Abstract
Few options exist for treatment of pervasive motoneuron death after spinal cord injury or in neurodegenerative diseases such as amyotrophic lateral sclerosis. Local transplantation of embryonic motoneurons into an axotomized peripheral nerve is a promising approach to arrest the atrophy of denervated muscles; however, muscle reinnervation is limited by poor motoneuron survival. The aim of the present study was to test whether acute electrical stimulation of transplanted embryonic neurons promotes motoneuron survival, axon growth, and muscle reinnervation. The sciatic nerve of adult Fischer rats was transected to mimic the widespread denervation seen after disease or injury. Acutely dissociated rat embryonic ventral spinal cord cells were transplanted into the distal tibial nerve stump as a neuron source for muscle reinnervation. Immediately post-transplantation, the cells were stimulated at 20 Hz for 1 h. Other groups were used to control for the cell transplantation and stimulation. When neurons were stimulated acutely, there were significantly more neurons, including cholinergic neurons, 10 weeks after transplantation. This led to enhanced numbers of myelinated axons, reinnervation of more muscle fibers, and more medial and lateral gastrocnemius muscles were functionally connected to the transplant. Reinnervation reduced muscle atrophy significantly. These data support the concept that electrical stimulation rescues transplanted motoneurons and facilitates muscle reinnervation.
Key words: axon regeneration, electrical stimulation, motoneuron transplantation, muscle denervation
Introduction
Motoneuron death is a significant trait of spinal cord trauma and several neurodegenerative diseases.1–5 In amyotrophic lateral sclerosis, for example, death of motoneurons occurs at supraspinal and spinal levels and the muscle denervation is severe enough to result in death within 5 years of diagnosis.6,7 Spinal cord contusion induces large cavities and motoneuron death. Ventral root degeneration can occur across several spinal segments.3,8–13 Denervation of triceps brachii, a muscle that is critical for reaching things and pushing a wheelchair, contributes to atrophy in 75 % of cases of cervical spinal cord injury. This motoneuron death routinely limits function.3,14 At present, there is no effective therapy to restore function to denervated muscles when motoneuron death is pervasive.15–18 And when muscles have no access to regenerating peripheral axons, neuron replacement is needed.
Early intervention is also needed to preserve nerve tissue and muscle targets after denervation. Deterioration is rapid.19–22 Collagenous material accumulates in intramuscular nerve sheaths. The sheaths collapse with time and few axons penetrate them.23 Schwann cells become unresponsive to growth signals and eventually die.24–26 Denervation-induced muscle wasting begins immediately and continues relentlessly unless arrested. Fibrous connective tissue builds up in muscle.27,28 Reconstructive surgery for nerve repair is often insufficient to achieve reinnervation of distal limb muscles, because axon growth is slow, and has to occur over long distances. Functional recovery is limited in humans, even at 6 months after peripheral nerve injury.19–22 Electrical stimulation to check or reverse deterioration of denervated muscle29 or to initiate limb movements is usually unworkable because of the large currents needed to activate denervated muscles.30,31 Therefore, reinnervation is central to rehabilitation and recovery of muscle function.
Our experimental strategy used neuron replacement to reinnervate hindlimb muscles that had no access to spinal motoneurons.32,33 In this model, neurons competent to innervate muscle were isolated from the embryonic ventral spinal cord and transplanted into peripheral nerve close to denervated muscles. The axons had a short distance to grow to muscle to re-establish neuromuscular connections, which provides neurotrophic support, and reduces atrophy. Muscle excitability was also restored as electromyography (EMG) and force were evoked by electrical stimulation of the transplanted neurons,33,34 but the extent of muscle reinnervation needs improvement. One explanation for some muscle fibers becoming reinnervated while others remain denervated is that the majority of transplanted neurons die soon after transplantation. This is the case when neurons are implanted into a peripheral nerve or the central nervous system.35–40 In support of this idea, motoneuron survival, muscle reinnervation, and motor unit function are all promoted when a combination of neurotrophic factors is included with the transplanted neurons.41,42
Neuron survival is also activity dependent,43 and developing neurons are excitable. In vitro-induced membrane depolarization improves neuron survival via calcium (Ca2+) regulated processes,44–48 but there are limits. If calcium is elevated beyond normal levels, neuron death will ensue.49–52 Inhibition of electrical activity also elevates neuron death, reduces axon growth, and retards motoneuron development.53–55 Electrical stimulation induces Ca2+ transients that are linked to L-type voltage-gated calcium channels, resulting in endogenous synthesis of cyclic adenosine monophosphate (cAMP) and neurotrophic factors,52,56 but the effects of electrical stimulation are not the same for all neurons. They are influenced by developmental stage and the experiences of the neurons. The mechanisms regulating neuron survival and axon growth are also different.43,56,57 Therefore, it is uncertain whether electrical stimulation of transplanted neurons would enhance neuron survival and/or axon growth, and lead to successful muscle reinnervation. In this study, we used a protocol that facilitated axon regeneration after femoral nerve section,58 but we applied the stimulation to a transplant of acutely dissociated neurons in the tibial nerve. Our objective was to test if acute electrical stimulation of neurons immediately after transplantation promoted neuron survival, axon regeneration, and muscle reinnervation 10 weeks later.
