Abstract
Objective
Cardiovascular risk remains high despite statin use. Overweight/obese diabetic persons usually have normal/low LDL-cholesterol but high C-reactive protein (CRP) levels. We aimed to examine the effects of intensive lifestyle intervention for weight loss (ILI) on CRP levels in overweight/obese diabetic individuals by statin use.
Design and Methods
Look AHEAD was a randomized trial in overweight/obese type 2 diabetic individuals testing whether ILI would reduce cardiovascular mortality, when compared to usual care. We evaluated CRP changes in 1,431 participants with biomarker levels, who remained on or off statin treatment for 1-year.
Results
The reduction in CRP levels with ILI at 1 year in men and women on statins was −44.9 and −42.3 %, respectively, compared to −13.7 and −21.0 % for those on statins and usual care (p<0.0001). At 1 year, median CRP levels were: 1.8 mg/L in participants randomized to ILI on statin therapy; 2.6 mg/L for those on statins randomized to usual care and 2.9 mg/L for participants not on statins but randomized to ILI. Weight loss was associated with 1-year CRP reduction (p<0.0001) in statin and non-statin users.
Conclusions
Our findings suggest that in overweight/obese diabetic persons, ILI and statin therapy may have substantial additive anti-inflammatory benefits.
INTRODUCTION
Cardiovascular disease (CVD) is the major cause of morbidity and mortality in persons with type 2 diabetes. Statin therapy is standard of care in the majority of diabetic adults for the primary and secondary prevention of cardiovascular events (CVE) (1,2). However, the residual risk of a CVE remains high even after the implementation of statin therapy in those with and without diabetes (3). Atherosclerosis progresses in about a third of individuals despite use of the most aggressive statin regimens (4). Treatment strategies aimed at decreasing CVD risk with the use of a second pharmacological agent, in addition to statin therapy, are used in clinical practice and have been the subject of multiple intervention trials (5–8), with potential benefits in certain individuals (7). On the other hand, the addition of intensive lifestyle intervention to a statin regimen remains underutilized in clinical care (9) and its benefits unexplored in clinical trials.
C-reactive protein (CRP) is a marker of chronic subclinical inflammation and is associated with atherosclerosis progression (10). It has been identified as a useful indicator of cardiovascular risk (11–13). CRP levels may be particularly useful in identifying individuals at risk for CVE in whom LDL-cholesterol (LDL-C) levels are not elevated (14–17). Persons with type 2 diabetes have characteristic low/average LDL-C levels but CRP levels that are significantly increased (18–20). Lifestyle intervention for weight loss reduces CRP levels in obese persons with and without type 2 diabetes (22–24), but its effects in addition to statin therapy have not been established. In this study we hypothesized that in individuals with type 2 diabetes and obesity, a combination regimen of intensive lifestyle intervention for weight loss (ILI) and statin would result in lower levels of CRP than either ILI or statin alone and that weight loss would remain a significant determinant of CRP change, regardless of statin use.
METHODS AND PROCEDURES
Study Design
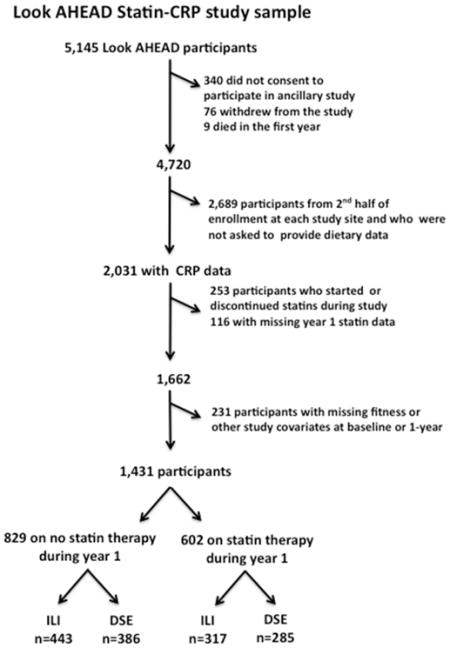
We evaluated a subset of 1,431 individuals, generally corresponding to the first half of Look AHEAD (Action for Health in Diabetes) participants from 15 of 16 clinic sites, who had CRP and fitness data at baseline and 1 year, and who remained on or off statin therapy for the duration of the study. Look AHEAD was a randomized clinical trial evaluating whether a behavioral lifestyle intervention for weight loss would reduce CVE in overweight/obese subjects with type 2 diabetes.
