Abstract
Prader-Willi syndrome (PWS) is a genomic imprinting disorder due to loss of paternally expressed genes in the 15q11-q13 region and characterized by hypotonia, a poor suck, failure to thrive, hypogonadism/hypogenitalism, growth hormone deficiency, learning and behavioral problems and hyperphagia leading to early childhood obesity. Growth hormone acts as a ligand for the growth hormone receptor (GHR) coded by a gene polymorphic for an exon-3 deletion (d3) seen in about 50% of Caucasians and associated with an increased response to growth hormone (GH) therapy. We examined 69 individuals with PWS (average age ± SD = 20.1 ± 12.8y). The GHR allele distribution in our PWS subjects was similar to reported data in the literature with no gender or PWS genetic subtype differences. A negative correlation was found with age for height standard deviational scores and a positive correlation with age for weight and BMI for non-GH treated PWS subjects. Adjusting for effects of age and gender, individuals with PWS and the d3/d3 allele showed a significant increase in BMI compared with those having the full length (fl) allele. In addition, 12 infants and children with PWS were examined when growth and GH data were available before and during GH treatment. A significant increase in growth rate (1.7 times) was noted in the presence of the d3 allele (fl/fl=0.87cm/month; fl/d3 or d3/d3=1.5 cm/month; p < 0.05). The presence of the d3 allele and its impact on growth and medical care of individuals with PWS while on GH therapy should be further investigated.
Keywords: Growth hormone receptor (GHR), Prader-Willi syndrome, growth hormone treatment, genotype, gene polymorphism
INTRODUCTION
Prader-Willi syndrome (PWS) is characterized by infantile hypotonia; failure to thrive, a poor suck and feeding difficulties; hypogonadism with genital hypoplasia; growth hormone deficiency with short stature and small hands and feet; hyperphagia leading to early childhood obesity; mild mental deficiency and behavioral problems [e.g., Butler et al., 2006; Butler, 2011; Cassidy et al., 2012]. PWS is due to loss of paternally expressed genes from the 15q11-q13 region [e.g., Butler, 1990; Bittel and Butler, 2005], usually from a de novo paternally derived deletion [Butler & Palmer, 1983]. When treated with growth hormone (GH), PWS individuals respond favorably in stature, lean body mass and physical activity, but are prone to developing scoliosis requiring braces or surgical intervention [Butler et al., 2006].
In randomized controlled studies reported in the literature, GH treatment increased the longitudinal growth rate in children with Prader-Willi syndrome [e.g., Oto et al., 2012]. Implementing a controlled diet along with GH therapy and exercise are key in the medical management of individuals afflicted with this condition. For example, Myers et al. [2000] reported that two years of growth hormone treatment in children with PWS was sufficient to reduce fat mass with a sustained increase in lean body mass. Additional long term studies, though limited, suggest that GH treatment for five years may not be sufficient to normalize the lean body mass [Eiholzer et al., 2000], but can help stabilize the body mass index [Lindgren et al., 1999; Tauber et al., 2000].
As the first step in growth hormone action, growth hormone acts as a ligand for the growth hormone receptor (GHR) consisting of an extracellular and cytoplasmic protein domain. GH binding is followed by the activation of the JAK-STAT pathway initiating an increase in expression of insulin-like growth factor I (IGF-I) and other growth hormone genes. The GHR gene contains 9 exons with exons 3–7 encoding the extracellular domain [Pantel et al., 2000; Dos Santos et al., 2004]. There are two recognized isoforms of the GHR gene in humans due to the presence or absence of exon 3. The exon-3 deletion (d3) occurs in about 50% of Caucasians in the general population [Dos Santos et al., 2004; Padidela et al., 2012] and results from exon skipping due to homologous recombination of two retroviral sequences flanking this exon that mimic an alternative splicing event [Pentel et al., 2000]. This exon deletion exhibits increased functional receptor activity by about 30% in transfection studies with an associated growth response to treatment in GH-deficient patients [Dos Santos et al., 2004]. Specifically, non-PWS children with short stature and d3 allele grew at 1.7 to 2 times faster growth rate when treated with GH.
Prior to growth hormone therapy, PWS individuals from South Korea with at least one d3 GHR allele exhibited greater height standard deviational scores than those who did not have this allele [Park et al., 2011]. In addition, a birth cohort studied from the general population in the United Kingdom with two wild type (fl/fl) alleles reported to have larger placentas and birth weights with increased intrauterine growth in late gestation [Padidela et al., 2012]. Hence, the aims for our study were to assess for an association between GHR allele subtypes and growth parameters before and during GH treatment in individuals with Prader-Willi syndrome.
