Summary
The anti-atherosclerotic effect of lacidipine has been attributed to its actions on cholesterol levels, lipid metabolism or oxidant stress in advanced disease. The purpose of the present experiments was to examine whether lacidipine is protective of intimal thickening and vascular dysfunction in early atherosclerosis in the absence of the hypertension and hypercholesterolemia. A second goal was to determine whether and to what extent MMP-9 and oxidant stress are involved in possible beneficial effects of lacidipine.
Lacidipine treatment (5 mg/kg/day, p.o. for 3 weeks) significantly prevented the collar-induced intimal thickening. MMP-9 expressions were increased by collar but not effected by lacidipine treatment. Nitrotyrosine staining, a marker for oxidant stress was not changed neither by collar nor lacidipine treatment in early atherosclerosis. The enhanced sensitivity to serotonine and diminished sensitivity to acetylcholine in collared arteries were restored to normal levels with treatment.
These results demonstrate that the lacidipine treatment prevents the collar-induced intimal thickening and accompanying vascular dysfunction in early atherosclerosis without cholesterol loading. These beneficial effects of lacidipine were not associated with changes in either MMP-9 expression or oxidant stress. However, enhanced endothelium dependent relaxations by lacidipine, suggest that vascular protective effects of nitric oxide may be at least partly, responsible from antiatherosclerotic effects of lacidipine
Keywords: Lacidipine, MMP-9, Collar-induced atherosclerosis, Nitric oxide, Vascular reactivity, 5-HT sensitivity, Intimal thickening, Rabbit carotid artery
INTRODUCTION
Atherosclerosis is a cardiovascular disease characterized by an inflammatory process (Ross, 1999) that may result from endothelial damage, leading to intimal thickening, altered production of extracellular matrix protein and lipid deposition. Lacidipine, a potent antihypertensive Ca++ channel blocker, has been shown to inhibit the development of atherosclerosis in hypercholesterolemic conditions (Bernini et al., 1993, P. Cristofori et al., 2004a, P. Cristofori et al., 2000, Soma et al., 1994, Soma et al., 1996, Zanchetti et al., 2002). Whether lacidipine prevents intimal thickening in the absence of cholesterol loading or altered lipid metabolism has not been reported yet.
In this study we induced early atherosclerosis in rabbit carotid arteries using a silicon collar - a widely accepted model which does not induce hypercholesterolemia and hypertension but causes biochemical, morphological and functional changes similar to those observed in the early stage of atherosclerosis in humans (Beesley J, 1992, Booth et al., 1989, Kerry et al., 2005, Yasa et al., 2007). The intimal thickening is generally accompanied by alterations in vascular reactivity, such as increased sensitivity to 5-hydroxytryptamine (5-HT) and decreased vasodilator responses to agonists (De Meyer et al., 1994, Kerry et al., 2005, Sobey et al., 1991, Ustunes et al., 1996, Yasa et al., 2007). The effect of lacidipine on vascular dysfunction in early atherosclerosis was unclear.
In previous studies the antiatherosclerotic effects of lacidipine has been attributed to its actions on modification of cholesterol metabolism (Bernini et al., 1991, Bernini et al., 1997, Bernini et al., 1993, Spieker & Zidek, 1995), amelioration of endothelial cell dysfunction (Zanchetti et al., 2004), enhanced activity of the NO system (P. G. Cristofori et al., 2004b), antioxidant properties (P. Cristofori et al., 2004a) or reduced expression of adhesion molecules (Zanchetti et al., 2004) or endothelin (P. Cristofori et al., 2000) in hypercholesterolemic conditions. Later it has been shown that lacidipine also decreases the MMP (matrix metalloproteinase)-9 expression in macrophages (Bellosta et al., 2001). Several studies demonstrated a role of MMP’s in atherogenesis and plaque stability (Brown et al., 1995, Galis et al., 1994). However, whether oxidant stress and MMP-9 is involved in possible antiatherosclerotic effects of lacidipine, in the absence of hypercholesterolemia in an early atherosclerosis model is not known.
Accordingly, the primary goal of the present study was to determine whether lacidipine prevents the intimal thickening and vascular dysfunction induced by collaring in the absence of hypertension and hypercholesterolemia. A second goal was to determine whether and to what extent MMP-9 and oxidant stress are involved in the possible beneficial effects of lacidipine.
MATERIALS AND METHODS
Treatment of animals
The animal experiments were carried out in accordance with guidelines described by the Ethics Committee of the Faculty of Pharmacy, Ege University. A total of 16 rabbits were used in this study. White rabbits (1.5–2 kg) of either sex were divided into 2 groups. The treatment protocol was as described previously (Ustunes et al., 1996). Briefly, the first group received a single administration of lacidipine (5 mg/kg/day, dissolved in 2.5 ml 0.5% aqueous solution of methylcellulose) via a gastric feeding tube. The second group (placebo) received only the vehicle (0.5% methylcellulose, 2.5 ml/kg/day) by the same administration route. Throughout the 3 week-treatment period, each rabbit was kept in a separate cage and allowed to access regular diet (standard rabbit chow and tap water ad libitum).
Surgical procedure for induction of intimal thickening
After the 7th day of lacidipine or placebo treatment, the rabbits were anesthetized with sodium pentobarbital (30 mg/kg, i.v.). Subsequently, the left carotid artery was surgically accessed and surrounded by a non-occlusive, flexible silicone collar (Ustunes et al., 1996, Yasa et al., 1999). The collar was 2 cm in length slightly touching the outer surface of the artery on each end. The right carotid artery was sham-operated (i.e. separated from surrounding connective tissue and vagus nerve and received a similar stretch as the contralateral collared artery). The carotid arteries were then replaced to their original positions and the incisions were sutured. Following recovery from the anesthesia, the animals were kept in their individual cages for a further 2 weeks prior to the tissue isolation.
