Abstract
Background
Corticosteroid excess is associated with declarative memory impairment and hippocampal atrophy. These findings are clinically important because approximately 1% of the population receives prescription corticosteroids at any time, and major depressive disorder is associated with elevated cortisol levels and hippocampal atrophy. In animals, hippocampal changes with corticosteroids are blocked by phenytoin. The objective of the current study was to extend these preclinical findings to humans. We examined whether phenytoin attenuated the effects of hydrocortisone on declarative memory. Functional magnetic resonance imaging (fMRI) assessed task-related hippocampal activation.
Methods
A randomized, double-blind, placebo-controlled, within-subject crossover study was conducted in 17 healthy adult volunteers. Participants received hydrocortisone (2.5 days), phenytoin (3.5 days), both medications together, or placebo, with 21-day washouts between conditions. Differences between treatments were estimated using a mixed-effects repeated measures analysis.
Results
Fifteen participants had data from at least two treatment conditions and were used in the analysis. Basal cortisol levels negatively correlated with fMRI BOLD activation in the para-hippocampus with a similar trend observed in the hippocampus. Decrease in declarative memory with hydrocortisone was blocked with concomitant phenytoin administration. Relative to the placebo condition, a significant decrease in hippocampal BOLD activation was observed with hydrocortisone and phenytoin alone, and the two medications in combination. Declarative memory did not show significant correlations with hippocampal activation.
Limitations
The modest sample size, which limited our statistical power, was a limitation.
Conclusions
Findings from this pilot study suggest phenytoin attenuated effects of corticosteroids memory in humans, but potentiated the reduction in hippocampal activation.
Keywords: anticonvulsant, corticosteroid, fMRI, cognition, hippocampus
Introduction
Corticosteroids (a.k.a glucocorticoids) are commonly prescribed medications (Fardet et al., 2011) that are associated with a variety of neuropsychiatric side effects (Fardet et al., 2012; Wolkowitz et al., 2009). The hippocampus appears to be a primary target for corticosteroids in the brain (Brown, 2009). Animal data suggest that exposure to high levels of corticosteroids in stress paradigms or through corticosterone administration is associated with changes in the hippocampus including dendritic remodeling (Magarinos et al., 1997; Vyas et al., 2002). In non-human primates, most (Sapolsky et al., 1990; Uno et al., 1994; Uno et al., 1989), but not all (Leverenz et al., 1999), studies suggest that stress or exogenous corticosteroid administration are associated with atrophy of the hippocampus.
In humans, acute corticosteroid administration is associated with a reversible decline in declarative memory performance in adults (Brown et al., 2006; de Quervain et al., 2000; Newcomer et al., 1999; Wolkowitz et al., 1990) and children (Bender et al., 1988). The magnitude of memory change with corticosteroids may be related to specific gene polymorphisms (Kumsta et al., 2010). Chronic endogenous hypercortisolism in Cushing’s syndrome is likewise associated with hippocampal atrophy and cognitive, particularly declarative memory, impairment (Starkman et al., 1992). At least partial recovery of brain atrophy and cognitive function occurs after treatment of Cushing’s syndrome (Starkman et al., 1999), but the extent of recovery declines with age (Hook et al., 2007), and neuropsychiatric dysfunction remains a major source of morbidity in this disease. Chronic exogenous corticosteroid use is also associated with structural and functional hippocampal changes. We reported that patients receiving long-term prescription corticosteroid therapy had poorer declarative memory, decreased hippocampal volume, and decreased temporal lobe levels of N-acetyl aspartate as compared to controls with similar medical histories but minimal lifetime corticosteroid exposure (Brown et al., 2007; Brown et al., 2004).
Only two studies have examined the impact of exogenous corticosteroids on functional magnetic resonance imaging (fMRI) in humans. Decreased activity in both the hippocampus and prefrontal-cortex was observed in healthy controls scanned before and one hour after oral hydrocortisone (Oei et al., 2007). In another report using healthy controls, hydrocortisone administration was associated with decreased activity in the hippocampus and amygdala, reaching a peak response minimum at approximately 30 minutes post-injection (Lovallo et al., 2010). Thus, hippocampal activation, as assessed by fMRI, appears to be decreased by acute administration of corticosteroids.
Stress and corticosteroids increase glutamate release in the hippocampus (Popoli et al., 2012). In animal models, the effects of stress or corticosteroids on the hippocampus are attenuated by agents that decrease glutamate release (e.g. phenytoin) or block the NMDA receptor (Magarinos et al., 1996). We reported significantly greater improvement in declarative memory with the NMDA receptor antagonist memantine than with placebo in patients receiving chronic corticosteroid therapy (Brown et al., 2008a). We observed significant improvement in declarative memory in corticosteroid-dependent patients receiving the glutamate release inhibitor lamotrigine.
In this report of a proof-of-concept study, we examine whether phenytoin prevents declarative memory changes with hydrocortisone (clinical aim). In addition, we explore the effect of hydrocortisone and phenytoin alone and in combination on hippocampal activation during a task (mechanistic aim).
Methods
The University of Texas Southwestern Medical Center Institutional Review Board (IRB) approved this study. All participants completed an IRB-approved written informed consent process at the Psychoneuroendocrine Research Program offices on the UT Southwestern campus. The study was registered at http://clinicaltrials.gov (NCT00591006).
