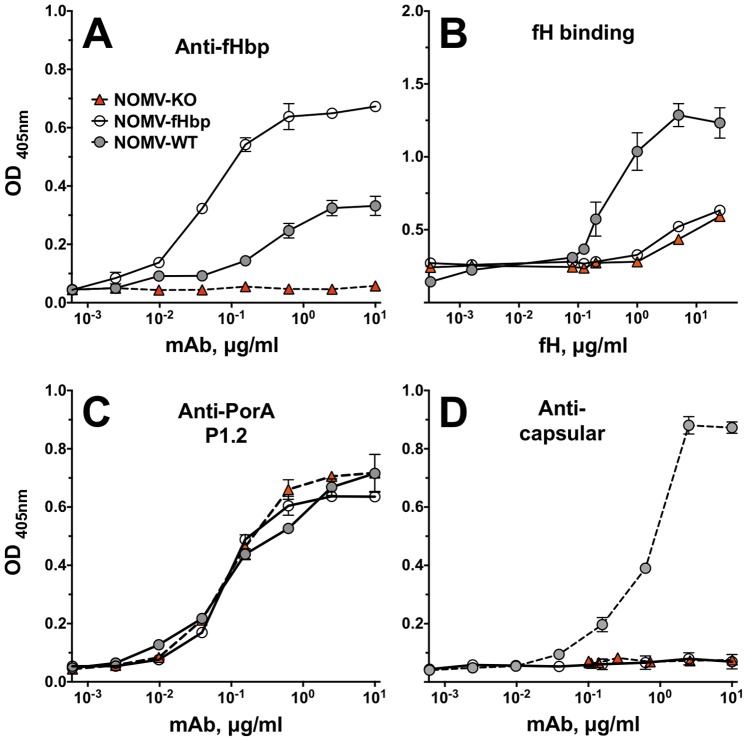
By ELISA, the anti-fHbp mAb showed >10-fold higher binding to the NOMV-fHbp vaccine than to a control NOMV vaccine prepared from the parent wildtype strain (Figure 4, Panel A). Despite higher amounts of fHbp in the NOMV-fHbp, there was less fH binding than to the control NOMV from the wild-type strain (Panel B), which resulted from the R41S amino acid substitution in fHbp expressed by the mutant vaccine strain [45]. The similar amount of fH-binding activity by the NOMV-fHbp as the NOMV-KO vaccine likely represented residual fH binding by ligands other than fHbp (for example, NspA) [63]. Both the NOMV-fHbp and the control NOMV-KO vaccine showed similar respective binding with an anti-P1.2 PorA mAb (Panel C). As expected, the NOMV-fHbp and control NOMV-KO were negative for binding with an anticapsular mAb specific for the MenW polysaccharide, since bothe vaccines were prepared from capsule knock-out mutants (Panel E).
Figure 4. Characterization of NOMV vaccines by ELISA.
Panel A. fHbp was measured using anti-fHbp mAb, JAR 5. Panel B. Binding of human fH to the NOMV vaccines was performed as previously described [43]. Panel C. PorA was measured using anti-PorA mAbs specific for P1.2 [92]. Panel D. MenW capsular polysaccharide was measured using anticapsular mAb, JW-W1.
The NOMV-fHbp Vaccine Elicits Broad Serum Bactericidal Activity against Epidemic Meningococcal Strains from Sub-Saharan Africa, and MenB Strains
We measured serum bactericidal antibody responses using a panel of 38 MenA, W, X and B strains. The reciprocal geometric mean titers (GMT) of two or three serum pools from mice immunized with the NOMV-fHbp or NOMV-fHbp KO vaccines against each of the strains are summarized in Table 1.
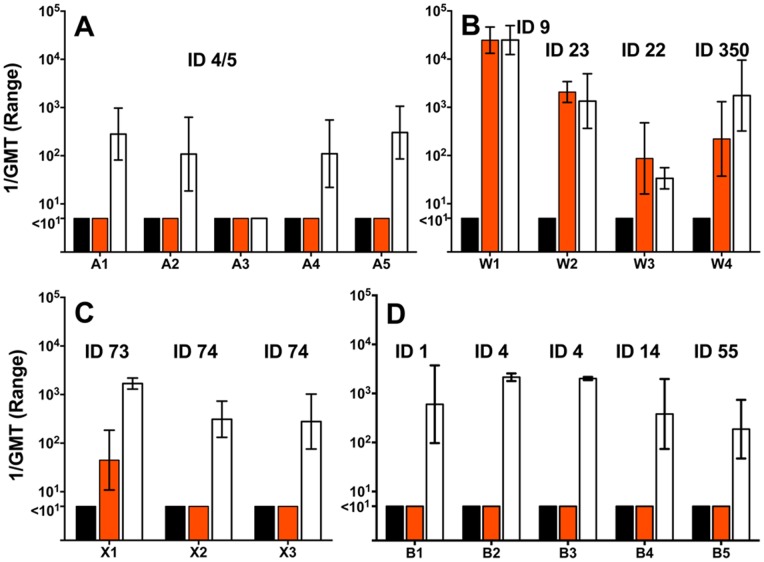
Mice immunized with the NOMV-fHbp vaccine alone developed serum bactericidal titers >1∶10 against 13 of 15 African MenA strains with fHbp ID 4 or 5 in variant group 1; 10 out of 10 African MenX strains with fHbp ID 73 or 74 in variant group 1, and 7 of 7 African MenW strains with fHbp ID 9, 23, or 349 in variant groups 1, 2, or 3 respectively. The NOMV-fHbp also elicited serum bactericidal titers >1∶10 against all six MenB strains tested from the US, Norway, New Zealand or The Gambia. All of these MenB strains were selected to have fHbp in variant group 1 (ID 1, 4, 14, 55 or 347) to match the variant group of the fHbp ID 9 in the NOMV-fHbp vaccine. For the susceptible MenA, W, X or B strains the GMT usually was >1∶100. The two exceptions were a MenA strain (A11, GMT 1∶71) and a MenW strain (W3, GMT 34). For representative strains tested with 3 serum pools per vaccine group, the reciprocal geometric mean titers (GMT) and ranges of the three titers are shown in Figure 5.
