Abstract
Purpose
The aim of this study was to assess the influence of age on the functional activity of the multidrug efflux transporter P-glycoprotein (P-gp) at the human blood-brain barrier.
Methods
7 young (mean age: 27±4 years) and 6 elderly (mean age: 69±9 years) healthy volunteers underwent dynamic (R)-[11C]verapamil (VPM) positron emission tomography (PET) scans and arterial blood sampling. Parametric distribution volume (DV) images were generated using Logan linearisation and age groups were compared with statistical parametric mapping (SPM). Brain regions that SPM analysis had shown to be most affected by age were analysed by a region of interest (ROI)-based approach using a maximum probability brain atlas, before and after partial volume correction (PVC).
Results
SPM analysis revealed significant clusters of DV increases in cerebellum, temporal and frontal lobe of elderly compared to younger subjects. In the ROI-based analysis, elderly subjects showed significant DV increases in amygdala (+30%), insula (+26%) and cerebellum (+25%), before PVC, and in insula (+33%), after PVC.
Conclusions
Increased VPM DV values in the brains of elderly subjects suggest a decrease in cerebral P-gp function with increasing age.
Keywords: P-glycoprotein, blood-brain barrier, age, (R)-[11C]verapamil, positron emission tomography
Introduction
The multidrug efflux pump P-glycoprotein (P-gp), which is highly expressed in the vessel wall of brain capillaries, protects the brain from the accumulation of blood-borne toxic substances [1]. Positron emission tomography (PET) with the radiolabelled P-gp substrate (R)-[11C]verapamil (VPM) is a validated technique to measure P-gp function in the blood-brain barrier (BBB) [2]. The distribution volume (DV) of VPM has been shown to inversely reflect cerebral P-gp function [3]. Previous data suggest decreased cerebral P-gp function in aged subjects [4-6] and Parkinson patients [7] and increased P-gp function in patients with epilepsy [8] and depression [9]. Toornvliet and colleagues found an 18% increase of VPM DVs in whole-brain grey matter of aged versus young subjects [5]. Bartels and colleagues extended their analytical approach to the description of regional differences in cerebral P-gp function [4].
In the present study, VPM PET scans were performed in aged (n=6) and young (n=7) subjects to further investigate the age dependency of regional cerebral P-gp function. In contrast to a previously published study that also investigated regional differences in cerebral P-gp function between young and elderly subjects with [11C]verapamil and PET [4], we used enantiomerically pure radiotracer, which has been found to be superior to racemic radiotracer for the kinetic modelling of PET data [2], corrected our PET data for partial volume effect and used a maximum probability atlas of the human brain for automated region of interest definition [10].
Material and Methods
The present study was performed at the Department of Clinical Pharmacology at the Medical University of Vienna. The study protocol was approved by the local Ethics Committee. All subjects were given a detailed description of the study and their written consent was obtained prior to the enrolment in the study. 7 young male subjects (mean age±standard deviation, SD, 27±4 years) and 6 elderly subjects (4 men, 2 women, mean age: 69±9 years) were included into the study. Intake of concomitant medication, known to interact with P-gp function, led to study exclusion.
After cannulation of the radial artery, VPM, which had been prepared as described earlier [11], was injected into the antecubital vein of each subject over 20 s. The mean injected dose was 370±21 MBq for the younger and 409±60 MBq for the elderly group. All subjects underwent a 1h dynamic PET scan consisting of 20 frames and serial arterial blood sampling. Arterial plasma samples were analysed for polar and lipophilic [11C]metabolites of VPM using a previously described combined solid-phase extraction/high-performance liquid chromatography assay [12]. An arterial input function was constructed by correcting total activity counts in arterial plasma for polar [11C]metabolites of VPM as described previously [8]. For image acquisition, PET and magnetic resonance imaging (MRI) protocols were used as described previously [8].
For data analysis, both a voxel-based parametric imaging approach and a region of interest (ROI)-based approach were used. Dynamic PET images were corrected for partial volume effect on a voxel-wise MRI basis by using the PVE routine implemented in PMOD v2.6 (PMOD Technologies, Ltd., Zurich, Switzerland) [13]. Parametric distribution volume (DV) maps were created for partial volume corrected (PVC) and non-PVC dynamic PET images and arterial blood activity data using the DV (Classic Logan Plot) routine in PMOD [14]. DV maps were analysed for age group differences with statistical parametric mapping (SPM) using a pixel-wise t-test. Using SPM5 (http://www.fil.ion.ucl.ac.uk/spm), DV maps were spatially normalised in Montreal Neurological Institute (MNI) space and smoothed by a Gaussian kernel of 8 mm. For visualisation, a probability threshold of p<0.005 family wise error corrected for multiple comparisons was applied.
