Abstract
Bacillus thuringiensis (Bt) bacteria are insect pathogens that rely on insecticidal pore forming proteins known as Cry and Cyt toxins to kill their insect larval hosts. At least four different non-structurally related families of proteins form the Cry toxin group of toxins. The expression of certain Cry toxins in transgenic crops has contributed to an efficient control of insect pests resulting in a significant reduction in chemical insecticide use. The mode of action of the three domain Cry toxin family involves sequential interaction of these toxins with several insect midgut proteins facilitating the formation of a pre-pore oligomer structure and subsequent membrane insertion that leads to the killing of midgut insect cells by osmotic shock. In this manuscript we review recent progress in understanding the mode of action of this family of proteins in lepidopteran, dipteran and coleopteran insects. Interestingly, similar Cry-binding proteins have been identified in the three insect orders, as cadherin, aminopeptidase-N and alkaline phosphatase suggesting a conserved mode of action. Also, recent data on insect responses to Cry toxin attack is discussed. Finally, we review the different Bt based products, including transgenic crops, that are currently used in agriculture.
Keywords: Bacillus thuringiensis, Cry toxins, Mode of action, Pore formation, Bt crops
1. Introduction
Control of insect pests in agriculture and of insect vectors of important human diseases is mainly achieved using chemical insecticides. However, the use of these chemical pesticides has led to several problems, including environmental pollution and increase in human health effects, such as cancer and several immune system disorders. The selection of insect resistant populations has also caused significant and major outbreaks of secondary pests (Devine and Furlong, 2007). Although microbial insecticide have been proposed as substitutes for chemicals their use is limited since most microbes show a narrow spectrum of activity that enables them to kill only certain insect species. Moreover, they have low environmental persistence and they require precise application practices, since many of these pathogens are specific to young insect larval stages or are sensitive to irradiation.
The most successful insect pathogen used for insect control is the bacterium Bacillus thuringiensis (Bt), which presently is ~2% of the total insecticidal market. Bt is almost exclusively active against larval stages of different insect orders and kills the insect by disruption of the midgut tissue followed by septicemia caused probably not only by Bt but probably also by other bacterial species (Raymond et al., 2010). Bt action relies on insecticidal toxins that are active during the pathogenic process but these bacteria also produce an array of virulence factors that contribute to insect killing (reviewed in Bravo et al., 2005). Upon sporulation, Bt produces insecticidal crystal inclusions that are formed by a variety of insecticidal proteins called Cry or Cyt toxins. These toxins show a highly selective spectrum of activity killing a narrow range of insect species. The Cry and Cyt toxins belong to a class of bacterial toxins known as pore forming toxins (PFT) that are secreted as water-soluble proteins that undergo conformational changes in order to insert into the membrane of their hosts. Despite the limited use of Bt products as sprayable insecticides, Cry toxins have been introduced into transgenic crops providing a more targeted and effective way to control insect pests in agriculture. Concomitantly, this approach has resulted in significant reduction in the use of chemical insecticides in places where this technology has been embraced (James, 2009).
The mode of action of Cry toxins has mostly been studied in lepidopteran insects and has been reviewed recently (Bravo et al., 2005, 2007). Also, the identification of insect midgut proteins that bind Cry toxins and mediate toxicity and insect specificity has also been previously reviewed (Pigott and Ellar, 2007). In this review we will summarize recent work regarding the mode of action of Cry toxins in different insect orders, the identification of new Cry toxin insect binding proteins and the binding of Cry toxins to insect midgut proteins depending on the oligomeric state of the toxin. Finally we discuss recent genetic studies of mechanisms of resistance to Cry toxins and their most important applications.
2. Cry toxins: a diverse and large family of insecticidal proteins
Cry toxins are classified by their primary amino acid sequence and more than 500 different cry gene sequences have been classified into 67 groups (Cry1–Cry67) (Crickmore et al., 2010). These cry gene sequences have been divided in to at least four phylogentically non-related protein families that may have different modes of action: the family of three domain Cry toxins (3D), the family of mosquitocidal Cry toxins (Mtx), the family of the binary-like (Bin) and the Cyt family of toxins (reviewed in Bravo et al., 2005). Also, some Bt strains produce additional insecticidal toxins named VIP. VIP toxins, in contrast to Cry, are produced during the vegetative growth phase. At least three VIP toxins have been characterized, VIP1/VIP2, a binary toxin, and VIP3 (Estruch et al., 1996; Warren, 1997).
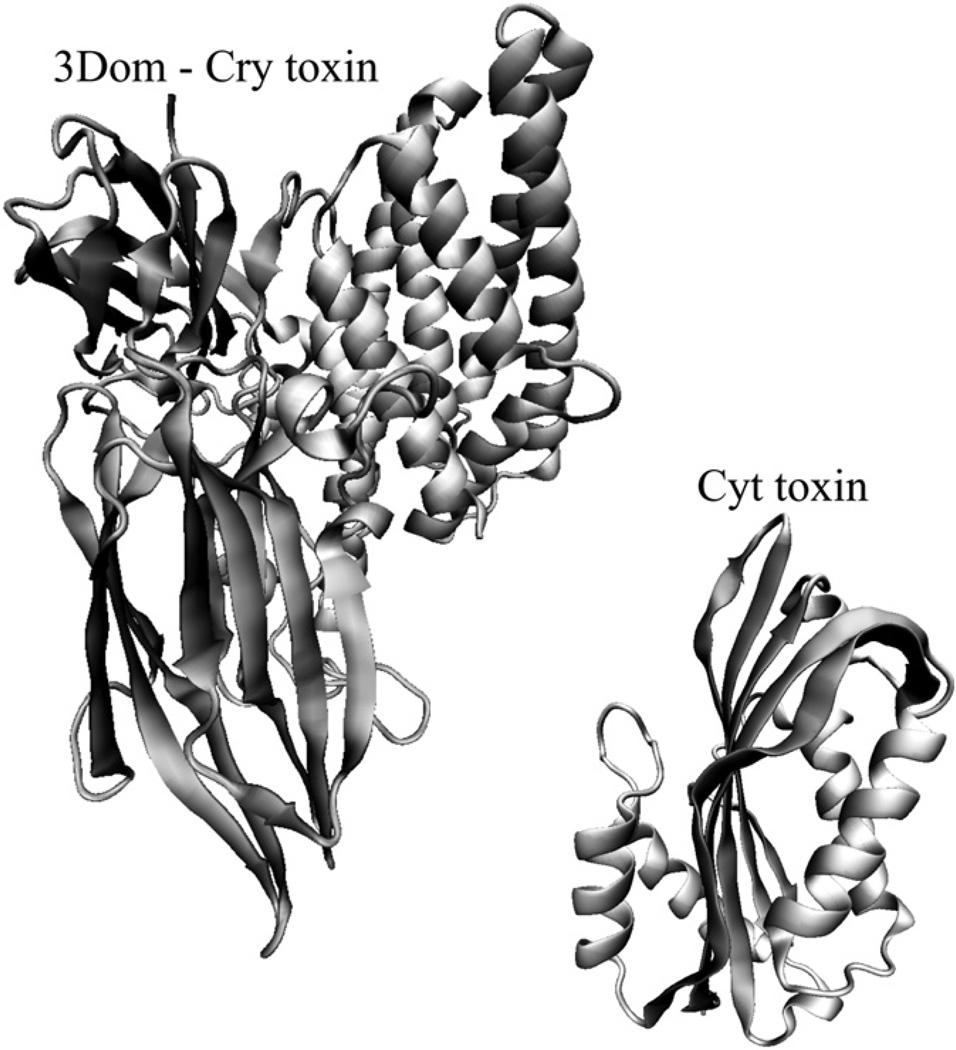
The largest Cry family is the 3D-Cry group that is formed by at least 40 different groups with more than 200 different gene sequences. The three dimensional structure of seven different 3D-Cry toxins have been solved, Cry1Aa, Cry2Aa, Cry3Aa, Cry3Ba, Cry4Aa, Cry4Ba and Cry8Ea (Li et al., 1991; Grochulski et al., 1995; Morse et al., 2001; Galitsky et al., 2001; Boonserm et al., 2005, 2006; Guo et al., 2009). Fig. 1 shows the three-dimensional structure of Cry8Ea that was the most recent structure released in the PDB database and has high similarity at the structural level with the other 3D-Cry that have been crystallized before (Guo et al., 2009). Domain I, a seven α-helix bundle, is implicated in membrane insertion, toxin oligomerization and pore formation. Domain II is a beta-prism of three anti-parallel β-sheets packed around a hydrophobic core with exposed loop regions that are involved in receptor recognition, and domain III, is a β-sandwich of two anti-parallel β-sheets. Both domain II and III are implicated in insect specificity by mediating specific interactions with different insect gut proteins (reviewed in Bravo et al., 2007). The three dimensional structure is conserved among members of the 3D-Cry family suggesting proteins from this family may share a similar mechanism of action even though they show very low amino acid sequence similarity. In Fig. 1 the three dimensional structure of Cyt2Ba toxin shows a single domain of two outer layers of α-helix hairpins wrapped around a β-sheet that is also highly similar to Cyt2Aa toxin that was previously crystallized (Cohen et al., 2008).