Methods
All procedures on animals adhered to the National Institutes of Health guidelines for care and use of animals and were approved by the University of Miami Animal Care and Use committee. All experiments were performed on female Fischer rats (140–160 g) under general anesthesia (40 mg/kg Nembutal, i.p.).
Experimental design and protocol
The experiments involved three steps (muscle denervation, transplantation, and physiological and anatomical assessments; Table 1). In the first surgery, the left sciatic nerve was transected near the hip to denervate many hindlimb muscles.33 The proximal nerve stump was sutured into hip muscles to prevent peripheral axons regenerating into the distal nerve stump. Tibial-innervated muscles were therefore disconnected from the spinal cord. One week later, 1,000,000 embryonic day 14–15 ventral spinal cord cells (or medium) were injected into the distal tibial nerve stump 10–15 mm proximal to its entry into the medial gastrocnemius muscle using a 26S gauge Hamilton Syringe. The transplanted cells were the only source of neurons available for muscle reinnervation. Cell transplantation was delayed because this improved axon regeneration and a delay is a clinically feasible approach.59 Physiological analysis of muscle function and tissue removal occurred 10 weeks after transplantation.
Table 1.
Experimental Design and Timetable
| Group | Week -1 Denervation | Week 0 Cell transplantation | Week 0 Treatment | Week 10 Assessments |
|---|---|---|---|---|
| Stimulation | + | + | 20 Hz, 1 h | Physiology; counts of motoneurons, myelinated axons; neuromuscular junctions, muscle fiber size, reinnervation |
| No stimulation | + | + | — | |
| No cells | + | — | — | |
| Uninjured | — | — | — |
Four groups of animals were used (Table 1). Animals in the stimulation group (n=16) underwent denervation, transplantation, and stimulation, as well as assessment. Immediately after injection of the cells into the tibial nerve, the transplant was laid over silver electrodes and stimulated at 20 Hz for 1 h (100 μs duration pulses, 3 V).58 Treatment of animals in the “no stimulation” group (n=14) was identical to that of the stimulation group except that no stimulation was delivered. Therefore, cells were injected into the nerve and the transplant was always placed onto the silver electrodes for 1 h, but the stimulator remained off. The “no cell” group had medium injected into the nerve without cells and no stimulation was given (n=5). Uninjured animals underwent physiological and morphological assessments only (n=5).
Preparation of the cell transplant
Day 14–15 embryos were placed in ice-cold phosphate buffered saline (PBS). Embryonic cords were freed of meninges, the ventral cord dissected free, and then exposed to 0.01% trypsin (Worthington Biochemical Corp Freehold, NJ) and 0.01% deoxyribonuclease (Sigma-Aldrich, St. Louis, MO) in Hank's balanced salt solution (HBSS) without calcium and magnesium. After 45 min at 37°C, fetal bovine serum was added to 10% to inhibit trypsin activity. The tissue was washed in HBSS. The ventral cords were dissociated by repeated passage through a fire-polished glass Pasteur pipette. The dispersed cell suspension was passed through a 70 μm nylon net and the cells were collected by centrifugation. The pellet was resuspended in culture medium and washed three times, and the cells were resuspended in Neurobasal medium (Invitrogen, Carlsbad, CA) with 2% B27 supplement, 3 mM glucose, 2 mM glutamate, 2 mM pyruvate, 5% rat serum and pencillin/streptomycin (100 U/mL and 100 μg/mL). No neurotrophic factors were added to the transplant medium, as our intent was to investigate the role of electrical stimulation on neuron survival and axon growth. Cell viability was determined by Trypan blue dye exclusion60 and averaged 96% immediately after ventral spinal cord dissociation. Cell viability decreased to 80–88% ∼2 h later, the time at which cell transplantation was complete.
