Abstract
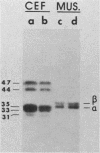
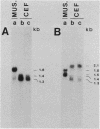
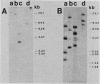
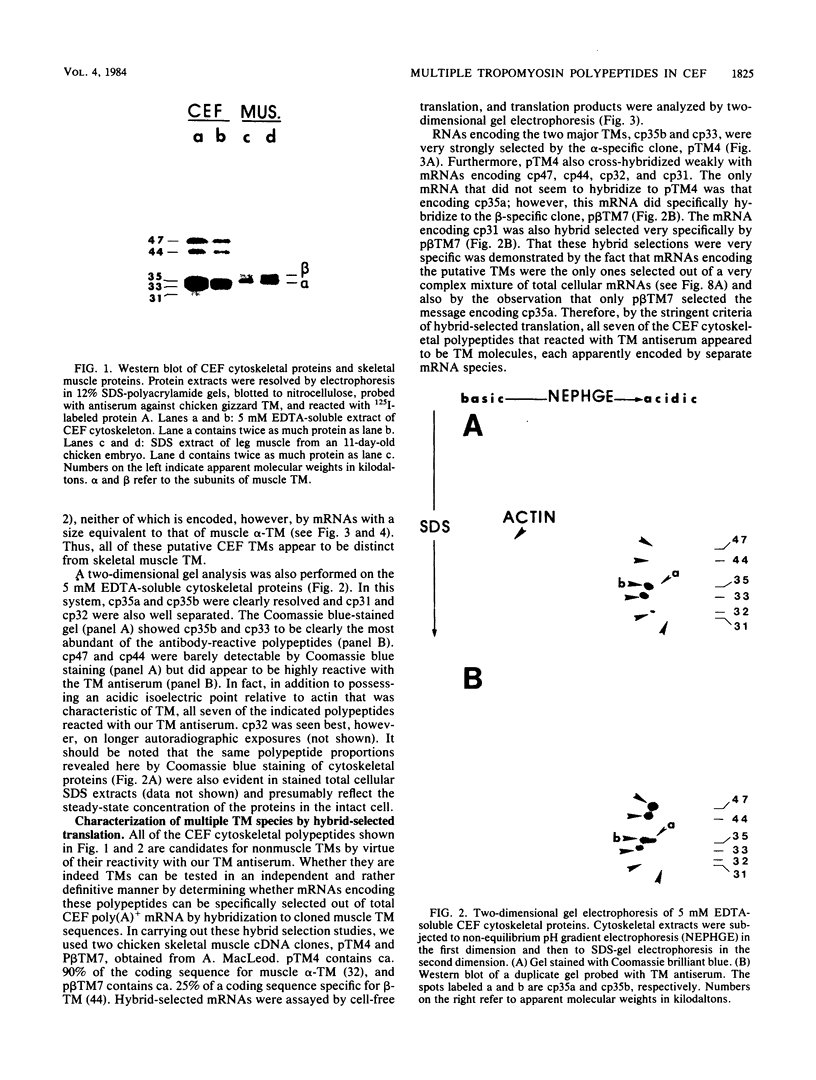
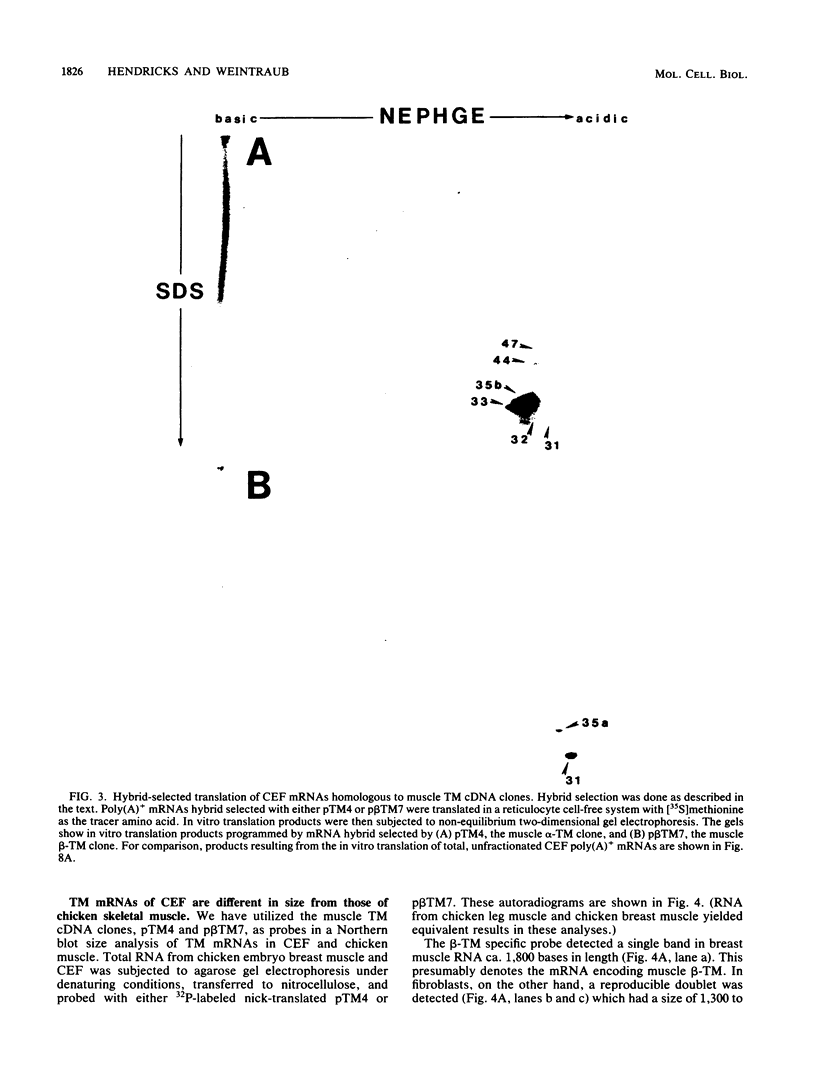
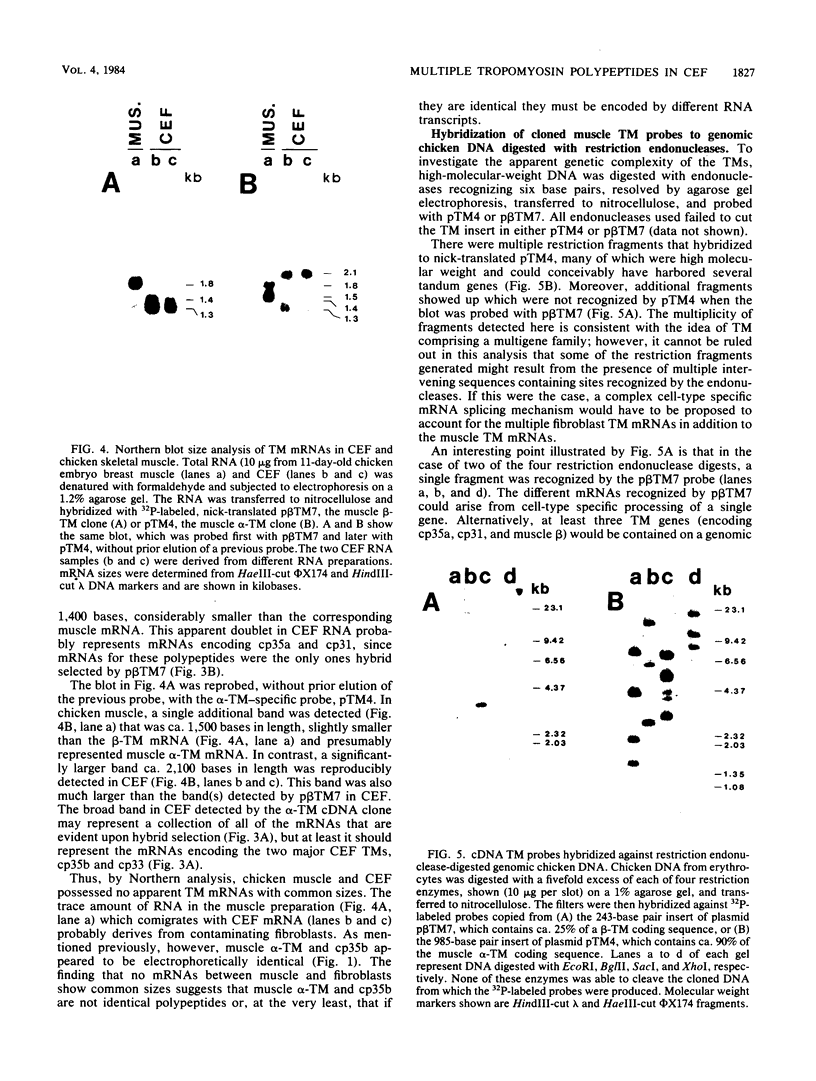
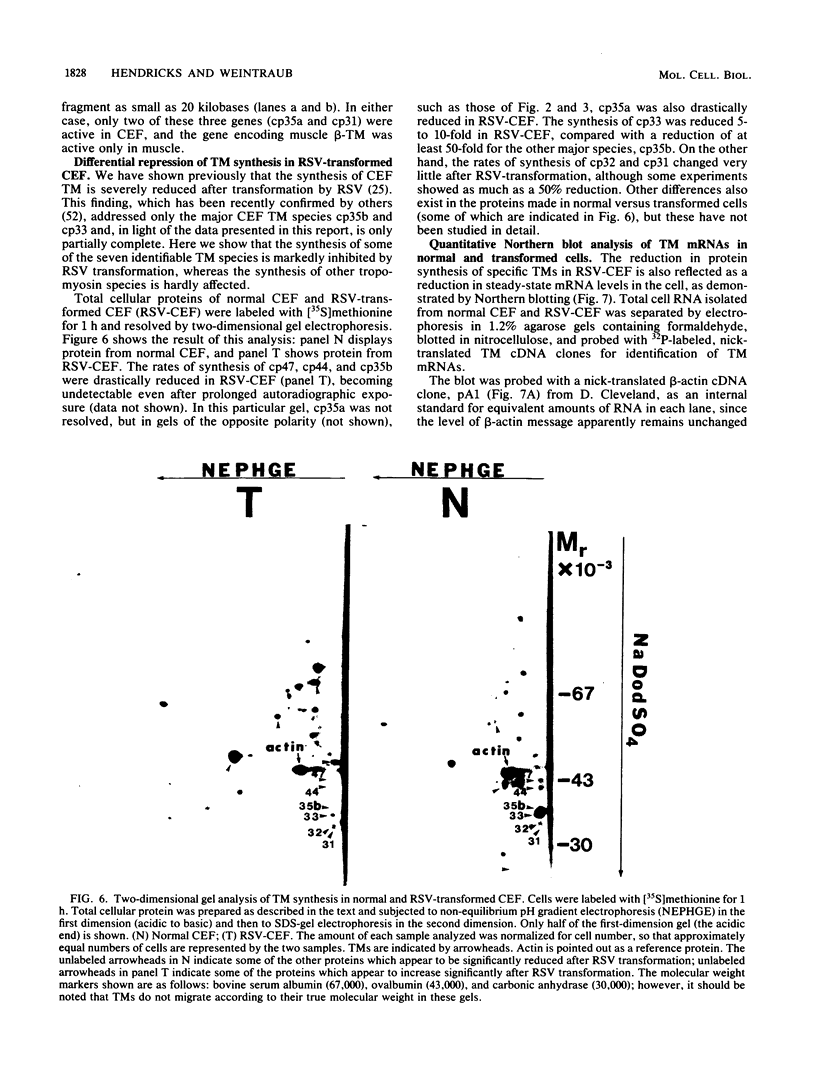
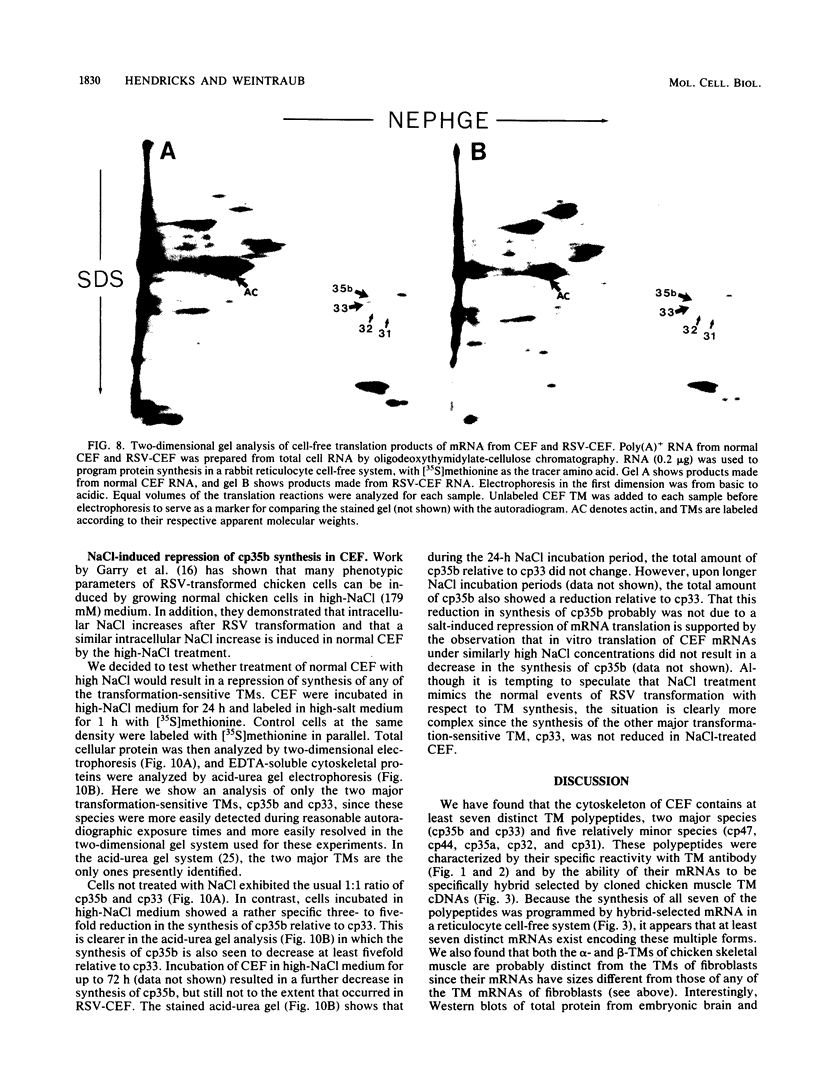
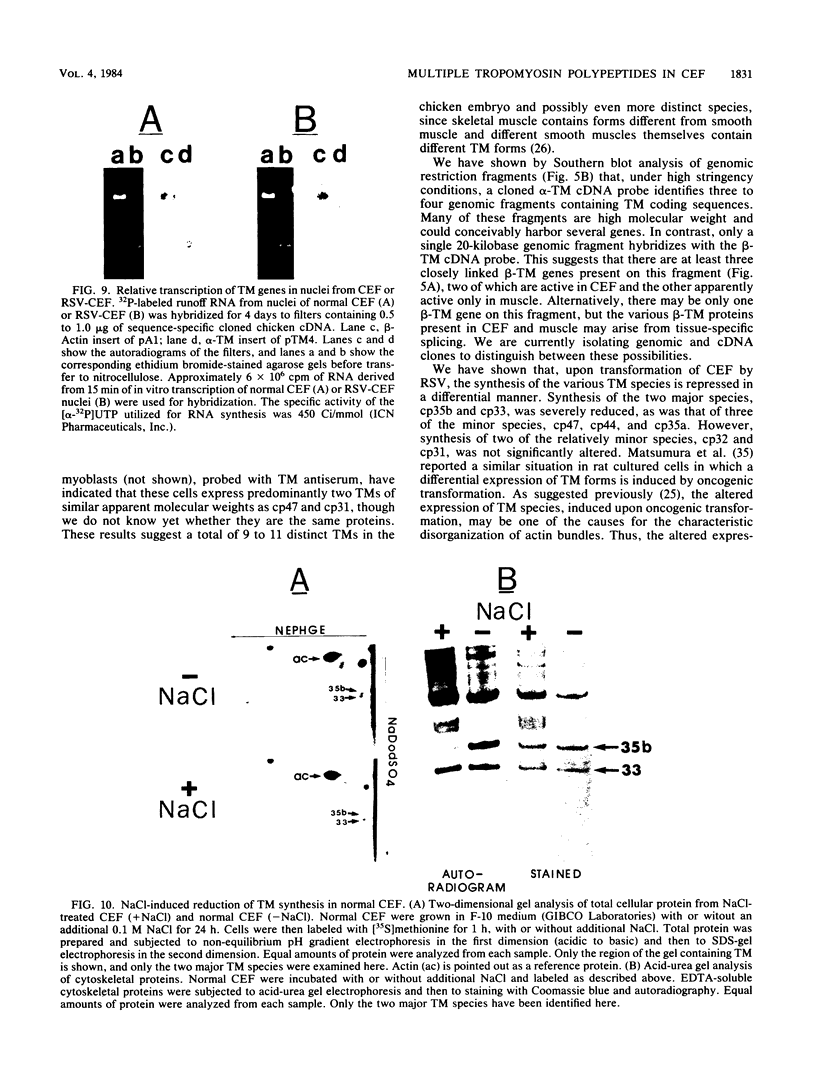
We have found that cytoskeletal extracts of cultured chicken embryo fibroblasts contain at least seven distinct polypeptides (two major and five minor) which cross-react with antiserum to chicken smooth muscle tropomyosin. These polypeptides range in apparent molecular weight from 31,000 to 47,000, and each is encoded by mRNAs which specifically hybridize to cloned muscle tropomyosin cDNAs. These nonmuscle tropomyosin species and their respective mRNAs are electrophoretically distinct from those of chicken skeletal muscle and appear by genomic DNA blotting to comprise a part of a multigene tropomyosin family. In Rous sarcoma virus-transformed chicken