MATERIALS AND METHODS
Participants
The study sample was comprised of 69 participants who were genetically confirmed with PWS and consisted of 30 males and 39 females with a mean age + SD = 20.1y ± 12.8y and age range of 2 mo to 50 yrs. Forty-one (58%) of the study participants had the typical 15q11-q13 deletion and the remaining 28 PWS subjects (42%) had maternal disomy 15 (UPD) or an imprinting center defect determined by established cytogenetic and molecular genetic techniques [Butler et al., 2008; Henkhaus et al., 2012]. Ninety percent of our PWS study subjects were Caucasian, 4% were African-American, 4% were Hispanic and 2% were Asian. Fifty-nine percent (N= 41) of our PWS study subjects were naïve to GH treatment while the remaining 41 percent (N=28) were undergoing GH treatment at the time of study or had been treated in the past. The study was conducted under the authority of the University of Kansas Medical Center Office of Research Compliance who reviewed the study protocol and monitored study activities to ensure that appropriate steps were taken to protect the rights and welfare of humans participating as research subjects.
Growth Hormone Receptor (GHR) Genotyping Method
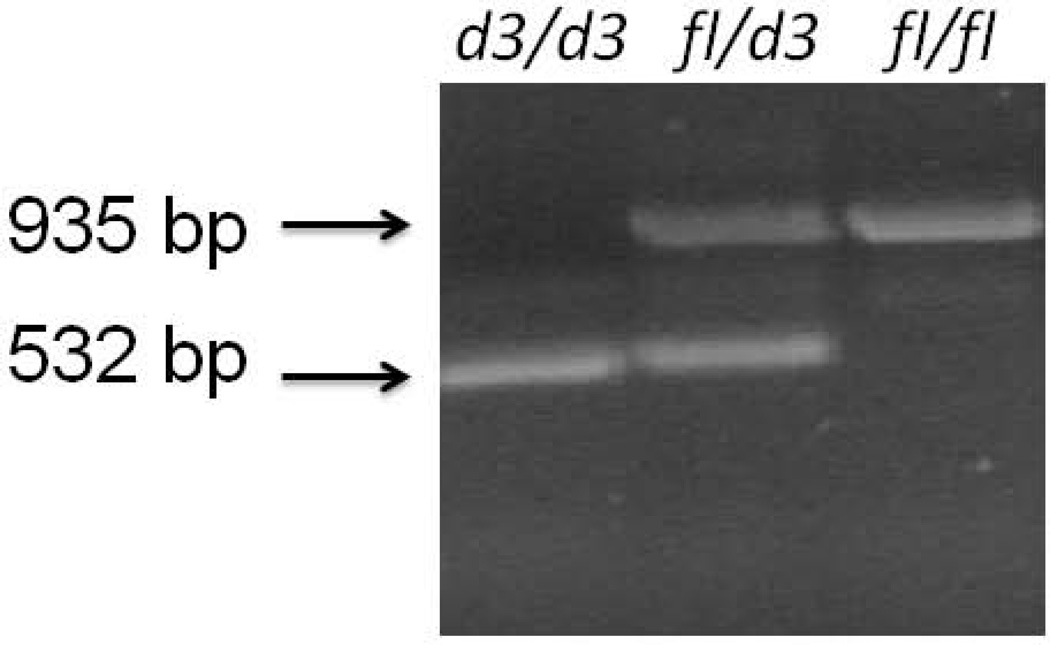
The GHR exon 3 deletion was identified using PCR-based technology with primers (G1, G2, and G3) described in GenBank, accession number AF155912. The GHR-exon 3 genotyping test was performed, to identify GHRfl and GHRd3 alleles, using a multiplex PCR procedure with primers G1 (5'-TGTGCTGGTCTGTTGGTCTG-3'), G2 (5'-AGTCGTTCCTGGGACAGAGA-3'), and G3 (5'-CCTGGATTAACACTTTGCAGACTC-3'). The following PCR cycle parameters were used as reported previously by Dos Santos et al. [2004]: the initial step of denaturation for five minutes at 94°C, then followed by 35 cycles with each cycle having a duration of 30 sec at 94°C, 30 sec at 60°C; and for 1 min 30 sec at 72°C, the extension period followed for 7 min at 72°C. Electrophoresis of the PCR fragments was performed with 1% agarose, and the gel stained with ethidium bromide. Primers for G1 and G3 generate a PCR fragment of 935 bp indicating the wild type full length (fl) allele while primers G1 and G2 generate a PCR fragment of 532 bp when the exon 3 deletion is present representing the d3 allele (Fig 1).
Figure 1.
Representative examples of growth hormone receptor gene (GHR) alleles using polymerase chain reaction to identify the wild type fl/fl; heterozygous fl/d3 and homozygous exon-3 deletion (d3/d3) alleles in Prader-Willi syndrome.
