Abstract
Background
Constitutive activation of nuclear factor κB (NF-κB) is associated with poor prognosis. Irinotecan demonstrates single-agent activity in head and neck cancer but activates NF-κB, promoting cell survival and resistance. Bortezomib is a proteasome inhibitor that inactivates NF-κB.
Patients and Methods
We performed a randomized phase II trial of bortezomib on days 1, 4, 8, and 11 and irinotecan on days 1 and 8 of each 21-day cycle or single-agent bortezomib on days 1, 4, 8, and 11 on a 21-day cycle. The addition of irinotecan to bortezomib was allowed in patients who progressed on bortezomib alone.
Results
The response rate of bortezomib and irinotecan was 13%. One patient had a partial response to bortezomib alone (response rate 3%). No responses were seen in patients with addition of irinotecan at time of progression on bortezomib.
Conclusions
The bortezomib-based regimens evaluated in this study have minimal activity in recurrent or metastatic head and neck cancer.
Keywords: head and neck cancer, chemotherapy, bortezomib, irinotecan, phase II
INTRODUCTION
Despite a greater understanding of the molecular underpinnings of head and neck cancer, the prognosis for recurrent or metastatic head and neck cancer remains poor, with median survival of less than 11 months. Cytotoxic agents demonstrate response rates of only 10% to 30%.1–3 The NF-κB/REL family of transcription factor proteins control the expression of genes that inhibit apoptosis, contributing to chemotherapy resistance in head and neck cancer.4–6 Additionally, NF-κB promotes the malignant phenotype through activation of genes that regulate cellular proliferation, angiogenesis, and inflammatory responses. Constitutive activation of NF-κB is an early event in carcinogenesis and is associated with poor prognosis. Moreover, chemotherapy further activates NF-κB, promoting cell survival and resistance.
The ubiquitin-proteosome pathway plays a critical role in cellular protein homeostasis, including degradation of the NF-κB inhibitory protein, inhibitor of κB (IκB), a requirement for nuclear translocation and activation of NF-κB. Conversely, inhibition of proteasome-dependent IκB degradation inhibits NF-κB, promoting apoptosis, including in head and neck cancer.6 Thus inhibition of the ubiquitin proteosome pathway represents a potential mechanism to overcome chemoresistance. Treatment of colorectal carcinoma cell lines with SN-38, the active metabolite of irinotecan, was shown to promote activation of NF-κB. However, pretreatment with the proteosome inhibitor bortezomib resulted in complete inhibition of activated NF-κB, enhanced stabilization of p21, p27, and p53, and chemosensitivity to SN-38.5,7
The topoisomerase I inhibitor irinotecan demonstrates a response rate of 20% in chemotherapy-naive head and neck cancer and has demonstrated activity in combination regimens.8–11 On the basis of the known dysregulation of NF-κB in head and neck cancer and the modest activity of irinotecan in this setting, we designed a phase II study to evaluate the activity of bortezomib administered before irinotecan, versus the activity of bortezomib alone, followed by the addition of irinotecan at the time of progression. We also evaluated the relationship between tumor response and pretreatment nuclear localization of NF-κB and NF-κB–regulated genes and proteins in blood.
PATIENTS AND METHODS
Eligible patients had histologically documented incurable, locally advanced, or metastatic squamous cell carcinoma of the head and neck. Patients were allowed up to 1 prior therapy for incurable, advanced disease, but treatment must have been completed at least 4 weeks before study entry. Patients could not have been previously treated with irinotecan or bortezomib. Other eligibility requirements included measurable disease by response evaluation criteria in solid tumors (RECIST) 1.0 criteria, Eastern Cooperative Oncology Group performance status (PS) of 0 to 1, age ≥18, leukocytes >3000/μL, absolute neutrophil count >1500/μL, platelets ≥100,000/μL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 institutional limits, normal bilirubin, creatinine within institutional limits, or creatinine clearance ≥60 mL/min/1.73 m2. Patients with known brain metastases or ≥ grade 2 peripheral neuropathy were excluded.