Healthy volunteers (n=17) were recruited through flyers and other forms of advertising. Included were men and women age 18–50, vision corrected to at least 20–40, education of ≥ 12 years, and baseline Rey Auditory Verbal Learning Test (RAVLT) (Ryan et al., 1986) total words recalled score ≥ 40 (to exclude baseline cognitive impairment). Excluded were those with a history of schizophrenia, major depressive, bipolar, posttraumatic stress, schizoaffective, panic or eating disorders, or drug/alcohol abuse/dependence, seizures, brain surgery, multiple sclerosis, Parkinson’s disease, taking CNS-acting medications, contraindications to phenytoin, hydrocortisone or MRI, significant medical conditions, or current tobacco use, pregnant/nursing women, prisoners, cognitive disorders, baseline 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) score > 7 (to exclude those with clinically significant depressive symptoms at baseline), current suicidal ideation or history of a suicide attempt, and history of systemic or past 14 day inhaled corticosteroid use. Participants and all persons with participant contact were blinded to treatment order. The Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) was used to rule out exclusionary psychiatric illnesses. Declarative memory was assessed with the RAVLT (Ryan et al., 1986). Alternative versions of the RAVLT (different words) were used to minimize learning effects from repeated administration.
Participants received each of the four medication conditions (hydrocortisone + placebo, phenytoin + placebo, hydrocortisone + phenytoin, placebo + placebo) and four fMRI scans using a randomized, crossover design with a 21-day washout between medication conditions. Medication and placebo were purchased from Abram’s Royal Pharmacy, Dallas, Texas. Three days prior to fMRI scans, participants took four capsules containing phenytoin (100 mg) or identical placebo by mouth at 2100 hours (400 mg/day) for a total of 3.5 days. Beginning 2 days prior to fMRI scans (day after initiating phenytoin or placebo), participants began taking four tablets containing hydrocortisone (20 mg) or placebo at 0900 hours and 2100 hours (160 mg/day) with the last dose at 0900 hours on the day of imaging. Thus, hydrocortisone was administered for a total of 2.5 days. Neuroimaging was performed at approximately 1300 hours after each exposure to medication. The RAVLT was administered at baseline and after each medication course. Blood was drawn at approximately 1400 hours at baseline and after each scan to assess cortisol and phenytoin levels. The samples were sent to the Immunopharmacology Laboratory at National Jewish Medical and Research Center in Denver, Colorado for cortisol analysis and Quest Diagnostics for phenytoin levels. Side effects/adverse events were assessed at each visit. Participants were paid for their participation.
Scan acquisition
Imaging was performed on a 3 Tesla MRI system (Philips Medical Systems, Best, The Netherlands). A body coil was used for radiofrequency transmission and an eightchannel sensitivity encoding (SENSE) head coil was used for receiving. Foam padding stabilized the head and minimized motion.
The fMRI image acquisition used Blood-Oxygenation-Level-Dependent (BOLD) pulse sequence with the following parameters: TR=1500 ms, flip angle 70 degrees (to allow for T1 relaxation and to reduce inflow effect), TE=30 ms, SENSE factor 2, field-of-view 220x220 mm2, matrix 64×64, 30 axial slices, voxel size 3.44×3.44×5 mm3 no gap, duration 6 minutes/run. A T1-weight anatomic image was acquired using a Magnetization-Prepared-Rapid-Acquisition-of-Gradient-Echo (MPRAGE) sequence with the following imaging parameters: TR=2100 ms, TE=3.8 ms, TI=1100 ms, field-of-view 256×256×160 mm3, matrix 256×256×160, voxel size 1×1×1 mm3, duration 3 minutes and 57 seconds. In addition, we acquired an fMRI correction factor to account for potential alterations in brain vascular physiology due to hydrocortisone or phenytoin. The fMRI signal is negatively modulated by baseline blood oxygenation independent of neural activity (Lu et al., 2008). The modulation effect can be appreciated by considering that the fMRI signal is based on changes in venous blood oxygenation (Yv) from resting to activated conditions and that Yv cannot exceed 100%. Therefore, a lower resting Yv means an increase in activation, potentially generating a greater fMRI signal. Differences in this parameter across individuals or sessions are a major source of variation in fMRI signal amplitude (Cohen et al., 2002; Lu et al., 2008). Medications used in our study may alter Yv and present a confounding factor in assessment of neural activity using fMRI. This effect was corrected by measuring Yv in each session using a T2-Relaxation-Under-Spin-Tagging (TRUST) MRI technique (Lu and Ge, 2008). The imaging parameters were: voxel size 3.44×3.44×5mm3, TR=8000ms, TI=1200ms, four TEs: 0ms, 40ms, 80ms and 160ms, measurement made in the superior sagittal sinus, duration 4.3 min.
Imaging Task
The fMRI experiments used a novelty detection task. Before entering the magnet, participants viewed two pictures, one indoor and one outdoor scene, for 20 seconds each. These were then considered as familiar pictures. For the in-scanner task, five fMRI runs were performed. Each run consisted of 40 stimulation trials with a stimulation period of 2 seconds followed by a fixation period of 4 seconds. During the stimulation period, one of four stimulus types (a familiar indoor, a familiar outdoor, a novel indoor, or a novel outdoor picture) was shown using a video projector located on the back of the magnet (visual angle=25°). Participants pressed a left-hand button for an indoor scene or right-hand button for an outdoor scene, regardless of familiarity. The purpose of the button press was to maintain attention. Each novel picture was used only once during the session. Additionally, periods of six-second fixation were introduced pseudorandomly (20/fMRI run) to vary the inter-trial interval.
Image processing
The fMRI images were preprocessed and analyzed using Statistical Parametric Mapping software (SPM5). All fMRI images were first realigned to correct for motion. The MPRAGE image was segmented into grey matter (GM) and white matter (WM) segments. The GM segment was normalized into Montreal Neurological Institute (MNI) space, and transformation parameters were applied to all images and the coil-sensitivity corrected MPRAGE image that was created during segmentation. After the normalization step, voxel size of all images was set to 3×3×3 mm3. All normalized functional images were then smoothed with an 8×8×8mm3 full width at half maximum (FWHM) Gaussian kernel.