Imaging data for ROI analysis were processed following previously published procedures [8]. The Hammersmith maximum probability brain atlas n20r49 [10] was used for defining a whole-brain grey matter ROI as well as brain regions that SPM analysis had shown to be most affected by ageing (amygdala, insula, cerebellum, frontal lobe, white matter), using both PVC and non PVC-data sets. A 1-tissue-2-rate-constant (1T2K) compartment model was used to estimate the influx (K1, [ml·ml−1·min−1]) and efflux (k2, min−1) rate constants of activity across the BBB [2, 8]. The distribution volume (DV), which is unitless, is defined as K1/k2 and can be considered as an estimate of the tissue-blood partition coefficient of activity at equilibrium. DVs for individual brain regions were estimated using Logan graphical analysis [8].
The dependency of model outcome parameters (DV, K1 and k2) on age was tested using the Pearson correlation coefficient (r). Due to the hypothesis of decreased P-gp activity in the elderly [4-6], age class differences for DV, K1 and k2 were analysed by one-sided t-tests. P values for multiple region testing were step-down bootstrap corrected. Age class differences in VPM metabolism were tested by comparing the area under the curve (AUC) values for the fractions of polar and lipophilic [11C]metabolites of VPM in arterial plasma over time with a two-sided t-test [12]. For all statistical computations the SAS System V9.2 (SAS Institute Inc., Cary, NC, USA) was used. The level of significance was set to 5%. The study had a power of 80% to detect a difference of 20% in DV values between groups (α error of 5%).
Results
Parametric imaging approach
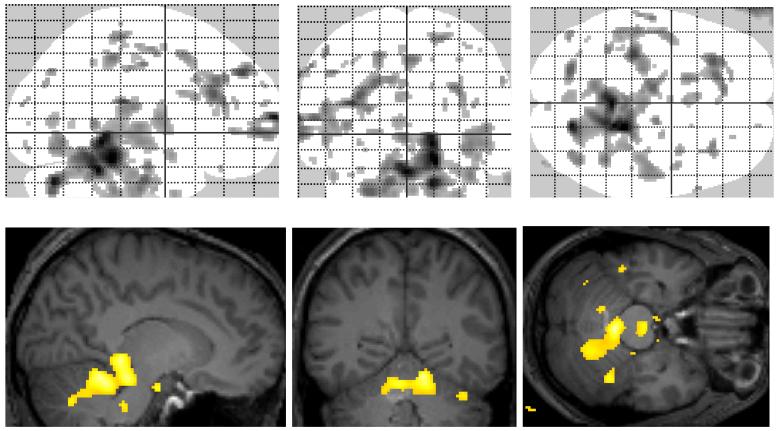
SPM analysis showed clusters of regionally increased DV values for the elderly compared to the younger group. The biggest differences were seen in cerebellum and temporal lobe regions, with some smaller clusters in frontal and temporal white matter. After partial volume correction (PVC), the global cluster size was reduced and the increases in temporal lobe and cerebellum remained the most pronounced (Fig. 1).
Fig. 1.
Statistical parametric map for the comparison of VPM DVs (after PVC) between elderly and younger subjects. Yellow colour indicates regions with p<0.005. The “glass brain” visualises DV increases in cerebellum, frontal and temporal lobe. Merged with a normalised MRI the major increase can be located in cerebellum.