Study design
The protocol was sponsored by the National Cancer Institute and the Eastern Cooperative Oncology Group and reviewed by national and local institutional review boards before study activation. Patients provided written informed consent and were equally randomized between 2 arms: combination bortezomib and irinotecan (arm A) or bortezomib alone (arm B), with the addition of irinotecan at time of progression. The primary end points were the response rates in each arm. A 2-stage design was used, with interim analysis on the basis of response rate. The minimum response criteria to proceed to Stage 2 were 4 (of 23 eligible patients) and 1 (of 12 eligible patients) objective responses for arm A and arm B, respectively. If at least 12 (arm A) and 4 (arm B) responses were observed in 55 (arm A) and 37 (arm B) eligible patients, respectively, the treatment regimen would be considered worthy of further study.
Secondary end points included the activity of irinotecan plus bortezomib after progression on bortezomib alone, toxicity, progression-free survival (PFS), and overall survival (OS) for each arm. Moreover, pilot biologic correlative studies including nuclear NF-κB RELA p65 subunit immunostaining and serum cytokines before and on treatment were performed for a subset of subjects in each arm.
Treatment
Patients randomized to arm A received bortezomib 1.3 mg/m2 intravenous push on days 1, 4, 8 and 11 and irinotecan 125 mg/m2 intravenously over 90 minutes, 1 hour after bortezomib infusion on days 1 and 8 of each 21-day cycle. Patients randomized to arm B received single-agent bortezomib 1.3 mg/m2 intravenous push on days 1, 4, 8, and 11 on a 21-day cycle. Patients with progression on arm B were eligible to have irinotecan added in an identical dose and schedule to arm A. This was called step 2. Patients were required to have no significant toxicity and a desire to continue in the study before irinotecan was added. Patients received dexamethasone 10 mg intravenously and a 5-HT3 receptor antagonist before chemotherapy. Therapy on protocol continued until disease progression (second progression for patients on step 2), unacceptable adverse event, change in patient condition prohibiting further drug administration, or patient desire to withdraw or decline further study treatment.
Safety and efficacy asessments
All toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 3.0). Dose modifications occurred for ≥grade 2 neuropathy and for nonhematologic and hematologic toxicity (thrombocytopenia or neutropenia) grade 3 or 4. Dose delays occurred if any of the following were noted on the day of scheduled treatment: ≥grade 1 neutropenia or thrombocytopenia, ≥grade 2 nonhematologic toxicity except bilirubin, which required holding of irinotecan only if grade 2, and both drugs if bilirubin grade 3 or higher. Bortezomib was held for ≥grade 2 peripheral neuropathy. Doses could be held for up to 14 days; if longer delays or more than 2 dose reductions were required, patients were removed from the study. Missed doses were not administered at a later date. No intrapatient dose escalation was allowed.
Response rate was determined with RECIST criteria. Patients underwent baseline imaging within 4 weeks before randomization, and this was repeated every 2 cycles. In patients deemed to have an objective response, complete response (CR) or partial response (PR), tumor measurements were repeated after 4 weeks to confirm response. OS was defined as the time from registration to death from any cause. Patients who were alive at the time of this analysis were censored at the date last known alive. PFS was defined as the time from registration to first documentation of disease progression or to death without documented progression. If date of death was greater than 3 months after date of last disease assessment, the patient was censored at the time of last disease assessment. Patients without documented progression were censored at the time they were last known to be free of progression. For arms A and B, OS and PFS were calculated starting from registration to trial, whereas, for arm B step 2, OS and PFS were calculated starting from registration onto step 2.
Correlative studies
Approximately 15 mL of peripheral blood was collected 1 hour after infusion of bortezomib at baseline, cycle 1 day 1 day 11 of each cycle of therapy to determine the effects on NF-κB regulated cytokines IL-6, IL-8, GRO-1, HGF, and VEGF, as previously described.12 When available, fixed paraffin block tumor tissue from the original or diagnostic biopsy specimen was examined for evidence of nuclear phospho-NF-κB RELA p65 activation as measured by immunohistochemistry.13
Statistical analysis
Descriptive statistics were used to characterize patient demographics, disease characteristics, and adverse events. Exact binomial 90% confidence intervals (CIs) were computed for the objective response rate (ORR, CR+PR) and the disease control rate (DCR; CR+PR+SD [stable disease]). The method of Atkinson and Brown was used to compute the CI of the ORR and DCR for the cohort with a 2-stage accrual. Fisher’s exact test was used to evaluate differences in response rate or disease control rate between groups. Kaplan-Meier estimates were used for event-time distributions, with differences assessed by the (stratified) log-rank test.