Morphometry
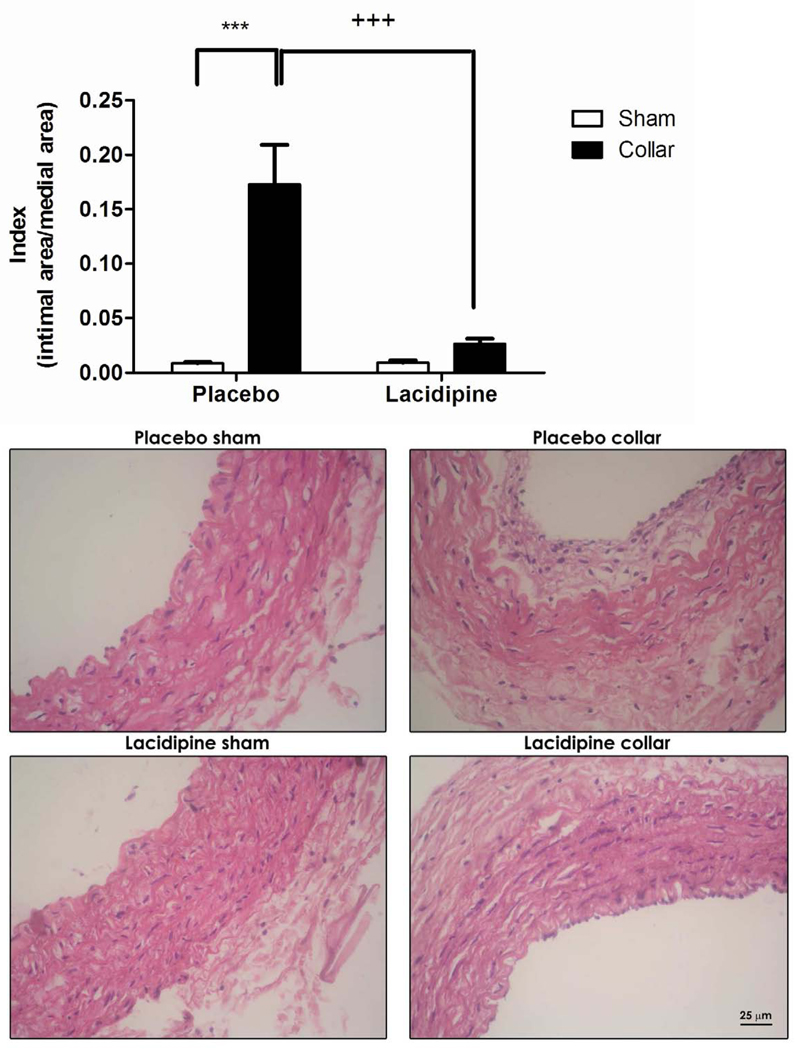
After anticoagulation with heparin (150 U/kg, i.v.), the rabbits were killed by sodium pentobarbital overdose. Tissue segments from both sham and collared arteries were isolated and cut into two 4 mm-long rings, one for morphometry and the other for organ bath experiments. The former ring was immediately placed in formalin fixative solution (0.4%) for 24 h, dehydrated in graded series of isopropyl alcohol (60 to 100%) followed by toluol before being embedded in paraffin. Transverse sections of 20 µm were cut and stained with sirius red haematoxylin. Randomly chosen two transverse sections from each artery ring were inspected via a light microscope (Olympus BH-2, Japan) and their video images recorded (JVC Color Video Camera, Head Model No. TK-890E, Japan; Sony VCR SL-C6E). Intimal and medial cross-sectional areas were marked (CorelDraw, Version 4.00.A5, Corel Corporation 1993, U.S.A.) and measured (AutoCAD, release 12-cl, 1993, Autodesk, Inc., U.S.A.). The ratios of intimal to medial cross-sectional areas (index) were also calculated.
Vascular reactivity
The two remaining rings from both the right (sham) and the left (collared) carotid arteries were used in organ chamber experiments to study vascular reactivity. Following careful removal of loose connective tissue, the arterial rings were suspended in organ chambers filled with 25 ml 37 °C physiological salt solution (PSS) continuously gassed with 95%O2–5%CO2 (7). PSS contained (in mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25; glucose, 11.1. Indomethacin (3×10−6 M) was included in all experiments to inhibit cyclooxygenase products. Isometric contractile force development was measured via Grass FT3 force transducer and recorded (Polywin95 1.0, Commat Ltd., Ankara, Turkey). Following a 15 min equilibration period, the resting tension was adjusted to 7 g, a previously determined optimal resting tension based on the length-tension relationship and tissues were allowed to equilibrate for additional 45 min. During the equilibration period, the bath solution was changed every 15 min. Following the equilibration period, arterial rings were contracted with phenylephrine (3×10−5 M) and acetylcholine (ACh) was added in a cumulative manner (10−9−10−4 M) to evaluate endothelium-dependent vasorelaxation. Later, the tissues were washed out three times with PSS and treated with cumulatively increased concentrations of 5-HT (10−9− 3×10−5 M). Each agonist was washed out by changing the bath solution three times within 30 min before addition of the next agonist. Endothelium-independent vasorelaxations were checked by cumulatively increased concentrations of nitroglycerine (NTG; 10−9−3×10−5 M) following by precontraction with phenylephrine (3×10−5 M). At the end, tissues were contracted by 120 mM KCl (with equimolar replacement of NaCl) to determine the maximal contractile force. The contractile responses are normalized relative to that elicited by KCl.
Immunohistochemistry
Dual immunostaining was performed on 20 µm sections using antibodies against MMP-9 (Calbiochem, Cambridge, MA, alkaline phosphatase conjugated), and nitrotyrosine (NY) (Upstate, Lake Placid, NY; horseradish peroxidase conjugated). Images were captured at 20× magnification using SPOT software (Diagnostic Instruments, MI). The vessel was divided into 4 quadrants, and each quadrant was captured as a separate image. Quantification of MMP-9 staining and NY was done for each quadrant of the vessel for all animals in a blinded fashion. Briefly, the regions spanning adventitia, media and intima were highlighted using a predefined same size box and the threshold area of this region was calculated as a percentage of the total area using Metamorph software (Molecular Devices, CA, USA).
Drugs
Reagent sources were as follows: Glaxo S.p.A. (Verona, Italy): lacidipine (gift); Merck, Sharp & Dohme (München, F.R.G.): indomethacin sodium; Nusil Silicone Technology (Anglet, France): silicone (MED-4011); Psyphac (Brussels, Belgium): sodium pentobarbital; Roche (Istanbul. Turkey): heparin solution; Sigma (St. Louis, MO, U.S.A.): acetylcholine hydrochloride, phenylephrine hydrochloride, 5-HT creatinine sulphate, nytroglycerine. 5-HT creatinine sulphate monohydrate was dissolved in aqueous solution of ascorbic acid (0.01%) and diluted in distilled water. The other drug solutions were prepared in distilled water.
Statistical analysis
Values are presented as means ± sem. Maximal tensions generated by the agonist [maximum effect (Emax)] and pD2 (−logEC50) were calculated by nonlinear regression analysis using the Graph Pad Prism computer program (version 5.0). Statistical comparisons were made by One- or Two-way ANOVA followed by Bonferroni's test, with the level of significance set at P<0.05 as described previously in literature (Chan et al., 2007, Fukada et al., 2004). Bonferroni’s post tests were performed for paired and unpaired comparisons when an interaction was observed.