Region of interest (ROI) approach
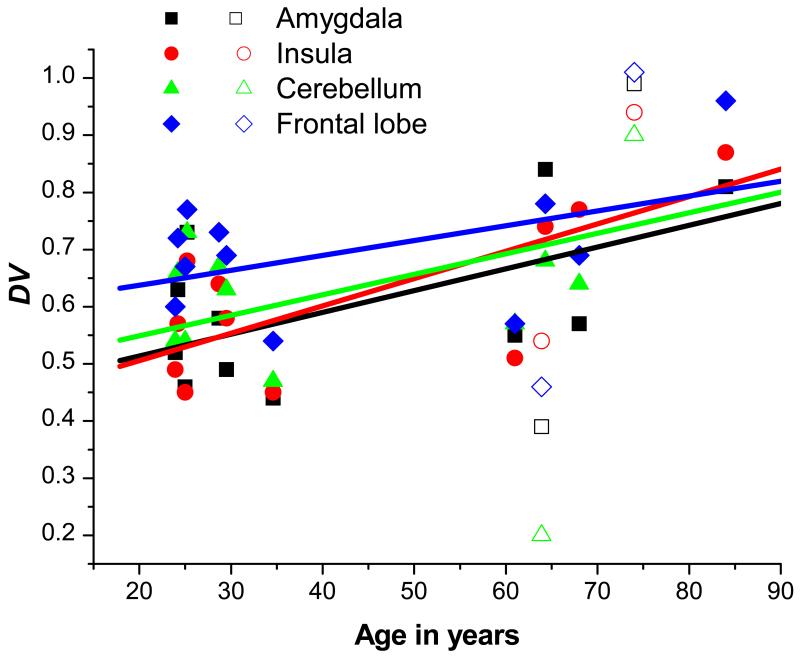
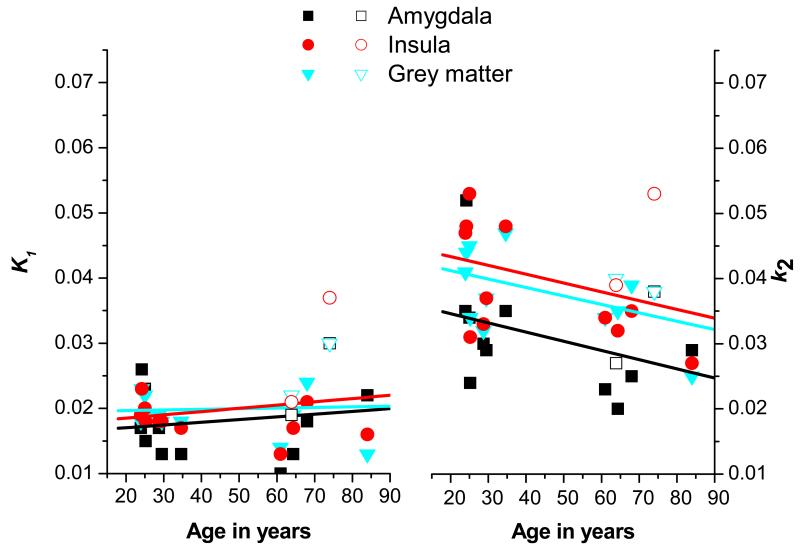
Brain regions that SPM analysis had shown to be most affected by age were analysed by the ROI-based approach using a maximum probability brain atlas. Table 1 displays mean DV values in different brain ROIs of both age groups, before and after PVC. Mean DV values were increased in the elderly, when compared to the young group (amygdala: +30%, insula: +26%, cerebellum: +25%, grey matter: +15%, white matter: +14%) (table 1). After PVC, the trend in DV increases remained consistent with the non-PVC data, although statistically significant increases were only detected for insula (+33%) (table 1). None of the analysed brain ROIs showed statistically significant differences in DV values after correction for multiple testing (table 1). Fig. 2 shows the dependency of DV on age for the ROIs amygdala, insula, cerebellum and frontal lobe (after PVC). A positive correlation between DV and age was determined for all 4 ROIs. Pearson correlation coefficients (r) for individual brain regions are displayed in table 1. Fig. 3 illustrates the dependency of the rate constants for VPM transport across the BBB on age. There was virtually no difference in the influx rate constant K1 between the two age groups for any of the regions analysed (amygdala: r=0.163, p=0.494; insula: r=0.196, p=0.470; whole-brain grey matter: r=0.051, p=0.494). The efflux rate constant k2, however, appeared to be negatively correlated with age. A clear trend towards decreased values in the elderly group was observed, although statistical significance was not reached in any of the analysed ROIs (amygdala: r=−0.384, p=0.172; insula: r=−0.343, p=0.272; whole-brain grey matter: r=−0.490, p=0.185).
Table 1.