The onset time for all pictures in the fMRI task was used as linear regressors for general linear model (GLM) analysis available in SPM5. A regressor for novel pictures and another for familiar pictures were specified in the GLM. Parameter estimates for the regressors were calculated and contrast images were generated for the novel pictures-familiar pictures contrast. The fMRI results presented were all based on the “novel pictures-familiar pictures” contrast.
Contrast images from individual subject analyses were then used in the group-level analysis with the flexible factorial statistical method in SPM5. Anatomic regions of interest (ROIs) in MNI space were created for the left and right hippocampus, and left and right para-hippocampal gyrus. These ROIs were created using WFU_PickAtlas software (Functional MRI Laboratory, Wake Forest University School of Medicine, NC). The average hemodynamic response function (HRF) for each regressor in the GLM design was then calculated from each ROI using a script from Marsbar toolbox (Brett et al., 2002). The peak stimulus response on HRF curve was seen between 4.5 and 9 seconds after stimulus onset, and these responses were summed to obtain integrated % signal change. The fMRI signal amplitude was corrected for Yv variance using a linear equation described previously (Lu et al., 2010): Scorr= Sorig-Yv*slope. The slope variable indicates the Yv modulation effect and represents the amount of signal variation for each unit of Yv difference when assuming that neural activity is identical. The slope was determined using the placebo/placebo data on a region specific basis.
Statistical Analysis
Of the 17 participants, two were excluded because data from at least two medication conditions were not available, leaving 15 evaluable participants. The participants received all four treatments except for three participants that each missed one treatment. The order of treatments was randomized by TJC (co-author) using a random number sequence and balanced such that every treatment followed every other treatment the same number of times. Statistical analysis was conducted for ROI results from the hippocampus and para-hippocampus. The fMRI signals, Scorr, of the left and right hemispheres were averaged for analysis as no significant lateralization in activation was observed. The differences between treatments were estimated with a mixed-effects repeated measures analysis (SAS Proc Mixed) to the Scorr measures. The model contained terms for treatment group, order of scans, and a term to allow for carry-over effects from prior treatment. Because of the cross-over design, all effects were within-subject. The effect sizes were computed by dividing the between-group difference estimated from the mixed-effects model by an estimate of the standard deviation of the raw data (Feingold, 2009; Raudenbush and Xiao-Feng, 2001). Standard deviation from the placebo/placebo condition was used to compute effect sizes.
Results
Demographic and baseline characteristics of the participants are given in Table 1. The sample was relatively young with normal baseline mood and declarative memory performance. Mean cortisol levels were higher during hydrocortisone administration (phenytoin + hydrocortisone 34.7±32.7, placebo + hydrocortisone 28.9±25.0, placebo + placebo 8.9±5.9, phenytoin + placebo 12.2±9.1). Phenytoin levels ranged from 2.6 to 15.8 (mean 8.6±3.5) during the phenytoin + hydrocortisone administration and 4.6 to 15.0 (mean 8.2±3.2) during the phenytoin + placebo administration. Phenytoin administration was not associated with significant differences in cortisol levels relative to placebo, and phenytoin levels did not correlate with cortisol levels.
Table 1.
Baseline Demographic characteristics of participants (N =15)
| Variable | Mean or % (N=15) |
S.D |
|---|---|---|
| Age (years) | 25.3 | 8.1 |
| Education (years) | 16.5 | 2.8 |
| Female (%) | 60.0 | |
| Race | ||
| Caucasian (%) | 73.3 | |
| African American (%) | 6.7 | |
| Hispanic (%) | 13.3 | |
| Native American (%) | 6.7 | |
| Right Handed (%) | 83.3 | |
| RAVLT Total T-Score | 53.8 | 8.1 |
Declarative memory data are provided in Table 2. Hydrocortisone was associated with a trend toward poorer performance than placebo on the RAVLT total (mean change −4.25, SE= 2.52 , p=.1, Cohen’s d 0.38). RAVLT total performance when phenytoin was given along with hydrocortisone was not significantly different than placebo alone and that was significantly better than with hydrocortisone alone (Cohen’s d 0.74).
Table 2.