Fig. 1.
Three dimensional structures of insecticidal toxins produced by Bacillus thuringiensis. The structure of the 3D-Cry toxin is that of Cry8Ea and the Cyt corresponds to Cyt2Ba.
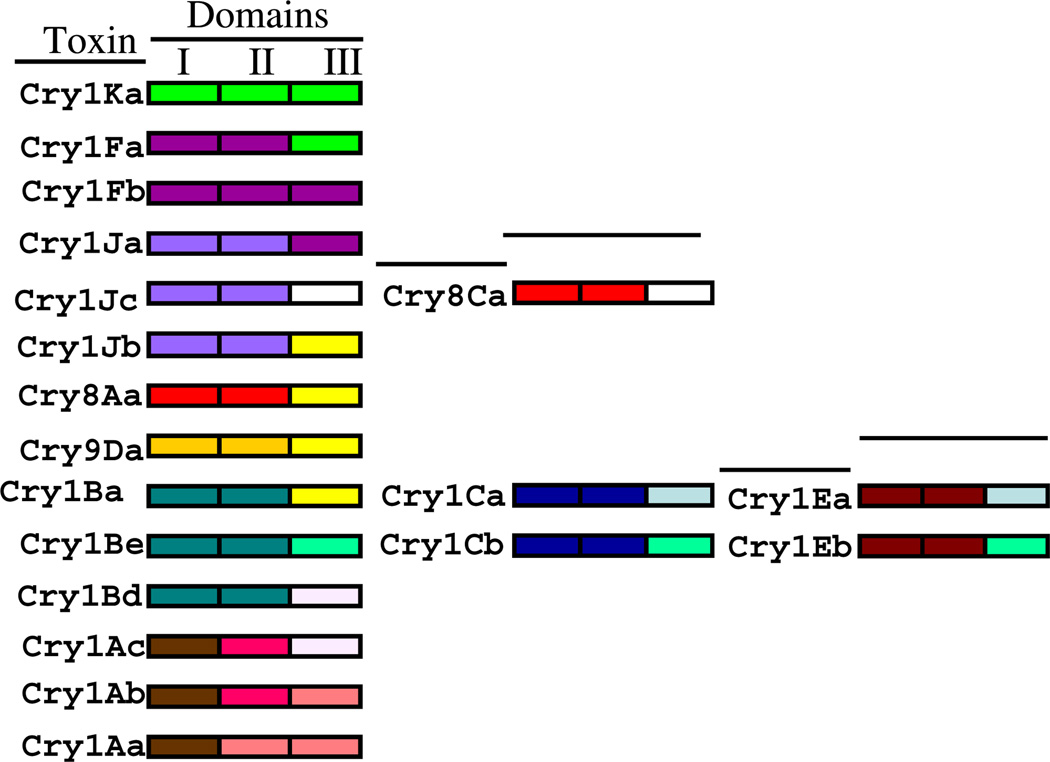
Phylogenetic analysis of the 3D-Cry toxin family revealed that Cry toxin variability evolved by two main processes; the independent evolution of the three functional domains and by domain III swapping among different toxins (Bravo, 1997; de Maagd et al., 2001). Fig. 2 shows several natural examples of domain III swapping, revealed by different toxins that share a domain III with highly similar amino acid sequence as Cry8Ca and Cry1Jc; Cry1Bd and Cry1Ac; Cry1Cb, Cry1Eb and Cry1Be; Cry8Aa, Cry1Jb, Cry1Ba and Cry9Da etc. Domains II and III are involved in binding of Cry toxins to insect midgut proteins, thus domain III swapping could have led to the selection of proteins with different insect specificities. In addition, in vitro construction of hybrid Cry proteins by interchanging domain III among different Cry toxins has been reported. An example of such in vitro novel-Cry constructions is the Cry1Ab hybrid toxin that contains the domain III of Cry1C toxin (1Ab-1Ab-1C). This hybrid toxin showed ten times increased insecticidal activity against Spodoptera exigua larvae than either of the parental proteins (de Maagd et al., 2000). More recently, a hybrid toxin containing domains I and II from Cry3Aa and domain III from Cry1Ab was shown to be toxic to Diabrotica virgifera virgifera in contrast to Cry3Aa and Cry1Ab that showed no toxicity to this insect (Walters et al., 2010).
Fig. 2.
Natural examples of domain III swapping among Bacillus thuringiensis Cry toxins. Colors represent similar amino acid sequences in the three domains of different 3D Cry toxins (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3. How Cry toxins kill their hosts
3.1. The case of lepidopteran insects
One of the most interesting features of Cry toxins is their insect specificity. Insect specificity is largely determined by the specific binding of Cry toxins to surface proteins located in the microvilli of larvae midgut cells. In the case of lepidopteran insects, Cry1 binding proteins have been identified as cadherin-like proteins, glycosylphophatidyl-inositol (GPI)-anchored aminopeptidase-N (APN), GPI-anchored alkaline phosphatase (ALP) a 270 kDa glycoconjugate and a 250 kDa protein named P252 (reviewed in Pigott and Ellar, 2007). In addition, glycolipids were proposed to act as Cry toxin receptors in lepidopteran insects as was demonstrated for the nematode Caenorhabditis elegans (Garczynski and Adang, 2000; Griffits et al., 2005). Cry1A toxins binds to cadherin proteins of at least six lepidopteran species, Manduca sexta, Bombyx mori, Heliothis virescens, Helicoverpa armigera, Pectinophora gossypiellaand Ostrinia nubilalis (reviewed in Pigott and Ellar, 2007). Insect cadherins that bind Cry toxins belong to a subset of the family of cadherin proteins and are composed of an ectodomain formed by 11 to 12 cadherin repeats (CR), a transmembrane domain and an intracellular domain (Bel and Escriche, 2006). In the case of APN, proteins belonging to at least five different subfamilies from B. mori, H. armigera. H. virescens, Lymantria dispar, M. sexta and Plutella xylostella have been found to bind Cry1 toxins (reviewed in Pigott and Ellar, 2007). ALP has been characterized as Cry1A binding protein in H. virescens and M. sexta (reviewed in Pigott and Ellar, 2007). The 270 kDa glycoconjugate was identified as Cry1Ac binding protein in L. dispar (reviewed in Pigott and Ellar, 2007). Recently B. mori P252 that binds Cry1Ac was identified as a choraphyllide-binding protein (Pandian et al., 2008). Table 1 shows the receptor molecules of Cry toxins identified in lepidopteran insects. Identification of Cry1Ac binding proteins by a proteomic approach after separation in 2D SDS-PAGE gels of brush border proteins from M. sexta and H. virescens, revealed that Cry1Ac also binds to V-ATPAse subunit A and actin indicating that the mode of action of Cry toxins may involve binding of the toxin with other components of the midgut cells but their role in the mechanism of action remains to be analyzed (McNall and Adang, 2003; Krishnamoorthy et al., 2007).
Table 1.
Midgut Cry toxin binding proteins in three different insect orders.
| Insect order | Insect speciesa | Cry-binding protein |
|---|---|---|
| Lepidoptera | Ms, Hv, On, Ha, Bm, Pg | Cadherin |
| Ld | 270 Glycoconjugate | |
| Bm | P252 | |
| Ms, Bm, Hv, Ld, Px | APN | |
| Ms, Hv | ALP | |
| Diptera | Ag, Ae | Cadherin |
| Ag, Ae, Aq | APN | |
| Ag, Ae, Aq | ALP | |
| Aa | alpha-glucosidase | |
| Coleoptera | Tm, Dv | Cadherin |
| Lde | ADAM 3 metalloprotease | |
| Ag | ALP |
Manduca sexta (Ms), Heliothis virscens (Hv), Ostrinia nubilalis (On), Helicoverpa armigera (Ha), Bombyx mori (Bm), Pectinophora gossypiella (Pg), Limantria dispar (Ld); Diptera, Anopheles gambiae (Ag), Anopheles quadrimaculatus (Aq), Anopheles albimanus (Aa), Aedes aegypti (Ae). Coleoptera, Tenebrio molitor (Tm), Diabrotica virgifera (Dv), Anthonomus grandis (Ag), Leptinotarsa decemlineata (Lde).
Although the binding kinetic parameters of the interaction of Cry1 proteins with the cadherin, APN and ALP binding proteins have not been fully analyzed, it is generally accepted that Cry1 toxins bind cadherin proteins with high affinity at the nM range; for instance Cry1Ab binds M. sexta cadherin with a 1 nM apparent binding affinity. In contrast, APN and ALP show lower binding affinities that are in the range of more than 100 nM binding affinity (reviewed in Pigott and Ellar, 2007).