Mean±SD DVs of VPM in different brain ROIs of young (n=7) and elderly (n=6) subjects before and after partial volume correction (PVC). In addition, p values for age group comparisons and Pearson correlation coefficients (r) for the correlation of DV with age are shown.
| Amygdala | Insula | Cerebellum | Grey matter | Frontal lobe | White matter a | |
|---|---|---|---|---|---|---|
| Mean±SD young (n=7) | 0.53±0.11 | 0.47±0.09 | 0.49±0.07 | 0.52±0.08 | 0.55±0.11 | 0.42±0.09 |
| Mean±SD old (n=6) | 0.69±0.14 | 0.59±0.11 | 0.61±0.12 | 0.60±0.12 | 0.51±0.17 | 0.48±0.10 |
| p value (1-sided t-test) | 0.027* | 0.034* | 0.040* | 0.098 | 0.325 | 0.174 |
| p value (step-down bootstrap corrected) | 0.079 | 0.083 | 0.091 | 0.168 | 0.325 | 0.267 |
| Pearson correlation coefficient (r) | 0.612 | 0.604 | 0.658 | 0.416 | −0.246 | 0.340 |
|
Amygdala
(PVC) |
Insula
(PVC) |
Cerebellum
(PVC) |
Grey matter
(PVC) |
Frontal lobe
(PVC) |
||
|---|---|---|---|---|---|---|
| Mean±SD young (n=7) | 0.55±0.10 | 0.55±0.09 | 0.61±0.09 | 0.65±0.08 | 0.67±0.08 | - |
| Mean±SD old (n=6) | 0.69±0.22 | 0.73±0.17 | 0.69±0.32 | 0.75±0.23 | 0.75±0.22 | - |
| p value (1-sided t-test) | 0.099 | 0.029* | 0.279 | 0.169 | 0.238 | - |
| p value (step-down bootstrap corrected) | 0.151 | 0.053 | 0.291 | 0.217 | 0.291 | - |
| Pearson correlation coefficient (r) | 0.483 | 0.683 | 0.367 | 0.446 | 0.380 | - |
Due to the small size of the white matter ROI, no reliable data could be obtained after PVC
statistically significant
Fig. 2.
Correlation of VPM DVs in different brain ROIs (after PVC) with age in 7 young and 6 elderly subjects (male subjects: closed symbols; female subjects: open symbols). Shown are the linear correlation fits. Refer to table 1 for individual Pearson correlation coefficients (r).
Fig. 3.
Correlation of the influx rate constant K1 (left graph) and the efflux rate constant k2 (right graph) of VPM in different brain regions with age in 7 young and 6 elderly subjects (male subjects: closed symbols; female subjects: open symbols). Shown are the linear correlation fits. The negative correlation of k2 with age points to decreased transporter-mediated efflux at the BBB in age.
Metabolism of VPM
VPM metabolism did not significantly differ between the young and the aged group (AUC lipophilic [11C]metabolite fraction (mean±SD): 11.8±1.9 and 11.4±1.9 min for the younger and elderly group, p=0.933; AUC polar [11C]metabolite fraction: 12.7±1.6 and 14.1±1.9 min for the younger and elderly group, p=0.110).
Discussion
Our results extend previous findings of decreased cerebral P-gp function in older aged subjects [4-6] in that we investigated the regional dependency of cerebral P-gp function on age. Our data indicate that, with increasing age, there is not only a global but also a regional decrease in cerebral P-gp function, suggesting that some brain areas might become more vulnerable to the accumulation of P-gp substrates, such as toxins and therapeutic drugs, than others. Thus, decreased cerebral P-gp function might account for central nervous system adverse effects of therapeutic drugs in the elderly and may constitute a risk factor for the development of neurodegenerative disease [4].
SPM analysis revealed significant clusters of regional DV increases in cerebellum, frontal and temporal lobe of elderly compared to younger subjects (Fig. 1). These increases were confirmed by using the ROI-based analysis approach (Fig. 2, table 1). However, the parametric analysis approach revealed limited sensitivity of the ROI-based analysis approach in cases where only part of a brain region is affected by significant DV changes (e.g. cerebellum).
Because aging leads to reductions in brain volume which possibly causes underestimation of DV values in certain brain regions, partial volume correction (PVC) was performed, as opposed to the two earlier studies that only reported non-PVC data [4, 5]. After PVC, DV values were still increased in the elderly as compared to the young group even though only the values for insula displayed statistically significant p values (table 1). Despite this lack of statistical significance, our data point to an age effect on cerebral P-gp function. Because several different methods for PVC have been described, further investigations are needed for selection of the most eligible approach in the present setting.