embryo fibroblasts, synthesis of the tropomyosins is differentially repressed such that the synthesis of the major species (cp35 and cp33, cytoskeletal proteins of molecular weight 35,000 and 33,000, respectively) and three minor species is drastically reduced, whereas the synthesis of two of the minor species (cp32 and cp31) remains essentially unchanged. Analysis of cellular mRNA and runoff nuclear transcription experiments indicate that the repression of tropomyosin synthesis by Rous sarcoma virus transformation occurs at the level of transcription. This repression of tropomyosin synthesis is partially mimicked in normal chicken embryo fibroblasts during incubation in high-NaCl medium, a condition in which chicken embryo fibroblasts acquire many characteristics of transformed cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Stromer M. H., Goll D. E., Robson R. M. Sythesis of tropomyosin in cultures of differentiating muscle cells. J Cell Biol. 1978 Jan;76(1):98–104. doi: 10.1083/jcb.76.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bernstein B. W., Bamburg J. R. Tropomyosin binding to F-actin protects the F-actin from disassembly by brain actin-depolymerizing factor (ADF). Cell Motil. 1982;2(1):1–8. doi: 10.1002/cm.970020102. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Human proteins sensitive to neoplastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982 Apr;28(4 Pt 2):949–954. [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Smillie L. B. The interaction of equine platelet tropomyosin with skeletal muscle actin. J Biol Chem. 1981 Jul 25;256(14):7257–7261. [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattoum A., Hartwig J. H., Stossel T. P. Isolation and some structural and functional properties of macrophage tropomyosin. Biochemistry. 1983 Mar 1;22(5):1187–1193. doi: 10.1021/bi00274a031. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L. A chemical comparison of tropomyosins from muscle and non-muscle tissues. J Mol Biol. 1975 Jul 5;95(3):447–454. doi: 10.1016/0022-2836(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L., Hitchcock