Changes in Declarative Memory between Treatment Conditions
| Measure | Difference | Mean | Std. Error |
D.F. | t-Value | P-Value | Effect Size |
|---|---|---|---|---|---|---|---|
| RAVLT Total T-Score | Phen+HC- Pbo+HC |
8.27 | 2.65 | 33.1 | 3.12 | <.01 | 0.74 |
| RAVLT Total T-Score | Phen+HC- Pbo+Pbo |
4.02 | 2.52 | 33.8 | 1.59 | .12 | 0.36 |
| RAVLT Total T-Score | Phen+Pbo Pbo+Pbo |
−3.46 | 2.37 | 32.7 | −1.46 | .15 | 0.31 |
| RAVLT Total T-Score | Pbo+HC- Pbo+Pbo |
−4.25 | 2.52 | 32.4 | −1.69 | .10 | 0.38 |
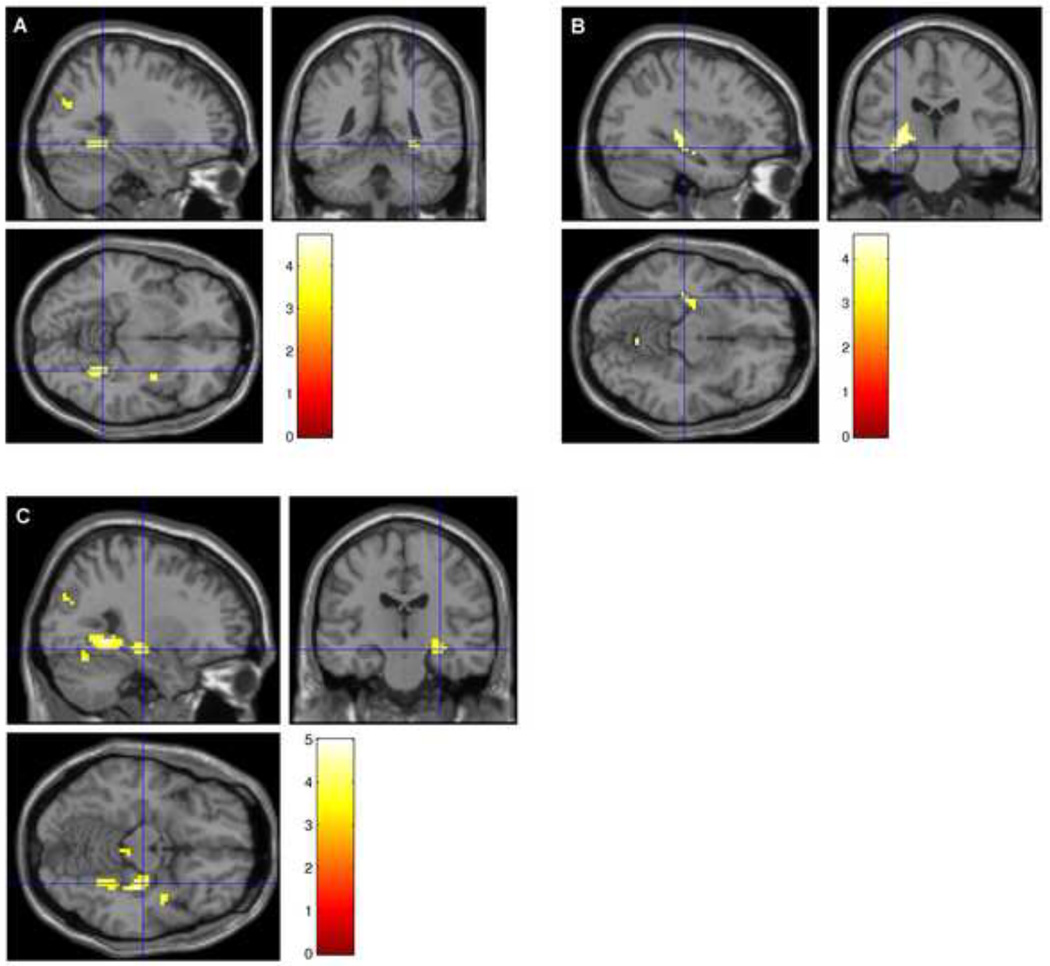
In an effort to better understand physiologic mechanisms for the observed memory changes, we performed fMRI in conjunction with declarative memory testing. Baseline venous oxygenation was decreased by hydrocortisone, but not by phenytoin (Yv values: placebo + hydrocortisone 60.0±4.6%, phenytoin + hydrocortisone 61.1±5.7%, placebo + placebo 63.8±5.6%, phenytoin + placebo 65.6±3.4%). Paired t-tests revealed that placebo + placebo vs. placebo + hydrocortisone Yv values (p=0.03) and phenytoin + placebo vs. phenytoin + hydrocortisone (p=0.007) were significantly different but the phenytoin + placebo vs. placebo + placebo comparison was not (p=0.68).The corrected fMRI ROI data are provided in Table 3, and a whole brain group comparison image of the placebo + placebo > phenytoin + hydrocortisone, placebo + placebo > phenytoin + placebo, and placebo + placebo > placebo + hydrocortisone in Figure 1. Task-related hippocampal activation as assessed by Scorr showed the following pattern: placebo + placebo > phenytoin + placebo and also placebo + placebo > hydrocortisone + placebo (both large effect sizes). Thus, the signal was similarly lower in the hippocampus with either hydrocortisone or phenytoin alone as compared to placebo. The combination of hydrocortisone and phenytoin was associated (at the trend level) with even lower activation than either medication alone. In the para-hippocampus, phenytoin and hydrocortisone alone were associated with non-significantly lower activation than placebo, while the combination was associated with significantly lower activation than placebo (large effect size).
Table 3.
Hippocampal and parahippocampal activation differences between treatment conditions
| Brain Region |
Difference | Mean | STD Error |
DF | T-value | P-Val | Effect Size |
|---|---|---|---|---|---|---|---|
| Hippocampus | Phen+HC- Pbo+HC |
−0.12 | 0.07 | 35.4 | −1.83 | .08 | 0.67 |
| Hippocampus | Phen+HC- Pbo+Pbo |
−0.28 | 0.06 | 34.9 | −4.43 | <.01 | 1.58 |
| Hippocampus | Phen+Pbo- Pbo/Pbo |
−0.17 | 0.06 | 33.0 | −2.76 | <.01 | 0.93 |
| Hippocampus | Pbo+HC- Pbo+Pbo |
−0.16 | 0.06 | 33.3 | −2.55 | .02 | 0.90 |
| Para-Hippocampus | Phen+HC- Pbo+HC |
−0.18 | 0.10 | 33.6 | −1.84 | .08 | 0.58 |
| Para-Hippocampus | Phen+HC- Pbo+Pbo |
−0.37 | 0.10 | 33.1 | −3.81 | <.01 | 1.17 |
| Para-Hippocampus | Phen+Pbo- Pbo+Pbo |
−0.15 | 0.09 | 31.1 | −1.62 | .11 | 0.47 |
| Para-Hippocampus | Pbo+HC- Pbo+Pbo |
−0.19 | 0.10 | 31.6 | −1.93 | .06 | 0.59 |
Figure 1.