Interestingly, the mean percent DV increase in whole-brain grey matter of elderly compared to young subjects seen in our study, both for non-PVC and PVC data (+15%), was very similar to the value reported by Toornvliet et al. (+18%) [5], but more than 3-fold lower than the value reported by Bartels et al. (+60%) [4]. The reasons for these differences are unclear at present but might be related to the fact that Bartels et al. used racemic [11C]verapamil, whose two enantiomers have been shown to possess different plasma pharmacokinetics and different degrees of plasma protein binding [15].
The elderly group investigated in this study included two female subjects, whereas the young group consisted only of male subjects. To date, very little is known about gender differences in BBB transport of verapamil. A preclinical study has found about 22% lower initial brain uptake of verapamil in female as compared to male mice, which pointed to modestly increased cerebral P-gp expression and/or function in female animals [16]. However, the sample size of the present study was too small to draw any conclusions about gender related differences in P-gp mediated transport of verapamil.
It remains a matter of debate which of the two rate constants for VPM transport across the BBB, i.e. the influx rate constant K1 or the efflux rate constant k2, is more sensitive to changes in cerebral P-gp function. Data from studies employing pharmacologic P-gp modulation with P-gp inhibitors, such as zosuquidar or tariquidar [3, 17], suggest that the influx rate constant K1 of VPM is increased under conditions of impaired P-gp function, which is in line with the concept of P-gp acting as a gate keeper in the BBB and preventing substrates from diffusing across the luminal endothelial cell membrane (“influx hindrance”) [18]. Our present data, however, suggest that the efflux rate constant k2 may also be sensitive to changes in P-gp function as reflected by an apparent negative correlation of k2 values with age and a lack of such a correlation for K1 values (Fig. 3). Under unblocked conditions, VPM and possibly its radiolabelled metabolites cross the BBB to some extent and are thereafter expelled from brain parenchyma by P-gp mediated efflux (“vacuum cleaner” function of P-gp), which manifests itself as a k2 effect. [18]. It thus appears likely that under physiologic conditions the efflux rate constant k2 is more sensitive to changes in cerebral P-gp function than the influx rate constant K1, whereas the opposite is true during pharmacologic P-gp blockade.
VPM is a radiotracer which is extensively metabolised in vivo [12]. The polar 11C-labelled metabolites of VPM have shown brain uptake in rodents that is independent of cerebral P-gp function [2]. Differences in peripheral radiotracer metabolism might therefore bias the data analysis by suggesting differences in cerebral P-gp function [12]. In our study, however, the fraction of polar [11C]metabolites in plasma did not differ between young and elderly subjects, thus arguing against the possibility that differences in VPM metabolism might have accounted for increased DV values in the elderly.
In conclusion, our study extends previous findings of increased VPM DV values in the brains of aged subjects in that we demonstrated regional variations in age dependency of cerebral P-gp function [4-6]. Our results indicate that this increase in DV is mainly due to a decrease in transporter-mediated efflux of VPM at the BBB, reflected by a negative correlation of the efflux rate constant k2 with age.
Acknowledgements
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 201380 and from the Austrian Science Fund (FWF) project “Transmembrane Transporters in Health and Disease” (SFB F35). This study would not have been possible without the excellent support of Rainer Bartosch (Department of Nuclear Medicine), and Edith Lackner and Johann Stanek (Department of Clinical Pharmacology). The authors wish to thank Gert Luurtsema, Mark Lubberink and Adriaan Lammertsma from the VU University Medical Center (Amsterdam, The Netherlands) for help with setting up the (R)-[11C]verapamil PET procedure in their laboratory. Alexander Hammers from the MRC Clinical Sciences Centre (London, UK), and Marie-Claude Asselin and Rainer Hinz from the Wolfson Molecular Imaging Centre (Manchester, UK) are gratefully acknowledged for providing help in establishing the brain atlas analysis approach.