Figure 5. Serum bactericidal antibody responses of mice immunized with NOMV-fHbp vaccine.
Panels A, B and C, MenA, W or X isolates, respectively, from cases of disease isolated during epidemics in sub-Saharan Africa. Panel D, MenB isolates from industrialized countries. The MenA, X and B strains had fHbp amino acid sequence variants in variant group 1 (See Table 1). The MenW stains had fHbp in variant group 1 (ID 9, ID 350) or variant group 2 (ID 23, ID 22). Bars represent the reciprocal GMT and ranges of three serum pools per vaccine group, and two pools for the adjuvant alone (four individual sera in each pool). Black bars, control mice immunized with aluminum hydroxide without a vaccine antigen; orange bars, control mice immunized with a NOMV-fHbp KO vaccine; white bars, mice immunized with the NOMV-fHbp vaccine.
The MenA and B strains had heterologous PorA VR types to the P1.5,2 in the NOMV vaccines. The titers elicited by the control NOMV-KO vaccine against these strains generally were <1∶10 (Figure 5 and Table 1). One notable exception was a MenA strain (A12) with a GMT of 1∶632 elicited by the NOMV-KO vaccine (Table 1), and 1∶1643 by the NOMV-fHbp vaccine; the antigenic target of the bactericidal antibodies elicited by the NOMV-KO vaccine was not identified. The four MenX strains with PorA 5-1,10-1, which was anticipated to be antigenically related to PorA P1.2,5 in the vaccine, were either resistant (N = 2 isolates, titers <1∶10), or only moderately susceptible to antibodies elicited by the NOMV-KO vaccine (N = 2 isolates, bactericidal titers of 1∶38 and 1∶93).
Against all 7 MenW test strains, there were no significant differences in bactericidal responses elicited by NOMV-fHbp or control NOMV-KO vaccines (Figure S3, respective GMTs of 1053 vs. 1139, P>0.8). For the five MenW strains with a homologous PorA VR type of P1.5,2 to the vaccine, the GMTs elicited by the NOMV-KO vaccine were >1000, which were within one-dilution of those elicited by the NOMV-fHbp vaccine. Two MenW strains had PorA VR types of P1.5–1,2–36 (W3) or P1.5–1,2–2 (W4), which were related to P1.5,2 in the vaccine. The GMTs elicited by the NOMV-KO vaccine against these strains were 1∶87 and 1∶221, respectively, compared to 1∶34 and 1∶1753 for the NOMV-fHbp vaccine (Figure 5). The higher GMT elicited by the NOMV-fHbp vaccine against strain W4 can be explained by the presence of fHbp ID 350 in variant group 1, which was similar to the one in the vaccine (ID 9), while the lower GMT against strain W3 reflected fHbp ID 22 in variant group 2.
The Combination NOMV-fHbp/MenA Conjugate Vaccine Elicits Enhanced IgG Antibody Responses and Enhanced Serum Bactericidal Activity
We used the liquid NOMV-fHbp that had been adsorbed with aluminum hydroxide to reconstitute a lyophilized MenA conjugate vaccine from Novartis (See methods). Control mice received the NOMV-fHbp vaccine alone, a European/U.S.-licensed quadrivalent A,C,Y,W conjugate vaccine (MCV4-CRM, Novartis), a NOMV-fHbp KO vaccine, or aluminum hydroxide without a vaccine antigen. For the combination NOMV-fHbp/MenA vaccine study, we measured titers in sera from individual mice to increase statistical power to detect significant group differences in the responses, if they were present.
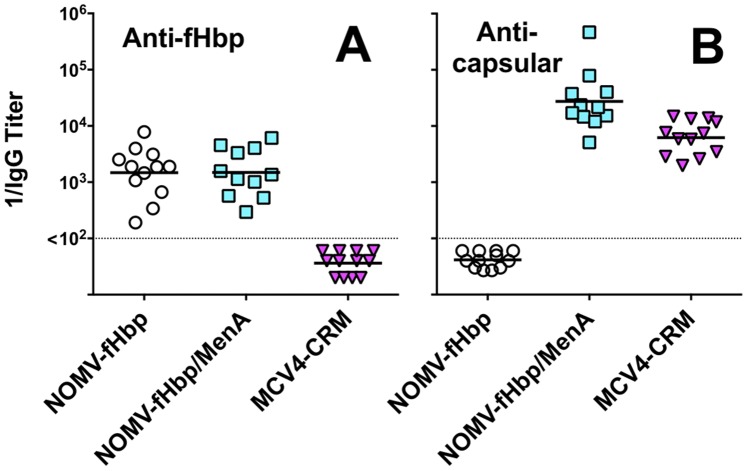
There were no significant differences between the serum IgG anti-fHbp titers of mice immunized with the combination NOMV-fHbp/MenA conjugate vaccine or the NOMV-fHbp vaccine alone (Figure 6, Panel A). Mice immunized with the combination vaccine had four-fold higher serum IgG group A anticapsular antibody titers than mice immunized with the control MCV4-CRM conjugate vaccine (P = 0.001, Panel B). Although we did not include a control group of mice immunized with the MenA conjugate component alone, the data suggested that there was no impairment of the group A anticapsular antibody responses elicited by the combination NOMV-fHbp/MenA conjugate vaccine.
Figure 6. Serum IgG antibody responses to vaccination as measured by ELISA.
Panel A. Anti-fHbp ID 9 titers. Panel B. Anticapsular antibody titers to MenA polysaccharide. Each symbol represents the reciprocal titer of an individual immunized mouse. Horizontal lines denote reciprocal geometric mean titers. There were no significant differences in the IgG anti-fHbp antibody titers elicited by the NOMV-fHbp or combination NOMV-fHbp/MenA conjugate vaccine (P>0.5). The serum IgG anticapsular titers were higher in mice immunized with the combination NOMV-fHbp/Men A conjugate vaccine than the control MCV4-CRM conjugate vaccine (P = 0.001).
