Abstract
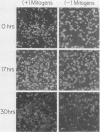
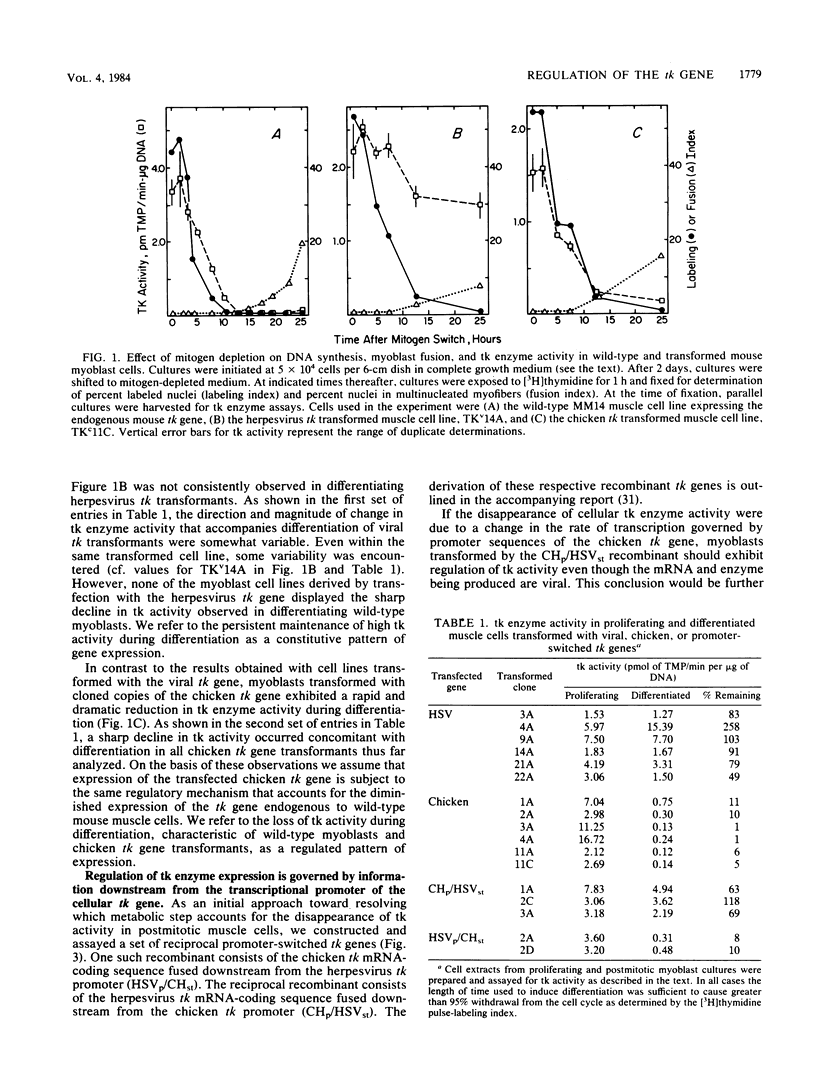
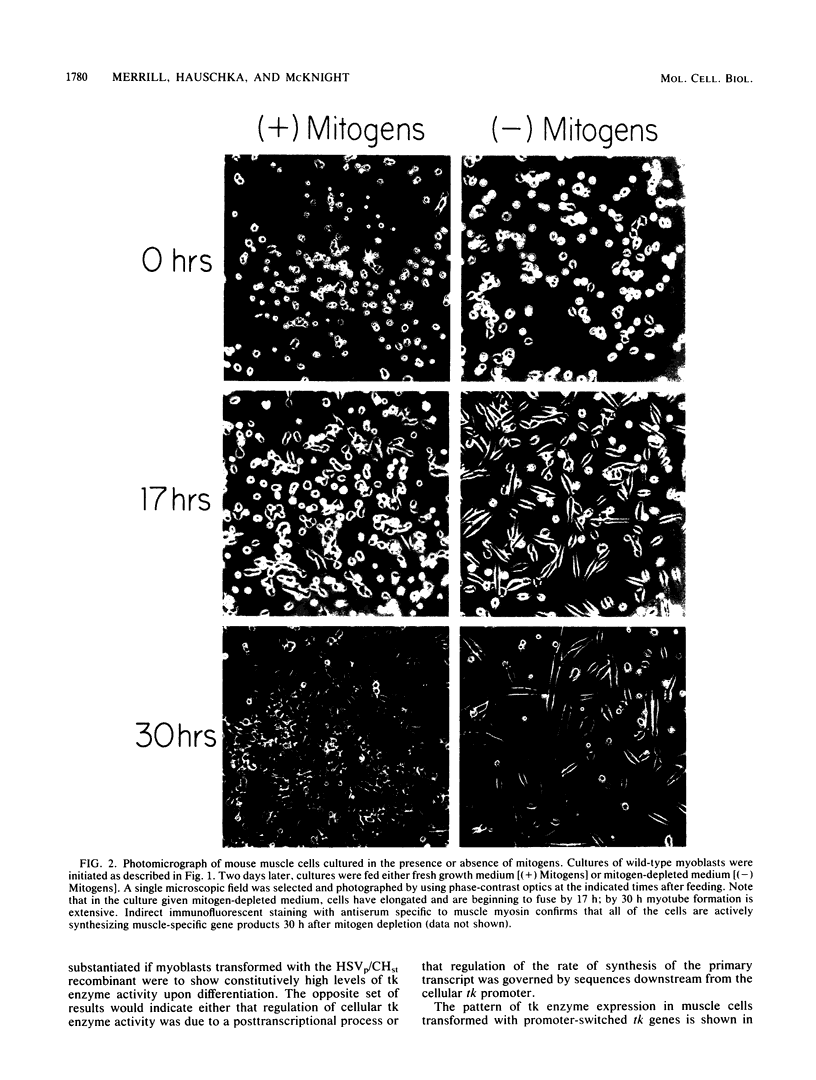
Thymidine kinase (tk) enzyme expression is shut down when cultured skeletal muscle cells terminally differentiate. This regulation is mediated by a rapid and specific decline in the abundance of cellular tk mRNA. tk-deficient mouse myoblasts were transformed to the tk-positive phenotype by using both the cellular tk gene of the chicken and the herpesvirus tk gene. Myoblasts transformed with the cellular tk gene effectively regulate tk enzyme activity upon terminal differentiation. Conversely, myoblasts transformed with the herpesvirus tk gene continue to express tk enzyme activity in postreplicative muscle cells. A regulated pattern of expression is retained when the promoter of the cellular tk gene is replaced by the promoter of the herpesvirus tk gene. Moreover, the cellular tk gene is appropriately regulated during terminal muscle differentiation when its 3' terminus is removed and replaced by the terminus of the viral tk gene. Thus, the element of the cellular tk gene sufficient to specify its regulation is entirely intragenic.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr Molecular cloning and cell cycle-specific regulation of a functional human thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5588–5591. doi: 10.1073/pnas.80.18.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Periodicity of DNA synthetic enzymes during the HeLa cell cycle. Cell Tissue Kinet. 1971 Jul;4(4):297–305. doi: 10.1111/j.1365-2184.1971.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guespin-Michel J. F., Menahem M., Monier F., Felenbok B. Kinetic of 5-bromodeoxyuridine action on Dictyostelium discoideum growth and development. Exp Cell Res. 1976 Mar 1;98(1):184–190. doi: 10.1016/0014-4827(76)90478-x. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Kressman A., Birnstiel M. L. A split promoter for a eucaryotic tRNA gene. Cell. 1981 May;24(2):573–585. doi: 10.1016/0092-8674(81)90348-2. [DOI] [PubMed] [Google Scholar]
- Howard D. K., Hay J., Melvin W. T., Durham J. P. Changes in DNA and RNA synthesis and associated enzyme activities after the stimulation of serum-depleted BHK21-C13 cells by the addition of serum. Exp Cell Res. 1974 May;86(1):31–42. doi: 10.1016/0014-4827(74)90643-0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Growth-dependent expression of dihydrofolate reductase mRNA from modular cDNA genes. Mol Cell Biol. 1983 Sep;3(9):1598–1608. doi: 10.1128/mcb.3.9.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg I. R. Diffusion-mediated control of myoblast fusion. Dev Biol. 1971 Sep;26(1):133–152. doi: 10.1016/0012-1606(71)90113-8. [DOI] [PubMed] [Google Scholar]