RESULTS
Intimal thickening
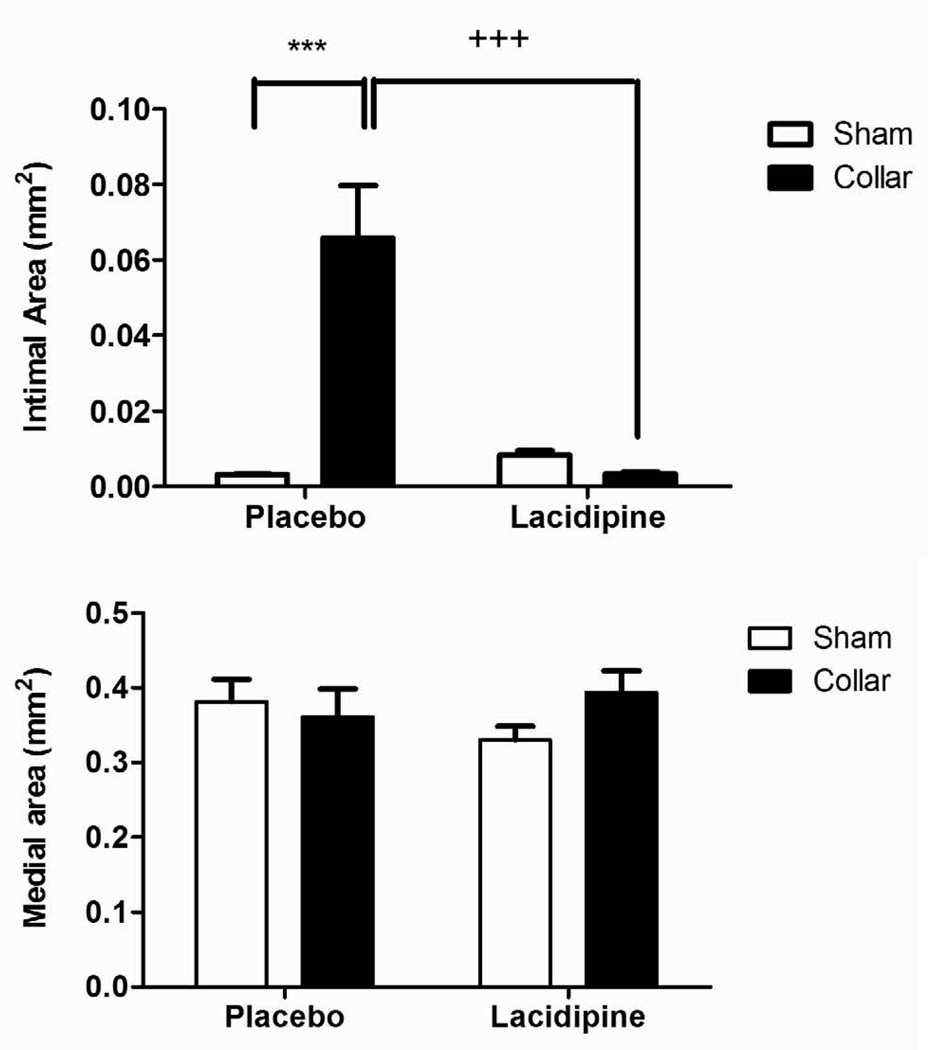
Intimal cross-sectional area and the ratio of intimal area to medial area (index) were significantly increased in collared arteries compared to those in sham-operated arteries (Fig. 1A, C and D n=8). Lacidipine treatment significantly inhibited both the intimal thickening and the index (Fig. 1A, C, D n=6). Collar or lacidipine treatment did not alter the medial cross-sectional area (Fig. 1B and D).
Figure 1. Effects of lacidipine treatment on collar-induced intimal thickening in rabbit carotid artery.
Cross-sectional areas of intima (A), media (B) index (intimal/medial area) (C) and photomicrographs of haematoxylin-eosin–stained rabbit carotid arteries (D) are shown. Intimal area and index were significantly increased in collared arteries in placebo group, *** P<0.001, n=8, sham vs. collar, two way ANOVA, Bonferroni posttest). Lacidipine treatment significantly inhibited the increase in intimal or intimal/medial area (+ ++ P<0.001, n=6, placebo vs. lacidipine, two way ANOVA, Bonferroni posttest). Collar or lacidipine treatment did not alter the medial cross-sectional area.
Immunohistochemistry
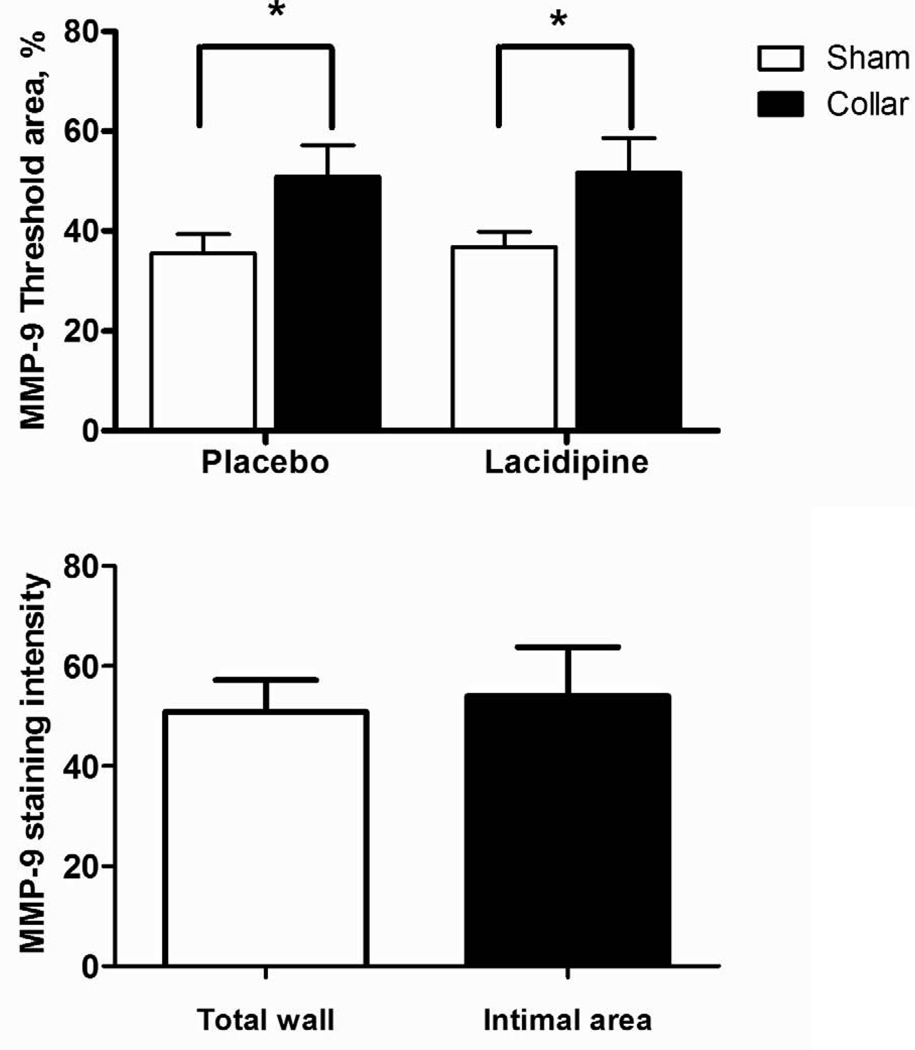
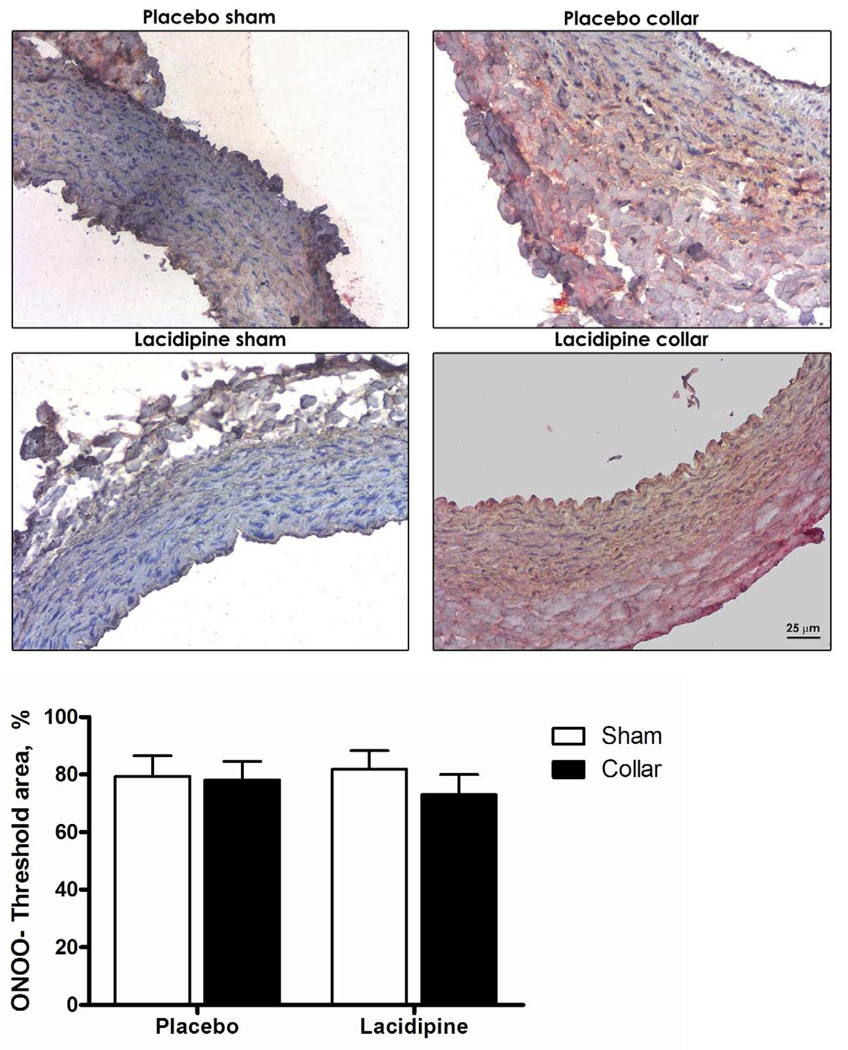
There was diffuse MMP-9 staining across the vessel wall. Collared arteries showed greater staining (Fig 2A and C) but this was not only in neointima but rather in all three layers (Fig 2B and C). Lacidipine treatment did not have an effect on staining intensity (Fig. 2A, C). There was no difference in staining pattern or intensity for NY (Fig. 2D).
Figure 2. Effects of collar and lacidipine treatment on MMP-9 expression and nitrotyrosine levels in rabbit carotid artery.
A. Collar induced an increase in MMP-9 staining in collared arteries both in placebo and lacidipine group (* P <0.05, sham vs. collar, n=5 and 4, respectively; each measurement was performed in duplicate, two way ANOVA, Bonferroni posttest). B. Comparison of the staining intensity of intimal area or total wall in collared arteries from placebo group for MMP-9. C. Photomicrographs of immunohistochemically stained rabbit carotid arteries for MMP-9. D. Nitrotyrosine staining was not changed neither by collar nor lacidipine treatment.