Physiological recording of muscle function
Ten weeks after transplantation, animals were re-anesthetized. The tibial nerve containing the cell transplant, as well as the medial and lateral gastrocnemii muscles (MG, LG) were dissected free of surrounding tissues. The rat was placed onto a heating pad with the left knee and ankle clamped. Skin was shaped into a pool to envelope the tissue with warm mineral oil. Two silver electrodes were placed on the surface of the LG muscle belly to record EMG signals. Silver electrodes were placed under the transplant to deliver stimulation. Single pulses of different durations (10–50 μs) and intensities (from 1 to 30V using either 0.1-V or 1-V steps) were delivered until maximal EMG was attained. The EMG signals were sampled online at 3200 Hz using SC/Zoom software (Umeå University, Sweden). Function of MG muscles was documented only by the presence of visible contractions in response to transplant stimulation. At the completion of the experiment, the tibial nerve was cut between the transplant and the MG and LG muscles. The transplant was stimulated again but no EMG or visible muscle contractions were evoked, which confirmed that the transplant was the source of muscle innervation. Off line, the peak-to-peak amplitude of the maximal evoked EMG recorded in LG was measured to estimate the amount of muscle reinnervation and function.
Analysis of neurons and motoneurons in the transplant
After the electrophysiology, the cell transplant was removed, oriented on Whatman Filter Paper and fixed overnight in Zamboni's fixative (2% paraformaldehyde, 15% picric acid in 0.1 M phosphate buffer, pH 7.3) at 4°C. The tissue was cryoprotected in graded sucrose solutions, frozen using dry-ice cooled isopentane, and stored at −80°C. The entire transplant was cut serially (25 μm thick). Sections were mounted onto gelatin-coated glass slides, dried, washed in 1.5T buffer, and permeabilized in methanol at −20°C for 30 min. A monoclonal antibody against NeuN, a neuron-specific nuclear protein expressed in the nucleus and cytoplasm of most neuronal cells, was used to detect neurons in the transplants (A60, catalog #MAB377, Millipore, Temecula, CA; diluted 1:200).61 An anticholine acetyltransferase (ChAT) goat antibody (Millipore; 1:110) was used to detect cholinergic neurons and fibers. A mouse monoclonal, SMI32, which recognizes heavy neurofilament chains, was used to detect mature neurons (Covance Inc, Emeryville, CA; 1:1000). The sections were washed three times for 15 min with 1.5T buffer. Sections were exposed to a combination of secondary antibodies that included Alexa 488 labeled donkey anti-goat IgG or Alexa 596 labeled donkey anti-mouse IgG (Invitrogen, diluted 1:300 in 1.5T containing 0.5% Triton X-100) and incubated for 2 h at room temperature in the dark.
The disector/fractionator method was used to estimate neuron survival 10 weeks after transplantation.62 Between 100 and 300 immunopositive neurons were counted in each transplant. All quantitative analysis was performed using an Olympus BX-60 fluorescent microscope. The optical images were captured using a digital camera (Cooke SensiCam-QE camera, Auburn Hills, MI), a StagePro controlled motorized stage, and ImagePro software (Media Cybernetics, Bethesda, MD).
Myelinated axon counts
Nerves to the MG, LG, and other tibial innervated muscles distal to the gastrocnemii were removed, fixed in 4% paraformaldehyde in phosphate buffer, then in 4% gluteraldehyde in phosphate buffer, post-fixed in 1% osmium tetroxide for 1 h, dehydrated in serial concentrations of ethanol, and embedded in epoxy resin.33 Cross-sections (1 μm) were stained with toluidine blue for light microscopy. Each nerve section was digitized and the diameter of all myelinated axons was measured using ImagePro Plus software (MediaCybernetics). These data provided the total number of myelinated axons that regenerated from the transplant and the number of axons that grew into the MG and LG nerve branches.