A description of Look AHEAD, its design and subject characteristics, has been published (23–24). Briefly, subjects were randomized to ILI, aiming for a 7% weight loss from baseline, or to a usual care (Diabetes support and education [DSE]) arm, which served as control. ILI participants attended group and individual sessions (3 group sessions and 1 individual encounter per month during the first 6 months of the study, followed by 2 group sessions and 1 individual appointment per month thereafter) in support of behavioral change to increase physical activity to 175 weekly minutes of moderate-intensity exercise and reduce caloric and saturated fat intake. The activity program relied on at home exercise, which for most participants consisted of brisk walking. Energy intake goal for persons <114 kg was 1200 −1500 kcal/day and 1500–1800 kcal/day for those ≥ 114 kg. DSE participants received 3 group health information sessions during the year. Participants in both groups continued clinical care with their primary providers. The institutional review boards of the participating centers approved Look AHEAD and this ancillary study.
Laboratory, Anthropometric and Fitness Determinations
CRP was measured using a high-sensitivity latex particle-enhanced immunoturbidimetric assay by Denka Seiken, C. Ltd. Intra- and interassay coefficients of variation were 3.5% and 5.6%, respectively, as previously reported (20,25). Fitness was measured in Look AHEAD using a graded exercise treadmill test following standard criteria from the American College of Sports Medicine. At baseline, the test was terminated when individuals reached ≥ 85% of age-predicted maximal heart rate (if on no beta blocker therapy) or when the rating of perceived exertion (RPE) reached 18, if on a beta-blocker. At 1-year, the treadmill test was performed at the same walking speed used at baseline, and terminated when participants achieved 80% of their maximal heart rate or an RPE of 16, as appropriate. To estimate change in fitness, the change in METS (mL/kg•min)/3.5 L/kg•min) achieved at sub-maximal effort (80% of maximal heart rate or an RPE of 16) from baseline to year 1 was determined (26). Procedures for obtaining anthropometric measures, hemoglobin A1c (HbA1c), glucose and lipids in Look AHEAD have been previously described (27).
Statistical Analysis
Descriptive statistics, including median and inter-quartile range (IQR), were determined for CRP levels at baseline and for their change from baseline to 1-year. Differences between the ILI and DSE arms in change variables over the first year were evaluated using either the two-sample t-test or the Wilcoxon rank sum test. The effects of changes in weight, waist and fitness by statin use on CRP change were evaluated in the overall group using multivariable regression analyses, with change in CRP (1 year minus baseline levels, log-transformed) as outcome. The models included a dichotomous indicator for treatment effect (ILI vs DSE) and adjusted for baseline CRP levels, age, gender, race/ethnicity, diabetes duration, smoking, cardiovascular disease, use of thiazolidinediones, insulin, hormone replacement therapy (HRT) in women and changes in HbA1c, HDL-cholesterol and triglycerides. The effects of gender, race/ethnicity and of HRT in women on the associations under study were evaluated with the use of interaction terms (ILI * gender, ILI* Race/ethnicity and ILI*HRT). Type I error rate was fixed at 0.05 for all analyses. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Participants were middle-aged, obese and sedentary (See Table 1). CRP levels (median [IQR]) were elevated in the overall group at baseline, with significantly higher levels in non-statin users, when compared to those on statin therapy (5.1 [2.4 to 10] and 3.1 [1.4, 7.0] mg/L, respectively). The differences in CRP levels between men and women, previously reported (20), remained despite statin use, with women having higher levels than men (2.7 [1.5 to 5.4] and 1.9 [1.0 to 4.3] mg/L, in female and male statin users, respectively). As expected, subjects on statin therapy represented a higher-risk group than those on no statin therapy, with more participants on statins having a positive history of CVD and a longer duration of diabetes than those not on statins. Participants on statin therapy were of slightly older age and included more males than those not treated with statins. There were also more Caucasians on statin therapy.
Table 1.