References
- 1.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Lubberink M, Luurtsema G, van Berckel BN, Boellaard R, Toornvliet R, Windhorst AD, Franssen EJ, Lammertsma AA. Evaluation of tracer kinetic models for quantification of P-glycoprotein function using (R)-[(11)C]verapamil and PET. J Cereb Blood Flow Metab. 2007;27:424–433. doi: 10.1038/sj.jcbfm.9600349. [DOI] [PubMed] [Google Scholar]
- 3.Bankstahl JP, Kuntner C, Abrahim A, Karch R, Stanek J, Wanek T, Wadsak W, Kletter K, Müller M, Löscher W, Langer O. Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J Nucl Med. 2008;49:1328–1335. doi: 10.2967/jnumed.108.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, van Oostrom JC, Leenders KL. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: A possible role in progressive neurodegeneration. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.02.002. Doi:10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Toornvliet R, van Berckel BN, Luurtsema G, Lubberink M, Geldof AA, Bosch TM, Oerlemans R, Lammertsma AA, Franssen EJ. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clin Pharmacol Ther. 2006;79:540–548. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.van Berckel BN, Lubberink M, Luurtsema G, Boellaard R, Lammertsma AA. Regional variations in age dependence of P-gp mediated transport across the blood-brain barrier measured using (R)-[11C]verapamil and positron emission tomography [symposium abstract] Alzheimers Dement. 2008;4:T95. [Google Scholar]
- 7.Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JC, Portman A, Leenders KL. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, Abrahim A, Luurtsema G, Brunner M, Sunder-Plassmann R, Zimprich F, Joukhadar C, Gentzsch S, Dudczak R, Kletter K, Müller M, Baumgartner C. Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[11C]verapamil. Epilepsia. 2007;48:1774–1784. doi: 10.1111/j.1528-1167.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 9.de Klerk OL, Willemsen AT, Roosink M, Bartels AL, Harry Hendrikse N, Bosker FJ, den Boer JA. Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood-brain barrier. Int J Neuropsychopharmacol. 2009 doi: 10.1017/S1461145709009894. Doi:10.1017/S1461145709009894. [DOI] [PubMed] [Google Scholar]
- 10.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunner M, Langer O, Sunder-Plassmann R, Dobrozemsky G, Müller U, Wadsak W, Krcal A, Karch R, Mannhalter C, Dudczak R, Kletter K, Steiner I, Baumgartner C, Müller M. Influence of functional haplotypes in the drug transporter gene ABCB1 on central nervous system drug distribution in humans. Clin Pharmacol Ther. 2005;78:182–190. doi: 10.1016/j.clpt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Abrahim A, Luurtsema G, Bauer M, Karch R, Lubberink M, Pataraia E, Joukhadar C, Kletter K, Lammertsma AA, Baumgartner C, Müller M, Langer O. Peripheral metabolism of (R)-[(11)C]verapamil in epilepsy patients. Eur J Nucl Med Mol Imaging. 2008;35:116–123. doi: 10.1007/s00259-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 13.Hurlemann R, Schlaepfer TE, Matusch A, Reich H, Shah NJ, Zilles K, Maier W, Bauer A. Reduced 5-HT2A receptor signaling following selective bilateral amygdala damage. Soc Cogn Affect Neurosci. 2009 doi: 10.1093/scan/nsn039. Doi:10.1093/scan/nsn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichelbaum M, Mikus G, Vogelgesang B. Pharmacokinetics of (+)-, (−)- and (+/−)-verapamil after intravenous administration. Br J Clin Pharmacol. 1984;17:453–458. doi: 10.1111/j.1365-2125.1984.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagenais C, Zong J, Ducharme J, Pollack GM. Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm Res. 2001;18:957–963. doi: 10.1023/a:1010984110732. [DOI] [PubMed] [Google Scholar]
- 17.Liow JS, Kreisl W, Zoghbi SS, Lazarova N, Seneca N, Gladding RL, Taku A, Herscovitch P, Pike VW, Innis RB. P-Glycoprotein Function at the Blood-Brain Barrier Imaged Using 11C-N-Desmethyl-Loperamide in Monkeys. J Nucl Med. 2009;50:108–115. doi: 10.2967/jnumed.108.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syvänen S, Xie R, Sahin S, Hammarlund-Udenaes M. Pharmacokinetic consequences of active drug efflux at the blood-brain barrier. Pharm Res. 2006;23:705–717. doi: 10.1007/s11095-006-9780-0. [DOI] [PubMed] [Google Scholar]