Statistical Analysis
Measurements of length (height) to the nearest 0.1 cm, weight to the nearest 0.1 kg, and head circumference to the nearest 0.1 cm were extracted from patient medical records and growth charts for all subjects along with growth hormone dosage and plasma IGF-I levels and reference ranges. Standard deviational scores were generated for the growth parameters from growth curves relative to normally developing children. Descriptive statistics are presented as means and standard deviations. One Way Analysis of Variance adjusted for age and gender was used to compare means by GHR subtype and correlation coefficients determined. Linear equations were used to determine the rate of change in growth parameters for each subject and the mean rate of change was compared by GHR subtype. We also examined the rate of height change during GH treatment in relationship to GHR allele subtype in a subset of 12 infants and children selected from our PWS cohort with available growth and GH treatment data.
RESULTS
Non-GH Treatment
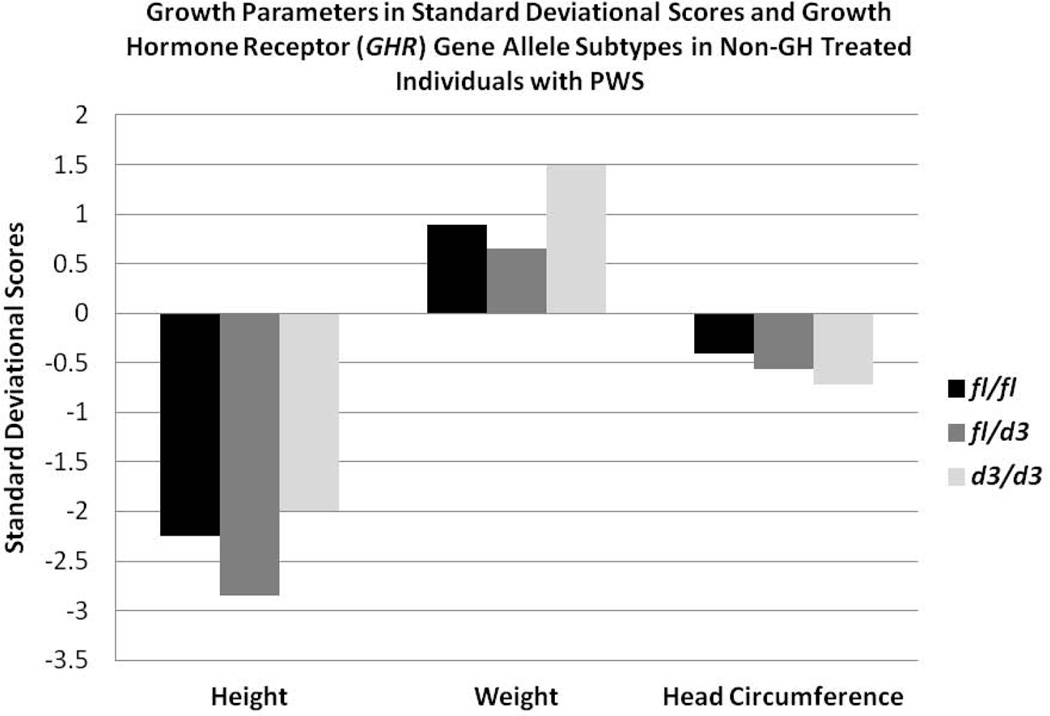
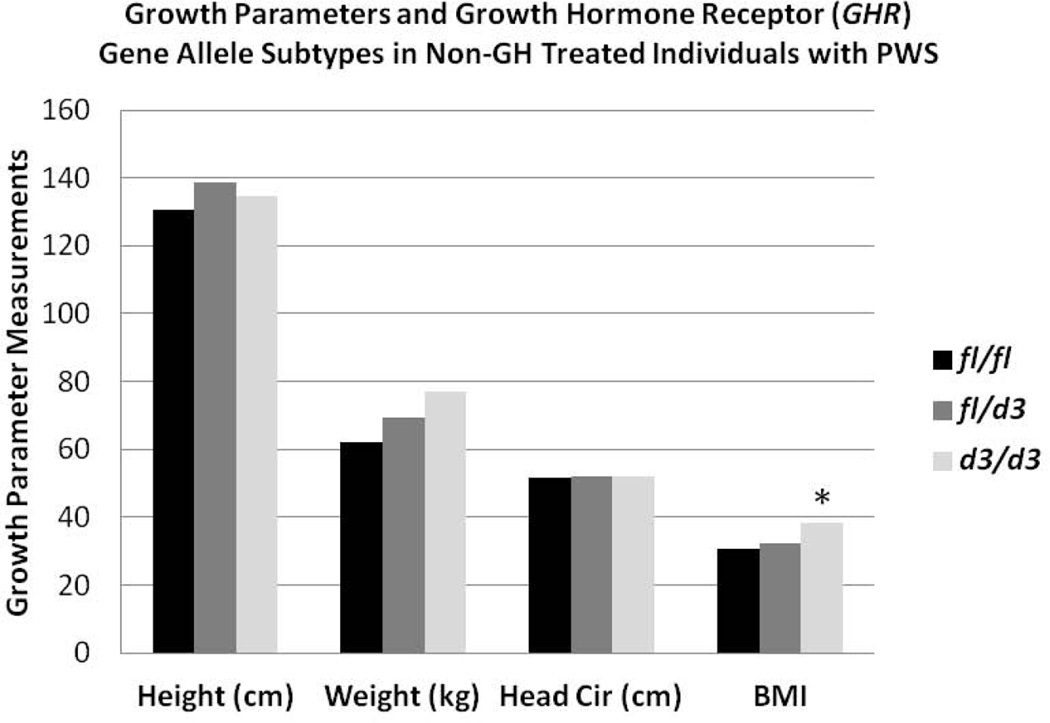
The distribution of GHR alleles (fl/fl, N=36 or 52%; fl/d3, N=25 or 36%; d3/d3, N=8 or 12%) in our 69 PWS patients was similar to reported data in Caucasian control participants. There were no gender or PWS genetic subtype (e.g., 15q11-q13 deletion, maternal disomy 15) differences identified in the distribution of GHR alleles or in the individual growth parameters studied. The association between GHR allele subtypes and growth parameters (height, weight, head circumference) obtained prior to GH treatment as well as body mass index (BMI) was examined in the PWS individuals. Prior to GH treatment, we found a negative