Baseline Characteristics
| Participants on statin therapy (n=602) | Participants not on statins (n= 829) | p-value | |
|---|---|---|---|
| Age, years | 58.6 ± 7.3 | 56.7 ± 7.1 | <0.0001 |
| Women, No. (%) | 312 (52%) | 521 (63%) | <0.0001 |
| Caucasian, No. (%) | 453 (75%) | 506 (61%) | <0.0001 |
| Diabetes Duration, years | 7.2 ± 6.7 | 5.9 ± 5.8 | <0.0001 |
| History of CVD, No. (%) | 129 (21%) | 42 (5%) | <0.0001 |
| Current smokers, No. (%) | 20 (3%) | 25 (3%) | 0.74 |
| On insulin, No. (%) | 105 (17%) | 100 (12%) | 0.004 |
| On thiazolidinediones, No. (%) | 181 (30%) | 182 (22%) | 0.0005 |
| Women on estrogen, No. (%) | 187 (60%) | 288 (55%) | 0.19 |
| Weight, kg | 101.7 ± 18.7 | 101.8 ± 19.6 | 0.99 |
| Waist, cm | 114.4 ± 14.3 | 114.3 ± 14.0 | 0.94 |
| BMI, kg/m2 | 35.8 ± 5.8 | 36.4 ± 6.2 | 0.062 |
| Fitness, submaximal METS | 5.2 ± 1.6 | 5.1 ± 1.6 | 0.39 |
| HbA1c, % | 7.3 ± 1.1 | 7.3 ± 1.3 | 0.26 |
| LDL-C, mg/dL | 101.3 ± 26.5 | 118.3 ± 31.2 | <0.0001 |
| HDL-C, mg/dL | 42.3 ± 10.7 | 43.1 ± 11.9 | 0.43 |
| Triglycerides, mg/dL (median [IQR]) | 159.5 [110, 227] | 153 [106, 222] | 0.13 |
| Triglyceride/HDL-C ratio (median [IQR]) | 4.0 [2.5, 6.0] | 3.6 [2.3, 6.0] | 0.08 |
| CRP, mg/L (median [IQR]) | 3.1 [1.4, 7.0] | 5.1 [2.4, 10.4] | <0.0001 |
| Men | 1.9 [1.0, 4.3] | 2.7 [1.5, 5.4] | 0.0004 |
| Women | 4.4 [2.3, 9.2] | 7.2 [3.7, 12.6] | <0.0001 |
All data expressed as frequency (percent, %), mean ± standard deviation or median [interquartile range].
No: number; kg: kilograms; cm: centimeters; m: meters; METS: metabolic equivalents; LDL-C: low density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; mg: milligrams; dL: deciliter; IQR: Interquartile range; CRP: C-reactive protein
Changes in statin and non-statin users after one year of intervention
ILI participants had improvements in adiposity, fitness, HDL-cholesterol (HDL-C), triglycerides, triglyceride/HDL-C ratio and in glucose control at 1 year, regardless of statin use, when compared to DSE (Table 2; p ≤0.0004 for ILI versus DSE; p≥0.09 for difference in statin effects for ILI and DSE participants). A significant reduction in LDL-C levels with ILI was observed in non-statin participants (p=0.004), whereas changes in LDL-C did not significantly differ by treatment arm in statin users. Relative and absolute reductions in CRP levels with ILI were greater than those achieved with DSE, in men and women (Table 3; all p<0.0001), regardless of statin use. Relative reductions in CRP levels with ILI, of ~40% from baseline, were observed in statin and non-statin users and in men and women (Table 3). The absolute reduction of CRP levels with ILI did not differ by statin use in men (Table 3, p=0.89 for statin effect for ILI). However, in women, there were greater absolute reductions of CRP levels with ILI in those not on statin therapy (median [IQR]) of − 2.1 [−5.0, −0.4] mg/dL), when compared to those on statins (−1.3 [−3.0, 0.0] mg/L; statin effect for ILI p<0.03).
Table 2.
Change in metabolic variables by treatment arm and statin use
| Variable change | Participants on statin therapy | Participants not on statins | Statin effect for ILI | Statin effect for DSE | ||||
|---|---|---|---|---|---|---|---|---|
| ILI (n=317) | DSE (n=285) | p | ILI (n=443) | DSE (n=386) | p | |||
| Δ in weight (kg) | −9.2 ±7.6 | −0.5 ±5.0 | <0.0001 | −9.1 ±7.9 | −0.9 ±5.1 | <0.0001 | 0.87 | 0.34 |
| Δ in waist (cm) | −7.6 ±8.3 | −0.8 ±9.1 | <0.0001 | −7.7 ±9.4 | −1.0 ±6.8 | <0.0001 | 0.89 | 0.75 |
| Δ in fitness (METS) | 1.1 ±1.3 | 0.3 ±1.0 | <0.0001 | 1.0 ±1.4 | 0.3 ±1.1 | <0.0001 | 0.17 | 0.89 |
| Δ in HbA1c (%) | −0.7 ±0.9 | −0.3 ±0.9 | <0.0001 | −0.7 ±1.0 | −0.1 ±0.9 | <0.0001 | 0.76 | 0.09 |
| Δ in LDL-C (mg/dL) | −3.4 ±24.9 | −3.2 ±26.5 | 0.92 | −4.1 ±22.5 | 0.4 ±21.0 | 0.004 | 0.70 | 0.06 |
| Δ in HDL-C (mg/dL) | 3.4 ± 7.2 | 1.2 ±6.2 | 0.0001 | 3.0 ±6.9 | 1.4 ±6.5 | 0.0004 | 0.55 | 0.74 |
| Δ in TG (mg/dL; median [IQR]) | −25 [−73, 12] | −5 [−43, 28] | 0.0003 | −18 [−61,15] | −3 [−37, 27] | <0.0001 | 0.36 | 0.72 |
| Δ in TG/HDL-C ratio (median [IQR]) | −0.7 [−2.2, 0.2] | −0.2 [−1.2, 0.7] | <0.0001 | −0.6 [−2.0, 0.3] | −0.1 [−1.1, 0.6] | <0.0001 | 0.19 | 0.73 |
All data expressed as mean ± standard deviation or median [interquartile range].