For cytokine measurements, the mean cytokine concentration determined from triplicate assay values was used for each patient at each time point. All cytokine concentration measurements were log-transformed (log10) before further data analysis. For a given cytokine, if a value was below the detection level, one half of the lower limit of the assay as specified by the manufacturer was imputed. Cox proportional hazards models were used to evaluate the relationship between pretreatment cytokine measurements (treated as on a continuous scale) and event-time distributions. A logistic regression model was used to examine the relationship between pretreatment cytokine measurements (treated as on a continuous scale) and DCR (CR+PR+SD).11
To evaluate the relationship between longitudinal changes in cytokine measurement and efficacy, a slope for each cytokine on every patient was estimated by fitting a least-squares regression line to longitudinal log-transformed cytokine measurements (for all measurements up to cycle 2, day 1). Landmark analysis was performed to evaluate the relationship between changes in these slopes and event-time distributions to minimize lead-time bias. Patients with events (either death or disease progression) occurring within 1 month after registration were excluded in this 1-month landmark analysis, and OS and PFS were computed forward from the landmark. This landmark was chosen because most patients had serum measurement up to this time point, and this period provided maximal time for first-cycle treatment to impact tumors. Cox proportional hazards models and logistic regression models were used to evaluate the relationship between changes in the slopes and event-time distributions and between changes in the slopes and disease control response, respectively. A log rank test was used to assess differences in event-time distributions between patients with ≥3 large longitudinal cytokine increases and those with ≤2 large increases. (A “large longitudinal increase” was defined as slope above the upper tercile (across patients). Because the analysis of correlative data was exploratory in nature, no statistical adjustments were made for tests on multiple biomarkers. All p values are 2-sided. A level of p < .05 is considered statistically significant.
RESULTS
Patients
Patient enrollment occurred between July 20, 2005, and September 24, 2008. Seventeen centers participated in the study. The study was suspended on September 29, 2006, because of neutropenia associated with the administration of irinotecan and reactivated on October 18, 2006, with the irinotecan dose reduced from 125 mg/m2 to 90 mg/m2. arm A (bortezomib plus irinotecan) was closed on November 13, 2008, after 27 patients were enrolled without meeting criteria to proceed to stage 2 of accrual. arm B (bortezomib alone) proceeded to the second stage of accrual with a total accrual of 44 patients. Among the 71 enrolled patients (27 on arm A and 44 on arm B), 3 patients on arm B were ineligible. Reasons for ineligibility included chemotherapy within 4 weeks of study entry and baseline scans more than 4 weeks before study entry. Four patients in arm A and 3 patients in arm B never received protocol treatment. arm A and B primary analysis thus was based on 61 treated and eligible patients (23 in arm A and 38 in arm B). All treated patients (regardless of eligibility status) were included in toxicity analysis. Twelve patients were reregistered into step 2: 1 never received crossover treatment, and another patient was ineligible (no disease progression in arm B). step 2 primary analysis was thus based on 10 treated and eligible patients, and toxicity analysis was based on 11 treated patients.
Baseline characteristics were balanced between the arms (Table 1). For all 61 eligible and treated patients, the median age was 61 years (range 36 to 85 years). All patients had squamous cell carcinoma of the head and neck. Most patients were male (85.2%), white (79.7%), and with PS 1 (54.1%). Most patients had undergone 1 prior chemotherapy (78.7%) or prior radiation therapy (86.9%). Most patients were not smoking (78.3%) or consuming alcohol (61.7%) at the time of study enrollment.
TABLE 1.
Patient demographics and disease characteristics at baseline (total N = 61) assigned treatment arm.
