Abstract
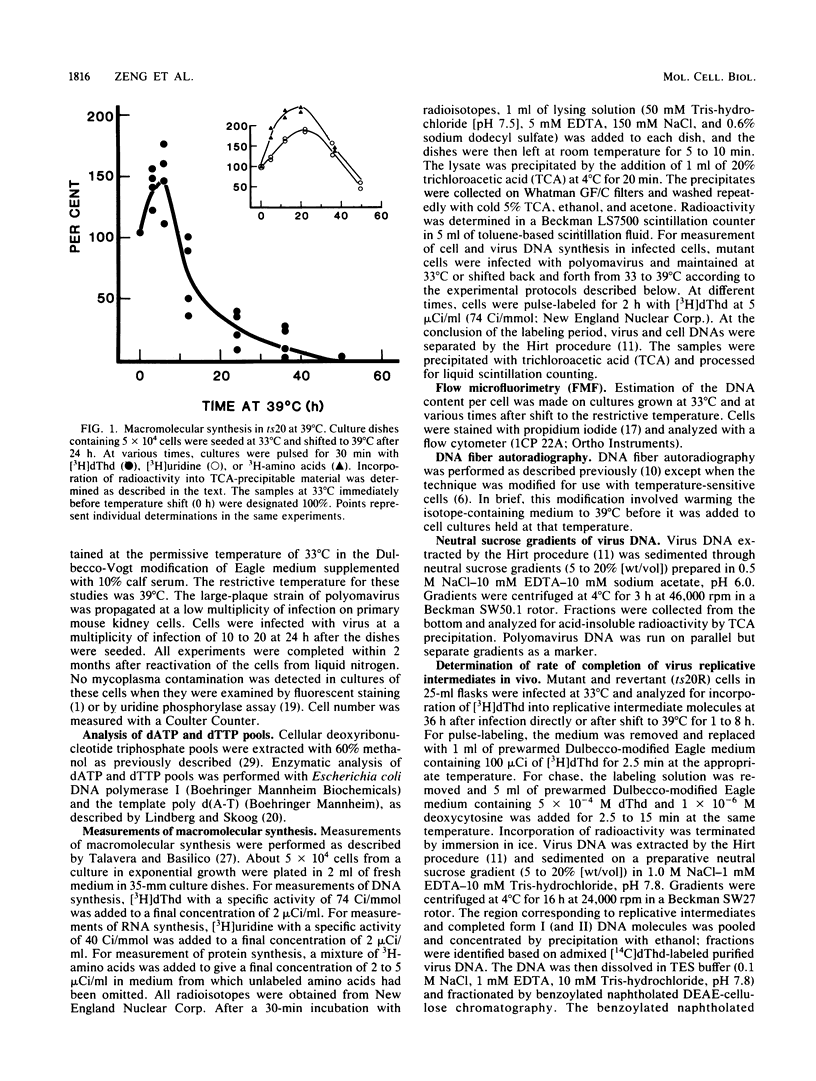
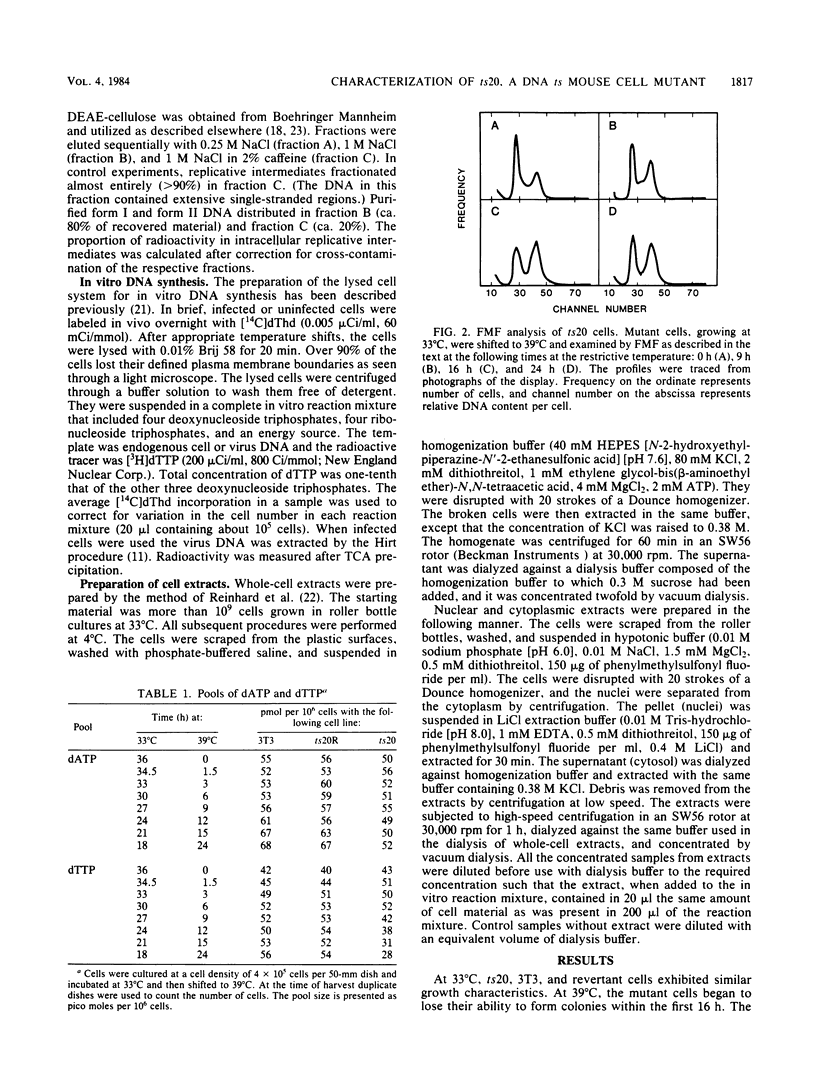
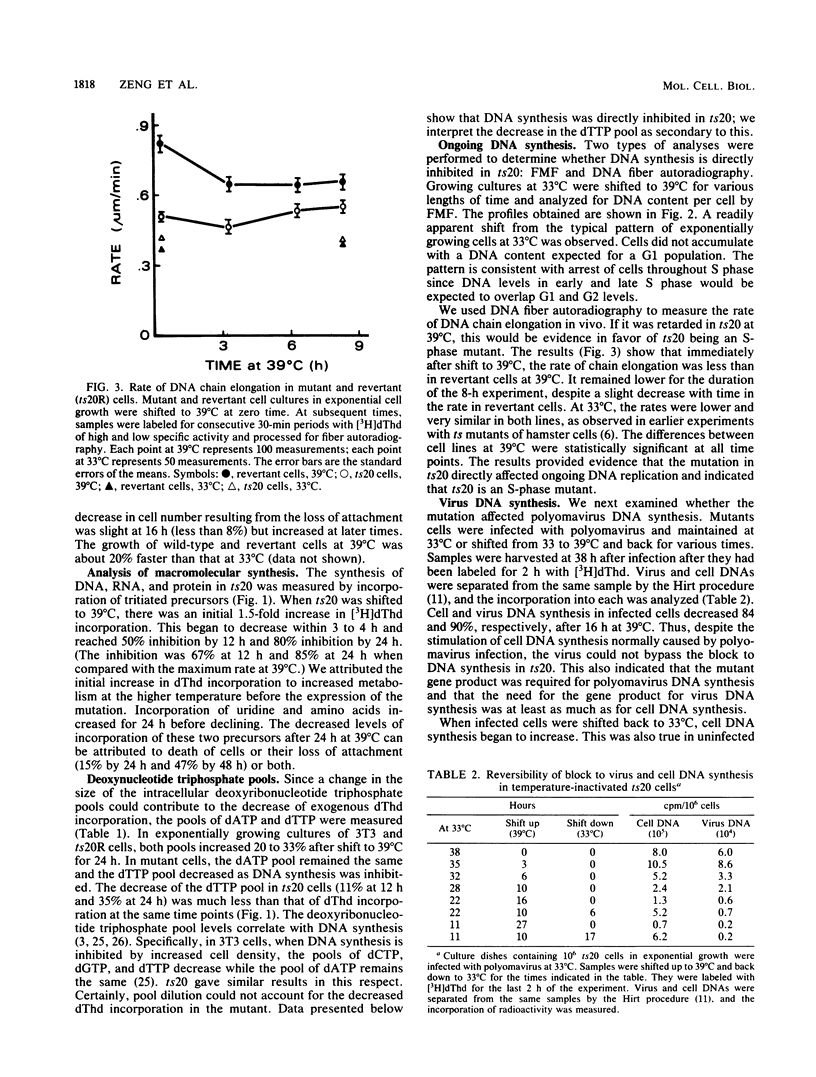
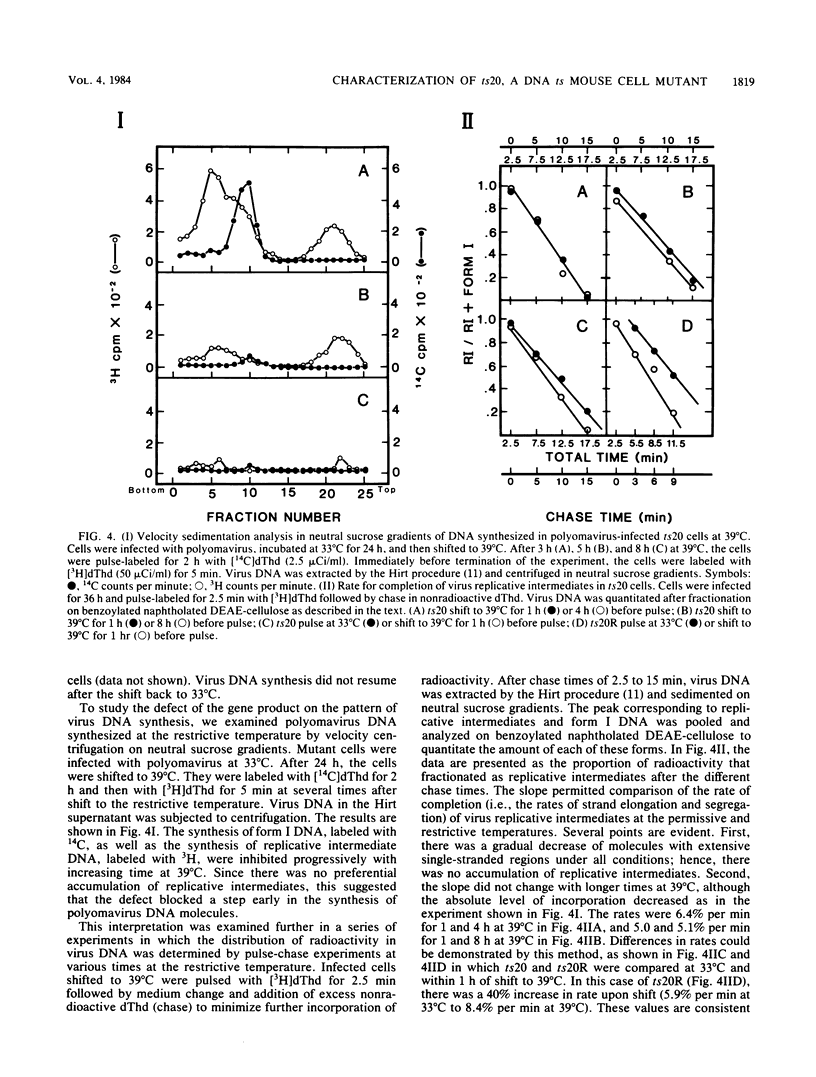
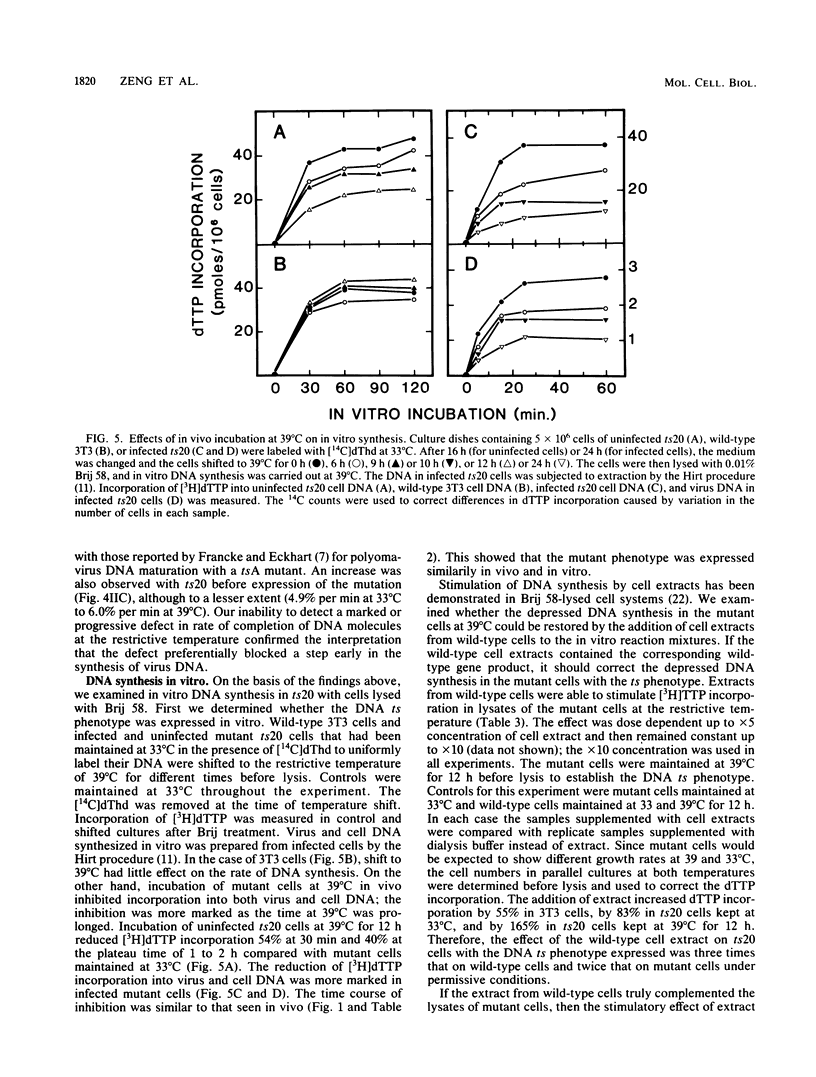
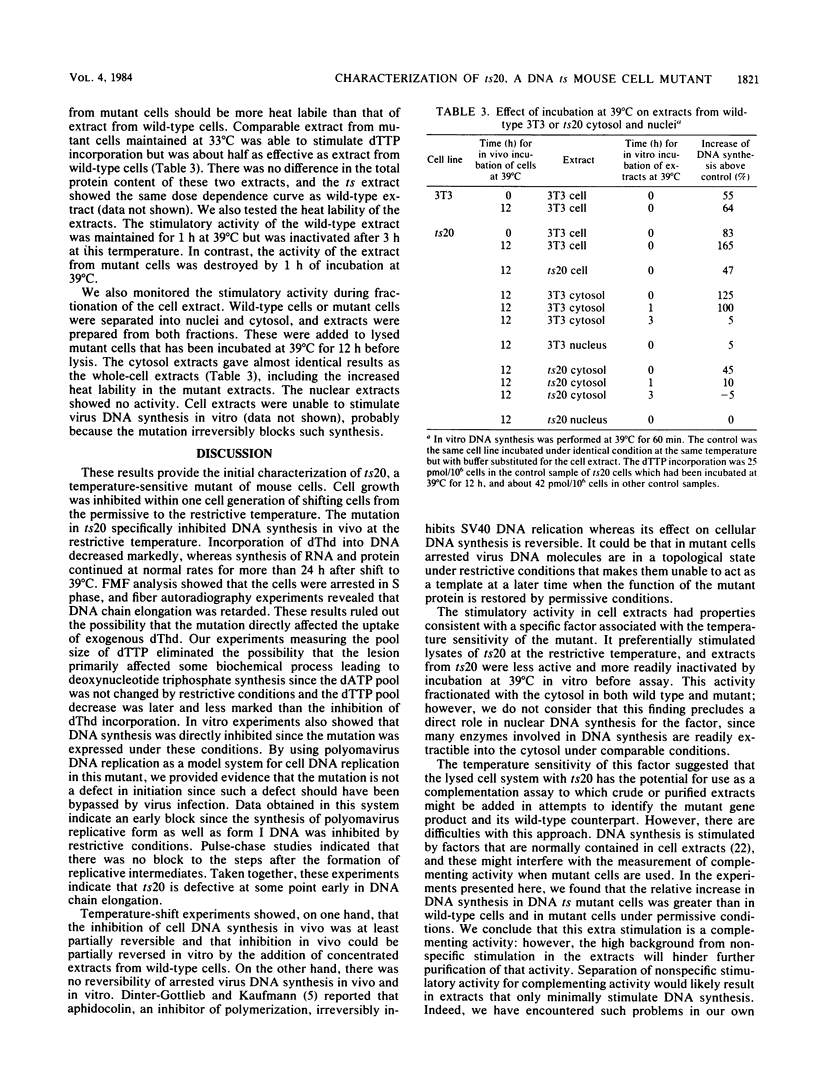
ts20 is a temperature-sensitive mutant cell line derived from BALB/3T3 cells. DNA synthesis in the mutant decreased progressively after an initial increase during the first 3 h at the restrictive temperature. RNA and protein synthesis increased for 20 h and remained at a high level for 40 h. Cells were arrested in S phase as determined by flow microfluorimetry, and DNA chain elongation was retarded as measured by fiber autoradiography. Infection with polyomavirus did not bypass the defect in cell DNA synthesis, and the mutant did not support virus DNA replication at the restrictive temperature. After shift down to the permissive temperature, cell DNA synthesis was restored whereas virus DNA synthesis was not. Analysis of virus DNA synthesized at the restrictive temperature showed that the synthesis of form I and replicative intermediate DNA decreased