Metabolic data
The body weight of animals in both groups did not change by treatment. Total cholesterol was within the normocholesterolemic range in all groups. Diastolic or systolic blood pressure levels did not change by either collar (systolic pressures; 76 ±10 vs. 81±10, diastolic pressures; 68±10 vs. 73±9 in placebo group, sham vs. collar respectively) or lacidipine treatment (systolic pressures; 77±7 v.s. 72±9 diastolic pressures; 65±6 vs. 62.±7 sham vs. collar, respectively)
Vascular reactivity
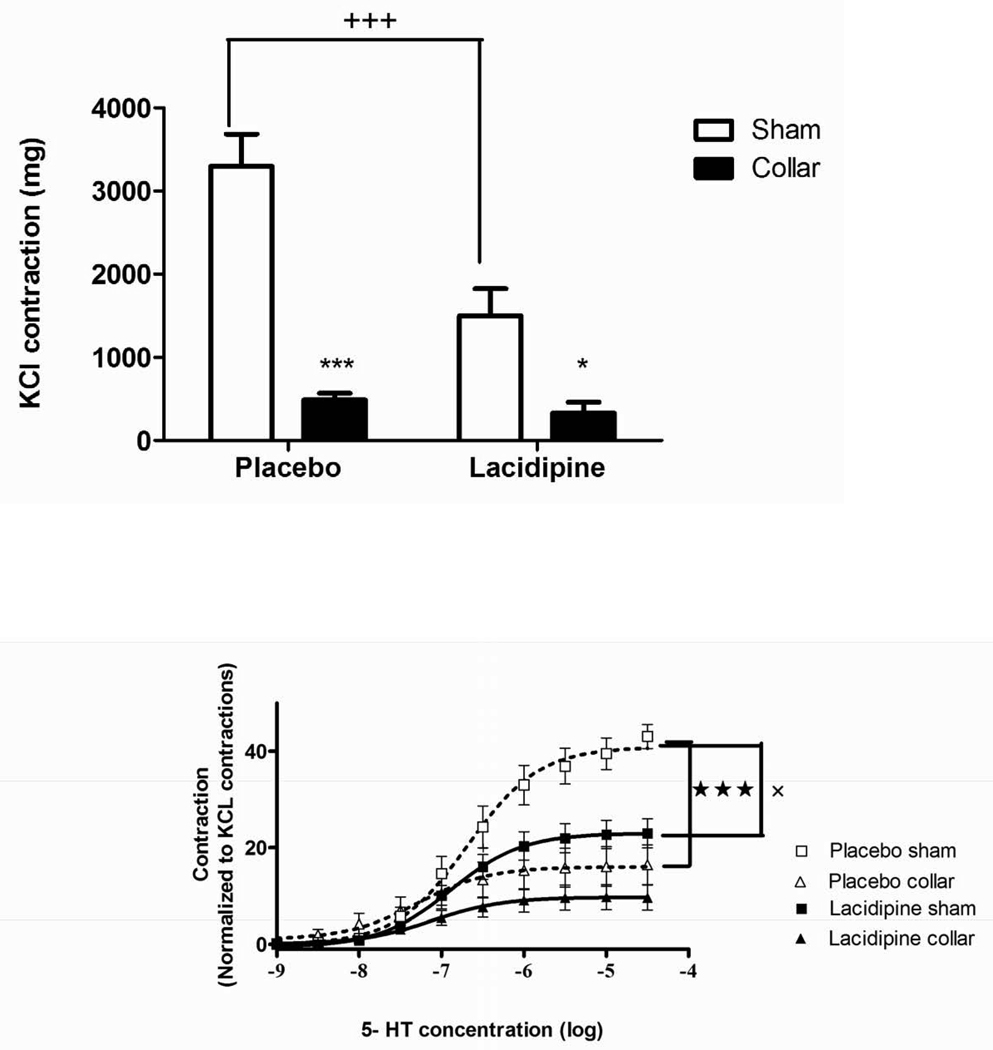
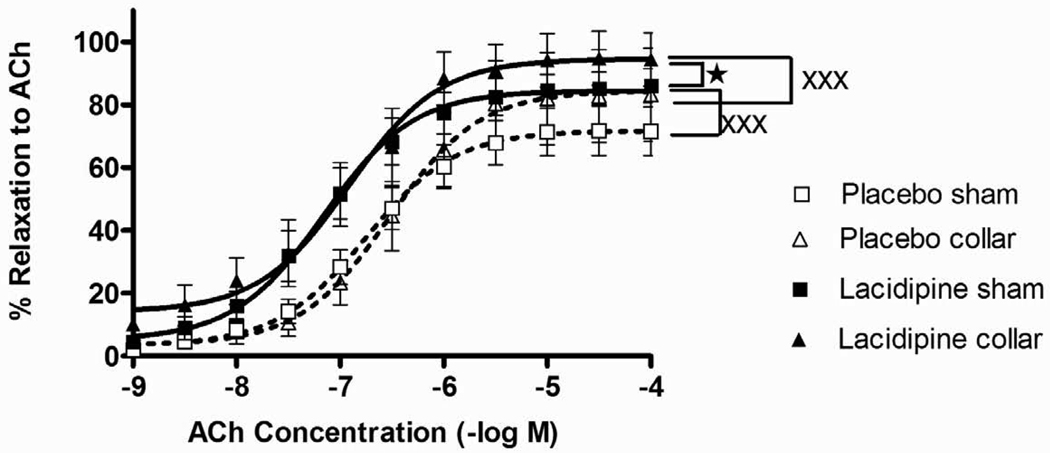
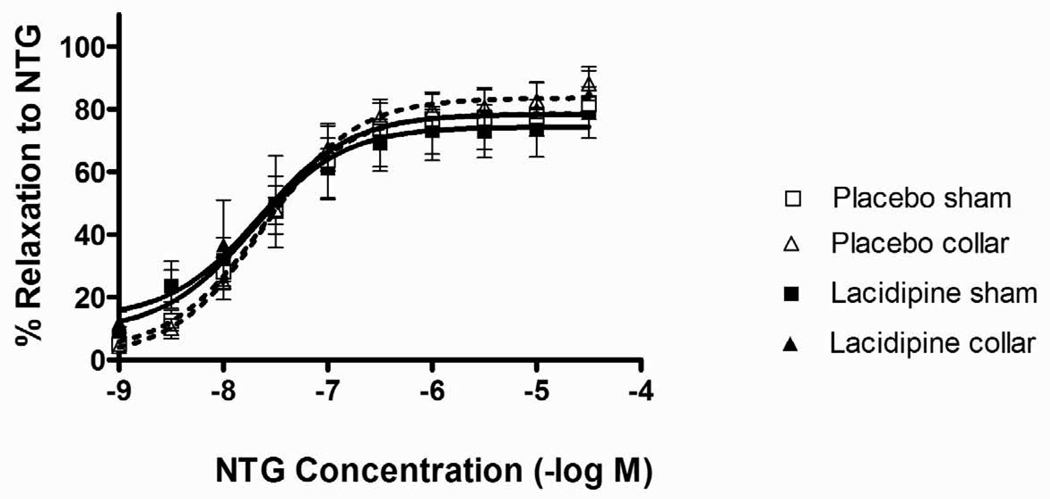
Both KCl and 5-HT-induced contractions were significantly diminished in collared arteries compared to sham (Figs. 3A and 3B). The inhibitory effect of lacidipine on both KCl and 5-HT-induced contractions were significant in sham arteries but not in collared arteries (Figs. 3A and 3B). The collar placement induced a significant increase in the pD2 values of 5-HT compare to sham arteries (Table 1). The increased sensitivity to 5-HT was normalized by lacidipine treatment (Table 1). ACh relaxed both sham and collared arteries pre-contracted with 3×10−5 M phenylephrine in a concentration dependent manner (Fig. 4). Lacidipine treatment significantly enhanced ACh-induced relaxations in both sham and collared arteries with a pronounced effect on the latter one (Fig. 4). The sensitivity to ACh-induced relaxations was decreased in collared arteries in a statistically nonsignificant manner (Table 1). Lacidipine treatment enhanced the sensitivity to ACh in collared arteries (Table 1). In addition, neither collar nor lacidipine treatment did not change endothelium independent vasorelaxations to nitroglycerine (Fig 5).