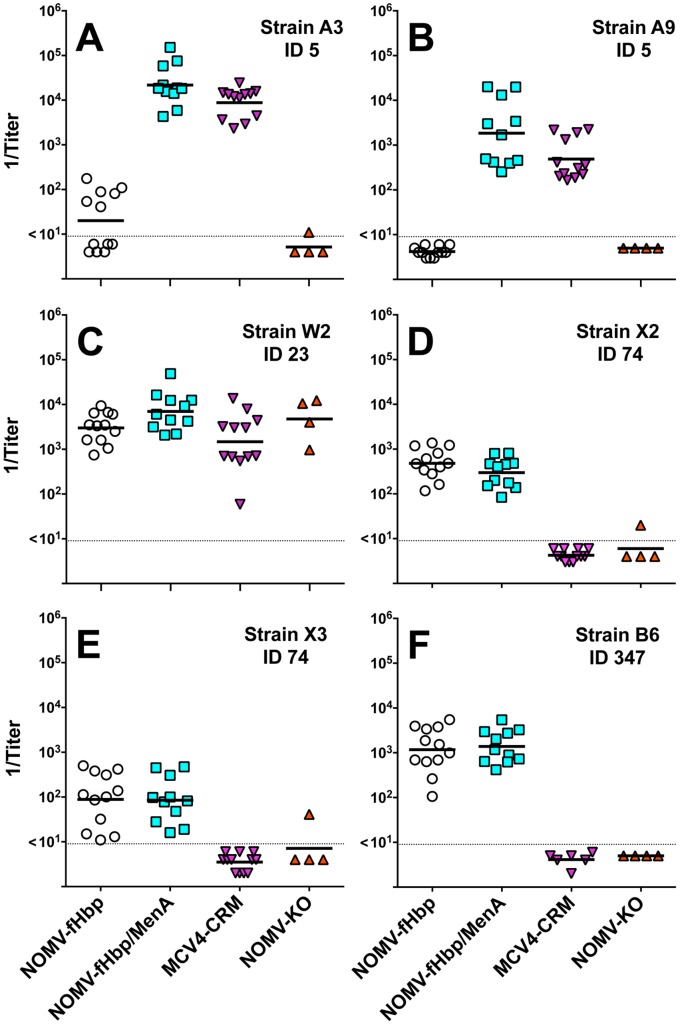
We chose strains A3 and A9 to assess MenA bactericidal responses because both strains were resistant to bactericidal activity of antibodies elicited by the NOMV-fHbp vaccine alone (GMT <1∶10, Table 1). For MenW, we chose strain W2 with fHbp ID 23 in variant group 2. Since the fHbp of this strain was in a different variant group than the variant group 1 fHbp antigen in the NOMV-fHbp vaccine, the bactericidal antibody responses largely reflected antibodies to non-fHbp antigens in the NOMV (the largest contributor likely being PorA). The serum bactericidal titers elicited by the combination NOMV-fHbp/MenA conjugate vaccine against these MenA or W strains were higher than to the NOMV-fHbp alone. (Figure 7, Panels A, B, and C, P<0.03). The serum bactericidal titers elicited by the combination NOMV-fHbp/MenA conjugate vaccine also were higher than the respective titers elicited by the control MCV4-CRM conjugate vaccine (P<0.03).
Figure 7. Serum bactericidal antibody responses of mice immunized with a combination NOMV-fHbp/MenA conjugate vaccine.
Test strains are described in Table 1, and strain selection criteria are described in the text. Panels A and B, MenA strains A3 and A9 with fHbp ID 5, respectively in variant group 1. Panel C, MenW strain, W2, with fHbp ID 23 in variant group 2. Panels D and E, MenX strains, X2 or X3, respectively, with fHbp ID 74 in variant group 1. Panel F, MenB strain, B6, from The Gambia, with fHbp ID 347 in variant group 1. Each symbol represents the reciprocal titer of an individual mouse; horizontal bars represent 1/GMT. The respective serum bactericidal titers elicited by the combination NOMV-fHbp/MenA vaccine were higher than the NOMV-fHbp alone against strains A3 and A9 (P<0.0001) and strain W2 (P = 0.03). For these three strains, the serum bactericidal titers elicited by the combination NOMV-fHbp/MenA conjugate vaccine also were higher than the respective titers elicited by control MCV4-CRM vaccine (P<0.03). For the two MenX strains and the MenB strain, the respective titers elicited by the combination NOMV-fHbp/MenA conjugate vaccine were not significantly different from those elicited by the NOMV-fHbp vaccine alone (P>0.14).
We also measured serum bactericidal titers against two representative MenX strains with fHbp ID 74 in variant group 1 (X2 and X3, Panels D and E, respectively), and a MenB strain from The Gambia with fHbp ID 347 in variant group 1 (B6, Panel F). These three strains had heterologous PorA to the vaccine strain and the serum bactericidal activity largely reflected antibody to fHbp. For all three strains, the respective titers elicited by the combination vaccine were not significantly different from the NOMV-fHbp vaccine alone P>0.14). As expected, none of the MenX or MenB strains was killed by complement together with sera from control mice immunized with the MCV4-CRM conjugate vaccine.