concurrently and that the rate of completion of virus DNA molecules remained constant with increasing time at the restrictive temperature. These studies indicated that the mutation inhibited ongoing DNA synthesis at a step early in elongation of nascent chains. The defect in virus and cell DNA synthesis was expressed in vitro. [3H]dTTP incorporation was reduced, consistent with the in vivo data. The addition of a high-salt extract prepared from wild-type 3T3 cells preferentially stimulated the incorporation of [3H]dTTP into the DNA of mutant cells at the restrictive temperature. A similar extract prepared from mutant cells was less effective and was more heat labile as incubation of it at the restrictive temperature for 1 h destroyed its ability to stimulate DNA synthesis in vitro, whereas wild-type extract was not inactivated until incubated at that temperature for 3 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C. Temperature-sensitive mutations in animal cells. Adv Cancer Res. 1977;24:223–266. doi: 10.1016/s0065-230x(08)61016-7. [DOI] [PubMed] [Google Scholar]
- Bjursell K. G., Reichard P. A., Skoog K. L. Deoxyribonucleoside triphosphate pools of normal and transformed baby-hamster-kidney cells. Eur J Biochem. 1972 Sep 18;29(2):348–352. doi: 10.1111/j.1432-1033.1972.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Eukaryotic DNA replication: viral and plasmid model systems. Annu Rev Biochem. 1982;51:901–934. doi: 10.1146/annurev.bi.51.070182.004345. [DOI] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Aphidicolin arrest irreversibly impairs replicating simian virus 40 chromosomes. J Biol Chem. 1983 Mar 25;258(6):3809–3812. [PubMed] [Google Scholar]
- Eilen E., Hand R., Basilico C. Decreased initiation of DNA synthesis in a temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Nov;105(2):259–266. doi: 10.1002/jcp.1041050209. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Burkhalter M., Reinhard P. Semiconservative DNA replication in vitro. II. Replicative intermediates of mouse P-815 cells. Biochim Biophys Acta. 1977 Feb 16;474(4):512–523. doi: 10.1016/0005-2787(77)90072-7. [DOI] [PubMed] [Google Scholar]