ILI: intensive lifestyle intervention for weight loss; DSE: Diabetes, Support and Education; Δ:1-year change; TG: triglycerides; other abbreviations as in Table 1.
Table 3.
1-year changes in CRP levels by gender and statin use
| Sex | Participants on statin therapy | Participants not on statins | Statin effect for ILI | Statin effect for DSE | ||||
|---|---|---|---|---|---|---|---|---|
| ILI (n=317) | DSE (n=285) | p | ILI (n=443) | DSE (n=386) | P | |||
| Relative Δ (%) | ||||||||
| Men | −44.9 (−63.0, −2.9) | −13.7 (−41.4, 27.0) | <0.0001 | −37.4 (−59.9, 4.5) | −0.9 (−27.0, 53.0) | <0.0001 | 0.29 | 0.09 |
| Women | −42.3 (−63.9, 0.0) | −21.0 (−46.8, 19.8) | <0.0001 | −40.0 (−58.9, −8.0) | −8.3 (−38.8,19.1) | <0.0001 | 0.72 | 0.11 |
| Absolute Δ (mg/L) | ||||||||
| Men | −0.7 (−2.0, 0.0) | −0.2 (−1.0, 0.4) | 0.0003 | −0.8 (−2.0, 0.1) | 0.0 (−0.9. 0.9) | <0.0001 | 0.89 | 0.25 |
| Women | −1.3 (−3.0, 0.0) | −0.7 (−3.0, 0.8) | 0.03 | −2.1 (−5.0, −0.4) | −0.5 (−3.0, 1.3) | <0.0001 | 0.03 | 0.35 |
CRP levels at 1-year post-randomization
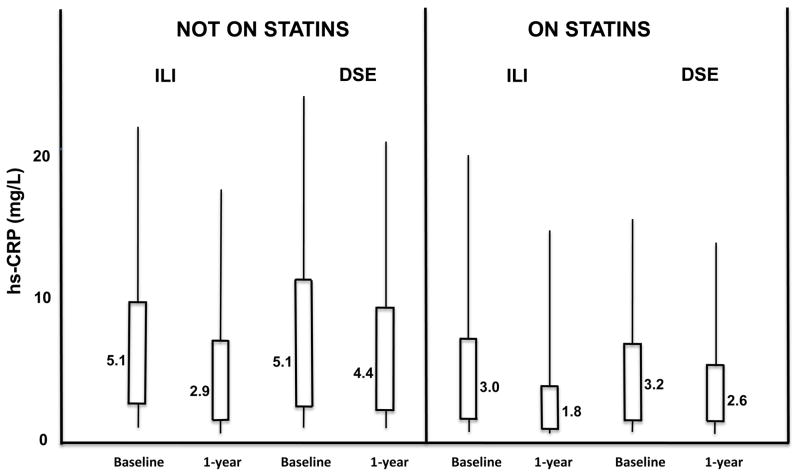
Overall, only participants on statin therapy in the ILI arm reached a 1-year median CRP level under 2.0 mg/L (Figure 1). When evaluating by gender, achievement of this goal was observed mainly in men, who started at lower baseline levels than did women, reaching a 1-year median level of 1.2 mg/L on combined ILI and statin therapy. In women, the 1-year median level of CRP with combination of ILI and statin was 2.6 mg/L, lower than the 1-year medians of 3.7 and 6.7 mg/L, observed in the DSE group on statin and on no statin therapy, respectively (Table 4).
Figure 1.
Baseline and 1-year CRP levels by treatment arm and statin use.
hs-CRP: high sensitivity C-reactive protein; ILI: intensive lifestyle intervention for weight loss; DSE: Diabetes, Support and Education. Values depicted next to each box plot represent median hs-CRP levels.
Table 4.
CRP levels at 1-year in men and women by statin use
| 1-year CRP levels (median [IQR]) | ||||||
|---|---|---|---|---|---|---|
| Participants on statin therapy | Participants not on statins | |||||
| ILI | DSE | p-value | ILI | DSE | p-value | |
| Men | 1.2 (0.6, 2.7) | 1.8 (1.0, 3.1) | 0.003 | 1.7 (1.0, 3.2) | 2.6 (1.5, 4.7) | 0.0003 |
| Women | 2.6 (1.0, 5.5) | 3.7 (2.1, 7.0) | 0.0006 | 4.1 (2.0, 8.2) | 6.7 (3.2,11.6) | <0.0001 |
Data expressed as median (interquartile range). Abbreviations as in Table 1.