Figure 3. Effects of collar and lacidipine treatment on maximal contractile responses (Emax) to KCl and 5-HT.
A. Collar inhibited maximal contraction to KCl both in placebo and lacidipine group (*** P < 0.001, n=8, * P < 0.05, n=5, sham vs. collar, two way ANOVA, Bonferroni post test). Lacidipine treatment significantly inhibited maximal contraction to KCl (+++ P <0.001, Placebo vs. lacidipine, two way ANOVA, Bonferroni post test) only in sham arteries compared to placebo. B. Collar inhibited 5-HT-induced contractions in the placebo group (★ ★ ★ P < 0.001, n=7 sham vs. collar in placebo group, one way ANOVA) but not in lacidipine group. Lacidipine induced inhibition of 5-HT-induced contractions was significant only in sham arteries (xx P <0.01, n=5, placebo vs. lacidipine in sham arteries, one way ANOVA).
Table 1.
Effects of collar and lacidipine on pD2 values for A) ACh- B) NTG- and C) 5-HT- induced vascular reactivity.
| Placebo sham | Placebo collar | Lacidipine sham | Lacidipine collar | |
|---|---|---|---|---|
| ACh | 6,965 ± 0,147 | 6,457 ± 0,186 | 7,161 ± 0,157 | 7,008 ± 0,149 + |
| NTG | 7,669 ± 0,078 | 7,591 ± 0,100 | 7,730 ± 0,120 | 7,695 ± 0,284 |
| 5-HT | 6,597 ± 0,1994 | 7,245 ± 0,194*** | 6,864 ± 0,0893 | 7,089 ± 0,089 |
A. Lacidipine increased pD2 values for ACh-induced relaxation significantly, only in collared arteries (+P <0.05, Lacidipine vs. placebo, two way ANOVA, Bonferroni posttest n=7). The collar-induced decrease in placebo group was not statistically significant (n=7).
B. The sensitivity to NTG- was not changed by placement of the collar or lacidipine (n=7)
C. The collar placement induced a significant increase in the pD2 values of 5-HT compare to sham arteries only in placebo group (*** P<0.001, n=7, sham vs. collar, two way ANOVA, Bonferroni post test) but not in lacidipine group (P>0.05, n=5, sham vs. collar, two way ANOVA, Bonferroni post test).
Figure 4. Effects of collar and lacidipine treatment on ACh-induced relaxations.