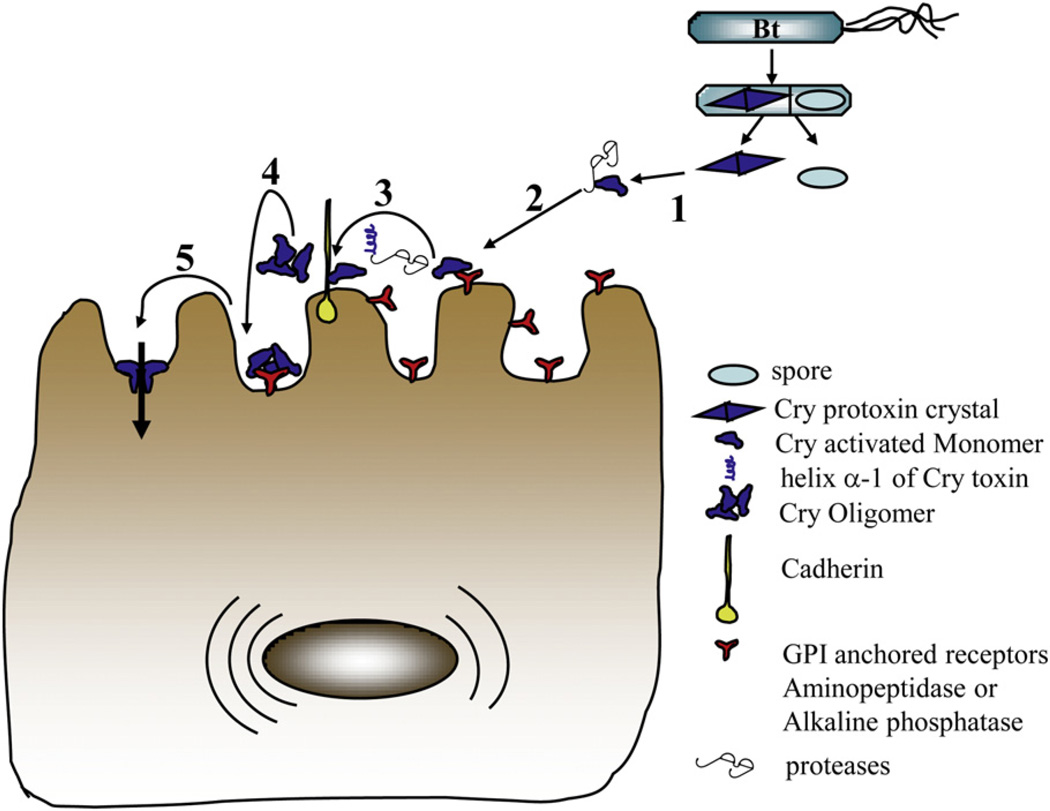
The mode of action of Cry1Ab toxin has been particularly well defined in M. sexta. The 3D-Cry toxins such as Cry1A are produced as protoxins that are dissolved and processed proteolytically by insect proteases releasing the active toxic fragment composed of the three domain structure as described in Fig. 1. Two groups of protoxins have been reported, large protoxins such as Cry1Aa of 130 kDa and short protoxins of 70 kDa such as Cry2Aa. Large protoxins lose half of the C-terminal end and 20 to 50 amino acids of the N-terminal end by proteolytical cleavages while short protoxins are processed primarily at the N-terminal end. The activated toxin goes through complex sequential binding events with the different insect gut Cry-binding proteins leading to membrane insertion and pore formation. The first binding interaction of the activated Cry1Ab toxin occurs through exposed amino acid regions of domain II (specifically through loop 3) and domain III (through strand β-16) of the toxin with M. sexta ALP and APN that are highly abundant low affinity binding sites for the toxin (Gómez et al., 2006; Pacheco et al., 2009b). These binding interactions concentrate the activated toxin in the microvilli membrane of the midgut cells, where the toxin then binds with high affinity through exposed domain II loops to the cadherin receptor including loops α-8, 2 and particularly loop 3 in M. sexta, H. virescens and B. mori (Xie et al., 2005; Gómez et al., 2006; Atsumi et al., 2008). This interaction with cadherin receptor facilitates further proteolytic cleavage of the N-terminal end including helix α-1 of domain I that induces the formation of a toxin pre-pore oligomer (Gómez et al., 2002; Atsumi et al., 2008). The oligomeric structure of the toxin showed an important increase of 200 fold in its affinity to GPI anchored receptors, ALP and APN, involving domain II loop 2 region (Arenas et al., 2010). The binding of the pre-pore to the GPI-anchored proteins leads finally to the insertion into the membrane causing pore-formation and cell lysis (Pardo et al., 2006). Recent data showed that formation of oligomers by Cry1Ab toxin is an essential step in the mode of action of this toxin. First, α-helix 3 of domain I was identified as a potential oligomerization region and point mutations in some residues of α-helix 3 resulted in complete loss of toxicity to M. sexta. It was shown that these non-toxic mutants were affected in oligomer formation indicating that oligomer formation is essential for toxicity (Jimenez-Juarez et al., 2007). In addition, characterization of domain I α-helix 4 mutants revealed that these mutants were also severely affected in toxicity against M. sexta but, in contrast to α-helix 3 mutants described above, the point mutations in α-helix 4 were able to form oligomeric structures that were affected in membrane insertion (Rodríguez-Almazan et al., 2009). Interestingly, the non toxic α-helix 4 mutants showed a dominant negative phenotype since they were capable of inhibiting Cry1Ab toxicity, membrane insertion and pore formation when mixed in low protein:protein ratios. The isolation of dominant negative phenotype indicates that the non-toxic α-helix 4 mutants were capable of forming hetero-oligomers with the wild type Cry1Ab toxin, inhibiting its insertion into the membrane (Rodríguez-Almazan et al., 2009). Also, it was shown that a M. sexta cadherin fragment containing a Cry1Ab binding site enhanced Cry1Ab toxicity when fed to the larvae along with the Cry1Ab protein (Chen et al., 2007). The enhancement in toxicity was later shown to correlate with Cry1Ab oligomer formation (Pacheco et al., 2009a). In the pore formation model of Cry toxin action, binding to cadherin facilitates the proteolytic removal of domain I α-helix 1 promoting oligomer formation. It was shown that genetically engineered Cry1Ab and Cry1Ac modified toxins (Cry1AbMod and Cry1AcMod) that were deleted at N-terminal region including domain I α-helix 1, were able to kill the P. gossypiella populations resistant to Cry1Ac toxin due to mutations in the cadherin gene (Soberón et al., 2007). These data show that even in the absence of cadherin protein the Cry1AMod toxins are active against resistant larvae and that the primary role of cadherin binding is the removal of α-helix 1 promoting oligomer formation (Soberón et al., 2007). Fig. 3 shows the molecular events that lead to Cry toxin membrane insertion and pore formation. As seen in Fig. 3, two GPI anchored receptors are involved in Cry1Ab toxicity to M. sexta. However, ALP seems to play a more important role in toxicity than APN since ALP is produced at higher levels at the first larval instars with low levels of expression in the fourth and fifth larval instars, while APN shows the opposite, low expression levels in the first larval instars and increased expression at the third until the fifth larval instars (Arenas et al., 2010). The expression profile of ALP correlates with the sensitivity of M. sexta larvae to Cry1Ab toxin since younger larvae are more sensitive to the toxin.
Fig. 3.
Mode of action of Cry1A toxins in the lepidopteran M. sexta. 1. Solubilization and proteolytic processing of Cry1A protoxin by midgut proteases. 2. Binding of monomeric 3D Cry1A to highly abundant GPI-anchored aminopeptidase-N and alkaline phosphatase. 3. Binding of monomeric Cry1A toxin to cadherin receptor and further proteolysis of domain I α-helix 1. 4. Oligomer formation and binding of the oligomeric structure to GPI-anchored aminopeptidase-N and alkaline phosphatase receptors. 5. Insertion of the oligomeric Cry1A structure into the membrane.
The Cry1Ab pre-pore oligomer was proposed to be a tetrameric structure based on the apparent molecular size of the oligomeric structure after SDS-PAGE electrophoresis (Gómez et al., 2002). Nevertheless, two dimensional array images of membrane inserted Cry4Ba and later of Cry1AbMod toxins revealed a trimeric organization (Ounjai et al., 2007; Muñoz-Garay et al., 2009). Interestingly, the three dimensional structure of Cry4Ba, obtained from a Cry4Ba truncated form that lacked domain I α-helices 1 and 2A, revealed a trimeric structure with a contact interface involving domain I α-helices 3, 4 and 6 (Boonserm et al., 2005). This structure agrees with the phenotype of α-helix 3 mutants that were affected in oligomer formation (Jiménez-Juárez et al., 2007). Recently, a structural model of Cry4Aa pre-pore trimer was reported and the authors proposed that the mechanism of membrane insertion may involve the insertion of three domain I α-helix 4-helix 5 hairpins to form a stable transmembrane pore (Taveecharoenkool et al., 2010).