S. E., Kaminer B. Tropomyosin in brain and growing neurones. Nat New Biol. 1973 Oct 10;245(145):182–186. doi: 10.1038/newbio245182a0. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Moyer M. P., Bishop J. M., Moyer R. C., Waite M. R. Transformation parameters induced in chick cells by incubation in media of altered NaCl concentration. Virology. 1981 Jun;111(2):427–439. doi: 10.1016/0042-6822(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Giometti C. S., Anderson N. L. A variant of human nonmuscle tropomyosin found in fibroblasts by using two-dimensional electrophoresis. J Biol Chem. 1981 Nov 25;256(22):11840–11846. [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C. L., Warren R. H., Rubin R. W. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. doi: 10.1016/0167-4889(82)90007-6. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R. Construction of bacterial plasmids containing sequences complementary to chicken alpha-tropomyosin mRNA. Nucleic Acids Res. 1981 Jun 25;9(12):2675–2689. doi: 10.1093/nar/9.12.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. R. Distinct alpha-tropomyosin mRNA sequences in chicken skeletal muscle. Eur J Biochem. 1982 Aug;126(2):293–297. doi: 10.1111/j.1432-1033.1982.tb06778.x. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Potter J. D., Sarkar S. Characterization of the Ca2+-regulatory complex of chick embryonic muscles: polymorphism of tropomyosin in adult and embryonic fibers. Biochem Biophys Res Commun. 1976 May 3;70(1):28–36. doi: 10.1016/0006-291x(76)91104-9. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Talbot K., MacLeod A. R. Novel form of non-muscle tropomyosin in human fibroblasts. J Mol Biol. 1983 Feb 15;164(1):159–174. doi: 10.1016/0022-2836(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Tyagi J. S., Hirano H., Merlino G. T., Pastan I. Transcriptional control of the fibronectin gene in chick embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1983 May 10;258(9):5787–5793. [PubMed] [Google Scholar]
- Vollet J. J., Brugge J. S., Noonan C. A., Butel J. S. The role of SV40 gene A in the alteration of microfilaments in transformed cells. Exp Cell Res. 1977 Mar 1;105(1):119–126. doi: 10.1016/0014-4827(77)90157-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Wehland J., Weber K. Distribution of fluorescently labeled actin and tropomyosin after microinjection in living tissue culture cells as observed with TV image intensification. Exp Cell Res. 1980 Jun;127(2):397–408. doi: 10.1016/0014-4827(80)90444-9. [DOI] [PubMed] [Google Scholar]
- Witt D. P., Brown D. J., Gordon J. A. Transformation-sensitive isoactin in passaged chick embryo fibroblasts transformed by Rous sarcoma virus. J Cell Biol. 1983 Jun;96(6):1766–1771. doi: 10.1083/jcb.96.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- der Terrossian E., Fuller S. D., Stewart M., Weeds A. G. Porcine platelet tropomyosin. Purification, characterization and paracrystal formation. J Mol Biol. 1981 Nov 25;153(1):147–167. doi: 10.1016/0022-2836(81)90531-3. [DOI] [PubMed] [Google Scholar]