Association of weight loss, and changes in waist and fitness with CRP change by statin use
Multivariable regression analyses showed that, after adjusting for multiple variables including fitness change, both change in weight and change in waist were significantly associated with CRP in subjects not on statin therapy (Table 5, p <0.0001), with weight change and waist change contributing similarly to the variance in CRP change. In participants on statins, weight change (p<0.0001), but not change in waist circumference (p=0.11), remained significantly associated with change in CRP. Like change in waist, change in fitness was a significant determinant of CRP change only in participants not on statin therapy (Table 5, p<0.047). No significant interaction effects were observed for gender or race/ethnicity in any of the models tested (Table 5, Models A and B, table legend). The interaction for ILI* HRT in women was also tested and found to be non-significant (Table 5, Models A: p= 0.83 in statin users and 0.96 in participants not on statin therapy; Models B: p= 0.97 in statin users and 0.71 in participants not on statin therapy).
Table 5.
Association of weight loss and fitness change with CRP change by statin use
| Change in CRP* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants on statin therapy | Participants not on statins | ||||||||
| Model A | B | SE | p-value | R2 | B | SE | p-value | R2 | |
| Δ in weight | 0.025 | 0.005 | <0.0001 | 0.589 | 0.020 | 0.004 | <0.0001 | 0.558 | |
| Δ in fitness | −0.016 | 0.029 | 0.58 | −0.045 | 0.022 | 0.0471 | |||
| Model B | Δ in waist | 0.006 | 0.004 | 0.1107 | 0.572 | 0.015 | 0.003 | <0.0001 | 0.558 |
| Δ in fitness | −0.051 | 0.028 | 0.0737 | −0.059 | 0.022 | 0.0067 | |||
CRP log-transformed. All models adjusted for baseline CRP level, treatment arm (ILI vs DSE), age, race/ethnicity, clinic site, history of CVD, diabetes duration, current smoking; use of insulin, thiazolidinediones, hormone replacement in women; changes in HbA1c, HDL-cholesterol and triglycerides.
Abbreviations as in Tables 1 and 2. For participants on statins and not on statins, respectively, the interaction effects of ILI* gender were p= 0.51 and 0.58 for Model A, and p=0.26 and 0.66 for Model B; for ILI* race/ethnicity: p= 0.41 and 0.51 for Model A, and p= 0.69 and 0.58 for Model B, in participants on and not on statins, respectively.
DISCUSSION
In this large sample of obese diabetic participants, ILI led to further reduction of CRP levels when added to statin therapy and usual care (DSE). The combination of statin and ILI, in the overall group, achieved a median CRP level at 1-year under 2.0 mg/L, whereas statin therapy or ILI alone did not. Relative changes in CRP with ILI were similar in men and women, regardless of statin use, but absolute changes in CRP were greater in women. Women started with higher baseline levels than did men and men achieved lower absolute levels at 1 year. Weight loss was significantly associated with a reduction in CRP levels in both statin and non-statin users, independently of demographics and medical history, baseline CRP levels and changes in other metabolic variables, including fitness.
CRP may be considered in the assessment of CVD risk and in the decision algorithm of statin initiation in individuals in the intermediate risk category (12). The indication of statin therapy in persons at increased risk is not usually questioned. However, in these individuals, CRP may provide useful information on the effectiveness of the intervention to prevent CVD events and delay atherosclerosis progression. Reductions in CRP levels with statins in persons with established coronary disease have been associated with a decrease in progression, and even with regression, of atherosclerotic disease (10). Patients with acute coronary syndrome who lowered their CRP levels with statin therapy below a median of 2.0 mg/L, had a decreased incidence of recurrent cardiovascular events when compared to those in whom the CRP level remained above 2.0 mg/L (11). Almost a fifth of participants in these trials had diabetes, suggesting that the benefits of CRP reduction could apply to them.
In this study, median CRP at baseline in participants treated with statins was 3.1 mg/L, with 25% of male and 50% of female statin users having CRP levels well above 4.0 mg/L. Those on no statins at baseline started at much higher levels, with a median of 2.7 mg/L in men and of 7.2 mg/L in women. At 1-year, ILI led to similar weight loss and improvements in fitness and glucose control in statin and non-statin users. The relative reduction in CRP, ~ 40%, was also similar in statin and non-statin users, and in men and women. In the overall sample, the combination of statin and ILI brought the 1-year median CRP level to 1.8 mg/L, whereas statin therapy and ILI alone achieved levels of 2.6 and 2.9 mg/L, respectively. A quarter of men and of women on ILI and statin combination were able to decrease median CRP levels to ≤ 1.0 mg/L.