Discussion
The MenA conjugate vaccine, MenAfriVac, introduced in sub-Sahara is safe and highly immunogenic [9] but coverage does not include MenW or MenX strains, which cause epidemics in this region [13]–[15]. One approach to expand protection to these serogroups would be a multivalent polysaccharide-protein conjugate vaccine, which also might include MenC and/or MenY. The technical feasibility of developing a multivalent meningococcal conjugate vaccine has been demonstrated by commercially available quadrivalent MenA,C,Y,W conjugate vaccines [16]–[19]. Vaccine grade MenX polysaccharide has been characterized [64], and favorable preliminary data on experimental MenX polysaccharide-protein conjugate vaccines have been presented (Romano, MR et al. Poster 150, XVIIIth International Pathogenic Neisseria Conference. Würzburg, Germany, September 2012, http://www.conventus.de/fileadmin/media/2012/ipnc/IPNC2012_Programme_and_Abstractbook_web.pdf). The cost of production, of a multivalent conjugate vaccine that includes MenA, W and X and, possibly, additional polysaccharides, is likely to be higher than the 1 US dollar per dose price point, which was considered essential for having a sustainable meningococcal vaccine immunization program in sub-Sahara [5], [65]. Also, the technical challenges required for development and licensure of a multivalent conjugate vaccine for Africa are greater than for a monovalent MenA conjugate vaccine. Specifically there is a high risk that one of the components of the multivalent conjugate will have suboptimal immunogenicity and require adjustment of dosage or formulation [66]–[68]. There also may be interference in immunogenicity between the individual components of the vaccine (the more components, the higher the risk; for a review please see [69]). With a multivalent conjugate vaccine there also is a risk that strains with serogroups not contained in the vaccine (for example, MenB), might emerge in Africa and cause epidemics. While the time required for development and licensure of a NOMV-fHbp/MenA conjugate vaccine also will be long, and the regulatory issues will be challenging, the uncertainties accompanying development of a multivalent conjugate vaccine underscore the need to investigate alternative vaccine approaches for preventing non-MenA epidemics in Africa.
Conventional detergent-treated outer membrane vesicle (dOMV) vaccines have been developed for prevention of MenB disease. The detergent treatment is used to decrease endotoxin activity but also extracts desirable antigens such fHbp [36]. dOMV vaccines have been given safely to millions of children and young adults [70], and have been demonstrated to be effective for control of epidemics caused by MenB strains [71], [72]. Recent pre-clinical studies also demonstrated the feasibility of developing a dOMV vaccine for prevention of MenA and MenW disease [73]. A major limitation of dOMV vaccines, however, is that the protective antibodies in infants and childrens are largely directed against PorA [74], which is antigenically variable [75], [76]. A dOMV vaccine for prevention of the main epidemic clones in the meningitis belt will need to include PorA antigens from prevalent MenA, MenW and MenX strains. While the PorA VR types of some of these clones (such as MenA) have been stable over the last decade [46], [77]–[79], dOMV vaccines likely will require frequent substitutions of the vaccine strains as the PorA of emerging strains changes over-time [80].
An important difference between NOMV and dOMV vaccines is that endotoxin activity of NOMV vaccines is decreased genetically by inactivating a gene (lpxL1) in lipid biosynthesis, which avoids the need for detergent treatment of the vesicles in the vaccine. In a phase 1 study in adults, a prototype meningococcal NOMV vaccine prepared from an lpxL1 knock-out strain appeared to be well tolerated [81]. The vaccine strain used to prepare an NOMV vaccine also can contain additional genetic modifications to increase expression of desirable antigens to enhance induction of protective antibodies, or deletion of unwanted molecules to increase safety, and/or lower to production costs (for example, by inactivating gna33, which increases release of vesicles from the bacteria [82]). NOMV vaccines also can elicit protective antibodies against more than one antigenic target, which may delay emergence of escape mutants.
In the present study, the NOMV-fHbp vaccine prepared from the mutant strain elicited broad serum bactericidal activity against a panel of Africa epidemic MenA, W and X strains and genetically diverse MenB strains with fHbp in variant group 1 from Norway, New Zealand, the U.S. and The Gambia. Of the 38 strains tested, two MenA strains were resistant to bactericidal activity of the NOMV-fHbp antisera (GMT <1∶10). By flow cytometry, the resistant strains had similar fHbp expression levels as MenA strains susceptible to anti-fHbp bactericidal activity (unpublished data). The basis of the resistance is therefore not known and is currently being investigated. This observation, however, together with our long-term goal to build upon the recent successful introduction of a MenA conjugate vaccine in Africa, suggested that combining the NOMV-fHbp vaccine with a MenA conjugate vaccine could augment protection against MenA strains. In mice, the resulting combination NOMV-fHbp/MenA conjugate vaccine elicited serum antibodies with greater bactericidal activity against MenA and W strains than the control MCV4-CRM vaccine. The combination NOMV-fHbp/MenA conjugate vaccine also provided coverage against MenX and B strains, which were not covered by the licensed meningococcal conjugate vaccine.
The reasons for the higher serum bactericidal antibody responses against the serogroup A and W strains in the NOMV-fHbp/MenA conjugate vaccine group, compared with the MCV4-CRM vaccine group, are not known. Conceivably, natural adjuvants in the NOMV-fHbp component such as PorB [83], [84] or LOS [49], [85] contributed to the higher antibody responses. An additional possibility is that anti-fHbp antibodies elicited by the NOMV-fHbp vaccine component acted synergistically with MenA anticapsular antibodies elicited by the MenA conjugate and resulted in higher MenA bactericidal activity than with either antibody individually [86]. Further studies are needed to elucidate the mechanism. Collectively, our results demonstrate that a combined NOMV-fHbp/MenA conjugate vaccine can provide broad coverage against African MenA, X and W strains, which are the principal causes of epidemics in the region, and also against MenB strains. Most likely the NOMV-fHbp/MenA vaccine would require two or three doses for maximum protection, which is compatible for routine vaccination of infants and toddlers, age groups at high risk for developing meningococcal disease. During epidemics, a single dose of the combination vaccine also could be used to boost immunity in older children or adults previously given the vaccine.