- Hand R., Gautschi J. R. Replication of mammalian DNA in vitro. Evidence for initiation from fiber autoradiography. J Cell Biol. 1979 Aug;82(2):485–493. doi: 10.1083/jcb.82.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Tamm I. DNA replication: direction and rate of chain growth in mammalian cells. J Cell Biol. 1973 Aug;58(2):410–418. doi: 10.1083/jcb.58.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hochstadt J., Ozer H. L., Shopsis C. Genetic alteration in animal cells in culture. Curr Top Microbiol Immunol. 1981;94-95:243–308. doi: 10.1007/978-3-642-68120-2_6. [DOI] [PubMed] [Google Scholar]
- Jha K. K., Ozer H. L. Expression of transformation in cell hybrids. I. Isolation and application of density-inhibited Balb/3T3 cells deficient in hypoxanthine phosphoribosyltransferase and resistant to ouabain. Somatic Cell Genet. 1976 May;2(3):215–223. doi: 10.1007/BF01538960. [DOI] [PubMed] [Google Scholar]
- Jha K. K., Siniscalco M., Ozer H. L. Temperature-sensitive mutants of BALB/3T3 cells. III. Hybrids between ts2 and other mouse mutant cells affected in DNA synthesis and correction of ts2 defect by human X chromosome. Somatic Cell Genet. 1980 Sep;6(5):603–614. doi: 10.1007/BF01538640. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Levine E. M. Mycoplasma contamination of animal cell cultures: a simple, rapid detection method. Exp Cell Res. 1972 Sep;74(1):99–109. doi: 10.1016/0014-4827(72)90484-3. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Reinhard P., Burkhalter M., Gautschi J. R. Semiconservative DNA replication in vitro. I. Properties of two systems derived from mouse P-815 cells by permeabilization or lysis with Brij-58. Biochim Biophys Acta. 1977 Feb 16;474(4):500–511. doi: 10.1016/0005-2787(77)90071-5. [DOI] [PubMed] [Google Scholar]
- Reinhard P., Maillart P., Schluchter M., Gautschi J. R., Schindler R. An assay system for factors involved in mammalian DNA replication. Biochim Biophys Acta. 1979 Aug 29;564(1):141–153. doi: 10.1016/0005-2787(79)90195-3. [DOI] [PubMed] [Google Scholar]
- Sadoff R. B., Cheevers W. P. Evidence for RNA-linked nascent strands in polyoma virus DNA replication. Biochem Biophys Res Commun. 1973 Aug 6;53(3):818–823. doi: 10.1016/0006-291x(73)90166-6. [DOI] [PubMed] [Google Scholar]
- Skoog K. L., Bjursell K. G., Nordenskjöld B. A. Cellular deoxyribonucleotide triphosphate pools levels and DNA synthesis. Adv Enzyme Regul. 1974;12:345–354. doi: 10.1016/0065-2571(74)90020-x. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Ozer H. L. Temperature-sensitive mutants of Balb/3T3 cells: description of a mutant affected in cellular and polyoma virus DNA synthesis. Cell. 1976 Feb;7(2):289–295. doi: 10.1016/0092-8674(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Söderhäll S. S., Larsson A., Skoog K. L. Deoxyribonucleotide pools during liver regeneration. Eur J Biochem. 1973 Feb 15;33(1):36–39. doi: 10.1111/j.1432-1033.1973.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]
- Wittes R. E., Ozer H. L. Characterization of a temperature-sensitive mutant of BALB-c 3T3 cells. Exp Cell Res. 1973 Jul;80(1):127–136. doi: 10.1016/0014-4827(73)90283-8. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Baumann E. A., Hand R. Effect of purine deprivation on DNA synthesis and deoxyribonucleoside triphosphate pools of a mammalian purine auxotrophic mutant cell line. J Biol Chem. 1980 Apr 10;255(7):3014–3019. [PubMed] [Google Scholar]