correlation (p<0.05) with age for height standard deviational scores (SDS) and a positive correlation (p<0.05) with age for weight and BMI regardless of GHR allele subtype. Adjusting for effects of age and gender, we found that individuals with PWS carrying the d3 allele showed a significantly increased mean BMI compared with those having the full length allele (F=3.9; p<0.02). However no differences were found in standard deviational scores for height, weight or head circumference by GHR subtype prior to GH treatment (Figs 2 and 3).
Figure 2.
Histograms of growth parameters (height, weight, head circumference) in standard deviational scores and GHR allele subtypes (fl/fl, fl/d3, d3/d3) in non-growth hormone (GH) treated individuals with Prader-Willi syndrome (PWS).
Figure 3.
Histograms of growth parameters (height, weight, head circumference) and body mass index (BMI) and GHR allele subtypes (fl/fl, fl/d3, d3/d3) in non-growth hormone (GH) treated individuals with Prader-Willi syndrome (PWS). A significant difference (F=3.7; p<0.05) was found for the d3/d3 allele (*) compared with the other GHR allele subtypes for BMI.
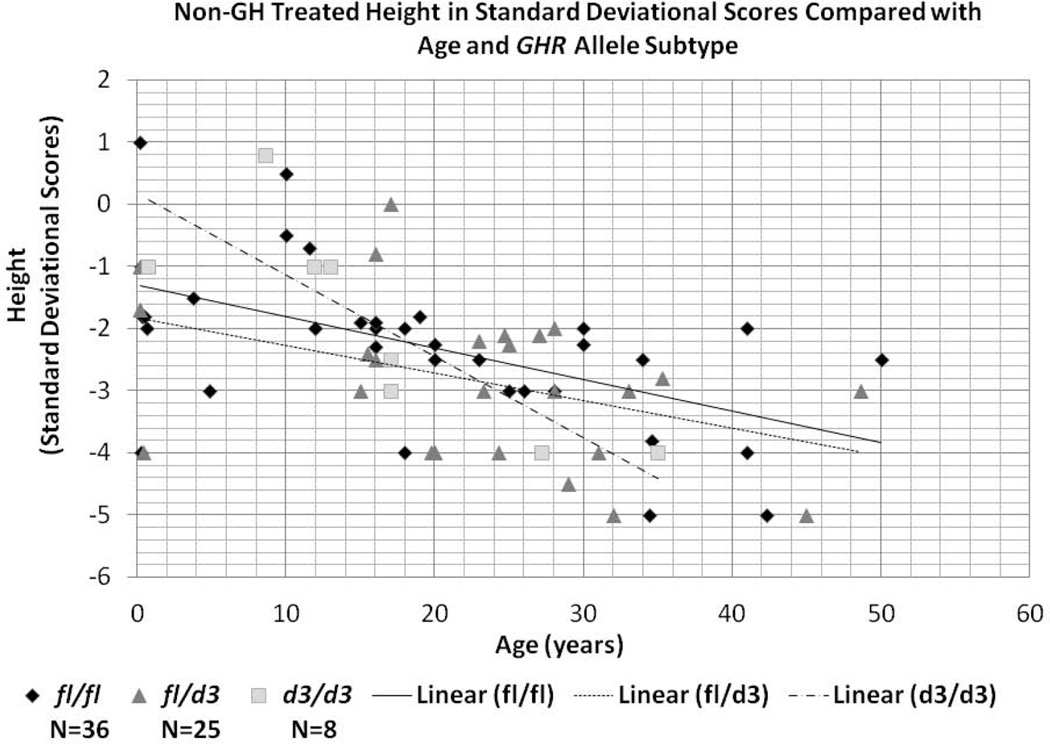
Height SDS as a function of age in the absence of GH treatment was compared to GHR allele subtype and shown in Figure 4. Linear regression analysis was significant (F= 4.1, p<0.05) and revealed a main effect of age (F=19.6; p<0.0001) as well as age by allele subtype interaction (F=3.8; p<0.05). Growth rate for the d3/d3 subtype showed a faster growth rate prior to 18 years of age and a slower growth rate over 20 years of age. The overall rate of decline in SDS for age was faster for the d3/d3 allele subtype in comparison with the fl/fl and fl/d3 subtypes.