ACh-induced relaxation was measured after pre-contraction with 3×10−5 M Phe. Relaxation data are expressed as percent of maximum Phe-induced contraction. Lacidipine increased ACh-induced relaxation significantly, both in sham and collared arteries (xxx P <0.001, lacidipine vs. placebo, one way ANOVA, n=7). Lacidipine induced a higher relaxation in collared arteries compared to lacidipine treated sham arteries (★ P <0.05, sham vs. collar, in lacidipine group, one way ANOVA, Bonferroni post test).
Figure 5. Effects of collar and lacidipine treatment on NTG-induced relaxations.
NTG-induced relaxation was measured after pre-contraction with 3×10−5 M Phe. Relaxation data are expressed as percent of maximum Phe-induced contraction. Neither collar nor FK409 treatment significantly influenced the NTG-induced relaxation significantly (P>0.05 One way ANOVA, Bonferroni post test, n=7)
DISCUSSION
The novel finding of the present study is that lacidipine is vasculoprotective by preventing intimal thickening (Fig 1A, C and D) and restoring vessel function (Table 1, Fig 4 and 3B) in an early model of atherosclerosis in the absence of risk factors commonly associated with atherosclerosis such as hypercholesterolemia, hypertension or oxidant stress. Antiatherosclerotic action of lacidipine is claimed to be independent of its antihypertensive action (Bond et al., 1994, Zanchetti et al., 2002, Zanchetti et al., 2004), however these studies were performed on patients with high levels of blood cholesterol and resulted in a reduction of cholesterol levels. Thus we do not know whether lacidipine has an effect on the development of atherosclerosis on those with normal levels of cholesterol.
In experimental models, antiatherosclerotic effects of lacidipine were again shown in hypercholesterolemic conditions (Bernini et al., 1991, P. Cristofori et al., 2004a, P. Cristofori et al., 2000, P. G. Cristofori et al., 2004b, Soma et al., 1994, Soma et al., 1996, Zanchetti et al., 2002, Zanchetti et al., 2004). Given that individuals can develop atherosclerosis despite normal or low blood cholesterol, this study is a further step of the previous studies to demonstrate the vasculoprotective effects of lacidipine both on intimal thickening and vascular reactivity in an early atherosclerosis model in the absence of hypercholesterolemia and hypertension.
Vascular oxidant stress and particularly interactions between NO and oxygen-derived radicals represent a common pathological mechanism present in many so-called risk factors for atherosclerosis (Kojda & Harrison, 1999). We used nitrotyrosine staining to detect whether oxidant stress play a role in the development of intimal thickening and antioxidant properties of lacidipine is involved in its potential antiatherosclerotic effect in early atherosclerosis. We did not find any increase in nitrotyrosine stainings, which are considered a footprint of the formation of peroxynitrite - a strong oxidant formed when NO reacts with superoxide anion, in arteries where intimal thickening was developed (Fig 2D). This finding is confirming another study where nitrotyrosine residuals were predominantly present in the advanced atherosclerotic plaques, but absent from the intimal thickening in human atherosclerotic carotid arteries (Cromheeke et al., 1999). This data may suggest that inhibition of intimal thickening by lacidipine is not related with its antioxidant properties in an early atherosclerosis. However it has to be recognized that further studies with different markers for oxidant stress should be investigated in this model as well.
Many MMP types (MMP-1, 2,3,7, 8, 9,10,11, 12,13 and 14) have been shown to be related with atherosclerosis (Back et al.). Of these types both MMP-2 and MMP-9 activity was indicated to increase in early atherosclerosis (Reel et al., 2009) and only MMP-9 has been shown to be inhibited by lacidipine. This relation was studied in macrophages and there is no study exploring the effect of lacidipine on MMP-9 in atherosclerotic vessels. Therefore we selected MMP-9 to investigate the effect of collaring and lacidipine on vessel, while other MMPs may be expressed in early atherosclerosis as well, and needs further studies. Our study showed a strong immunoreactivity to MMP-9 in collared arteries (Fig 2A and C). However in a previous study which enhanced activity of MMP-2 and MMP-9 has been reported (Reel et al., 2009), authors were not able to detect expression of MMP-9 in collared arteries. This was linked to antibody problems by authors. Therefore we suggest that increased MMP-9 activity may be related to the increased MMP-9 expressions in early atherosclerosis. However we could not exclude that decreased expressions of TIMP-1 (an endogenous inhibitor of MMP-9) may also contribute to increased MMP-9 activity. MMP-9 overexpression was not inhibited by lacidipine in our study in the absence of the oxidant stress. However lacidipine inhibited the increase of MMP-9 expressions in macrophages (Bellosta et al., 2001) by TNF alpha or phorbol ester, which induces reactive oxygen species (ROS) (Datta et al., 2000) and MMP expressions via ROS (Awad et al.). The discrepancy between the two studies may result from involvement of the oxidant stress-induced MMP overexpression (Nelson & Melendez, 2004) and antioxidant effect of lacidipine. It may also result from using different cell types. We suggest that the inhibitory effect of lacidipine on MMP-9 could be limited to macrophages as noted by others (Bellosta et al., 2001), but does not extend to VSMC.
In atherosclerosis not only structural but also functional changes in the vasculature are important for disease pathology and often present before any structural changes occur. Accordingly, we studied vascular contraction and relaxation. For the first time, we showed that lacidipine could reverse the increased sensitivity to 5-HT in early atherosclerosis. The increased expression of 5-HT-1B receptor is reported to be involved in hypersensitivity to 5HT-induced contraction in collared arteries (Geerts et al., 2000, Geerts et al., 1999) as in pathological conditions (Kaumann & Levy, 2006) and stimulation of the migration and proliferation of SMC (Fanburg BL, 1997, Sharma et al., 1999). 5HT-1B receptor induced hypersensitivity is reported due to sensitization of the contractile myofilaments to Ca++ via voltage-dependent Ca++ channels (Hill et al., 2000). Therefore calcium channel blocker activity with the high partition coefficient of the lacidipine (Herbette et al., 1994) could contribute to the reversing effect of lacidipine on both increased sensitivity to 5-HT and intimal thickening.
Both KCl- and 5-HT- induced maximal contractions were attenuated in collared rabbit carotid arteries (Beesley J, 1992, De Meyer et al., 1994, Sozmen et al., 2000, Ustunes et al., 1996, Van Put et al., 1995, Yasa et al., 1999). Consistent with other studies (De Meyer et al., 1992) collar placement induced a slight decrease in the sensitivity to ACh, but it did not reach to a statistically significant level. Lacidipine significantly increased ACh-induced maximal relaxation in both sham and collared arteries and normalized the decreased sensitivity in collared arteries. Our preliminary data (data not shown) support that lacidipine increases eNOS expression in rabbit carotid arteries as parallel to literature (Krenek et al., 2001). On the other hand NTG-induced vasorelaxation was not changed neither by collar (Fig. 5)- correlated with previous studies- (De Meyer et al., 1992, Kerry et al., 1999, Verbeuren et al., 1986, Yasa et al., 1999) nor by lacidipine. Based on these data we suggest that increased vasorelaxation to ACh by lacidipine was due to enhanced production of NO.