Limitations of the Study
The African continent is vast, and the epidemiology of meningococcal disease is complex with major seasonal and temporal changes in incidence rates, and geographical differences in strain prevalence, which remain poorly understood [1], [46], [77], [87], [88]. Further, the number of isolates investigated from different epidemics represents only a small fraction of the total number of cases [46]. Our previous data from studies of MenA strains collected from epidemics spanning 50 years indicated that only two fHbp variants, ID 4 or 5, which differed from each other by only one amino acid, were present in all isolates. The available data, however, on fHbp sequence variants of MenX and MenW isolates from Africa are more limited and it is possible that there is greater fHbp diversity among strains with these serogroups than MenA strains. Thus, for a broadly protective NOMV-fHbp vaccine intended for all countries in sub-Sahara, additional strain characterization and epidemiology data are needed, particularly for MenX and MenW strains. It is also is possible that additional fHbp sequence variants and/or other antigens might be needed in the NOMV vaccine to ensure optimal coverage, particularly against MenB strains with fHbp in variant groups 2 or 3, should serogroup B strains emerge to cause epidemics in Africa.
A second limitation of our study was the small number of isolates tested for bactericidal activity using sera from individual mice immunized with the NOMV-fHbp/MenA conjugate vaccine (Figure 7). However, we tested susceptibility of a much larger strain panel using pooled serum from immunized mice (33 isolates from 13 different Sub-Saharan countries, and South Africa, including 8 different clonal complexes, Table 1). All but two of the group A strains were susceptible to bactericidal activity of pooled antisera from mice immunized with the NOMV-fHbp vaccine. Further, these two serogroup A strains, A3 and A9, were tested for bactericidal activity of sera from individual mice immunized with the NOMV-fHbp/MenA conjugate combination vaccine and were highly susceptible.
A third limitation of our study is lack of information on whether or not NOMV-fHbp immunization will decrease asymptomatic carriage and transmission of the N. meningitidis in the population. One of the strengths of polysaccharide conjugate vaccines is their ability to induce herd immunity by decreasing asymptomatic carriage and transmission in the population [12], [89]. This question is important for an NOMV-fHbp vaccine for Africa, but is difficult to investigate experimentally in mice because receptors that are important for nasopharyngeal colonization of N. meningitidis, such as CEACAM, are human specific [90], [91].
For testing proof of principal of a combination NOMV-fHbp/MenA conjugate vaccine, we used a lyophilized MenA conjugate vaccine from Novartis, which is likely to be too expensive for inclusion in a vaccine intended for Africa. The feasibility of combining the NOMV-fHbp vaccine with the lyophilized MenA conjugate vaccine being used in Africa (MenAfriVac), therefore should be investigated. For our study with the Novartis MCV4-CRM conjugate vaccine we used one-tenth of the human dose in the mice. Although this dose elicited high titers of serum anticapsular antibodies (Figure 6), the dose may not have been optimally immunogenic. For this reason the superior serogroup A and W bactericidal antibody responses elicited by the NOMV-fHbp/MenA conjugate vaccine when compared to the MCV4-CRM vaccine, needs to be interpreted with caution.
The cost of production of an NOMV-fHbp vaccine currently is uncertain and will depend on the growth characteristics of the final mutant strain selected and large scale manufacturing processes that will need to be developed. Low cost dOMV-based vaccines, however, have been manufactured in Cuba and Brazil, and the processes used are within the technical abilities of developing country manufacturers. Finally, a vaccine intended for Africa must be conveniently administered and stable under stressful environmental conditions. Our studies did not investigate these questions, which will require additional studies. Despite these limitations, the excellent breadth of protective antibodies elicited in mice by the prototype NOMV-fHbp/MenA conjugate vaccine investigated in the present study provide ample justification for conducting additional studies to address these technical questions as well as advancing the vaccine for testing proof of principle in a human vaccine trial.
Supporting Information
Network analysis of prominent individual fHbp sequence variants of meningococcal isolates from Africa. The analysis was generated using SplitsTree, version 4.0 (http://www.splitstree.org/). Data shown are for the five predominant fHbp sequence variants and two related sequences with one amino acid differences, from 124 fHbp African isolates investigated in a previous study [42]. The network, using the hamming distance, represents how similar (closer in the network) or different (far) are the fHbp sequences present among African isolates. The scale bar refers to 0.01 differences per unit of length. Numbers represent the specific fHbp ID designation for each sequence. ID 4 and 5 and found among serogroup A isolates; ID 73 and ID 74 are found among serogroup X isolates, and ID 9, ID 22 and ID 23 are found among serogroup W isolates. Note that fHbp ID 4 and ID 5 differ from each other by one amino acid, and fHbp ID 22 and ID 23 differ from each other by one amino acid.
(DOCX)
Detection of major proteins in NOMV vaccines. Major proteins in NOMV vaccines as visualized by Coomassie-stained SDS-PAGE. rfHbp, recombinant fHbp ID 9 control. OE, NOMV-fHbp vaccine with over-expressed R41S mutant fHbp prepared from the mutant vaccine strain with Δlpxl1 and Δcapsule; KO, NOMV prepared from a triple knockout (ΔfHbp, Δlpxl1, and Δcapsule). A total of 5 µg of NOMV was loaded in each lane, and 0.25 µg of the recombinant fHbp.
(DOCX)
Comparison of serum bactericidal antibody responses of mice immunized with NOMV-fHbp or NOMV-fHbp KO vaccines. Serum bactericidal antibody responses were measured against seven serogroup W isolates with porA VR types related to the PorA contained in both NOMV vaccines. The antibody responses elicited by the NOMV-fHbp KO vaccine are mostly directed at PorA. Bars represent the reciprocal GMT of two or three serum pools. Orange bars, control mice immunized with an NOMV vaccine prepared from the mutant vaccine strain with Δlpxl1 and Δcapsule in which the gene for fHbp had been inactivated; white bars, mice immunized with the NOMV vaccine with Δlpxl1 and Δcapsule and over-expressed fHbp. There were no significant differences in bactericidal responses elicited by two vaccines (respective GMTs of 1053 vs. 1139, P>0.8 by paired T test).