Figure 4.
Scatterplot of non-growth hormone (GH) treatment data for height in standard deviational scores compared with age (in years) and GHR allele subtypes for all individuals studied with Prader-Willi syndrome.
GH Treatment
The rate of height change during GH treatment was examined in relationship to GHR allele subtype in a subset of 12 infants and children from our PWS cohort (Table I). The GHR allele subtype for this group included eight patients with fl/fl (four males and four females), three with fl/d3 (two males and one female) and one infant female with the GHR d3/d3 allele subtype. Age at pre-GH treatment measurements for height and weight ranged from 1 month to 19 months while measurements during the GH treatment phase ranged from 6 months to 7 years of age at the time when GH dosage and plasma IGF-I levels were within therapeutic range for age. Individuals with the fl/fl subtype had a height increase of 0.87 cm/month or 0.03 SDS/month while individuals possessing the d3 allele (i.e., fl/d3 or d3/d3) had a significantly faster rate of height increase of 1.5 cm/month or 0.16 SDS/month (t-test; p<0.05) during GH treatment compared with −.11 SDS/month for fl/fl and 0.08 SDS/month for those with the d3 allele during the pre-GH treatment period. Figure 5 illustrates a representative example of a PWS female infant (Patient 5, Table I) with the GHR fl/fl allele subtype and maternal disomy 15. Figure 6 illustrates a representative example of a PWS female infant (Patient 9, Table I) with the GHR fl/d3 allele subtype and the 15q11-q13 deletion.
Table 1.
Clinical Summary of 12 Infants with Prader-Willi Syndrome before and during Growth Hormone Therapy
| Genetic Subtype |
Birth Measures |
Average Height (SDS)/ Interval Studied (months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Race | PWS | GHR | Pregnancy/Birth | Weight [kg (SDS)] |
Length [cm (SDS)] |
History of G-tube |
Scoliosis (>10°) |
Age GHT Initiated (months) |
Pre-GHT | GHT | Other |
| 1 | F | C | 15q11-q13 type I deletion |
fl/fl | 34 week gestation, C-section, polyhydramnios, pre-eclampsia, dizygotic twin birth |
1.8 kg (−1.3) | 45 cm (−0.7) | Yes | No | 9.6 | −1.9/3.0 | −1.3/2.0 | |
| 2 | F | A/C | 15q11-q13 type II deletion |
fl/fl | 34 week gestation, PROM, emergency C-section |