Despite the use of the most aggressive statin regimens available, atherosclerosis progresses in about a third of individuals (4) and the relative risk of a cardiovascular event is reduced only approximately by a third (3,9). This residual risk points to the need of identifying alternative treatment approaches, targeting mechanisms independent of HMG-CoA reductase inhibition. Clinical efforts have focused on adding a second agent to a statin, aiming for further reduction of LDL-cholesterol or for an improvement in HDL-cholesterol or triglyceride levels (7,8) with a suggestion of benefit in those with low HDL- cholesterol and elevated triglycerides (7).
Modification of lifestyle behaviors to achieve moderate weight loss improves adipose tissue function and leads to a significant reduction in CRP levels (20–22). A decrease in CRP levels has been shown to occur in persons with type 2 diabetes in association, not only with reduced adiposity, but also with improvements in fitness, glucose and lipid control (20]. The mechanisms through which lifestyle behavior change influence adipose tissue are multiple and affect tissue macrophage and lymphocyte recruitment, macrophage activation and the balance of pro and anti-inflammatory adipocytokine production (28–31). In this study we show that the association of weight loss with CRP change is present in obese individuals with diabetes, regardless of statin use and that increased fitness and waist reduction relate to CRP change, in individuals on no statin therapy.
We speculate that the greater reduction in CRP levels obtained when ILI and statin therapy are combined may be consequent to distinct and complementary effects of statins and ILI on CRP production. Although statins may have a predominant effect on hepatic production of CRP (32) and ILI a potentially greater impact on adipose-derived CRP in the setting of obesity (33,34), it is possible that statin use and ILI affect production at both sites (35–38). The fact that waist and fitness changes did not reach statistical significance in their association with CRP change in statin users does not exclude the possibility that greater changes in waist or in fitness, or similar changes in less obese individuals treated with statins, may be associated with a lowering of CRP levels. A differential effect of statins on visceral and subcutaneous adipose depots, could also contribute to the observed differences in the association of waist change and CRP change by statin use. Additional studies evaluating the effects of statins on adipose tissue function are needed.
This study is unique in that it uses a large randomized lifestyle intervention trial to address a question of clinical significance to a growing population of overweight/obese individuals with diabetes. Our results suggest that ILI and statin therapy may result in further improvement of subclinical inflammation, when compared to statin therapy or ILI alone. However, due to its design, certain limitations need to be addressed: Although statin use was clearly documented during the year of follow-up and was used to determine eligibility to this study, information on statin dose and/or type and on medication adherence was not available and could not be accounted for. This limitation is mitigated by the fact that Look AHEAD participants in both treatment arms (ILI and DSE) were under the care of their primary physician, with freedom to adjust statin doses, statin type and to support adherence. Furthermore, the significant changes in LDL-C from baseline to year-1 observed in statin users in the DSE arm, but not in ILI participants, suggest that statin therapy was adjusted more aggressively in statin users in the DSE arm than in the ILI arm, potentially making our observations on the added benefit of ILI on CRP change even more significant. In addition, due to sample size limitations in certain race/ethnic groups when stratifying by statin use, we are unable to confirm that the study’s results are applicable to non-Caucasian populations.
Our findings from Look AHEAD suggest that in overweight/obese diabetic persons, ILI and statin therapy may have substantial additive anti-inflammatory benefits and that weight loss is significantly associated with CRP lowering regardless of statin use. Analyses of cardiovascular outcome data from Look AHEAD by statin use will inform on the implications of our findings on incident events.
What is known about this subject
The residual risk of heart disease remains high despite the use of statins
Elevated CRP levels are associated with an increased risk of cardiovascular disease
Statins lower CRP and reduce cardiovascular disease risk
CRP levels are elevated in obese individuals with diabetes
What this study adds
Intensive lifestyle intervention for weight loss may significantly reduce CRP levels in overweight/obese diabetic men and women regardless of statin use, when compared to usual care
Greater reductions in CRP levels are seen in obese diabetic persons who receive statin therapy and participate in an intensive lifestyle intervention for weight loss, when compared to individuals who receive either statin therapy or weight loss intervention alone.
Acknowledgments
Members of the Look AHEAD Research Study Group are listed in the Online Appendix. The authors thank Charles E. Rhodes for technical support with the CRP assays. Look AHEAD is sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, co-sponsored by the National, Heart, Lung and Blood Institute (NHLBI), National Institute of Nursing Research, Office of Research on Women’s Health, National Center on Minority Health and Health Disparities and Centers for Disease Control and Prevention. Additional Look AHEAD funding is listed online. This work was supported by NHLBI Grants HL090514-02S1 (LMB) and HL090514 (CMB).