| Arm A (n = 23) | Arm B (n = 38) | Totals | |
|---|---|---|---|
| Age, yr | |||
| Median | 61 | 61.5 | 61 |
| Range | 36–85 | 45–78 | 36–85 |
| Sex | |||
| Male | 19 (82.6%) | 33 (86.8%) | 52 (85.2%) |
| Female | 4 (17.4%) | 5 (13.2%) | 9 (14.8%) |
| PS | |||
| 0 | 11 (47.8%) | 17 (44.7%) | 28 (45.9%) |
| 1 | 12 (52.2%) | 21 (55.3%) | 33 (54.1%) |
| Primary site | |||
| Oral cavity | 5 (21.71%) | 11 (28.94%) | 16 (26.2%) |
| Oropharynx | 5 (21.7%) | 9 (23.61%) | 14 (22.9%) |
| Hypopharynx | 0 (21.7%) | 2 (5.2%) | 2 (3.2%) |
| Larynx | 11 (47.8%) | 12 (31.6%) | 23 (37.1%) |
| Other | 2 (8.6%) | 4 (10.1%) | 6 (9.8%) |
Treatment
Among the 23 eligible and treated patients assigned to arm A, 13 (56.5%) started at an irinotecan dose of 125 mg/m2 and 9 (39.1%) at 90 mg/m2. One patient started irinotecan at the dose of 76 mg/m2 because of grade 3 vomiting. Table 2 shows the number of treatment cycles administered and the reasons off treatment by arm (A or B). One patient in arm B received 10 cycles of treatment until disease progression. Most patients discontinued treatment because of disease progression (52.2% in arm A and 60.5% in arm B). Three patients were removed from the study for other reasons, namely noncompliance, symptomatic deterioration, and leukocytosis and hypercalcemia consistent with disease progression.
TABLE 2.
The number of treatment cycles and reasons off treatment (arms A and B).
| Reasons off treatment arm | Total number of cycles
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | |
| Disease progression | |||||||||||
| A | 3 | 7 | 1 | — | 1 | — | — | — | — | — | 12 |
| B | — | 17 | — | 3 | 1 | 1 | — | — | — | 1 | 23 |
| Toxicity | |||||||||||
| A | — | 1 | 1 | — | — | — | — | 1 | — | — | 3 |
| B | 3 | 3 | — | 2 | — | 1 | — | — | — | — | 9 |
| Death on study | |||||||||||
| A | 2 | 1 | — | — | — | — | — | — | — | — | 3 |
| B | 1 | 1 | — | — | — | — | — | — | — | — | 2 |
| Patient withdrawal/refusal | |||||||||||
| A | — | 1 | — | — | — | 1 | — | — | — | — | 2 |
| B | 1 | 1 | 1 | — | — | — | — | — | — | — | 3 |
| Alternative therapy | |||||||||||
| A | — | — | — | — | — | — | 1 | — | — | — | 1 |
| Other | |||||||||||
| A | — | — | 1 | — | — | — | 1 | — | — | — | 2 |
| B | 1 | — | — | — | — | — | — | — | — | — | 1 |
| Total | 11 | 32 | 4 | 5 | 2 | 3 | 2 | 1 | — | — | 61 |
The number of cycles administered in step 2 was 9 (range 1–7). The reasons off step 2 treatment for the 10 eligible and treated patients were as follow: 9 of 10 patients came off protocol treatment because of disease progression. Again, most patients (7 patients, 70%) received 2 cycles of treatment in step 2; 1 patient received 6 cycles; 2 patients received only 1 cycle of protocol treatment (1 because of disease progression and the other because of treatment delay over 14 days). Three of these patients received the irinotecan dose at 125 mg/m2 during step 2 treatment.
Efficacy
Table 3 provides a summary of the best overall response in arms A and B and in step 2. No CR was observed in either arm A or B. Three PRs (13.1%, all with a starting irinotecan dose = 125 mg/m2) were reported in arm A and 1 PR (2.6%) was seen in arm B. Five patients (21.7%) with SD and 11 patients (47.8%) with PD as the best response were noted on arm A. In contrast, 9 patients (23.7%) with SD and 19 patients (50.0%) with PD as the best response were observed in arm B.
TABLE 3.
Best overall response.
| Step 1
|
Step 2 | |||
|---|---|---|---|---|
| Arm A | Arm B | Total | ||
| Partial response | 3 (13.1%) | 1 (2.6%) | 4 (6.6%) | 0 (0.0) |
| Stable disease | 5 (21.7%) | 9 (23.7%) | 14 (22.9%) | 1 (10.0%) |
| Progression | 11 (47.8%) | 19 (50%) | 30 (49.2%) | 8 (80.0%) |
| Unevaluable | 4 (17.4%) | 9 (23.7%) | 13 (21.3%) | 1 (10.0%) |
| Total | 23 (100.0%) | 38 (100.0%) | 61 (100.0%) | 10 (100.0%) |
The ORR were 13.1% (90% CI of 3.6%–30.3%) and 2.6% (90% CI of 0.4%–22.1%) for arm A and arm B, respectively. The DCR are 34.8% (90% CI of 18.6%–54.1%) and 26.3% (90% CI of 15.1%–40.5%) for arm A and arm B, respectively.