- Kwoh T. J., Zipser D., Wigler M. Mutational analysis of the cloned chicken thymidine kinase gene. J Mol Appl Genet. 1983;2(2):191–200. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Landy-Otsuka F., Scheffler I. E. Enzyme induction in a temperature-sensitive cell cycle mutant of Chinese hamster fibroblasts. J Cell Physiol. 1980 Nov;105(2):209–220. doi: 10.1002/jcp.1041050204. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Shimizu K., Zipser D. Isolation and preliminary characterization of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1983 Oct;3(10):1815–1823. doi: 10.1128/mcb.3.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. W., Hauschka S. D. A rapid decrease in epidermal growth factor-binding capacity accompanies the terminal differentiation of mouse myoblasts in vitro. J Cell Biol. 1984 Feb;98(2):739–747. doi: 10.1083/jcb.98.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschka S. D. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struct. 1980;14(4):483–498. doi: 10.1002/jss.400140407. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Harland R. M., Groudine M., McKnight S. L. Genetic and physical analysis of the chicken tk gene. Mol Cell Biol. 1984 Sep;4(9):1769–1776. doi: 10.1128/mcb.4.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Witter E. B., Hauschka S. D. Differentiation of thymidine kinase deficient mouse myoblasts in the presence of 5'-bromodeoxyuridine. Exp Cell Res. 1980 Sep;129(1):191–199. doi: 10.1016/0014-4827(80)90342-0. [DOI] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- O'Neill M., Strohman R. C. Changes in DNA polymerase activity associated with cell fusion in cultures of embryonic muscle. J Cell Physiol. 1969 Feb;73(1):61–68. doi: 10.1002/jcp.1040730109. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Hereford L. Identification of a sequence responsible for periodic synthesis of yeast histone 2A mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7689–7693. doi: 10.1073/pnas.79.24.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980 Nov;22(2 Pt 2):629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Turner M. K., Abrams R., Lieberman I. Levels of ribonucleotide reductase activity during the division cycle of the L cell. J Biol Chem. 1968 Jul 10;243(13):3725–3728. [PubMed] [Google Scholar]
- WESSELLS N. K. DNA SYNTHESIS, MITOSIS, AND DIFFERENTIATION IN PANCREATIC ACINAR CELLS IN VITRO. J Cell Biol. 1964 Mar;20:415–433. doi: 10.1083/jcb.20.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Campbell G. L., Holtzer H. Identification of a developmental program using bromodeoxyuridine. J Mol Biol. 1972 Sep 28;70(2):337–350. doi: 10.1016/0022-2836(72)90543-8. [DOI] [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]