As mentioned previously there is a significant consensus that Bt Cry toxins are pore forming proteins that cause cell lysis by producing an osmotic shock. Nevertheless, an alternative model of the mode of action of Cry toxins was proposed based on data obtained with transfected H5 Tricoplusia ni cultured cells expressing the M. sexta cadherin gene (Zhang et al., 2006). This model proposes that insect cell death is triggered by the binding of monomeric Cry1Ab toxin to cadherin receptor resulting in increased cAMP cellular levels by activation of adenylyl cyclase. Then, cAMP activates protein kinase-A resulting in cell death related to oncosis (Zhang et al., 2006). In this signal transduction model, neither GPI-anchored receptors nor oligomer formation are involved in Cry toxicity (Zhang et al., 2006).
3.2. The case of dipteran insects
There is an increasing interest in determining the mode of action of Cry toxins in mosquitoes since these insects are important vectors of human diseases such as dengue, yellow fever and malaria among others. Interestingly there are multiple Cry toxins with low primary sequence similarities that show toxicity against mosquitoes like, Cry1, Cry2, Cry4, Cry11, Cry29 etc. However, one particular Bt strain has been used worldwide for the control of mosquitoes, Bt var israelensis (Bti). Bti produces crystal inclusions formed principally by Cry4Aa, Cry4Ba, Cry11Aa and Cyt1Aa (Margalith and Ben-Dov, 2000). Bti shows high toxicity to Aedes aegypti vector of dengue and yellow fevers and Culex sp. species but moderate toxicity to Anopheles gambiae, a vector of malaria (Margalith and Ben-Dov, 2000). One interesting feature of these four Bti toxins is that they show a synergistic effect. The toxicity of the whole Bti crystal inclusion is much higher than the sum of the individual toxicities of each one of the Cry and Cyt proteins in this crystal (reviewed in Bravo et al., 2005). Cyt1Aawas shown to synergize the activity of the three Cry toxins and to overcome resistance of the Culex sp. populations to the Cry toxins in Culex sp. (Khasdan et al., 2001; Wirth et al., 1997).
Receptor identification of mosquitocidal proteins has been performed primarily in Ae. aegypti, An. gambiae, An. quadrimaculatus and An. albimanus. As in lepidopteran insects, cadherin proteins have been identified in Ae. aegypti and An. gambiae showing binding to Cry11Aa and Cry4Ba respectively (Hua et al., 2008; Chen et al., 2009b). In Ae. aegypti, cadherin also serves a receptor of Cry11Ba toxin that was isolated from the Bt var jegathesan strain but showed lower affinity to Cry4Ba protein (Chen et al., 2009b; Likitvivatanavong et al., 2010). An An. gambiae cadherin fragment containing the Cry4Ba binding site enhanced the toxicity of Cry4Ba in both An. gambiae and Ae. aegypti larvae suggesting its active role as a receptor of Cry4Ba in these mosquitoes species (Hua et al., 2008; Park et al., 2009a). In the case of Ae. aegypti cadherin, it was shown that an anti-cadherin antibody competed binding of Cry11Aa to Ae. aegypti BBMV (Chen et al., 2009b). In both Ae. aegypti and An. gambiae, cadherin is located in the microvilli of the caeca and in the microvilli of the posterior gut cells, that are the same sites where Cry11Aa and Cry4Ba bind (Hua et al., 2008; Chen et al., 2009b). Regarding GPI anchored proteins, both APN and ALP have been identified in An. gambiae, An. quadrimaculatus and Ae. aegypti as Cry4Ba, Cry11Aa and Cry11Ba binding proteins (Fernández et al., 2006; Abdullah et al., 2006; Zhang et al., 2008; Hua et al., 2009; Chen et al., 2009a; Likitvivatanavong et al., 2010). Interestingly, a GPI-anchored α-glucosidasewas identified as a Cry4Ba binding molecule in A. albimanus larvae (Fernández-Luna et al., 2010). The Ae. aegypti ALP is involved in Cry11Aa toxicity since a peptide-phage (P1-BBMV) that bound ALP, competed the binding and toxicity of Cry11Aa (Fernández et al., 2006). The ALP1 isoform was identified as the Cry11Aa receptor and two ALP Cry11Aa binding sites in this receptor were shown to bind domain III561RVQSQNSGNN570 and domain II loop α-8 regions of Cry11Aa toxin (Fernández et al., 2009). Previous work identified Cry11Aa loop α-8 as an important toxin region involved in Cry11Aa binding to Ae. aegypti BBMV and toxicity and more recently with the cadherin receptor (Fernández et al., 2005; Chen et al., 2009b). ALP1 was later shown to bind Cry4Ba and Cry11Ba (Fernández et al., 2009; Likitvivatanavong et al., 2010). In A. gambiae an ALP was also identified as Cry11Ba binding protein (Hua et al., 2009).
Two APN isoforms (AaeAPN1 and AaeAPN2) were identified in Ae. aegypti by Cry11Aa pull down experiments (Chen et al., 2009a). Protein fragments from both APN isoforms were produced in E. coli and shown to inhibit binding of Cry11Aa to BBMV, suggesting their active role in Cry11Aa binding to insect membranes (Chen et al., 2009a). In the case of An. gambiae and An. quadrimaculatus larvae, two APN’s were also identified as Cry11Ba binding proteins (Abdullah et al., 2006; Zhang et al., 2008). Interestingly, Cry11Ba binds both An. quadrimaculatus and An. gambiae APN molecules with a very high binding affinity of 0.56 nM and 6.4 nM respectively (Abdullah et al., 2006; Zhang et al., 2008). These results suggest that APN may have a more important role in the toxicity of Cry11Ba in these two Anopheline species. In fact, it was recently shown that certain A. gambiae APN protein fragments enhanced Cry11Ba toxicity as has been shown for cadherin protein fragments (Zhang et al., 2010).
Table 1 shows the Cry toxin receptors identified in different mosquito species. The fact that similar Cry binding proteins are involved in the mechanism of action of Cry toxins in both lepidopteran and dipteran insects suggests the Cry toxins have a conserved mode of action. However, the precise role of the Cry toxins receptors identified in mosquitoes in the mode of action of Cry toxins still remains to be determined. As in lepidopteran insects cadherin binding might facilitate oligomer formation while binding of Cry oligomer to GPI-anchored ALP or APN receptors might be necessary to facilitate membrane insertion. Nevertheless, the high binding affinity of Anopheline APN’s to Cry11Ba is substantially different from what has been reported in lepidopteran insects and further studies on the differential role of APN and cadherin in monomer/oligomer binding in mosquitoes are necessary to determine the precise role of toxin binding to these receptor molecules.
The analysis of Cry4Ba binding proteins by mass spectrometry in Ae. aegypti BBMV, revealed two lipid rafts associated proteins, flotillin and prohibitin, as well as cytoplasmic actin, besides ALP and APN, thus suggesting that additional proteins as well as intracellular proteins may have an active role in the mode of action of Cry toxins in mosquitoes (Bayyareddy et al., 2009).
One of the most interesting features of Bti toxins is the synergistic effect of Cyt1Aa on Cry4Aa, Cry4Ba and Cry11Aa toxins activity. Cry4Ba and Cry11Aa bind Cyt1Aa through domain II loop regions that are involved in receptor interaction (Pérez et al., 2005; Cantón et al., 2011). Moreover single point mutations in the Cyt1Aa binding epitopes involved in the binding interaction with Cry4Ba and Cry11Aa toxins, showed a correlation between Cyt1Aa binding to these toxins and synergism (Pérez et al., 2005; Cantón et al., 2011). These results suggest that Cyt1Aa functions as a surrogate receptor for Cry4Ba and Cry11Aa. In the case of Cry11Aa, it was shown that its binding to Cyt1Aa facilitates the formation of a 250 kDa oligomeric structure of Cry11Aa, which is competent in pore formation suggesting that Cyt1Aa fulfills at least the role of a cadherin receptor regarding oligomer formation (Pérez et al., 2007).
3.3. The case of coleopteran insects
In the case of Cry toxins active against coleopteran insects, Cry binding proteins have been identified in Tenebrio molitor, Diabrotica virgifera virgifera, Leptinotarsa decemlineata and Anthonomus grandis (Fabrick et al., 2009; Park et al., 2009b; Ochoa-Campuzano et al., 2007; Martins et al., 2010). A cadherin protein from T. molitor was identified as a Cry3Aa binding protein, and it was shown to facilitate Cry3Aa oligomer formation. Moreover, silencing of the cadherin gene by feeding dsRNA showed that the silenced beetles were resistant to Cry3Aa indicating an active role of cadherin on Cry3Aa toxicity (Fabrick et al., 2009). A cadherin protein was also identified as a Cry3Aa receptor in Diabrotica virgifera virgifera. A fragment of this cadherin protein containing the membrane proximal cadherin repeats 8–10 bound Cry3Aa and Cry3Bb toxins with high affinity (Kd of 12 and 1.4 nM, respectively) and enhanced Cry3Aa and Cry3Bb toxicity to different coleopteran insects (Park et al., 2009b). In the case of L. decemlineata, an ADAM 3 metalloprotease was identified as Cry3Aa receptor. Binding of Cry3Aa to ADAM-3 through domain II loop 1 enhanced Cry3Aa pore-formation activity suggesting that this binding interaction is important for Cry3Aa toxicity (Ochoa-Campuzano et al., 2007). The only GPI-anchored protein identified in coleopteran insects as a putative Cry receptor was an ALP from A. grandis that bound Cry1B toxin (Martins et al., 2010). Table 1 shows the Cry toxin receptors identified in different coleopteran pests.