(DOCX)
Acknowledgments
We are grateful to the following individuals at Children’s Hospital Oakland Research Institute for important contributions to this study. Peter Beernink, PhD, and Greg Moe, PhD provided helpful discussions and critical review of the manuscript. Emily Braga, Serena Giuntini, PhD, and David Vu, MD, provided expert technical assistance.
Funding Statement
This work was supported by Public Health Service grants R01 AI 046464 and AI 082263 (to D.M.G.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). D.M.G. also is supported in part by an endowment established by the Clorox Company. The laboratory work at Children’s Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant C06 RR 016226 from the National Center for Research Resources, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Greenwood B (1999) Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 93: 341–353. [DOI] [PubMed] [Google Scholar]
- 2. Harrison LH (2010) Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis 50 Suppl 2S37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, et al. (2010) Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 50: 184–191. [DOI] [PubMed] [Google Scholar]
- 4. Harrison LH, Trotter CL, Ramsay ME (2009) Global epidemiology of meningococcal disease. Vaccine 27 Suppl 2B51–63. [DOI] [PubMed] [Google Scholar]
- 5. Jodar L, LaForce FM, Ceccarini C, Aguado T, Granoff DM (2003) Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 361: 1902–1904. [DOI] [PubMed] [Google Scholar]
- 6. Lee CH, Kuo WC, Beri S, Kapre S, Joshi JS, et al. (2009) Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine 27: 726–732. [DOI] [PubMed] [Google Scholar]
- 7. Bishai DM, Champion C, Steele ME, Thompson L (2011) Product development partnerships hit their stride: lessons from developing a meningitis vaccine for Africa. Health affairs 30: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 8. Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S (2009) Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine 27 Suppl 2B13–19. [DOI] [PubMed] [Google Scholar]
- 9. Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, et al. (2011) Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med 364: 2293–2304. [DOI] [PubMed] [Google Scholar]
- 10. Djingarey MH, Barry R, Bonkoungou M, Tiendrebeogo S, Sebgo R, et al. (2012) Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine 30 Suppl 2B40–45. [DOI] [PubMed] [Google Scholar]
- 11. Novak RT, Kambou JL, Diomande FV, Tarbangdo TF, Ouedraogo-Traore R, et al. (2012) Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis 12: 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, et al.. (2012) Impact of the Serogroup A Meningococcal Conjugate Vaccine, MenAfriVac, on Carriage and Herd Immunity. Clin Infect Dis DOI 10.1093/cid/cis892 [DOI] [PubMed]
- 13. Mueller JE, Borrow R, Gessner BD (2006) Meningococcal serogroup W135 in the African meningitis belt: epidemiology, immunity and vaccines. Expert Rev Vaccines 5: 319–336. [DOI] [PubMed] [Google Scholar]
- 14. Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, et al. (2007) Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis 44: 657–663. [DOI] [PubMed] [Google Scholar]
- 15. Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, et al. (2012) The changing and dynamic epidemiology of meningococcal disease. Vaccine 30 Suppl 2B26–36. [DOI] [PubMed] [Google Scholar]
- 16. Pace D (2009) MenACWY-CRM, a novel quadrivalent glycoconjugate vaccine against Neisseria meningitidis for the prevention of meningococcal infection. Curr Opin Mol Ther 11: 692–706. [PubMed] [Google Scholar]
- 17. Gasparini R, Panatto D (2011) Meningococcal glycoconjugate vaccines. Hum Vaccin 7: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison LH, Mohan N, Kirkpatrick P (2010) Meningococcal group A, C, Y and W-135 conjugate vaccine. Nat Rev Drug Discov 9: 429–430. [DOI] [PubMed] [Google Scholar]
- 19. Harrison LH (2008) A multivalent conjugate vaccine for prevention of meningococcal disease in infants. JAMA 299: 217–219. [DOI] [PubMed] [Google Scholar]
- 20. Finne J, Leinonen M, Makela PH (1983) Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 2: 355–357. [DOI] [PubMed] [Google Scholar]
- 21. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, et al. (2003) Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 197: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, et al. (2004) Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 72: 2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, et al. (2006) The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 177: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welsch JA, Ram S (2008) Factor H and Neisserial pathogenesis. Vaccine 26 Suppl 8I40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mothibeli KM, du Plessis M, von Gottberg A, Murphy E, Hoiseth SK, et al. (2011) Distribution of factor H binding protein beyond serogroup B: variation among five serogroups of invasive Neisseria meningitidis in South Africa. Vaccine 29: 2187–2192. [DOI] [PubMed] [Google Scholar]
- 26. Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, et al. (2009) Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis . J Infect Dis 200: 379–389. [DOI] [PubMed] [Google Scholar]
- 27. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, et al. (2010) Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 51: 1127–1137. [DOI] [PubMed] [Google Scholar]
- 28. Snape MD, Dawson T, Oster P, Evans A, John TM, et al. (2010) Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J 29: e71–79. [DOI] [PubMed] [Google Scholar]
- 29. Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, et al. (2012) A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: Results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 30: 6163–6174. [DOI] [PubMed] [Google Scholar]
- 30. Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, et al. (2012) Safety and Immunogenicity of a Meningococcal B Bivalent rLP2086 Vaccine in Healthy Toddlers Aged 18–36 Months: A Phase 1 Randomized-controlled Clinical Trial. Pediatr Infect Dis J 31: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 31. Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, et al. (2012) Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 12: 597–607. [DOI] [PubMed] [Google Scholar]
- 32.Granoff DM (2012) European Medicines Agency Recommends Approval of a Broadly Protective Vaccine against Serogroup B Meningococcal Disease. Pediatr Infect Dis J DOI: 10.1097/INF.0b013e318282942f [DOI] [PubMed]
- 33. Scarselli M, Arico B, Brunelli B, Savino S, Di Marcello F, et al. (2011) Rational design of a meningococcal antigen inducing broad protective immunity. Sci Transl Med 3: 91ra62. [DOI] [PubMed] [Google Scholar]