1.9 kg (−1.3) | 49 cm (0.7) | No | No | 3.6 | −1.3/1.0 | −1/27.0 | |
| 3 | M | C | 15q11-q13 type II deletion |
fl/fl | 38 week gestation, C-section |
2.3 kg (−1.9) | 49 cm (−0.7) | No | No | 20.4 | −0.8/7.0 | 0.9/9.0 | |
| 4 | F | C | 15q11-q14 atypical deletion |
fl/fl | Term | 2.7 kg (−1.6) | 51 cm (0.3) | Yes | No | 6.0 | 0.5/3.5 | 0.4/25 | Coarctation of aorta, PDA, ear tags |
| 5 | F | C | UPD | fl/fl | Term | 2.7 kg (−1.6) | 48 cm (−1.3) | Yes | No | 10.8 | −1.0/5.0 | 0.1/5.0 | Hip subluxation |
| 6 | M | C/AA | UPD | fl/fl | 31 week gestation, maternal hypertension, abnormal placenta, oligohydramnios |
0.9 kg (−1.9) | 35 cm (−1.9) | Yes | No | 6.0 | −6.7/2.5 | −1.6/12.0 | Adrenal insufficiency, pulmonary hypertension, hypospadias, inguinal hernia |
| 7 | M | C | UPD | fl/fl | 38 week gestation | 2.5 kg (−1.3) | 50 cm (0) | No | No | 12.0 | −2.2/8.0 | 0.2/ 81.0 | |
| 8 | M | C | UPD | fl/fl | Term, polyhydramnios, gestational diabetes |
4.2 kg (1.3) | 55 cm (1.9) | Yes | No | 7.2 | 0.7/4.5 | 0.5/22 | NF1 |
| 9 | F | C | 15q11-q13 type I deletion |
fl/d3 | 35 week gestation | 1.9 kg (−1.6) | 46 cm (−0.7) | Yes | No | 6.0 | −3.1/3.0 | −1.0/3.0 | |
| 10 | M | C | 15q11-q13 type II deletion |
fl/d3 | Term | 2.3 kg (−2.3) | 48 cm (−1.3) | Yes | No | 0.6 | NA | −0.8/8.0 | |
| 11 | M | C | UPD | fl/d3 | 37 week gestation | 2.0 kg (−2.3) | 46 cm (−1.3) | No | No | 7.2 | −3.6/2.5 | −0.2/62.0 | Multiple bone fractures |
| 12 | F | C | 15q11-q13 type II deletion |
d3/d3 | 38 week gestation, C-section |
2.4 kg (−1.6) | 48 cm (−0.7) | Yes | No | 9.6 | −0.9/5.0 | 0.1/11.0 | Horseshoe kidney, hip dysplasia |
M= Male; F= Female; AA= African-American; A= Asian; C= Caucasian; PWS= Prader-Willi syndrome; GHR= Growth hormone receptor gene; UPD= Uniparental maternal disomy 15; PROM= Premature rupture of membranes; SDS= Standard deviational score; GHT= Growth hormone therapy; NF1= Neurofibromatosis 1; PDA= Patent ductus arteriosus; NA= Not applicable
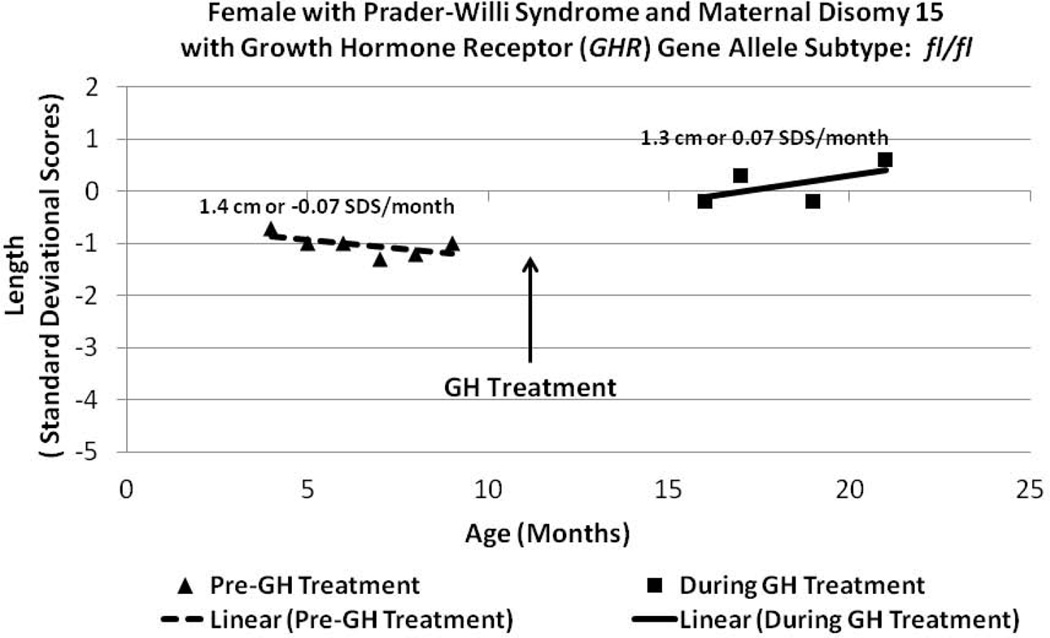
Figure 5.
Length data plotted from a Prader-Willi syndrome (PWS) female with maternal disomy 15 (Patient 5, Table I) before and during GH treatment within the therapeutic range monitored by plasma IGF-I levels and having the GHR fl/fl allele subtype.