Group subtraction results that survived an uncorrected threshold of p=0.001 for A Placebo + Placebo − Hydrocortisone + Placebo, B Placebo + Placebo − Phenytoin + Placebo, and C Placebo + Placebo − Phenytoin + Hydrocortisone.
No significant correlations were observed between changes in BOLD activation and changes in RAVLT total scores (all p > .1), except for a trend for a negative relationship between change in RAVLT score and change in activation between phenytoin + hydrocortisone and placebo + placebo conditions (r=−0.510, p=.090). Cortisol level showed a significant negative correlation with BOLD activation in the para-hippocampus in the placebo + placebo condition (r=−0.541, p=.037), and a trend toward negative correlations in the hippocampus in the phenytoin + placebo (r=−0.475, p=.073) and placebo + placebo (r=−0.448, p=.094) conditions. Cortisol level was not associated with BOLD activation during either hydrocortisone administration condition (both p>.1). Phenytoin levels were not associated with BOLD activation during either phenytoin administration condition (both p>.1)
All of the treatment conditions were well tolerated. Two adverse events were reported during the study. One participant, during the placebo + hydrocortisone condition, experienced difficulty breathing, tremors in his left hand and disorientation to time while undergoing the fMRI procedure. The participant recovered in a few minutes, and was discontinued from the study. In another participant, mega cisterna magna was diagnosed as a benign and incidental finding on the MRI scans.
Discussion
We observed a reduction in declarative memory performance with hydrocortisone. Although this decline in RAVLT scores during hydrocortisone administration had a medium effect size, the findings did not reach statistical significance (p=0.1) perhaps due to the modest sample size in this pilot study. The finding is consistent with our prior research (Brown et al., 2006) and research by other investigators (de Quervain et al., 2000; Newcomer et al., 1999; Wolkowitz et al., 1990) suggesting reversible decline in declarative memory with brief corticosteroid administration. We also observed a reduction in baseline venous oxygenation with hydrocortisone. This finding suggests that hydrocortisone has effects on brain vasculature. Previous studies have reported a negative relationship between baseline cortisol levels and resting regional cerebral perfusion in the medial temporal lobe of patients with posttraumatic stress disorder (Bonne et al., 2003). In healthy controls, diurnal cortisol variability was negatively associated with hippocampal and para-hippocampal BOLD signal during a stressful stimulus (Cunningham-Bussel et al., 2009). We observed that basal cortisol levels were negatively associated with hippocampal activation during a task that was not stressful or emotionally charged, but, perhaps due to the overall increase in levels and differences in metabolism, this relationship disappeared following hydrocortisone administration. During hydrocortisone administration, a reduction in the BOLD signal was observed. These imaging results are consistent with two prior fMRI reports of decreases in hippocampal activation with hydrocortisone (Lovallo et al., 2010; Oei et al., 2007). The current study design used a longer exposure to hydrocortisone than did prior studies. Thus, the findings are novel in that we assessed the impact of hydrocortisone over repeated administration, not after a single dose.
Despite many decades of use for seizures, no previous studies have examined the effects of phenytoin on hippocampal activation. The findings suggest that phenytoin is associated with a decrease in hippocampal BOLD activation. This is similar to what has been observed in previous studies using the anticonvulsant lamotrigine (Brown et al., 2010; Deakin et al., 2008). No significant change in venous oxygenation was observed with phenytoin alone as compared to placebo. This is in contrast to the reduction in venous oxygenation with hydrocortisone. To our knowledge, prior studies have not examined the impact of phenytoin, as compared to placebo, on declarative memory. We found a non-significant decline in performance on the RAVLT compared to placebo when phenytoin was administered alone. Consistent with our findings, one report in healthy controls observed a non-significant decline in performance on a declarative memory task from baseline with phenytoin administration (Meador et al., 1995).
The primary aim of the study was to examine the effects of the combination of phenytoin and hydrocortisone on declarative memory. Based on the preclinical literature, we hypothesized that corticosteroid effects on human declarative memory could be prevented with phenytoin. Our findings support this hypothesis. RAVLT performance with the combination of phenytoin and hydrocortisone was significantly better than with hydrocortisone alone and was not significantly different from the placebo condition. The ability of phenytoin to prevent the effects of hydrocortisone on declarative memory is important because declarative memory impairment is perhaps the most common and clinically relevant feature of hippocampal insult or dysfunction. The finding is consistent with preclinical data suggesting that phenytoin prevents stress-induced hippocampal CA3 region apical dendritic atrophy in subordinate tree shrews (Magarinos et al., 1996). Thus, the current data translate the pre-clinical data into humans and suggest that phenytoin can prevent the memory effects of hydrocortisone in humans as well as histological changes in the animal hippocampus. Improvement in memory was only observed when phenytoin was given in combination with hydrocortisone. This finding is consistent with what we observed with the glutamate release inhibitor lamotrigine. When administered to chronic corticosteroid-treated patients, lamotrigine was associated with a significant improvement in declarative memory as compared to placebo (Brown et al., 2008b)). However, in a group of patients with bipolar disorder, we observed a slight decline in declarative memory with lamotrigine alone (Osuji et al., 2008).
The memory findings in this report extend our previous research demonstrating improvement in declarative memory with lamotrigine in corticosteroid-treated patients (Brown et al., 2008b). Declarative memory changes appear to be both prevented and reversed by medications that, among other mechanisms, decrease glutamate release. Clinically, the current findings suggest that phenytoin may attenuate the effects of acute, high-dose corticosteroid therapy on declarative memory. However, we do not know whether phenytoin, like lamotrigine, reverses memory impairment associated with long-term corticosteroid therapy.