In previous studies the antiatherosclerotic effects of lacidipine in hypercholesterolemic conditions have been attributed to its actions on modification of cholesterol metabolism (Bernini et al., 1991, Bernini et al., 1997, Bernini et al., 1993, Spieker & Zidek, 1995), amelioration of endothelial cell dysfunction (Zanchetti et al., 2004), enhanced activity of the NO system (P. G. Cristofori et al., 2004b), antioxidant properties (P. Cristofori et al., 2004a) or reduced expression of adhesion molecules (Zanchetti et al., 2004) or endothelin (P. Cristofori et al., 2000). However in our study the beneficial effect of lacidipine was not related to effect of lacidipine on cholesterol metabolism, oxidant stress or MMP-9 but possibly linked to the protective role of NO and the inhibition of the Ca++-dependent subcellular processes involved in on SMC migration and/or proliferation as reported.
In summary our study demonstrated that lacidipine could inhibit intimal thickening also in the absence of the other risk factor such as hypercholesterolemia or hypertension in the early atherosclerosis. Further, the restoration of vascular function by lacidipine may be critical for development of atherosclerotic changes. Taken all together lacidipine may be evaluated as a therapeutic option in clinical conditions such as early atherosclerosis and other occlusive vascular disorders.
Acknowledgment
We would like to thank Dr. Arnold Herman (University of Antwerp, Belgium) for his help in providing lacidipine. A part of this work was supported by a grant from Ege University Scientific Research Fund, 1995/ECZ/01 (G.Y.) and NIH, DK074385 (A.E.)
This study was supported by grants from the Turkish Academy of Sciences (G.Y.A/TÜBA-GEBIP/2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner. Am J Physiol Cell Physiol. 298:C679–C692. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 52:410–428. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Beesley JHA, Martin J. Ultrastructural assessment of lesion development in the collared rabbit carotid artery model. Cells materials. 1992;2:201–203. [Google Scholar]
- Bellosta S, Canavesi M, Favari E, Cominacini L, Gaviraghi G, Fumagalli R, Paoletti R, Bernini F. Lacidipine [correction of Lalsoacidipine] modulates the secretion of matrix metalloproteinase-9 by human macrophages. J Pharmacol Exp Ther. 2001;296:736–743. [PubMed] [Google Scholar]
- Bernini F, Bellosta S, Didoni G, Fumagalli R. Calcium antagonists and cholesteryl ester metabolism in macrophages. J Cardiovasc Pharmacol. 1991;18(Suppl 10):S42–S45. [PubMed] [Google Scholar]
- Bernini F, Canavesi M, Bernardini E, Scurati N, Bellosta S, Fumagalli R. Effect of lacidipine on cholesterol esterification: in vivo and in vitro studies. Br J Pharmacol. 1997;122:1209–1215. doi: 10.1038/sj.bjp.0701469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernini F, Corsini A, Raiteri M, Soma MR, Paoletti R. Effects of lacidipine on experimental models of atherosclerosis. J Hypertens Suppl. 1993;11:S61–S66. doi: 10.1097/00004872-199303001-00011. [DOI] [PubMed] [Google Scholar]
- Bond G, Dal Palu C, Hansson L, Magnani B, Mancia G, Neiss A, Rahn KH, Reid JL, Rodicio JL, Safar M, et al. The E.L.S.A. trial: protocol of a randomized trial to explore the differential effect of antihypertensive drugs on atherosclerosis in hypertension. J Cardiovasc Pharmacol. 1994;23(Suppl 5):S85–S87. [PubMed] [Google Scholar]
- Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Rennick RE, Kalevitch SG, Campbell GR. Heparan sulfate-degrading enzymes induce modulation of smooth muscle phenotype. Exp Cell Res. 1992;200:156–167. doi: 10.1016/s0014-4827(05)80084-9. [DOI] [PubMed] [Google Scholar]
- Chan EC, Datla SR, Dilley R, Hickey H, Drummond GR, Dusting GJ. Adventitial application of the NADPH oxidase inhibitor apocynin in vivo reduces neointima formation and endothelial dysfunction in rabbits. Cardiovasc Res. 2007;75:710–718. doi: 10.1016/j.cardiores.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40:1001–1010. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Cristofori P, Crivellente F, Campagnola M, Pasini AF, Garbin U, Rigoni A, Tosetti M, Turton J, Faustinelli I, Cominacini L. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice treated with lacidipine is associated with a decreased susceptibility of low-density lipoprotein to oxidation. Int J Exp Pathol. 2004a;85:105–114. doi: 10.1111/j.0959-9673.2004.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofori P, Lanzoni A, Quartaroli M, Pastorino AM, Zancanaro C, Cominacini L, Gaviraghi G, Turton J. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE-deficient mouse. J Hypertens. 2000;18:1429–1436. doi: 10.1097/00004872-200018100-00010. [DOI] [PubMed] [Google Scholar]
- Cristofori PG, Crivellente FA, Faustinelli I, Lanzoni AR, Lazzarini C, Vecchiato E, Andreoli M, Turton JA, Zancanaro C, Crespi FM. Involvement of the nitric oxide system in the anti-atherosclerotic potential of lacidipine in the ApoE-deficient mouse: a morphological, functional, and electrochemical study. Toxicol Pathol. 2004b;32:493–499. doi: 10.1080/01926230490483351. [DOI] [PubMed] [Google Scholar]
- Cromheeke KM, Kockx MM, De Meyer GR, Bosmans JM, Bult H, Beelaerts WJ, Vrints CJ, Herman AG. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc Res. 1999;43:744–754. doi: 10.1016/s0008-6363(99)00148-0. [DOI] [PubMed] [Google Scholar]
- Datta R, Yoshinaga K, Kaneki M, Pandey P, Kufe D. Phorbol ester-induced generation of reactive oxygen species is protein kinase cbeta -dependent and required for SAPK activation. J Biol Chem. 2000;275:41000–41003. doi: 10.1074/jbc.M009322200. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Bult H, Herman AG. Selective muscarinic alterations of nitric oxide-mediated relaxations by neointima. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S205–S207. doi: 10.1097/00005344-199204002-00058. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Bult H, Martin JF, Van Hoydonck AE, Herman AG. The effect of a developing neo-intima on serotonergic and adrenergic contractions. Eur J Pharmacol. 1990;187:519–524. doi: 10.1016/0014-2999(90)90380-o. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Bult H, Ustunes L, Kockx M, Jordaens FH, Zonnekeyn LL, Herman AG. Vasoconstrictor responses after neo-intima formation and endothelial removal in the rabbit carotid artery. Br J Pharmacol. 1994;112:471–476. doi: 10.1111/j.1476-5381.1994.tb13097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusting GJ, Curcio A, Harris PJ, Lima B, Zambetis M, Martin JF. Supersensitivity to vasoconstrictor action of serotonin precedes the development of atheroma-like lesions in the rabbit. J Cardiovasc Pharmacol. 1990;16:667–674. doi: 10.1097/00005344-199010000-00021. [DOI] [PubMed] [Google Scholar]