- 34. Beernink PT, Granoff DM (2008) Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect Immun 76: 2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, et al. (2006) A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 103: 10834–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koeberling O, Seubert A, Granoff DM (2008) Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis 198: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, et al. (2011) Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29: 4728–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pajon R, Beernink PT, Harrison LH, Granoff DM (2010) Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 28: 2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seib KL, Brunelli B, Brogioni B, Palumbo E, Bambini S, et al. (2011) Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infect Immun 79: 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brunelli B, Del Tordello E, Palumbo E, Biolchi A, Bambini S, et al. (2011) Influence of sequence variability on bactericidal activity sera induced by Factor H binding protein variant 1.1. Vaccine 29: 1072–1081. [DOI] [PubMed] [Google Scholar]
- 41. Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, et al. (2010) Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28: 6086–6093. [DOI] [PubMed] [Google Scholar]
- 42. Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM (2011) Meningococcal factor h binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl Trop Dis 5: e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, et al. (2012) The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog 8: e1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, et al. (2011) A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186: 3606–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pajon R, Beernink PT, Granoff DM (2012) Design of meningococcal factor H binding protein mutant vaccines that do not bind human complement factor H. Infect Immun. 80: 2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caugant DA, Kristiansen PA, Wang X, Mayer LW, Taha MK, et al. (2012) Molecular Characterization of Invasive Meningococcal Isolates from Countries in the African Meningitis Belt before Introduction of a Serogroup A Conjugate Vaccine. PLoS One 7: e46019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beernink PT, LoPasso C, Angiolillo A, Felici F, Granoff D (2009) A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol Immunol 46: 1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giuntini S, Reason DC, Granoff DM (2011) Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 79: 3751–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steeghs L, Tommassen J, Leusen JH, van de Winkel JG, van der Ley P (2004) Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J Endotoxin Res 10: 113–119. [DOI] [PubMed] [Google Scholar]
- 50. Koeberling O, Giuntini S, Seubert A, Granoff DM (2009) Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol 16: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zollinger WD, Donets MA, Schmiel DH, Pinto VB, Labrie J, et al. (2010) Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine 28: 5057–5067. [DOI] [PubMed] [Google Scholar]
- 52. Findlow H, Sow S, Borrow R, Tapia M, Haidara FC, et al. (2011) Meningococcal group C and W135 immunological hyporesponsiveness in african toddlers. Clin Vaccine Immunol 18: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, et al. (2005) Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med 159: 907–913. [DOI] [PubMed] [Google Scholar]
- 54. Welsch JA, Rossi R, Comanducci M, Granoff DM (2004) Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol 172: 5606–5615. [DOI] [PubMed] [Google Scholar]
- 55. Granoff DM, Maslanka SE, Carlone GM, Plikaytis BD, Santos GF, et al. (1998) A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol 5: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kogan G, Uhrin D, Brisson JR, Jennings HJ (1997) Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr Res 298: 191–199. [DOI] [PubMed] [Google Scholar]
- 57. Tsang RS, Law DK, Tsai C, Ng L (2001) Detection of the lst gene in different serogroups and LOS immunotypes of Neisseria meningitidis . FEMS Microbiol Lett 199: 203–206. [DOI] [PubMed] [Google Scholar]
- 58. Smith H, Parsons NJ, Cole JA (1995) Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog 19: 365–377. [DOI] [PubMed] [Google Scholar]
- 59. Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM (2009) Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J Infect Dis 199: 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang JF, Caugant DA, Li X, Hu X, Poolman JT, et al. (1992) Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People’s Republic of China. Infect Immun 60: 5267–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dempsey JA, Wallace AB, Cannon JG (1995) The physical map of the chromosome of a serogroup A strain of Neisseria meningitidis shows complex rearrangements relative to the chromosomes of the two mapped strains of the closely related species N. gonorrhoeae . J Bacteriol 177: 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koeberling O, Delany I, Granoff DM (2011) A critical threshold of meningococcal factor h binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol 18: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, et al. (2010) The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog 6: e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie O, Bolgiano B, Gao F, Lockyer K, Swann C, et al. (2012) Characterization of size, structure and purity of serogroup X Neisseria meningitidis polysaccharide, and development of an assay for quantification of human antibodies. Vaccine 30: 5812–5823. [DOI] [PubMed] [Google Scholar]
- 65. LaForce FM, Konde K, Viviani S, Preziosi MP (2007) The Meningitis Vaccine Project. Vaccine 25 Suppl 1A97–100. [DOI] [PubMed] [Google Scholar]
- 66. Broker M, Dull PM, Rappuoli R, Costantino P (2009) Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine 27: 5574–5580. [DOI] [PubMed] [Google Scholar]
- 67.Jackson LA, Jacobson RM, Reisinger KS, Anemona A, Danzig LE, et al.. (2009) A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J: 86–91. [DOI] [PubMed] [Google Scholar]
- 68. Perrett KP, Snape MD, Ford KJ, John TM, Yu LM, et al. (2009) Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J 28: 186–193. [DOI] [PubMed] [Google Scholar]
- 69. Dagan R, Poolman J, Siegrist CA (2010) Glycoconjugate vaccines and immune interference: A review. Vaccine 28: 5513–5523. [DOI] [PubMed] [Google Scholar]
- 70. Nokleby H, Aavitsland P, O’Hallahan J, Feiring B, Tilman S, et al. (2007) Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine 25: 3080–3084. [DOI] [PubMed] [Google Scholar]