Our observation of an additive effect between phenytoin and hydrocortisone on BOLD signal but a counteracting effect between them on memory is one of the most intriguing findings of this study. We hypothesize the reason to be that each drug can alter (at least) two aspects of the brain physiology, baseline glutamate level and neuronal excitation, and the BOLD and memory performance changes reflect different aspects of these effects. Stress and administration of corticosteroids are associated with increases in baseline glutamate in the hippocampus (Chen et al., 1998; Ioannou et al., 2003; Lowy et al., 1993; Moghaddam et al., 1994). Higher-than-normal glutamate release in the hippocampus apparently has a negative impact on memory as studies have shown that normalization of glutamate release using an NMDA receptor antagonist [27] or glutamate release inhibitor (Brown et al., 2008b)) can restore memory performance. Therefore, we hypothesize that the reduction in memory performance due to hydrocortisone administration primarily reflects its enhancement effect on glutamate release. On the other hand, hydrocortisone also has the effect of reducing neuronal excitability (Hamzei et al., 2002). At the dose used in the present study, hydrocortisone binds primarily to the glucocorticoid receptor (GR), the activation of which is associated with decreased neuronal excitability (Prager et al., 2010). Decreased neuronal excitability is expected to decrease the BOLD signal, as we have observed in our experiment.
The effect of phenytoin on glutamate levels is opposite to that of hydrocortisone. Phenytoin is associated with a reduction in baseline glutamate release (Magarinos et al., 1996) and upregulation of gene expression associated with glutamate degradation (Mariotti et al., 2010). Therefore, the effect of phenytoin on memory is also expected to be opposite to that of hydrocortisone (i.e. restoring glutamate levels and memory performance). In terms of the effect of phenytoin on neuronal excitability, interestingly it is similar to the effect of hydrocortisone (i.e. decreasing excitability). Assuming that the neuronal excitability is directly related to the BOLD signal, phenytoin is expected to decrease hippocampal activation (Greenhill and Jones, 2010), as our experimental data have shown. In short, the two drugs seem to have opposite effects in terms of baseline glutamate level, but similar effects on neuronal excitability. Our data therefore suggest that the memory effects of the drugs are most reflective of their influence on baseline glutamate level whereas the alterations in BOLD fMRI are most reflective of the changes in neuronal excitability due to the drugs. It should be noted that a decrease in baseline glutamate level does not always cause memory improvement and an increase does not always cause memory impairment. Instead, it is possible that brain’s baseline glutamate level has an optimal level under which the memory performance is at its best. Therefore, both an increase (e.g. due to hydrocortisone alone) and a decrease (due to phenytoin alone) from that optimal level are detrimental (see Table 2). However, when the two are used in conjunction, the memory performance may be restored.
The current report found that changes in declarative memory did not correlate with changes in hippocampal BOLD activation. The literature on the relationship between task-related hippocampal activation and declarative memory performance is also mixed. A positive correlation between memory and hippocampal activation (Johnson et al., 2001) as well as no correlation (Petrella et al., 2007) are reported. Perhaps due to a compensatory mechanism, greater hippocampal activation in cognitive impaired subjects than in controls has also been reported (Schwarze et al., 2009; Thomaes et al., 2009) and is suggestive of a negative relationship between hippocampal BOLD signal and memory performance. Given these findings, one explanation for attenuation of the effects of hydrocortisone on the RAVLT and enhancement of its effect on BOLD signal by phenytoin is that declarative memory and the hippocampal BOLD signal are not closely related in the present intervention study using multiple medications. The improvement in memory performance associated with phenytoin administration could be due to the reduction in baseline glutamate release, resulting in a greater potential for neural response with a task. The BOLD signal did not reflect this change because the fMRI signal is additionally affected by the effect of phenytoin on vascular reserve. Therefore, the discrepancy between memory performance and BOLD signal could be due to a difference between neural-based signal and vasculature-based signal. Both findings are potentially important. Memory is an observable process mediated by the hippocampus that can impact daily functioning. On the other hand, the BOLD signal may reflect important mechanisms within the hippocampus. Future research should use techniques, such as magnetic resonance spectroscopy and basal cerebral blood flow measurement, which can measure basal glutamate concentrations and neural activity more directly and correlate these changes with declarative memory performance.
The study has several limitations. The sample size in this pilot study was modest limiting our ability to detect between-group differences. Thus, the findings must be interpreted with caution. However, the statistical power was increased by the crossover design in which the subjects served as their own controls. Perhaps due to the sample size in this study, hydrocortisone alone was associated with a reduction in RAVLT performance that did not reach statistical significance (p=0.10). The duration of exposure to hydrocortisone was subacute, but longer than in any other fMRI studies. Consequently, the findings might not be generalizable to settings with longer exposures to corticosteroids or phenytoin. In many cases the participants did not reach therapeutic phenytoin levels for seizures. However, the concentration needed to attenuate the effects of hydrocortisone on the hippocampus may not be the same as that needed for seizure control. Finally, it is important to note that phenytoin has a complex mechanism of action that, in addition to reducing glutamate release, includes decreasing sodium influx through inhibition of sodium channels, decrease in calcium influx, enhancement of ligand binding at sigma sites, and enhancement of GABA neurotransmission (Catterall, 1999; Tunnicliff, 1996). Thus, one cannot assume that all of the observed effects of phenytoin in this study were related to glutamate.