Analysis of muscle fiber areas
At the completion of the electrophysiology, the left MG and LG muscles were harvested, weighed, promptly frozen in dry-ice cooled isopentane, and stored at −80°C.33 Transverse sections (8 μm) were cut at the midbelly of the LG muscle and air dried. To visualize the basic structure of the muscle, sections were stained with Mayer's hematoxylin and eosin (H&E) and mounted in Depex medium. The areas of 500 fibers were measured per muscle using MetaMorph software (Molecular Devices Corporation, Sunnyvale, CA). Fibers from up to 100 different areas were sampled from the entire muscle section. Muscle fiber area was used to discriminate reinnervated fibers from denervated ones. Fibers with an area ≥498 μm2 were considered reinnervated, because 95 % of the fibers in denervated muscles had areas smaller than this value, verified by comparing the sizes of glycogen-depleted fibers in reinnervated muscles to fibers in denervated muscles.63
Immunostaining of motor endplates
The MG muscle was cut longitudinally. Sections (65 μm thick) were dried onto glass slides coated with ethylenediaminetetraacetic acid and gelatin. Neuromuscular junctions were detected using a monoclonal to neurofilament (SMI31; 1:1000, Covance). Acetylcholine receptors at motor end plates were detected with Alexa Fluor 568 conjugated α-Bungarotoxin (Invitrogen).59
Statistical analysis
Medians and 25th and 75th percentiles are given. ANOVA on ranks was used to compare neuron, motoneuron, and axon counts, as well as muscle areas across groups. The percentage of reinnervated muscle fibers and the percentage of muscles functionally connected to the transplant were compared across groups using χ2 tests. Statistical significance was p<0.05.
Results
Electrical stimulation of transplanted neurons promoted survival of neurons and motoneurons
Ten weeks after cell transplantation, both NeuN and ChAT immunoreactive neurons were dispersed over a ∼2 mm length of nerve. ChAT-positive neurons differed in size, morphology, and distribution, occurring alone or in groups (Fig. 1A). Many ChAT-positive axons coursed through the transplants (Fig. 1B). No neurons were detected in the tibial nerve of animals injected with medium but no cells.
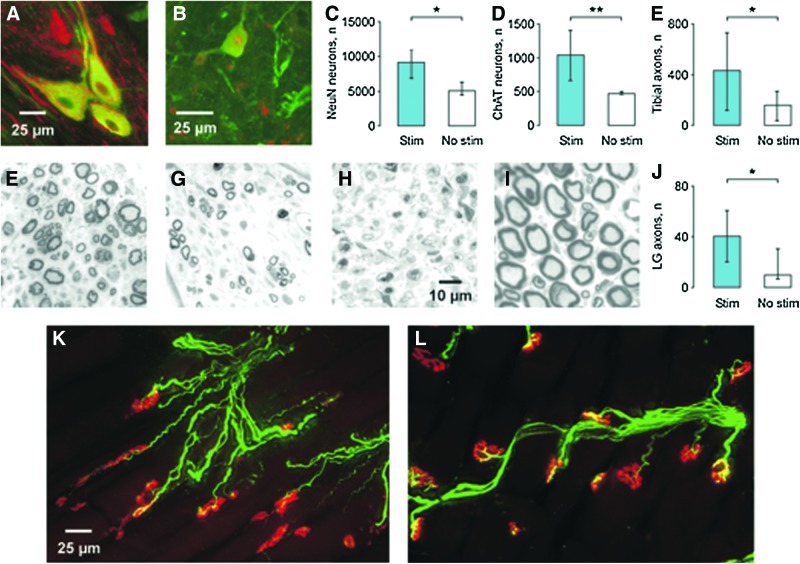
FIG. 1.
(A) Longitudinal section of a tibial nerve with neurons stained with antibodies to choline acetyltransferase (ChAT) (green) and neurofilament (red) 10 weeks after transplantation of acutely dissociated ventral spinal cord cells. (B) Transverse transplant section shows neurons express ChAT (green; goat antibody) and/or neuron-specific nuclear protein NeuN (red; mouse antibody). Median (25th, 75th percentile) NeuN-positive (C), ChAT-positive (D), and myelinated axon counts in tibial (E) and lateral gastrocnemii (LG) nerves (J) for stimulation and no stimulation groups. In injured animals, we estimated the median number of myelinated axons in the tibial and LG nerves to be 1127 and 112, respectively. Cross-sections of toluidine-blue stained myelinated axons in LG nerves from an animal in the stimulation (F), no stimulation (G), no cell (H), and uninjured groups (I), respectively. Longitudinal sections of medial gastrocnemii (MG) muscles with axons immunostained for neurofilament (SMI31), monoclonal anti-neurofilament (68 kDa), and synaptophysin (all green) coursing across fibers and terminating at motor end plates (red; Alexa 596 conjugated-bungarotoxin) after cell transplantation of E14 cells and acute stimulation (K), and in muscle from uninjured animals (L). Statistical significance is shown with asterisk *p<0.05; **p<0.01. Color image is available online at www.liebertpub.com/neu
Acute stimulation of the transplant increased the median number of NeuN-positive neurons after 10 weeks (9132 vs. 5082 for the no stimulation group, p=0.026; Fig. 1C). We next examined the possibility that acute stimulation affected cholinergic neuronal survival. In the stimulation group, 1035 of 9132 NeuN-positive neurons were ChAT positive (11.3%) versus 469 of 5082 neurons (9.3%) in the no stimulation group (Fig. 1D). Significantly more motoneurons survived with stimulation (p=0.008). However, similar percentages of cholinergic neurons suggest that acute stimulation did not change motoneuron differentiation.