Appendix 1
Appendix 2. Clinical Sites
The Johns Hopkins Medical Institution:s Frederick L. Brancati, MD, MHS1; Jeff Honas, MS2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jeanne Charleston, RN; Kathy Horak, RD; Pennington Biomedical Research Center: George A. Bray, MD1; Kristi Rau2; Allison Strate, RN2; Brandi Armand, LPN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Amy Bachand; Michelle Begnaud; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas; David Creel; Diane Crow; Helen Guay; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker; The University of Alabama at Birmingham: Cora E. Lewis, MD, MSPH1; Sheikilya Thomas MPH2; Monika Safford, MD3; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD; Sara Hannum, MA; Anne Hubbell, MS; Jennifer Jones, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; Karen Marshall; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Janet Truman; Nita Webb, MA; Audrey Wrenn, MAEd; Harvard Center: Massachusetts General Hospital: David M. Nathan, MD1; Heather Turgeon, RN, BS, CDE2; Kristina Schumann, BA2; Enrico Cagliero, MD3; Linda Delahanty, MS, RD3; Kathryn Hayward, MD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Richard Ginsburg, PhD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Charles McKitrick, RN, BSN, CDE; Alan McNamara, BS; Theresa Michel, DPT, DSc CCS; Alexi Poulos, BA; Barbara Steiner, EdM; Joclyn Tosch, BA; Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Kristinia Day, RD; Ann McNamara, RN; University of Colorado Health Sciences Center: James O. Hill, PhD1; Marsha Miller, MS, RD2; JoAnn Phillipp, MS2; Robert Schwartz, MD3; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Salma Benchekroun MS; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Elizabeth Daeninck, MS, RD; Amy Fields, MPH; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Michael McDermott, MD; Lindsey Munkwitz, BS; Loretta Rome, TRS; Kristin Wallace, MPH; Terra Worley, BA; Baylor College of Medicine: John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Peter Jones, MD3; Michele Burrington, RD; Chu-Huang Chen, MD, PhD; Allyson Clark, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Patricia Pace, RD: Julieta Palencia, RN; Olga Satterwhite, RD; Jennifer Schmidt; Devin Volding, LMSW; Carolyn White; University of California at Los Angeles School of Medicine: Mohammed F. Saad, MD1; Siran Ghazarian Sengardi, MD2; Ken C. Chiu, MD3; Medhat Botrous; Michelle Chan, BS; Kati Konersman, MA, RD, CDE; Magpuri Perpetua, RD; The University of Tennessee Health Science Center: University of Tennessee East. Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Stephanie Connelly, MD, MPH3; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA; University of Tennessee Downtown: Abbas E. Kitabchi, PhD, MD1; Helen Lambeth, RN, BSN2; Debra Clark, LPN; Andrea Crisler, MT; Gracie Cunningham; Donna Green, RN; Debra Force, MS, RD, LDN; Robert Kores, PhD; Renate Rosenthal PhD; Elizabeth Smith, MS, RD, LDN; and Maria Sun, MS, RD, LDN; and Judith Soberman, MD3; University of Minnesota: Robert W. Jeffery, PhD1; Carolyn Thorson, CCRP2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott Crow, MD3; Susan K Raatz, PhD, RD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Jeanne Carls, MEd; Tara Carmean-Mihm, BA; Emily Finch, MA; Anna Fox, MA; Elizabeth Hoelscher, MPH, RD, CHES; La Donna James; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh CHES; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS, RD; St. Luke’s Roosevelt Hospital Center: Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Stanley Heshka, PhD3; Carmen Pal, MD3; Lynn Allen, MD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; University of Pennsylvania: Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2; Stanley Schwartz, MD3; Gary D. Foster, PhD3; Robert I. Berkowitz, MD3; Henry Glick, PhD3; Shiriki K. Kumanyika, PhD, RD, MPH3; Johanna Brock; Helen Chomentowski; Vicki Clark; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Louise Hesson, MSN; Stephanie Krauthamer-Ewing, MPH; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, MS, RD; Leslie Womble, PhD, MS; Nayyar Iqbal, MD; University of Pittsburgh: David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis Kuller, MD, DrPH3; Andrea Kriska, PhD3; Janet Bonk, RN, MPH; Rebecca Danchenko, BS; Daniel Edmundowicz, MD3; Mary L. Klem, PhD, MLIS3; Monica E. Yamamoto, DrPH, RD, FADA 3; Barb Elnyczky, MA; George A. Grove, MS; Pat Harper, MS, RD, LDN; Janet Krulia, RN, BSN, CDE; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, MS, RD, LDN; Tracey Y. Murray, BS; Joan R. Ritchea; Jennifer Rush, MPH; Karen Vujevich, RN-BC, MSN, CRNP; Donna Wolf, MS; The Miriam Hospital/Brown Medical School: Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS; The University of Texas Health Science Center at San Antonio: Steven M. Haffner, MD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; University of Washington/VA Puget Sound Health Care System Steven Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD3; Matthew L. Maciejewski, PhD3; Dace Trence, MD3; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; April Thomas, MPH, RD; Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico: William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Justin Glass, MD3; Sara Michaels, MD3; Peter H. Bennett, MB, FRCP3; Tina Morgan3; Shandiin Begay, MPH; Bernadita Fallis RN, RHIT, CCS; Jeanette Hermes, MS, RD; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Nancy Scurlock, MSN, ANP-C, CDE; Leigh A. Shovestull, RD, CDE; Janelia Smiley; Katie Toledo, MS, LPC; Christina Tomchee, BA; Darryl Tonemah PhD; University of Southern California: Anne Peters, MD1; Valerie Ruelas, MSW, LCSW2; Siran Ghazarian Sengardi, MD2; Kathryn Graves, MPH, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan.