In summary, our findings suggest that this paradigm can be used to assess the effects of corticosteroids on the human hippocampus. These results also suggest that both declarative memory and hippocampal activation, as assessed with the BOLD signal, are sensitive to the effects of brief exogenous corticosteroid administration. In addition, the results extend findings with phenytoin in animal models to humans and suggest that phenytoin may attenuate the impact of acute corticosteroid exposure on declarative memory (the memory process mediated by the hippocampus). Phenytoin has already demonstrated potential beyond seizure control (e.g. treatment of mania in bipolar disorder) (Mishory et al., 2000). The current findings suggest that phenytoin may also block the effects of stress or corticosteroids on declarative memory. Future research will include a larger trial of phenytoin to confirm these findings, use of other medications in the paradigm, use of other neuroimaging techniques, and exploration of hippocampal activation with hydrocortisone in clinical populations associated with elevated cortisol, such as major depressive disorder and Cushing’s disease.
Acknowledgments
We would like to thank Kimberly Anderson who assisted with the preparation and proof-reading of the manuscript.
Financial support: Funded by NIH grant MH078182 to ESB and MH084021 to HL.
I would like to disclose that I have research support from NIAAA, NIDA, NIMH, NHLBI, Stanley Medical Research Institute, Forest and Sunovion.
Dr. Carmody has provided consulting services for Cyberonics, Inc.
Dr. Auchus reports research support from NIGMS, the Burroughs Wellcome Fund, the Thrasher Foundation, the American Cancer Society, and the Partnership for Clean Competition; he participates in contracted research with Novartis Pharmaceuticals, Janssen Pharmaceuticals, and Corcept Therapeutics; he is or was a paid consultant for Janssen Pharmaceuticals/Johnson & Johnson, Viamet Pharmaceuticals, Orphagen Pharmaceuticals, Bristol Myers Squibb, and BioMarin Pharmaceuticals.
Dr. Tamminga works as an ad hoc consultant for Alexza Pharmaceuticals Inc., Astellas, Axion Advisors, LLC, Bradley, Arant, Boult and Cummings, LLC (Pfizer), Eli Lilly Pharmaceuticals, and Merck and serves as the deputy editor of the American Psychiatric Association, is on the advisory board of Intracellular Therapies (ITI, Inc.), and is a Council Member with NAMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
ES Brown served as the PI of the study and participated in the design of the study, supervision of data collection and manuscript writing. H Lu participated in the design of the study, data collection and analysis, writing and editing of the manuscript. D Denniston participated in data collection and editing of the manuscript. J Uh participated in data collection and analysis, writing and editing of manuscript. BP Thomas participated in data collection and analysis, writing and editing of manuscript. TJ Carmody participated in data analysis and writing and editing of the manuscript. RJ Auchus participated in data interpretation and writing and editing of manuscript. R Diaz-Arrastia participated in the design of the study and reviewed manuscript drafts. C Tamminga participated in the design of the study, supervised data collection and analysis, and edited manuscript drafts. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No other authors have disclosures to make.
References
- 1.Bender BG, Lerner JA, Kollasch E. Mood and memory changes in asthmatic children receiving corticosteroids. J Am Acad Child Adolesc Psychiatry. 1988;27:720–725. doi: 10.1097/00004583-198811000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Bonne O, Gilboa A, Louzoun Y, Brandes D, Yona I, Lester H, Barkai G, Freedman N, Chisin R, Shalev AY. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biological psychiatry. 2003;54:1077–1086. doi: 10.1016/s0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- 3.Brett M, Anton J, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan: 2002. [Google Scholar]
- 4.Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown ES, Beard L, Frol AB, Rush AJ. Effect of two prednisone exposures on mood and declarative memory. Neurobiol Learn Mem. 2006;86:28–34. doi: 10.1016/j.nlm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Brown ES, Vazquez M, Nakamura A. Randomized, placebo-controlled, crossover trial of memantine for cognitive changes with corticosteroid therapy. Biol Psychiatry. 2008a;64:727–729. doi: 10.1016/j.biopsych.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Brown ES, Vera E, Frol AB, Woolston DJ, Johnson B. Effects of chronic prednisone therapy on mood and memory. J Affect Disord. 2007;99:279–283. doi: 10.1016/j.jad.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown ES, Wolfshohl J, Shad MU, Vazquez M, Osuji IJ. Attenuation of the effects of corticosteroids on declarative memory with lamotrigine. Neuropsychopharmacology. 2008b;33:2376–2383. doi: 10.1038/sj.npp.1301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown ES, Woolston JD, Frol A, Bobadilla L, Khan DA, Hanczyc M, Rush AJ, Fleckenstein J, Babcock E, Cullum CM. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Brown ES, Zaidel L, Allen G, McColl R, Vazquez M, Ringe WK. Effects of lamotrigine on hippocampal activation in corticosteroid-treated patients. J Affect Disorders. 2010;126:415–419. doi: 10.1016/j.jad.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catterall WA. Molecular properties of brain sodium channels: an important target for anticonvulsant drugs. Adv Neurol. 1999;79:441–456. [PubMed] [Google Scholar]
- 12.Chen J, Adachi N, Tsubota S, Nagaro T, Arai T. Dexamethasone augments ischemia- induced extracellular accumulation of glutamate in gerbil hippocampus. Eur J Pharmacol. 1998;347:67–70. doi: 10.1016/s0014-2999(98)00198-8. [DOI] [PubMed] [Google Scholar]
- 13.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, Weisholtz DS, Pavony M, Silverman ME, Goldstein MS, Altemus M, Cloitre M, Ledoux J, McEwen B, Stern E, Silbersweig D. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology. 2009;34:694–704. doi: 10.1016/j.psyneuen.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 16.Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 17.Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford) 2011;50:1982–1990. doi: 10.1093/rheumatology/ker017. [DOI] [PubMed] [Google Scholar]