Overall, the identification of similar Cry binding proteins in the three different insect orders and the fact that Cry toxins share a similar three domain fold, suggests that the mode of action of Cry toxins is conserved in different insect orders.
4. Opposing the attack of Cry toxins
The identification of cellular components involved in a defense response to Cry toxins could provide tools for enhancing Cry toxicity against certain insects. The most important contributions on the identification of these cellular responses were achieved in C. elegans that is sensitive to Cry5B and Cry21 toxins. Nevertheless, it should be pointed out that although Cry5B and Cry21 are members of the 3D Cry toxin family, it has been shown that Cry5Ba is internalized into the host cell cytoplasm (Griffitts et al., 2003) and no pore formation activity of these toxins has been documented until today indicating that probably the mode of action of Cry toxins in this organism may be different from that of Cry toxins in insects. A microarray analysis of C. elegans gene expression in the presence of Cry5B toxin, revealed the mRNA up-regulation of MAPK p38 (PMK-1), SEK-1 (a MAPKK immediately upstream of p38) and JNK kinases (Huffman et al., 2004). JNK and p38 MAPK pathways are mainly associated to stress-associated stimuli, and they are collectively termed stress-activated protein kinases.
The relevance of SEK-1 and PMK-1 in the defense pathway was demonstrated by feeding C. elegans sek-1 or pmk-1 mutants animals with Cry5B showing a hypersensitive response, indicating that SEK-1 and PMK-1 kinases participates in the protection of nematodes against Cry5B toxin action (Huffman et al., 2004).
To identify downstream targets of the p38 pathway that are specifically activated in response to Cry5B, differences in transcript levels C. elegans wild type or p38 silenced animals in the presence of Cry5B revealed two p38 dependent transcripts named ttm-1 and ttm-2 (Huffman et al., 2004). The role of these proteins in Cry5B defense was determined by analyzing silenced animals using dsRNA and again they were hypersensitivity to Cry5B intoxication (Huffman et al., 2004). The ttm-1 gene shares homology with the human zinc transporter ZnT-3, suggesting a possible role in removing cytotoxic cations (Huffman et al., 2004). Additional genes identified as targets of p38 pathway after Cry5B action in C. elegans showed that the stress response to the unfolded proteins of the endoplasmic reticulum (UPR) resulted in the hypersensitive phenotype to Cry5B exposure (Bischof et al., 2008). Recently, a whole genome approach in the nematode led to the identification of hypoxia response and the signal transduction ERK pathway as additional responses to Cry5B intoxication (Bellier et al., 2009; Chen Cha et al., 2010).
In the case of insects, the role of p38 pathway on Cry toxin defense was reported in the lepidopteran M. sexta and the dipteran Ae. aegypti (Cancino-Rodezno et al., 2010). Treatment of M. sexta or Ae. aegypti larvae with a medium lethal concentration dose of Cry1Ab or Cry11Aa, respectively, resulted in a fast activation of p38 by phosphorylation. The activation of p38 was not observed when the insect larvae were treated with non-toxic Cry1Ab- or Cry11Aa-mutants affected in pore formation, indicating that p38 phosphorylation is triggered after pore formation by Cry toxin and not by the interaction with receptor proteins (Cancino-Rodezno et al., 2010). Finally, silencing of p38 protein by feeding dsRNA in M. sexta and Ae aegypti, resulted in hypersensitivity of both insect larvae to Cry toxin intoxication, supporting again that p38 pathway has a protective function in insects (Cancino-Rodezno et al., 2010).
5. Cry toxins as bioinsecticide products
Different Bt products have been developed for insect control in agriculture and also against mosquitoes species. Most of these products are based on spore-crystal preparations derived from a few wild-type strains such as B. thuringiensis var. kurstaki (Btk) HD1 that express Cry1Aa, Cry1Ab, Cry1Ac and Cry2Aa proteins or HD73 that produces Cry1Ac; B. thuringiensis var. aizawai HD137 which produces slightly different Cry toxins such as Cry1Aa, Cry1B,a Cry1Ca and Cry1Da; B. thuringiensis var. san diego and B. thuringiensis var. tenebrionis, which produce Cry3Aa toxin and Bti containing Cry4A, Cry4B, Cry11A and Cyt1Aa toxins. Btk products are effective in controlling many leaf-feeding lepidopterans that are important crop pests or forest pest defoliators (reviewed in Soberón et al., 2009). Bt aizawai based products are especially active against lepidopteran larvae that feed on stored grains. Bt san diego and Bt tenebrionis based products, are suited for beetle pests in agriculture. Finally, Bti based products are used for the control of mosquitoes that are vector of human diseases as dengue fever and malaria (reviewed in Soberón et al., 2009).
Bt based sprayable products have limited use in agriculture since Cry toxins are specific to young larval stages, are sensitive to sun radiation and have a limited activity against borer insects. Nevertheless, an important breakthrough in the reduction of chemical insecticides in agriculture came with the development of transgenic crops that are able to express Cry toxins (James, 2009). In transgenic plants the Cry protein is produced continuously, protecting the insecticidal toxin from UV degradation and specifically targets chewing and boring insects. In 2009 more than 40 million hectares of Bt-crops were grown world-wide resulting in a significant reduction on the use of chemical insecticides and contributing in some cases to the suppression of certain insect pests like P. gossypiella, a pest of cotton (James, 2009; Tabashnik et al., 2010). Presently the most important Bt-crops are soya, corn, cotton and canola (James, 2009). Commercial Bt cotton expresses the Cry1Ac protein for the control of lepidopteran pests as H. zea and P. gossypiella among others. A second generation Bt-cotton produces Cry2Ab besides Cry1Ac as a resistance managing mechanism (see below). Bt-corn expressing Cry1Ac effectively controls lepidopteran pests as H. virescens and O. nubilalis (Christou et al., 2006). The next generation of commercial Bt-corn express a series of toxin including Cry34Ab/Cry35Ab binary toxin and Cry3Bb to control coleopteran pests such as Diabrotica virgifera and also Cry1A, Cry2Ab and Cry1F for the control of lepidopteran pests including also Spodoptera frugiperda (Christou et al., 2006). Although not commercially available yet, VIP3 has been also successfully produced in transgenic corn (Christou et al., 2006).
6. A threat to the technology: resistance to Cry toxins
The major threat to the use of Bt crops is the appearance of insect resistance. Resistance to Cry toxins can be developed by mutations in the insect pests that affect any of the steps of the mode of action of Cry toxins. Laboratory selected resistant insect populations have shown that resistance can be developed by different mechanisms including alteration of Cry toxins activation (Oppert et al., 1997), sequestering the toxin by lipophorin (Ma et al., 2005) or esterases (Gunning et al., 2005), by elevated immune response (Hernández-Martínez et al., 2010) and by alteration of toxin receptors resulting in reduced binding to insect gut membranes (reviewed in Griffits and Aroian, 2005). In the case of C. elegans mutations affecting glycolipid biosynthesis resulted in resistance to Cry5 toxin (Griffits et al., 2005). The most common mechanism of toxin resistance in insect pests until now is the reduction in toxin binding to midgut cells, that in different insect species include mutations in Cry toxin receptors as cadherin, ALP or APN (Gahan et al., 2001; Morin et al., 2003; Herrero et al., 2005; Jurat-Fuentes et al., 2004; Zhang et al., 2009). Recently, a resistant allele of a H. virescens resistant population was identified as a mutation in a gene coding for an ABC transporter molecule. This mutation affected binding of Cry1A toxin to brush border membrane vesicles indicating that this ABC transporter molecule is a novel Cry1A toxin receptor probably involved in the later stages of oligomer membrane insertion (Gahan et al., 2010). In fact, the most frequently phenotype of insect resistance, denoted as “Mode 1 of Resistance”, is characterized by the reduction of one Cry1A toxin binding, cross resistance of Cry1Aa, Cry1Ab and Cry1Ac and lack of resistance to Cry1C. In several lepidopteran insects, the mode 1 of resistance is linked to mutations in the cadherin gene (Gahan et al., 2001; Morin et al., 2003; Xu et al., 2005). In field conditions three lepidopteran insect pests have evolved resistance to formulated Bt products, Plodia interpunctella, Plutella xylostella and T. ni (McGaughey, 1985; Tabashnik, 1994; Janmaat and Myers, 2003). In recent years, at least four cases of resistance to Bt crops have been documented, H. zea to Bt-cotton expressing Cry1Ac in United States (Tabashnik et al., 2008), S. frugiperda to Bt-corn expressing Cry1F in Puerto Rico (Storer et al., 2010), Busseola fusca to Bt-corn expressing Cry1Ab in South Africa (van Rensburg, 2007), and P. gossypiella to Bt-cotton expressing Cry1Ac in India (Bagla, 2010).