- Fanburg Bl LS. A new role for an old molecule: serotonin as a mitogen. Am. J. Physiol. 1997;16:L795–L806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Hayward IP, Thomas AC, Campbell GR, Campbell JH. Matrix metalloproteinase can facilitate the heparanase-induced promotion of phenotype change in vascular smooth muscle cells. Atherosclerosis. 1999;145:97–106. doi: 10.1016/s0021-9150(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Fukada SY, Correa FM, Ramalho LN, Mizusaki CI, De Oliveira AM. Perivascular injury leads to a reduction in vascular reactivity of the collared and to an enhancement on contralateral carotid artery of rats. Cardiovasc Pathol. 2004;13:251–259. doi: 10.1016/j.carpath.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts IS, De Meyer GR, Bult H. Collar-induced elevation of mRNA and functional activity of 5-HT(1B) receptor in the rabbit carotid artery. Br J Pharmacol. 2000;131:1723–1731. doi: 10.1038/sj.bjp.0703732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts IS, Matthys KE, Herman AG, Bult H. Involvement of 5-HT1B receptors in collar-induced hypersensitivity to 5-hydroxytryptamine of the rabbit carotid artery. Br J Pharmacol. 1999;127:1327–1336. doi: 10.1038/sj.bjp.0702684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette LG, Mason PE, Gaviraghi G, Tulenko TN, Mason RP. The molecular basis for lacidipine's unique pharmacokinetics: optimal hydrophobicity results in membrane interactions that may facilitate the treatment of atherosclerosis. J Cardiovasc Pharmacol. 1994;23(Suppl 5):S16–S25. [PubMed] [Google Scholar]
- Hill PB, Dora KA, Hughes AD, Garland CJ. The involvement of intracellular Ca(2+) in 5-HT(1B/1D) receptor-mediated contraction of the rabbit isolated renal artery. Br J Pharmacol. 2000;130:835–842. doi: 10.1038/sj.bjp.0703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kerry Z, Yasa M, Akpinar R, Sevin G, Yetik G, Tosun M, Ozdemir N, Erhan Y, Ustunes L, Ozer A. Effects of nicardipine on collar-induced intimal thickening and vascular reactivity in the rabbit. J Pharm Pharmacol. 1999;51:441–447. doi: 10.1211/0022357991772493. [DOI] [PubMed] [Google Scholar]
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Krenek P, Salomone S, Kyselovic J, Wibo M, Morel N, Godfraind T. Lacidipine prevents endothelial dysfunction in salt-loaded stroke-prone hypertensive rats. Hypertension. 2001;37:1124–1128. doi: 10.1161/01.hyp.37.4.1124. [DOI] [PubMed] [Google Scholar]
- Kyselovic J, Martinka P, Batova Z, Gazova A, Godfraind T. Calcium channel blocker inhibits Western-type diet-evoked atherosclerosis development in ApoE-deficient mice. J Pharmacol Exp Ther. 2005;315:320–328. doi: 10.1124/jpet.105.089847. [DOI] [PubMed] [Google Scholar]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Reel B, Oktay G, Ozkal S, Islekel H, Ozer E, Ozsarlak-Sozer G, Cavdar Z, Akhisaroglu ST, Kerry Z. MMP-2 and MMP-9 alteration in response to collaring in rabbits: the effects of endothelin receptor antagonism. J Cardiovasc Pharmacol Ther. 2009;14:292–301. doi: 10.1177/1074248409343690. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Zahradka P, Chapman D, Kumamoto H, Takeda N, Dhalla NS. Inhibition of serotonin-induced vascular smooth muscle cell proliferation by sarpogrelate. J Pharmacol Exp Ther. 1999;290:1475–1481. [PubMed] [Google Scholar]
- Sobal G, Menzel EJ, Sinzinger H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem Pharmacol. 2001;61:373–379. doi: 10.1016/s0006-2952(00)00548-7. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Dusting GJ, Woodman OL. Enhanced vasoconstriction by serotonin in rabbit carotid arteries with atheroma-like lesions in vivo. Clin Exp Pharmacol Physiol. 1991;18:367–370. doi: 10.1111/j.1440-1681.1991.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Soma MR, Donetti E, Parolini C, Barberi L, Paoletti R, Fumagalli R, Catapano AL. Effect of lacidipine on the carotid intimal hyperplasia induced by cuff injury. J Cardiovasc Pharmacol. 1994;23(Suppl 5):S71–S74. doi: 10.1097/00005344-199423005-00015. [DOI] [PubMed] [Google Scholar]
- Soma MR, Donetti E, Seregni R, Barberi L, Fumagalli R, Paoletti R, Catapano AL. Effect of lacidipine on fatty and proliferative lesions induced in hypercholesterolaemic rabbits. Br J Pharmacol. 1996;118:215–219. doi: 10.1111/j.1476-5381.1996.tb15389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EY, Kerry Z, Uysal F, Yetik G, Yasa M, Ustunes L, Onat T. Antioxidant enzyme activities and total nitrite/nitrate levels in the collar model. Effect of nicardipine. Clin Chem Lab Med. 2000;38:21–25. doi: 10.1515/CCLM.2000.004. [DOI] [PubMed] [Google Scholar]
- Spieker C, Zidek W. The impact of lacidipine, a novel dihydropyridine calcium antagonist, on carbohydrate and lipid metabolism. J Cardiovasc Pharmacol. 1995;25(Suppl 3):S23–S26. [PubMed] [Google Scholar]
- Ustunes L, Yasa M, Kerry Z, Ozdemir N, Berkan T, Erhan Y, Ozer A. Effect of verapamil on intimal thickening and vascular reactivity in the collared carotid artery of the rabbit. Br J Pharmacol. 1996;118:1681–1688. doi: 10.1111/j.1476-5381.1996.tb15592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Put DJ, Van Hove CE, De Meyer GR, Wuyts F, Herman AG, Bult H. Dexamethasone influences intimal thickening and vascular reactivity in the rabbit collared carotid artery. Eur J Pharmacol. 1995;294:753–761. doi: 10.1016/0014-2999(95)00635-4. [DOI] [PubMed] [Google Scholar]
- Verbeuren TJ, Jordaens FH, Zonnekeyn LL, Van Hove CE, Coene MC, Herman AG. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986;58:552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Yasa M, Kerry Z, Yetik G, Sevin G, Reel B, Ozdemir N, Erhan Y, Ustunes L, Berkan T, Ozer A. Effects of treatment with FK409, a nitric oxide donor, on collar-induced intimal thickening and vascular reactivity. Eur J Pharmacol. 1999;374:33–39. doi: 10.1016/s0014-2999(99)00236-8. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palu C, Hansson L, Magnani B, Rahn KH, Reid JL, Rodicio J, Safar M, Eckes L, Rizzini P. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation. 2002;106:2422–2427. doi: 10.1161/01.cir.0000039288.86470.dd. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, Tang R, Hollweck R, Mancia G, Eckes L, Micheli D. Absolute and relative changes in carotid intima-media thickness and atherosclerotic plaques during long-term antihypertensive treatment: further results of the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2004;22:1201–1212. doi: 10.1097/00004872-200406000-00022. [DOI] [PubMed] [Google Scholar]