In contrast to arm B, arm A did not demonstrate the minimum response criteria to proceed to stage 2. For the 11 patients on step 2, neither CR nor PR was observed out of the 10 eligible and treated patients. One patient showed SD as the best overall response; 8 had disease progression as the best overall response; 1 patient was not evaluable for response. The objective response rate on step 2 was 0.0% (90% CI, 0.0%–25.9%). The disease control rate in step 2 was 10% (90% CI, 5.2%–39.4%).
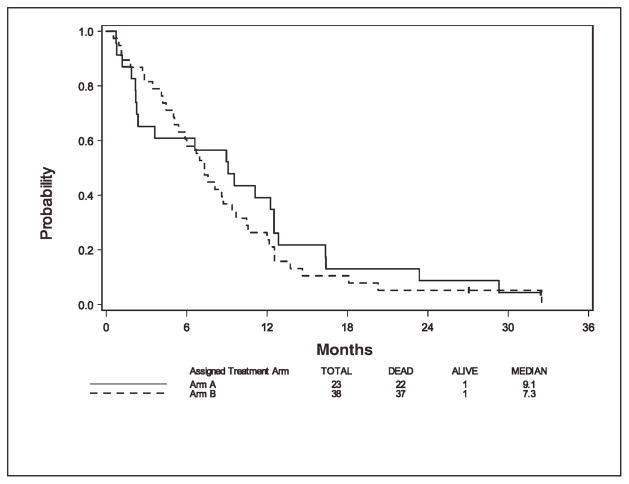
Among 61 eligible and treated patients, 59 patients had died at the time of this analysis. For the 2 patients still alive, the follow-up times are 32.4 months (on arm A) and 27.1 months (on arm B). Figure 1 shows survival curves for arms A and B. The median overall survival was 9.1 months (95% CI, 2.3–12.5 months) and 7.3 months (95% CI, 5.1–9.4 months) for arm A and arm B, respectively. For arm A, the 6-month OS rate and 1-year OS rate were 0.61 (95% CI, 0.38–0.77) and 0.39 (95% CI, 0.20–0.58), respectively. For arm B, the 6-month OS rate and 1-year OS rate were 0.61 (95% CI, 0.43–0.74) and 0.24 (95% CI, 0.12–0.38), respectively.
FIGURE 1.
Overall survival by arm.
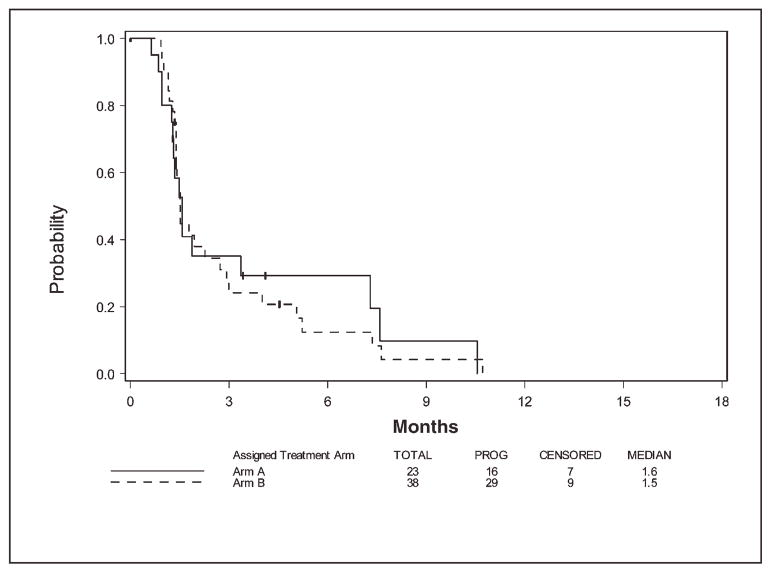
Figure 2 displays PFS for the 2 arms. The median PFS was 1.6 months (95% CI, 1.2–7.3 months) and 1.5 months (95% CI, 1.4–2.7 months) for arm A and arm B, respectively. At the time of this analysis, 16 and 29 patients on arm A and arm B, respectively, have progressed. For arm A, the 3-month PFS rate and 6-month PFS rate were 0.35 (95% CI, 0.15–0.56) and 0.29 (95% CI, 0.11–0.51), respectively. For arm B, the 3-month PFS rate and 6-month PFS rate were 0.24 (95% CI, 0.11–0.40) and 0.17 (95% CI, 0.06–0.32), respectively. No difference in OS or PFS between the 2 treatment arms was observed, but the study was not powered to detect such a difference.