Appearance of insect resistance to Cry toxins in transgenic crops has been delayed by the so called “High Dose Refuge Strategy”. This strategy entails planting a significant percentage of non-Bt plants in the proximity of Bt-crops that express a high dose of Cry toxin. Non-Bt plants refuges are intended to maintain a population of susceptible insects. Susceptible insects to Cry toxins mate with resistant individuals that are potentially selected on Bt-plants producing susceptible offspring due to the recessive characteristic of resistant alleles (Tabashnik et al., 2008). Modeling studies have shown that refuge strategy has been successful in delaying appearance of resistance of P. gosypiella to Bt-cotton in the United States and explains the reason for the appearance of resistance of the same insect species to Bt-cotton in India (Tabashnik et al., 2010). Recently it has been shown that the release of sterile P. gosypiella females in an eradication program along with the use of Bt-cotton can efficiently slow down the frequency of resistant alleles in the field (Tabashnik et al., 2010). This strategy can be used instead of the refuge strategy to avoid significant crop losses since non-Bt plants will suffer damage from insect attack. This strategy may be particularly relevant in countries where the refuge strategy is difficult to implement.
Other strategies to cope with the appearance of insect resistance is the use of gene stacking of different Cry toxins with different mode of action in the same plant (reviewed in Bravo and Soberón, 2008). This includes for instance the Bt-cotton Bollgard II expressing Cry1Ac and Cry2Ab proteins that bind to different receptor molecules. Also Bt-corn plant expressing Vip3 along with Cry1Ab and Bt-corn with three Cry toxins against lepidopteran insects (Cry1A1.05, Cry2Ab and Cry1F) and two Cry toxins against coleopteran insects (Cry34Ab/Cry35Ab and Cry3Bb). As mentioned previously Cry1AMod toxins skip cadherin receptor and have been shown to be able to kill P. gossypiella resistant population that is linked to mutations in the cadherin gene. Cry1AMod toxins also killed M. sexta larvae that were silenced of the cadherin gene expression by dsRNA and that showed high tolerance to Cry1Ab intoxication (Soberón et al., 2007). More recently, Cry1AcMod was shown to be effective against the T. ni field-resistant population indicating that this resistant population may be linked to mutations in the cadherin gene (Franklin et al., 2009).
7. The future
Bt Cry toxins have been shown to be a valuable tool for insect control, especially with the development of transgenic plants expressing Cry toxins. This technology has been shown successful in diminishing the use of chemical insecticides (James, 2009). As pointed out earlier, the appearance of resistant insects could threaten this technology. However, only a limited number of Cry proteins are now produced in transgenic crops. New Cry proteins that are active against important pests will be introduced to transgenic crops diminishing the possibility for the appearance of resistant insects. In fact the next generation Bt crops produce more multiple Cry toxins reducing the possibility of the development of insect resistance while controlling different insect order species as coleopteran and lepidopteran insects. Gene stacking in crops will continue with the introduction of novel Cry genes identified by screening novel Bt isolates or by introducing novel Cry genes engineered to have improved insecticidal activities against important insect pests. Understanding the mode of action of these toxins and how insects respond to the attack of Cry proteins will allow the development of new, more efficient Bt crops and spray products. Therefore, we foresee a brilliant future in the use Bt Cry proteins to control important insect pests in agriculture reducing to a greater extent the dependence in chemical insecticides and having a positive impact helping to conserve a healthier environment.
References
- Abdullah MA, Valaitis AP, Dean DH. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 2006;22:7–16. doi: 10.1186/1471-2091-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S, Inoue Y, Ishizaka T, Mizuno E, Yoshizawa Y, Kitami M, Sato R. Location of the Bombyx mori 175kDa cadherin-like protein-binding site on Bacillus thuringiensis Cry1Aa toxin. FEBS. J. 2008;275:4913–4926. doi: 10.1111/j.1742-4658.2008.06634.x. [DOI] [PubMed] [Google Scholar]
- Arenas I, Bravo A, Soberon M, Gomez I. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 2010;285:12497–12503. doi: 10.1074/jbc.M109.085266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagla P. Hardy cotton-munching pests are latest blow to GM crops. Science. 2010;327:1439. doi: 10.1126/science.327.5972.1439. [DOI] [PubMed] [Google Scholar]
- Bayyareddy K, Andacht TM, Abdullah MA, Adang MJ. Proteomic identification of Bacillus thuringiensis subsp. israelensis toxin Cry4Ba binding proteins in midgut membranes from Aedes (Stegomyia) aegypti Linnaeus (Diptera, Culicidae) larvae. Insect Biochem. Mol. Biol. 2009;39:279–286. doi: 10.1016/j.ibmb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bel Y, Escriche B. Common genomic structure for the Lepidoptera cadherin-like genes. Gene. 2006;381:71–80. doi: 10.1016/j.gene.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Bellier A, Chen Ch-S, Kao Ch-Y, Cinar HN, Aroian RV. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLoS Pathogen. 2009;5(12):e1000689. doi: 10.1371/journal.ppat.1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof LJ, Kao Ch-Y, Los FCO, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathogens. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonserm P, Davis P, Ellar DJ, Li J. Crystal Structure of the Mosquitolarvicidal Toxin Cry4Ba and Its biological implications. J. Mol. Biol. 2005;348:363–382. doi: 10.1016/j.jmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Boonserm P, Mo M, Angsuthanasombat Ch, Lescar J. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-Å resolution. J. Bacteriol. 2006;188:3391–3401. doi: 10.1128/JB.188.9.3391-3401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A. Phylogenetic relationships of Bacillus thuringiensis d-endotoxin family proteins and their functional domains. J. Bacteriol. 1997;179:2793–2801. doi: 10.1128/jb.179.9.2793-2801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, Gill SS, Soberón M. Bacillus thuringiensis mechanisms and use. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecluar Insect Science. Elsevier BV; 2005. pp. 175–206. ISBN 0-44-451516-X. [Google Scholar]
- Bravo A, Gill SS, Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, Soberón M. How to cope with resistance to Bt toxins? Trends Biotechnol. 2008;26:573–579. doi: 10.1016/j.tibtech.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Cancino-Rodezno A, Alexander C, Villaseñor R, Pacheco S, Porta H, Pauchet Y, Gill SS, Soberón M, Bravo A. The mitogen-activated protein kinase p38 p.thway is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2010;40:58–63. doi: 10.1016/j.ibmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantón PE, Reyes EZ, Ruiz I, Bravo A, Soberón M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides. 2011;32:595–600. doi: 10.1016/j.peptides.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hua G, Jurat-Fuentes JL, Abdullah MA, Adang MJ. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13901–13906. doi: 10.1073/pnas.0706011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Aimanova KG, Pan S, Gill SS. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem. Mol. Biol. 2009a;39:688–696. doi: 10.1016/j.ibmb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Aimanova KG, Fernandez LE, Bravo A, Soberón M, Gill SS. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. Israelensis. Biochem. J. 2009b;424:191–200. doi: 10.1042/BJ20090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Cha S, Bellier A, Kao Ch-Y, Yang Y-L, Chen H-D, Los FCO, Aroian RV. WWP-1 is a novel modulator of the DAF-2 insulin-like signaling network involved in pore-forming toxin cellular defenses in Caenorhabditis elegans. PLoS One. 2010;5:e9494. doi: 10.1371/journal.pone.0009494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Dym O, Albeck S, Ben-Dov E, Cahan R, Firer M, Zaritsky A. High-resolution crystal of activated Cyt2Ba monomer from Bacillus thuringiensis subs. Israelensis. J. Mol. Biol. 2008;380:820–827. doi: 10.1016/j.jmb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Crickmore N, Zeigler DR, Schnepf E, Van Rie J, Lereclus D, Baum J, Bravo A, Dean DH. Bacillus thuringiensis toxin nomenclature. 2010 http://www.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/index.html.
- Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AM. Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci. 2006;11:302–308. doi: 10.1016/j.tplants.2006.04.001. [DOI] [PubMed] [Google Scholar]
- de Maagd RA, Weemen-Hendriks M, Stiekema W, Bosch D. Domain III substitution in Bacillus thuringiensis delta-endotoxin Cry1C domain III can function as a specific determinant for Spodoptera exigua in different, but not all, Cry1-Cry1C hybrids. Appl. Environ. Microbiol. 2000;66:1559–1563. doi: 10.1128/aem.66.4.1559-1563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd RA, Bravo A, Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001;17:193–199. doi: 10.1016/s0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- Devine GJ, Furlong MJ. Insecticide use: contexts and ecological consequences. Agr. Hum. Values. 2007;24:281–306. [Google Scholar]
- Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Sci. U.S.A. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrick J, Oppert C, Lorenzen MD, Morris K, Oppert B, Jurat-Fuentes JL. A novel Tenebrio molitor cadherin is a functional receptor for Bacillus thuringiensis Cry3Aa toxin. J. Biol. Chem. 2009;284:18401–18410. doi: 10.1074/jbc.M109.001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández LE, Perez C, Segovia L, Rodriguez MH, Gill SS, Bravo A, Soberón M. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop α-8 of domain II. FEBS Lett. 2005;579:3508–3514. doi: 10.1016/j.febslet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Fernández LE, Aimanova KG, Gill SS, Bravo A, Soberón M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem. J. 2006;394:77–84. doi: 10.1042/BJ20051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández LE, Martinez-Anaya C, Lira E, Chen J, Evans J, Hernández-Martínez S, Lanz-Mendoza H, Bravo A, Gill SS, Soberón M. Cloning and epitope mapping of Cry11Aa-binding sites in the Cry11Aa-receptor alkaline phosphatase from Aedes aegypti. Biochemistry. 2009;48:8899–8907. doi: 10.1021/bi900979b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Luna MT, Lanz-Mendoza H, Gill SS, Bravo A, Soberón M, Miranda-Rios J. An α-amylase is a novel receptor for Bacillus thuringiensis subsp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae) Environ. Microbiol. 2010;12:746–757. doi: 10.1111/j.1462-2920.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MT, Nieman CL, Janmaat AF, Soberón M, Bravo A, Tabashnik BE, Myers JH. Modified Bacillus thuringiensis toxins and a hybrid B. thuringiensis strain counter greenhouse-selected resistance in Trichoplusia ni. Appl. Environ. Microbiol. 2009;75:5739–5741. doi: 10.1128/AEM.00664-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- Gahan LJ, Pauchet Y, Vogel H, Heckel DG. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010;6:e1001248. doi: 10.1371/journal.pgen.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitsky N, Cody V, Wojtczak A, Ghosh D, Luft JR, Pangborn W, English L. Structure of the insecticidal bacterial d-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Cryst. 2001;D57:1101–1109. doi: 10.1107/s0907444901008186. [DOI] [PubMed] [Google Scholar]
- Garczynski SF, Adang MJ. Investigations of Bacillus thuringiensis Cry1 toxin receptor structure and function. In: Charles JF, Delécluse A, Nielsen-LeRoux C, editors. Entomopathogenic Bacteria, from Laboratory to Field Application. Kluwer Academic Publishers; 2000. pp. 181–197. [Google Scholar]
- Gómez I, Sanchez J, Miranda R, Bravo A, Soberon M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix alpha-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 2002;513:242–246. doi: 10.1016/s0014-5793(02)02321-9. [DOI] [PubMed] [Google Scholar]
- Gómez I, Arenas I, Benitez I, Miranda-Ríos J, Becerril B, Grande G, Almagro JC, Bravo A, Soberón M. Specific epitopes of Domains II and III of Bacillus thuringiensis Cry1Ab toxin involved in the sequential interaction with cadherin and aminopeptidase-N receptors in Manduca sexta. J. Biol. Chem. 2006;281:34032–34039. doi: 10.1074/jbc.M604721200. [DOI] [PubMed] [Google Scholar]
- Griffitts JS, Huffman DL, Whitacre JL, Barrows BD, Marroquin LD, Müller R, Brown JR, Hennet T, Esko JD, Aroian RV. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxinehost interactions. J. Biol. Chem. 2003;278:45594–45602. doi: 10.1074/jbc.M308142200. [DOI] [PubMed] [Google Scholar]
- Griffits JS, Haslam SM, Yang T, Garczynski SF, Mulloy B, Morris H, Cremer PS, Dell A, Adang MJ, Aroian RV. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- Griffits J, Aroian RV. Many roads to resistance: how invertebrates adapt to Bt toxins. BioEssays. 2005;27:614–624. doi: 10.1002/bies.20239. [DOI] [PubMed] [Google Scholar]
- Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL, Brousseau R, Cygler M. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 1995;254:447–464. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- Gunning RV, Dang HT, Kemp FC, Nicholson IC, Moores GD. New resistance mechanism in Helicoverpa armigera threatens transgenic crops expressing Bacillus thuringiensis Cry1Ac toxin. Appl. Environ. Microbiol. 2005;71:2558–2563. doi: 10.1128/AEM.71.5.2558-2563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Ye S, Liu Y, Wei L, Xue J, Wu H, Song F, Zhang J, Wu X, Huang D, Rao Z. Crystal structure of Bacillus thuringiensis Cry8Ea1: An insecticidal toxin toxic to underground pests, the larvae of Holotrichia parallela. J. Struct. Biol. 2009;168:259. doi: 10.1016/j.jsb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Hernández-Martínez P, Navarro-Cerrillo G, Caccia S, de Maagd RA, Moar WJ, Ferré J, Escriche B, Herrero S. Constitutive activation of the midgut response to Bacillus thuringiensis in Bt resistant Spodoptera exigua. PLoS One. 2010;5:e12795. doi: 10.1371/journal.pone.0012795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero S, Gechev T, Bakker PL, Moar WJ, de Maagd RA. Bacillus thuringiensis Cry1Ca-resistant Spodoptera exigua lacks expression of one of four Aminopeptidase N genes. BMC Genomics. 2005;24:6–96. doi: 10.1186/1471-2164-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Zhang R, Abdullah MA, Adang MJ. Anopheles gambiae cadherin AgCad1 binds the Cry4Ba toxin of Bacillus thuringiensis israelensis and a fragment of AgCad1 synergizes toxicity. Biochemistry. 2008;47:5101–5110. doi: 10.1021/bi7023578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Zhang R, Bayyareddy K, Adang MJ. Anopheles gambiae alkaline phosphatase is a functional receptor of Bacillus thuringiensis jegathesan Cry11Ba toxin. Biochemistry. 2009;48:9785–9793. doi: 10.1021/bi9014538. [DOI] [PubMed] [Google Scholar]
- Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Mitogen-activated protein kinase pathways defend against bacterial poreforming toxins. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. Global Status of Commercialized Biotech/GM Crops: 2009. ISAAA Brief No. 41. Ithaca, NY: ISAAA; 2009. [Google Scholar]
- Janmaat AF, Myers JH. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Tricoplusia ni. Proc. R. Soc. Lond. 2003;B270:2263–2270. doi: 10.1098/rspb.2003.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Juárez A, Muñoz-Garay C, Gómez I, Saab-Rincon G, Damian-Alamazo JY, Gill SS, Soberón M, Bravo A. Bacillus thuringiensis Cry1Ab mutants affecting oligomer formation are non-toxic to Manduca sexta larvae. J. Biol. Chem. 2007;282:21222–21229. doi: 10.1074/jbc.M701314200. [DOI] [PubMed] [Google Scholar]
- Jurat-Fuentes JL, Gahan LJ, Gould FL, Heckel DG, Adang MJ. The HevCaLP protein mediates binding specificity of the Cry1A class of Bacillus thuringiensis toxins in Heliothis virescens. Biochemistry. 2004;43:14299–14305. doi: 10.1021/bi048500i. [DOI] [PubMed] [Google Scholar]
- Khasdan V, Ben-Dov E, Manasherob R, Boussiba S, Zaritsky A. Toxicity and synergism in transgenic Escheichia coli expressing four genes from Bacillus thuringiensis subsp. israeliensis. Environ. Microbiol. 2001;3:798–806. doi: 10.1046/j.1462-2920.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy M, Jurat-Fuentes JL, McNall RJ, Andacht T, Adang MJ. Identification of novel Cry1Ac binding proteins in midgut membranes from Heliothis virescens using proteomic analyses. Insect Biochem. Mol. Biol. 2007;37:189–201. doi: 10.1016/j.ibmb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Li J, Carrol J, Ellar DJ. Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5 Å resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Likitvivatanavong S, Chen J, Bravo A, Soberón M, Gill SS. Role of cadherin, alkaline phosphatase and aminopeptidase N as receptors of Cry11Ba toxin from Bacillus thuringiensis jegathesan in Aedes aegypti. Appl. Environ. Microbiol. 2010 doi: 10.1128/AEM.01852-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Roberts H, Sarjan M, Featherstone N, Lahnstein J, Akhurst R, Schmidt O. Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant Helicoverpa armigera larvae? Insect Biochem. Mol. Biol. 2005;35:729–739. doi: 10.1016/j.ibmb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Margalith Y, Ben-Dov E. Biological control by Bacillus thuringiensis subsp. israeliensis. In: Rechcigl JE, Rechcigl NA, editors. Insect Pest Management: Techniques for Environmental Protection. CRC Press; 2000. p. 243. [Google Scholar]