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1; Judy L. Bahnson, BA2; Lynne Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain Bertoni, MD, MPH3; Wei Lang, PhD3; Gary Miller, PhD3; David Lefkowitz, MD3; Patrick S. Reynolds, MD3; Paul Ribisl, PhD3; Mara Vitolins, DrPH3; Michael Booth, MBA2; Kathy M. Dotson, BA2; Amelia Hodges, BS2; Carrie C. Williams, BS2; Jerry M. Barnes, MA; Patricia A. Feeney, MS; Jason Griffin, BS; Lea Harvin, BS; William Herman, MD, MPH; Patricia Hogan, MS; Sarah Jaramillo, MS; Mark King, BS; Kathy Lane, BS; Rebecca Neiberg, MS; Andrea Ruggiero, MS; Christian Speas, BS; Michael P. Walkup, MS; Karen Wall, AAS; Michelle Ward; Delia S. West, PhD; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco: Michael Nevitt, PhD1; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH; Lisa Palermo, MS, MA; Michaela Rahorst; Ann Schwartz, PhD; John Shepherd, PhD; Central Laboratory, Northwest Lipid Research Laboratories: Santica M. Marcovina, PhD, ScD1; Greg Strylewicz, MS; ECG Reading Center, EPICARE, Wake Forest University School of Medicine: RonaldJ. Prineas, MD, PhD1; Teresa Alexander; Lisa Billings; Charles Campbell, AAS, BS; Sharon Hall; Susan Hensley; Yabing Li, MD; Zhu-Ming Zhang, MD; Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities Elizabeth J Mayer-Davis, PhD1; Robert Moran, PhD; Hall-Foushee Communications, Inc.:Richard Foushee, PhD; Nancy J. Hall, MA.
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases: Barbara Harrison, MS; Van S. Hubbard, MD PhD; Susan Z. Yanovski, MD; National Heart, Lung, and Blood Institute: Lawton S. Cooper, MD, MPH; Jeffrey Cutler, MD, MPH; Eva Obarzanek, PhD, MPH, RD; Centers for Disease Control and Prevention: Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD.
Funding and Support
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056 44) and NIH grant (DK 046204); and the University of Washington/VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations committed contributions to Look AHEAD: Federal Express; Health Management Resources; Johnson & Johnson, LifeScan Inc.; Optifast-Novartis Nutrition; Roche Pharmaceuticals; Ross Product Division of Abbott Laboratories; Slim-Fast Foods Company; and Unilever.
Footnotes
Principal Investigator
Program Coordinator
Co-Investigator
DISCLOSURE
Authors do not have any conflicts of interest to disclose.
Trial Registration: clinicaltrials.gov Identifier: NCT00017953
All other Look AHEAD staff members are listed alphabetically by site.
References
- 1.American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, et al. Cholesterol Treatment Trialist’s (CTT) Collaboration. Efficacy and safety of more intense lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg HN, Elam MB, et al. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden WE, Probstfield JL, et al. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 9.Fruchart JC, Sacks F, Hermans MP, et al. The residual risk reduction initiative: A call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 12.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 13.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 15.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: The cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Zinman B, Haffner SM, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 20.Belalcazar LM, Reboussin DM, Haffner SM, et al. A one-year lifestyle intervention for weight loss in persons with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change, from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33:2297–303. doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 22.Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Wadden TA, West DS, et al. Look AHEAD Research Group. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 26.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: Results from the Look AHEAD study. Int J Obes (Lond) 2009;33:305–316. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi-Sunyer X, Blackburn G, et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaud C, Burger F, Steffens S, Veilllard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes. New evidence for direct anti-inflammatory effects of statins. ATVB. 2005;25:1231–1236. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 34.Anty R, Bekri S, Luciani N, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–1833. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 35.Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196:114–121. doi: 10.1016/j.atherosclerosis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Le Lay S, Krief S, Farnier C, et al. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 37.Horton JD, Shimomura I, Ikemoto S, Bashmakov Y, Hammer RE. Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J Biol Chem. 2003;278:36652–36660. doi: 10.1074/jbc.M306540200. [DOI] [PubMed] [Google Scholar]
- 38.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]