FIGURE 2.
Progression-free survival by arm.
For the 10 treated and eligible patients in step 2, all of them had died at the time of this analysis. The median OS was 7.4 months (95% CI, 1.2–10.3 months). The median PFS was 1.3 months (95% CI, 0.8–1.6 months).
Toxicity
Among possibly treatment-related toxicities, grade 5 toxicities (hypoxia, aspiration, and death not otherwise specified) were observed in 3 patients (all treated with irinotecan 125 mg/m2 in arm A). The most common grade 3 toxicities for arm A and arm B were diarrhea without prior colostomy (n = 5) and fatigue (n = 7), respectively. step 2 treatment-related toxicities were reported for all 11 treated patients. See Table 4 for toxicities by arm, step, and irinotecan starting dose. Because of neutropenia associated with the administration of irinotecan, the irinotecan dose was reduced during the study accrual.
TABLE 4.
Treatment-related toxicity by step, arm, and irinotecan starting dose.
| Toxicity Type | No. by step, arm, dose, and toxicity grade
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1, Arm A*
|
Step 2
|
Arm B (n = 41) | |||||||||||||
| 125 mg/m2 (n = 13)
|
90 mg/m2 (n = 9)
|
125 mg/m2 (n = 3)
|
90 mg/m2 (n = 8)
|
||||||||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | |
| Hemoglobin | 1 | — | — | 3 | — | — | — | — | — | — | — | — | 2 | — | — |
| Leukocytes | 4 | 1 | — | — | — | — | — | — | — | 2 | — | — | 1 | — | — |
| Lymphopenia | 1 | — | — | — | — | — | — | — | — | — | 1 | — | 4 | — | — |
| Neutrophils | 3 | 1 | — | — | — | — | — | — | — | 1 | — | — | 1 | — | — |
| Platelets | 1 | — | — | 3 | — | — | — | — | — | 1 | — | — | — | 1 | — |
| Hypotension | 1 | 1 | — | — | — | — | — | — | — | — | — | — | 2 | — | — |
| Fatigue | — | — | — | — | — | — | 1 | — | — | — | — | — | 7 | — | — |
| Fever without neutropenia | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Insomnia | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Constitutional, other | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Death NOS | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Anorexia | 2 | — | — | 1 | — | — | — | — | — | 2 | — | — | — | — | — |
| Constipation | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Dehydration | 2 | 1 | — | 1 | — | — | 1 | — | — | 2 | — | — | — | — | — |
| Diarrhea without prior colostomy | 2 | — | — | 3 | — | — | — | — | — | 1 | — | — | 1 | — | — |
| Nausea | 1 | — | — | 2 | — | — | — | — | — | — | — | — | — | — | — |
| Vomiting | 2 | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Obstruction, small bowel NOS | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Stomach, hemorrhage | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Infection w/gr3-4 neut, lung | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Infection Gr0-2 neut, lung | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Infection Gr0-2 neut, trachea | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Infection Gr0-2 neut, blood | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Infection w/unk ANC foreign body | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Hypoalbuminemia | 1 | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Glomerular filtration rate | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Hypophosphatemia | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — |
| Hyperkalemia | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Hypokalemia | 1 | — | — | 2 | — | — | — | — | — | 1 | — | — | 2 | — | — |
| Hyponatremia | 3 | — | — | 1 | — | — | — | — | — | — | — | — | 1 | — | — |
| Nonneuropathic generalized weakness | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Dizziness | — | — | — | — | — | — | — | — | — | — | — | — | 3 | 1 | — |
| Neuropathy-sensory | — | — | — | — | — | — | — | — | — | — | — | — | 2 | 1 | — |
| Syncope | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Abdomen, pain | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Aspiration | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Dyspnea | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Hypoxia | — | — | 1 | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Pneumonitis/pulmonary infiltrates | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — |
| Vessel injury–carotid | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — |
One patient started irinotecan at dose 76 mg/m2 and was reported with grade 3 vomiting.