- Martins ES, Monnerat RG, Queiroz PR, Dumas VF, Braz SV, de Souza Aguiar RW, Gomes AC, Sánchez J, Bravo A, Ribeiro BM. Midgut GPI-anchored proteins with alkaline phosphatase activity from the cotton boll weevil (Anthonomus grandis) are putative receptors for the Cry1B protein of Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2010;40:138–145. doi: 10.1016/j.ibmb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- McGaughey WH. Insect resistance to the biological insecticide Bacillus thuringiensis. Science. 1985;229:193–195. doi: 10.1126/science.229.4709.193. [DOI] [PubMed] [Google Scholar]
- McNall RJ, Adang MJ. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem. Mol. Biol. 2003;33:999–1010. doi: 10.1016/s0965-1748(03)00114-0. [DOI] [PubMed] [Google Scholar]
- Morin S, Biggs RW, Shriver L, Ellers-Kirk C, Higginson D, Holley D, GahanHeckel DG, Carriere Y, Dennehy TJ, Brown JK, Tabashnik BE. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Nat. Acad. Sci. U.S.A. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse RJ, Yamamoto T, Stroud RM. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure. 2001;9:409–417. doi: 10.1016/s0969-2126(01)00601-3. [DOI] [PubMed] [Google Scholar]
- Muñoz-Garay C, Portugal L, Pardo-López L, Jiménez-Juárez N, Arenas I, Gómez I, Sánchez-López R, Arroyo R, Holzenburg A, Savva CG, Soberón M, Bravo A. Characterization of the mechanism of action of the genetically modified Cry1AbMod toxin that is active against Cry1Ab-resistant insects. Biochim. Biophys. Acta. Biomemb. 2009;1788:2229–2237. doi: 10.1016/j.bbamem.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Ochoa-Campuzano C, Real MD, Martínez-Ramírez AC, Bravo A, Rausell C. An ADAM metalloprotease is a Cry3Aa Bacillus thuringiensis toxin receptor. Biochem. Biophys. Res. Commun. 2007;362:437–442. doi: 10.1016/j.bbrc.2007.07.197. [DOI] [PubMed] [Google Scholar]
- Oppert B, Kramer KJ, Beeman RW, Johnson D, McGaughey WH. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J. Biol. Chem. 1997;272:23473–23476. doi: 10.1074/jbc.272.38.23473. [DOI] [PubMed] [Google Scholar]
- Ounjai P, Unger VM, Sigworth FJ, Angsuthanasombat C. Two conformational states of the membrane-associated Bacillus thuringiensis Cry4Ba deltaendotoxin complex revealed by electron crystallography: implications for toxin-pore formation. Biochem. Biophys. Res. Commun. 2007;361:890–895. doi: 10.1016/j.bbrc.2007.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco S, Gómez I, Gill SS, Bravo A, Soberón M. Enhancement of insecticidal activity of Bacillus thuringiensis Cry1A toxins by fragments of a toxin-binding cadherin correlates with oligomer formation. Peptides. 2009a;30:583–588. doi: 10.1016/j.peptides.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco S, Gomez I, Arenas I, Saab-Rincon G, Rodriguez-Almazan C, Gill SS, Bravo A, Soberon M. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping-pong” binding mechanism with Manduca sexta aminopetidase-N and cadherin receptors. J. Biol. Chem. 2009b;284:32750–32757. doi: 10.1074/jbc.M109.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandian NG, Ishikawa T, Togashi M, Shitomi Y, Haginoya K, Yamamoto K, Nishiumi T, Hori H. Bombyx mori midgut membrane protein P252 which binds to Cry1A of Bacillus thuringiensis is a chlorophyllide binding protein and its resulting complex has antimicrobial activity. Appl. Environ. Microbiol. 2008;74:1324–1331. doi: 10.1128/AEM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-López L, Gómez I, Rausell C, Sánchez J, Soberón M, Bravo A. Structural changes of the Cry1Ac oligomeric pre-pore from Bacillus thuringiensis induced by N-acetylgalactosamine facilitates toxin membrane insertion. Biochemistry. 2006;45:10329–10336. doi: 10.1021/bi060297z. [DOI] [PubMed] [Google Scholar]
- Park Y, Hua G, Abdullah MA, Rahman K, Adang MJ. Cadherin fragments from Anopheles gambiae synergize Bacillus thuringiensis Cry4Ba’s toxicity against Aedes aegypti larvae. Appl. Environ. Microbiol. 2009a;75:7280–7282. doi: 10.1128/AEM.01870-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Abdullah MA, Taylor MD, Rahman K, Adang MJ. Enhancement of Bacillus thuringiensis Cry3Aa and Cry3Bb toxicities to coleopteran larvae by a toxin-binding fragment of an insect cadherin. Appl. Environ. Microbiol. 2009b;75:3086–3092. doi: 10.1128/AEM.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C, Fernández LE, Sun J, Folch JL, Gill SS, Soberón M, Bravo A. Bacillus thuringiensis subsp. israeliensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C, Muñoz-Garay CC, Portugal L, Sánchez J, Gill SS, Soberón M, Bravo A. Bacillus thuringiensis subsp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell. Microbiol. 2007;9:2931–2937. doi: 10.1111/j.1462-5822.2007.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 2010;18:189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Almazan CR, Zavala LE, Muñoz-Garay C, Jiménez-Juárez N, Pacheco S, Masson L, Soberón M, Bravo A. Dominant negative mutants of Bacillus thuringiensis Cry1Ab toxin function as anti-toxins: demonstration of the role of oligomerization in toxicity. PLoS One. 2009;4:e5545. doi: 10.1371/journal.pone.0005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón M, Pardo-López L, López I, Gómez I, Tabashnik B, Bravo A. Engineering modified Bt toxins to counter insect resistance. Science. 2007;318:1640–1642. doi: 10.1126/science.1146453. [DOI] [PubMed] [Google Scholar]
- Soberón M, Gill SS, Bravo A. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 2009;66:1337–1349. doi: 10.1007/s00018-008-8330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, Huckaba RM. Discovery and characterization of field resistance to Bt Maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010;103:1031–1038. doi: 10.1603/ec10040. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994;39:47–49. [Google Scholar]
- Tabashnik BE, Gassman AJ, Crowdwer DW, Carriere Y. Insect resistance to Bt crops: evidence versus theory. Nat. Biotechnol. 2008;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L, Liesner L, Whitlow M, Staten RT, Fabrick JA, Unnithan GC, Yelich AJ, Ellers-Kirk C, Harpold VS, Li X, Carriere Y. Supressing resistance to Bt cotton with sterile insect releases. Nat. Biotechnol. 2010 doi: 10.1038/nbt.1704. [DOI] [PubMed] [Google Scholar]
- Taveecharoenkool T, Angsuthanasombat Ch, Kantchanawarin Ch. Combined molecular dynamics and continuumsolvent Studies of the pre-pore Cry4Aa trimer suggest its stability in solution and how it may forma pore. PMC Biophys. 2010;3:1–16. doi: 10.1186/1757-5036-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg JBJ. First report of field resistance by stem borer Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil. 2007;24:147–151. [Google Scholar]
- Walters FS, deFontes ChM, Hart H, Warren GW, Chen JS. Lepidopteran-active variable-region sequence imparts coleopteran activity in eCry3.1Ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Appl. Environ. Microbiol. 2010;76:3082–3088. doi: 10.1128/AEM.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Vegetative insecticidal proteins: novel proteins for control of corn pests. In: Carozzi N, Koziel M, editors. Advances in Insect Control: The Role of Transgenic Plants. Taylor & Francis Ltd; 1997. p. 109. [Google Scholar]
- Wirth MC, Georghiou GP, Federeci BA. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10536–10540. doi: 10.1073/pnas.94.20.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Zhuang M, Ross LS, Gómez I, Oltean DI, Bravo A, Soberón M, Gill SS. Single amino acid mutations in the cadherin receptor from Heliothis virescens affect its toxin binding ability to Cry1A toxins. J. Biol. Chem. 2005;280:8416–8425. doi: 10.1074/jbc.M408403200. [DOI] [PubMed] [Google Scholar]
- Xu X, Yu L, Wu Y. Disruption of a cadherin gene associated with resistance to Cry1Ac delta-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 2005;71:948–954. doi: 10.1128/AEM.71.2.948-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Hua G, Andacht TM, Adang MJ. A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry. 2008;47:11263–11272. doi: 10.1021/bi801181g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Hua G, Urbauer JL, Adang MJ. Synergistic and inhibitory effects of aminopeptidase peptides on Bacillus thuringiensis Cry11Ba toxicity in the mosquito Anopheles gambiae. Biochemistry. 2010 doi: 10.1021/bi1009908. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cheng H, Gao Y, Wang G, Liang G, Wu K. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem. Mol. Biol. 2009;39:421–429. doi: 10.1016/j.ibmb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Candas M, Griko NB, Taussig R, Bulla LA., Jr A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9897–9902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]