- 18.Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following gluco-corticoid therapy in primary care. Am J Psychiatry. 2012;169:491–497. doi: 10.1176/appi.ajp.2011.11071009. [DOI] [PubMed] [Google Scholar]
- 19.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First M, Spitzer R, Gibbon M, JBW W. Structured Clinical Interview for DSM-IV Axis I Disorders. Columbia University, New York: Biometrics Research Department, New York State Psychiatric Institute, Department of Psychiatry; 1995. [Google Scholar]
- 21.Greenhill SD, Jones RS. Diverse antiepileptic drugs increase the ratio of background synaptic inhibition to excitation and decrease neuronal excitability in neurones of the rat entorhinal cortex in vitro. Neuroscience. 2010;167:456–474. doi: 10.1016/j.neuroscience.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C. Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage. 2002;17:490–496. doi: 10.1006/nimg.2002.1077. [DOI] [PubMed] [Google Scholar]
- 24.Hook JN, Giordani B, Schteingart DE, Guire K, Giles J, Ryan K, Gebarski SS, Langenecker SA, Starkman MN. Patterns of cognitive change over time and relationship to age following successful treatment of Cushing's disease. J Int Neuropsychol Soc. 2007;13:21–29. doi: 10.1017/S1355617707070051. [DOI] [PubMed] [Google Scholar]
- 25.Ioannou N, Liapi C, Sekeris CE, Palaiologos G. Effects of dexamethasone on K(+)-evoked glutamate release from rat hippocampal slices. Neurochemical research. 2003;28:875–881. doi: 10.1023/a:1023271325728. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SC, Saykin AJ, Flashman LA, McAllister TW, Sparling MB. Brain activation on fMRI and verbal memory ability: functional neuroanatomic correlates of CVLT performance. Journal of the International Neuropsychological Society : JINS. 2001;7:55–62. doi: 10.1017/s135561770171106x. [DOI] [PubMed] [Google Scholar]
- 27.Kumsta R, Entringer S, Koper JW, van Rossum EF, Hellhammer DH, Wust S. Working memory performance is associated with common glucocorticoid receptor gene polymorphisms. Neuropsychobiology. 2010;61:49–56. doi: 10.1159/000262180. [DOI] [PubMed] [Google Scholar]
- 28.Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovallo WR, Robinson JL, Glahn DC, Fox PT. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology. 2010;35:15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation- Under-Spin-Tagging MRI. Magn Reson Med. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Yezhuvath US, Xiao G. Improving fMRI sensitivity by normalization of basal physiologic state. Hum Brain Mapp. 2010;31:80–87. doi: 10.1002/hbm.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Zhao C, Ge Y, Lewis-Amezcua K. Baseline blood oxygenation modulates response amplitude: Physiologic basis for intersubject variations in functional MRI signals. Magn Reson Med. 2008;60:364–372. doi: 10.1002/mrm.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariotti V, Melissari E, Amar S, Conte A, Belmaker R, Agam G, Pellegrini S. Effect of prolonged phenytoin administration on rat brain gene expression assessed by DNA microarrays. Experimental Biology and Medicine. 2010;235:300–310. doi: 10.1258/ebm.2009.009225. [DOI] [PubMed] [Google Scholar]
- 37.Meador KJ, Loring DW, Moore EE, Thompson WO, Nichols ME, Oberzan RE, Durkin MW, Gallagher BB, King DW. Comparative cognitive effects of phenobarbital, phenytoin, and valproate in healthy adults. Neurology. 1995;45:1494–1499. doi: 10.1212/wnl.45.8.1494. [DOI] [PubMed] [Google Scholar]
- 38.Mishory A, Yaroslavsky Y, Bersudsky Y, Belmaker RH. Phenytoin as an antimanic anticonvulsant: a controlled study. Am J Psychiatry. 2000;157:463–465. doi: 10.1176/appi.ajp.157.3.463. [DOI] [PubMed] [Google Scholar]
- 39.Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 40.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 41.Oei NY, Elzinga BM, Wolf OT, de Ruiter MB, Damoiseaux JS, Kuijer JP, Veltman DJ, Scheltens P, Rombouts SA. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osuji IJ, Tucker E, Brown ES. Declarative memory in patients with bipolar disorder and stimulant abuse given lamotrigine. Journal of Dual Diagnosis. 2008;4:303–319. [Google Scholar]
- 43.Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 44.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR. Localization of mineralocorticoid receptors at mammalian synapses. PLoS One. 2010;5:el4344. doi: 10.1371/journal.pone.0014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychol Methods. 2001;6:387–401. [PubMed] [Google Scholar]
- 47.Ryan JJ, Geisser ME, Randall DM, Georgemiller RJ. Alternate form reliability and equivalency of the Rey Auditory Verbal Learning Test. J Clin Exp Neuropsychol. 1986;8:611–616. doi: 10.1080/01688638608405179. [DOI] [PubMed] [Google Scholar]
- 48.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarze U, Hahn C, Bengner T, Stodieck S, Buchel C, Sommer T. Enhanced activity during associative encoding in the affected hippocampus in right temporal lobe epilepsy patients. Brain research. 2009;1297:112–117. doi: 10.1016/j.brainres.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 50.Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 51.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 52.Thomaes K, Dorrepaal E, Draijer NP, de Ruiter MB, Elzinga BM, van Balkom AJ, Smoor PL, Smit J, Veltman DJ. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiat Res. 2009;171:44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Tunnicliff G. Basis of the antiseizure action of phenytoin. Gen Pharmacol. 1996;27:1091–1097. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- 54.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 55.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- 58.Wolkowitz OM, Reus VI, Weingartner H, Thompson K, Breier A, Doran A, Rubinow D, Pickar D. Cognitive effects of corticosteroids. The American journal of psychiatry. 1990;147:1297–1303. doi: 10.1176/ajp.147.10.1297. [DOI] [PubMed] [Google Scholar]