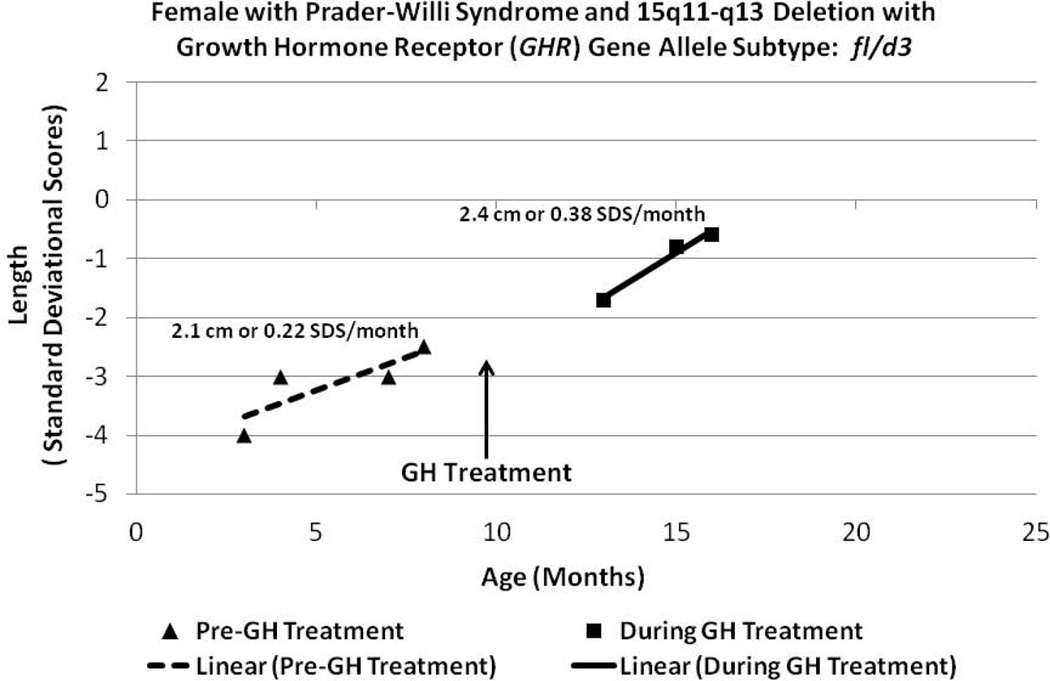
Figure 6.
Length data plotted from a Prader-Willi syndrome (PWS) female with the 15q11-q13 deletion (Patient 9, Table I) before and during GH treatment within the therapeutic range monitored by plasma IGF-I levels and having the GHR fl/d3 allele subtype.
DISCUSSION
Our study showed that the distribution of GHR alleles in individuals with PWS was not different from the expected frequency reported in non-PWS controls from the general population. In addition, our study of growth parameters in PWS subjects confirmed the well-known pattern of growth in this obesity-related syndrome with decreased height but increased weight and BMI with advancing age relative to normally developing children without PWS and in the absence of GH therapy [Myers et al., 2000; Burman et al., 2001; Butler et al., 2006; Carrel et al., 2010]. Examination of the influence of GHR allele subtypes in PWS found an association between the GHR subtype and the rate of change of height SDS in the presence and absence of exogenous GH treatment. Without GH treatment, subjects homozygous for the d3 allele showed an accelerated growth rate in infancy and childhood compared with both heterozygous (fl/d3) subjects and homozygous (fl/fl) subjects suggesting that the d3 allele influences height without GH treatment supporting increased sensitivity to GH and with more responsiveness to GH secretions even if below normal range with GH deficiency being common in PWS. This observation was also reported by Park et al. [2011] in patients with PWS from South Korea in which the d3 allele was associated with increased height and IGF-I levels before GH therapy and thus the d3 allele influences height through GH secretion sensitivity. If endogenous GH levels decrease with age in infants and children with PWS, then the rate of growth (e.g., length) would significantly decrease relative to a normal growth rate particularly in those the fl/fl allele.
Growth rates for PWS subjects with the d3/d3 subtype decreased sharply after puberty if GH treatment is not administered. When treated with growth hormone, all PWS individuals respond favorably in stature, with decreased fat, increased muscle mass and physical activity. Heterozygous (fl/d3) individuals and homozygous (d3/d3) individuals showed a higher sensitivity to GH treatment than homozygous fl/fl subjects as indicated by greater acceleration in growth rate in PWS subjects with the fl/d3 or d3/d3 alleles. PWS infants with the fl/fl GHR allele subtype in our study were responsive to GH treatment but did not appear to be as sensitive as those possessing the d3 allele. We estimated that the presence of the d3 allele increased height change by 1.7 times in comparison to the response of the full length allele as similarly seen in the literature in GH treated non-PWS children [Dos Santos et al., 2004].
In summary, the d3 allele was associated with significantly increased BMI in our cohort of PWS study participants prior to GH treatment but not for height or weight. The presence of the d3 allele was associated with an increased rate of height change compared to the response of the full length (fl) allele as similarly seen in GH treated non-PWS children. The sensitivity to GH treatment and relationship to GHR allele subtype could influence management decisions about GH dosage by following IGF-I levels more closely (e.g., 3 month intervals) and for monitoring for scoliosis. The presence of the d3 allele and its impact on management decisions and caring for individuals with PWS while on GH therapy should be addressed in future studies with a larger sample of PWS subjects for investigation and for an extended period of observational time.