Cytokine expression
Among 61 eligible and treated patients, 23 patients gave consent for the correlative study and had pretreatment serum cytokine measurements available for data analysis. Because no differences in OS, PFS, or disease control response was noted between arms in our proceeding clinical data analysis, the 23 patients with laboratory data were combined across arms for cytokine data analysis.
Results from logistic regression analysis (with the cytokine concentration as a continuous variable) indicate that there is no significant relationship between differences in baseline concentration and disease control response for the sample size evaluated (Table 5). Longitudinal changes in each cytokine were examined up to cycle 2, day 1 treatment (1 hour after infusion). There is no relationship between the longitudinal changes in the cytokine expressions (treated as a continuous predictor) and disease control response for any cytokine.
TABLE 5.
Hazard ratio (with a unit increase in the form of log10 transformation) and 95% confidence interval by pretreatment cytokine (n = 23).
| Efficacy | Cytokine | HR (95% CI) | p value* |
|---|---|---|---|
| OS | |||
| GRO-1 | 0.75 (0.35, 1.62) | .47 | |
| HGF | 4.07 (1.21, 13.67) | .02 | |
| VEGF | 1.35 (0.58, 3.14) | .48 | |
| IL-6 | 1.13 (0.62, 2.07) | .69 | |
| IL-8 | 2.08 (0.47, 9.31) | .34 | |
| PFS | |||
| GRO-1 | 1.07 (0.39, 2.96) | .90 | |
| HGF | 1.50 (0.38, 5.91) | .56 | |
| VEGF | 1.16 (0.47, 2.86) | .75 | |
| IL-6 | 1.23 (0.55, 2.74) | .62 | |
| IL-8 | 2.31 (0.33, 16.2) | .40 | |
Via the univariate Cox proportional hazards model.
We further evaluated the hypothesis that an individual patient with longitudinal changes in 3 or more cytokines could predict OS or PFS, on the basis of a previous study demonstrating a relationship between these parameters for patients receiving chemoradiotherapy.10 Any slope above the upper tercile (across patients) was defined as a large change for each cytokine. No significant correlation was observed between the number of large increases (<3 vs ≥3) and DCR.
NF-κB nuclear staining
For the limited number of 10 tumor samples obtained from the study (5 before and 5 after registration), 7 showed intermediate (2+) or strongly positive (3+) nuclear staining, and 3 showed negative/weak 0–1+ staining for NF-κB RELA subunit p65, the intended target for proteasome inhibition. However, of the patients for which there were tumor biopsy specimens receiving bortezomib alone or with irinotecan, only 1 of 10 exhibited stable disease, providing insufficient response to determine any relationship between study groups or staining intensity.
DISCUSSION
This randomized phase II study was conducted to evaluate the clinical antitumor activity of bortezomib in combination with irinotecan or bortezomib as a single agent in patients with SCCHN. The objective response rates in this study were 13.1% (a 90% CI of 3.6%–30.3%) with irinotecan and bortezomib (arm A) and 2.6% (a 90% CI of 0.4%–22.1%) with bortezomib alone (arm B). For either arm, the observed response rate was not different than the null hypothesis (15% and 5% for arm A and arm B, respectively). Although the 90% confidence intervals for either arm include the targeted true response rates, the wide interval is probably due to the small number of patients on either arm.