Electrical stimulation of transplanted neurons increased axon number
Myelinated axons were found in all nerve trunks distal to the cell transplants (Fig. 1F, G) but were absent following injection of medium without cells (Fig. 1H). Acute electrical stimulation of the cell transplant increased the total myelinated axon count significantly (median stimulation vs. no stimulation: 433 vs.159; p=0.04) although both counts were lower than the 1127 myelinated axons found in nerves of uninjured animals (Fig. 1E, I). The corresponding axon counts for the LG nerve were 41, 10, and 112, respectively (p=0.04; Fig. 1J). Axons that grew from the transplants coursed across muscles and formed neuromuscular junctions (Fig. 1K) but the motor endplates were small, less complex, and immature compared with the pretzel-shaped endplates in uninjured muscles (Fig. 1L).
Electrical stimulation increased muscle reinnervation, which in turn, reduced atrophy
Acute transplant stimulation increased the percentage of reinnervated fibers in LG muscles compared with no stimulation (median: 56 % vs. 41 %, p=0.048; Fig. 2A, B, E). Only some muscle fibers were reinnervated 10 weeks after cell transplantation, which is illustrated by large (reinnervated) fibers close in size to fibers of uninjured muscles (Fig. 2D) dispersed among relatively small fibers, as seen in denervated muscles (Fig. 2C).
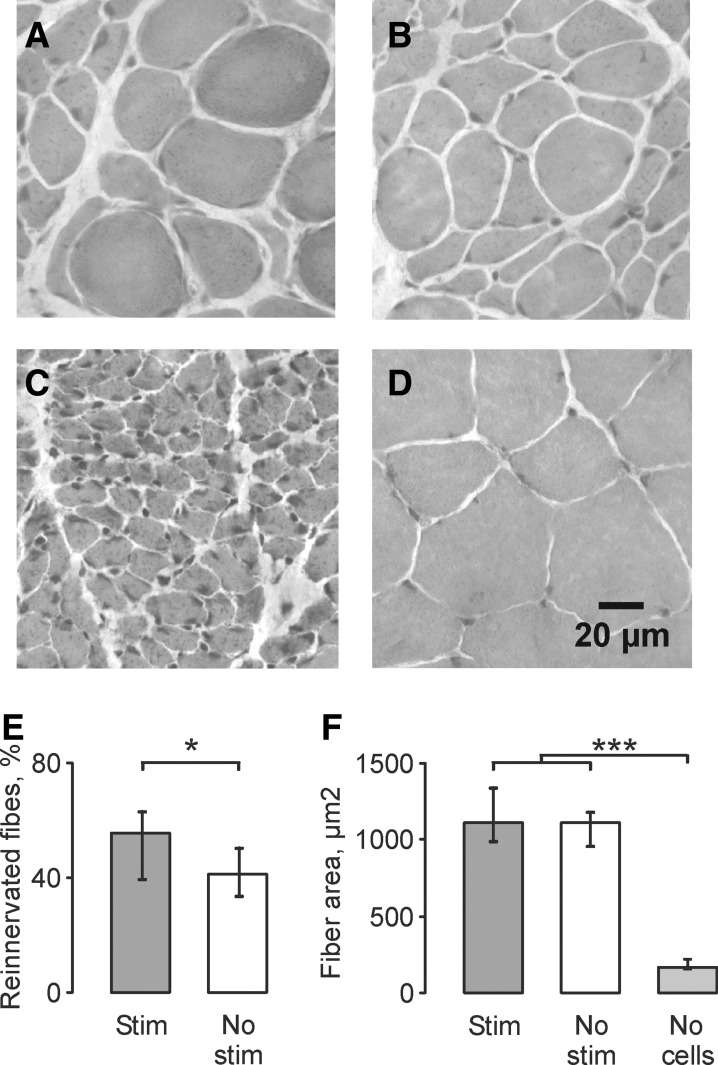
FIG. 2.