ACKNOWLEDGMENTS
Funding for this project was supported by the NIH U54 grant HD061222 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and RR019478 (NCRR) from the NIH Office of Rare Diseases Research as well as NICHD grant HD002528. In addition, we thank the families and individuals with PWS who participated in this study.
REFERENCES
- Bittel DC, Butler MG. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med. 2005;7(14):1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman P, Ritzen EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: A review with special reference to GH. Endocr Rev. 2001;22:787–799. doi: 10.1210/edrv.22.6.0447. [DOI] [PubMed] [Google Scholar]
- Butler MG, Palmer CG. Parental origin of chromosome 15 deletion in Prader-Willi syndrome. Lancet. 1983;1(8336):1285–1286. doi: 10.1016/s0140-6736(83)92745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J. Med Genet. 1990;35(3):319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Lee PDK, Whitman BY. Management of Prader-Willi Syndrome. New York: Springer; 2006. [Google Scholar]
- Butler MG, Fischer W, Kibiryeva N, Bittel DC. Array comparative genomic hybridization (aCGH) analysis in Prader-Willi syndrome. Am J Med Genet A. 2008;146(7):854–860. doi: 10.1002/ajmg.a.32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr Genomics. 2011;12(3):204–215. doi: 10.2174/138920211795677877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2010;95(3):1131–1136. doi: 10.1210/jc.2009-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- Dos Santos C, Esioux L, Teinturier C, Tauber M, Goffin V, Bougneres P. A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat Genet. 2004;36:720–724. doi: 10.1038/ng1379. [DOI] [PubMed] [Google Scholar]
- Eiholzer U, l’Allemand D, van der Sluis I, Steinhert H, Gasser T, Ellis K. Body composition abnormalities in children with Prader-Willi syndrome and long-term effects of growth hormone therapy. Horm Res. 2000;53:200–287. doi: 10.1159/000023567. [DOI] [PubMed] [Google Scholar]
- Henkhaus RS, Kim SJ, Kimonis VE, Gold JA, Dykens EM, Driscoll DJ, Butler MG. Methylation-specific multiplex ligation-dependent probe amplification and identification of deletion genetic subtypes in Prader-Willi syndrome. Genet Test Mol Biomarkers. 2012;16(3):178–186. doi: 10.1089/gtmb.2011.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren AC, Ritzen EM. Five years of growth hormone treatment in children with Prader-Willi syndrome. Acta Paediatr Suppl. 1999;433:109–111. doi: 10.1111/j.1651-2227.1999.tb14416.x. [DOI] [PubMed] [Google Scholar]
- Myers SE, Carrel AL, Whitman BY, Allen DB. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome. J Pediatr. 2000;137:42–49. doi: 10.1067/mpd.2000.105369. [DOI] [PubMed] [Google Scholar]
- Oto Y, Obata K, Matsubara K, Kozu Y, Tsuchiya T, Sakazume S, Yoshino A, Murakami N, Ogata T, Nagai T. Growth hormone secretion and its effect on height in pediatric patients with different genotypes of Prader-Willi syndrome. Am J Med Genet A. 2012;158A(6):1477–1480. doi: 10.1002/ajmg.a.35378. [DOI] [PubMed] [Google Scholar]
- Padidela R, Bryan SM, Abu-Amero S, Hudson-Davies RE, Achermann JC, Moore GE, Hindmarsh PC. The growth hormone receptor gene deleted for exon three (GHRd3) polymorphism is associated with birth and placental weight. Clinical Endocrinol. 2012;76:236–240. doi: 10.1111/j.1365-2265.2011.04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel J, Machinis K, Sobrier ML, Duquesnoy P, Goossens M, Amselem S. Species-Specific alternative splice mimicry at the growth hormone receptor locus revealed by the lineage of retroelments during primate evolution. J Biol Chem. 2000;275:18664–18669. doi: 10.1074/jbc.M001615200. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee S-T, Sohn YB, Kim SH, Sho S-Y, Ko A-r, Ji S-T, Kwon J-Y, Yeau S, Paik K-H, Kim J-W, Jin D-K. A Polymorphism in the growth hormone receptor is associated with height in children with Prader-Willi syndrome. Am J Med Genet Part A. 2011;155:2970–2973. doi: 10.1002/ajmg.a.34309. [DOI] [PubMed] [Google Scholar]
- Tauber M, Barbeau C, Jouret B, Pienkowski C, Malzac P, Moncla A, Rochiccioli O. Auxological and endocrine evolution of 28 children with Prader-Willi syndrome: effect of GH therapy in 14 children. Horm Res. 2000;53:279–287. doi: 10.1159/000053184. [DOI] [PubMed] [Google Scholar]