Although patients receiving bortezomib together with the higher irinotecan dose demonstrated a superior response rate than those receiving the combined regimen with a lower irinotecan dose (n = 9) (23% vs 0%, non-significant), 3 grade 5 treatment-related adverse events were reported among the former. Further, the median overall survivals of 9.1 months (95% CI, 2.3–12.5 months) and 7.3 months (95% CI, 5.1–9.4 months) for arm A and arm B were not significantly greater than the median survival of approximately 11 months observed historically. Thus bortezomib in combination with a tolerable schedule of irinotecan or alone may not be worthy of further study in the general population of patients with recurrent or metastatic SCCHN. However, preclinical data show that the human papillomavirus E7 protein causes proteolytic degredation of the tumor suppressor Rb via a ubiquitin-dependent mechanism. Proteasome inhibition blocked the proteolysis of both E7 and Rb, suggesting that this pathway may be a unique target in human papillomavirus–associated malignancy.10
Head and neck cancer is associated with production of proangiogenic and proinflammatory cytokines. Thus inhibiting production of these cytokines might have therapeutic application. Our study did not demonstrate a pharmacodynamic relationship between cytokine response and tumor response in the setting of bortezomib in a significant cohort of patients. This finding has been noted in previous investigations as well.11 Several possible explanations for this observation include the following: (1) NF-κB may not be a “driver” event in head and neck cancer; (2) some tumors demonstrate robust expression of multiple NF-κB family members and related genes, which bortezomib may not be able to adequately inhibit; or (3) upregulation of alternative pathways in the setting of proteasome inhibition leads to bortezomib resistance.14,15 Recent studies suggest that combination of bortezomib with docetaxel, or with cetuximab and radiotherapy, may result in reduced PFS or OS.16,17 In the latter case, bortezomib was shown to antagonize cetuximab or radiotherapy-mediated degradation of epidermal growth factor receptor, a proteasome-dependent event implicated in sensitivity to cetuximab, chemotherapy or radiotherapy. It is unknown whether combination of bortezomib with other agents that target alternate mitogen activated protein kinase (MAPK) or signal transducer and activator of transcription 3 (STAT3) pathways downstream of EGFR and augment bortezomib cytotoxicity in experimental studies,13,14,18 could overcome such resistance in the clinical setting.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA39229, CA49957, CA14958, CA16116 and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Langer CI. Targeted therapy in head and neck cancer: state of the art 2007 and review of clinical applications. Cancer. 2008;112:2635–2645. doi: 10.1002/cncr.23521. [DOI] [PubMed] [Google Scholar]
- 2.Gibson MK, Li Y, Murphy B, et al. Randomized Phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, Ang KK, Brizel DM, et al. Head and Neck Cancers. J Natl Compr Cancer Netw. 2011;9:596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 4.Van Waes C. Nuclear factor-kappaB in development, prevention and therapy of cancer. Clin Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 5.Cusack JC, Liu R, Houston Am, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 6.Allen CT, Ricker JL, Chen Z, Van Waes C. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:959–971. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 7.Desai SD, Li TK, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61:5926–5932. [PubMed] [Google Scholar]
- 8.Murphy BA, Cmelak A, Burkey B, et al. Topoisomerase I inhibitors in the treatment of head and neck cancer. Oncology. 2001;15:47–52. [PubMed] [Google Scholar]
- 9.Gilbert J, Cmelak A, Shyr Y, et al. Phase II trial of irinotecan plus cisplatin in patients with recurrent or metastatic squamous carcinoma of the head and neck. Cancer. 2008;113:186–192. doi: 10.1002/cncr.23545. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Sampath A, Raychaudhuri P, Bachi S. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene. 2001:4740–4749. doi: 10.1038/sj.onc.1204655. [DOI] [PubMed] [Google Scholar]
- 11.Argiris A, Buchanan A, Brockstein B, et al. Docetaxel and iriontecan in recurrent or metastatic head and neck cancer: a phase 2 trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4504–4513. doi: 10.1002/cncr.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen C, Duffy S, Teknos T, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:182–190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 13.Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C. Nuclear NF-kappaB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res. 2009;15:5974–5984. doi: 10.1158/1078-0432.CCR-09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Ricker JL, Malhotra PS, et al. Differential bortezomib sensitivity in head and neck cancer lines corresponds to proteasome, nuclear factor-kappaB and activator protein-I related mechanisms. Mol Cancer Ther. 2008;7:1949–1960. doi: 10.1158/1535-7163.MCT-07-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloss CM, Wang F, Liu R, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14:5116–5123. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung CH, Aulino J, Muldowney NJ, et al. Nuclear factor-kappa B pathway and response in bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21:864–870. doi: 10.1093/annonc/mdp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argiris A, Duffy A, Kummar S, et al. Early tumor progression associated with enhanced EGFR signaling with bortezomib, cetuximab and radiotherapy for head and neck cancer. Clin Cancer Res. 2011;17:5755–5764. doi: 10.1158/1078-0432.CCR-11-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Zang Y, Sen M, Leeman-Neill RJ, et al. Bortezomib up-regulates activated signal transducer and activator of transcription-3 and synergizes with inhibitors of signal transducer and activator of transcription-3 to promote head and neck squamous cell carcinoma cell death. Mol Cancer Ther. 2009;8:2211–20. doi: 10.1158/1535-7163.MCT-09-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]