- 71.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, et al.. (1991) Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14: 195–207; discussion 208–110. [PubMed] [Google Scholar]
- 72. Galloway Y, Stehr-Green P, McNicholas A, O’Hallahan J (2009) Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol 38: 413–418. [DOI] [PubMed] [Google Scholar]
- 73. Norheim G, Tunheim G, Naess LM, Kristiansen PA, Caugant DA, et al. (2012) An outer membrane vesicle vaccine for prevention of serogroup A and W-135 meningococcal disease in the African meningitis belt. Scand J Immunol 76: 99–107. [DOI] [PubMed] [Google Scholar]
- 74. Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, et al. (1999) Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281: 1520–1527. [DOI] [PubMed] [Google Scholar]
- 75. Suker J, Feavers IM, Achtman M, Morelli G, Wang JF, et al. (1994) The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol 12: 253–265. [DOI] [PubMed] [Google Scholar]
- 76. Feavers IM, Heath AB, Bygraves JA, Maiden MC (1992) Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis . Mol Microbiol 6: 489–495. [DOI] [PubMed] [Google Scholar]
- 77. Caugant DA, Nicolas P (2007) Molecular surveillance of meningococcal meningitis in Africa. Vaccine 25 Suppl 1A8–11. [DOI] [PubMed] [Google Scholar]
- 78. Norheim G, Rosenqvist E, Aseffa A, Yassin MA, Mengistu G, et al. (2006) Characterization of Neisseria meningitidis isolates from recent outbreaks in Ethiopia and comparison with those recovered during the epidemic of 1988 to 1989. J Clin Microbiol 44: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nicolas P, Norheim G, Garnotel E, Djibo S, Caugant DA (2005) Molecular epidemiology of Neisseria meningitidis isolated in the African Meningitis Belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J Clin Microbiol 43: 5129–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jelfs J, Munro R, Wedege E, Caugant DA (2000) Sequence variation in the porA gene of a clone of Neisseria meningitidis during epidemic spread. Clin Diagn Lab Immunol 7: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, et al. (2011) A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29: 1423–1420. [DOI] [PubMed] [Google Scholar]
- 82. Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, et al. (2006) Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6: 1856–1866. [DOI] [PubMed] [Google Scholar]
- 83. Chiavolini D, Weir S, Murphy JR, Wetzler LM (2008) Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the capacity of Francisella tularensis lipopolysaccharide to protect mice against experimental tularemia. Clin Vaccine Immunol 15: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wetzler LM (2010) Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol 5: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zughaier S, Steeghs L, van der Ley P, Stephens DS (2007) TLR4-dependent adjuvant activity of Neisseria meningitidis lipid A. Vaccine. 25: 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vu DM, Wong TT, Granoff DM (2011) Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and Neisserial heparin binding antigen. Vaccine 29: 1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Greenwood B (2007) The changing face of meningococcal disease in West Africa. Epidemiol Infect 135: 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stephens DS, Greenwood B, Brandtzaeg P (2007) Epidemic meningitis, meningococcaemia, and Neisseria meningitidis . Lancet 369: 2196–2210. [DOI] [PubMed] [Google Scholar]
- 89. Ibarz-Pavon AB, Morais L, Sigauque B, Mandomando I, Bassat Q, et al. (2011) Epidemiology, molecular characterization and antibiotic resistance of Neisseria meningitidis from patients </ = 15 years in Manhica, rural Mozambique. PLoS One 6: e19717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gray-Owen SD (2003) Neisserial Opa proteins: impact on colonization, dissemination and immunity. Scand J Infect Dis 35: 614–618. [DOI] [PubMed] [Google Scholar]
- 91. Popp A, Dehio C, Grunert F, Meyer TF, Gray-Owen SD (1999) Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol 1: 169–181. [DOI] [PubMed] [Google Scholar]
- 92. Poolman JT, Kriz-Kuzemenska P, Ashton F, Bibb W, Dankert J, et al. (1995) Serotypes and subtypes of Neisseria meningitidis: results of an international study comparing sensitivities and specificities of monoclonal antibodies. Clin Diagn Lab Immunol 2: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, et al. (1998) Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jolley KA, Maiden MC (2010) BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Network analysis of prominent individual fHbp sequence variants of meningococcal isolates from Africa. The analysis was generated using SplitsTree, version 4.0 (http://www.splitstree.org/). Data shown are for the five predominant fHbp sequence variants and two related sequences with one amino acid differences, from 124 fHbp African isolates investigated in a previous study [42]. The network, using the hamming distance, represents how similar (closer in the network) or different (far) are the fHbp sequences present among African isolates. The scale bar refers to 0.01 differences per unit of length. Numbers represent the specific fHbp ID designation for each sequence. ID 4 and 5 and found among serogroup A isolates; ID 73 and ID 74 are found among serogroup X isolates, and ID 9, ID 22 and ID 23 are found among serogroup W isolates. Note that fHbp ID 4 and ID 5 differ from each other by one amino acid, and fHbp ID 22 and ID 23 differ from each other by one amino acid.
(DOCX)
Detection of major proteins in NOMV vaccines. Major proteins in NOMV vaccines as visualized by Coomassie-stained SDS-PAGE. rfHbp, recombinant fHbp ID 9 control. OE, NOMV-fHbp vaccine with over-expressed R41S mutant fHbp prepared from the mutant vaccine strain with Δlpxl1 and Δcapsule; KO, NOMV prepared from a triple knockout (ΔfHbp, Δlpxl1, and Δcapsule). A total of 5 µg of NOMV was loaded in each lane, and 0.25 µg of the recombinant fHbp.
(DOCX)
Comparison of serum bactericidal antibody responses of mice immunized with NOMV-fHbp or NOMV-fHbp KO vaccines. Serum bactericidal antibody responses were measured against seven serogroup W isolates with porA VR types related to the PorA contained in both NOMV vaccines. The antibody responses elicited by the NOMV-fHbp KO vaccine are mostly directed at PorA. Bars represent the reciprocal GMT of two or three serum pools. Orange bars, control mice immunized with an NOMV vaccine prepared from the mutant vaccine strain with Δlpxl1 and Δcapsule in which the gene for fHbp had been inactivated; white bars, mice immunized with the NOMV vaccine with Δlpxl1 and Δcapsule and over-expressed fHbp. There were no significant differences in bactericidal responses elicited by two vaccines (respective GMTs of 1053 vs. 1139, P>0.8 by paired T test).
(DOCX)