Hematoxylin and eosin (H&E)-stained cross-sections of lateral gastrocnemii (LG) muscles from an animal in the stimulation (A), no stimulation (B), no cell (C), and uninjured (D) groups. Median (25th, 75th percentile) percentage of reinnervated muscles fibers (E) and reinnervated muscle fiber areas (F) for the stimulation, no stimulation, and no cells groups. Statistical significance is shown with asterisk *p<0.05; ***p<0.001.
Formation of neuromuscular junctions reduced fiber atrophy significantly in both the stimulation and no stimulation groups compared with fibers in muscles that had been denervated for 11 weeks. Median fiber area for the stimulation, no stimulation and no cells (denervated) groups were 1115 μm2, 1112 μm2, and 171 μm2, respectively (p<0.001; Fig. 2F), all of which were smaller than fibers in uninjured control muscles (median: 2783 μm2).
Electrical stimulation increased the number of reinnervated muscles that functioned
Ten weeks after cell transplantation, electrical stimulation of the transplants produced contractions in 91% of the MG and LG muscles in the stimulation group, but in only 57% of muscles in the no stimulation group (p<0.001). The median EMG recorded from LG muscles was similar for both the stimulation and no stimulation groups (both 14% uninjured; Fig. 3). It is possible that muscle surface recordings do not capture signals from all of the reinnervated fibers. No EMG was evoked in LG muscles of animals in which medium was transplanted without cells, even with 150 V and 1 ms duration pulses. No visible contractions occurred in the MG muscles of these animals either. These data and the absence of myelinated axons in the nerves (Fig. 1H) confirm that these muscles remained denervated after nerve transection.
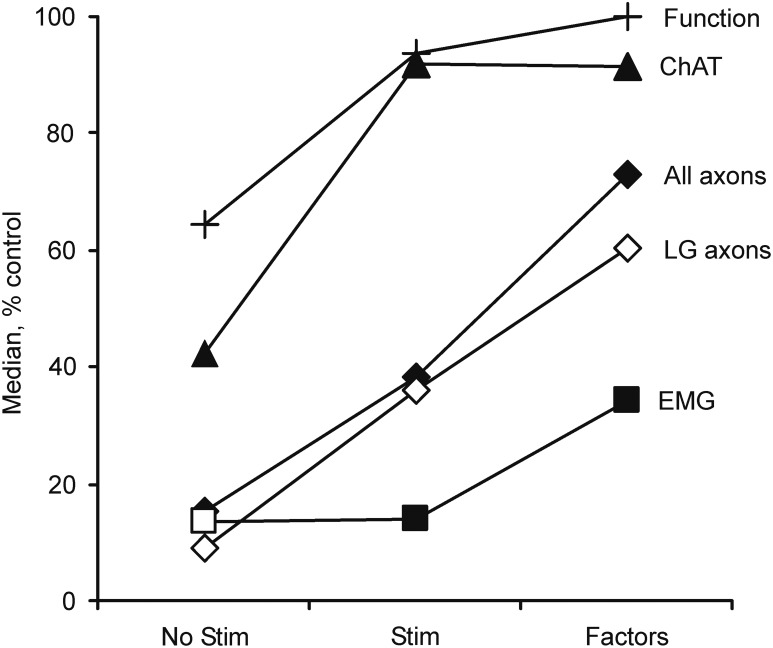
FIG. 3.
Median choline acetyltransferase (ChAT)-positive neuron survival, total (All) and lateral gastrocnemii (LG) myelinated axon counts, percentage of functional LG muscles (Function), and evoked LG electromyography (EMG) for animals in the current no stimulation and stimulation groups, in which no neurotrophic factors were used compared with published data,42 in which three neurotrophic factors were included with the cells at transplantation (glial cell-derived neurotrophic factor [GDNF], hepatocyte growth factor [HGF], and insulin-like growth factor 1 [IGF-1]; each factor, 1 ng/mL) but no stimulation was delivered. All data are expressed relative to uninjured values.
Discussion
The present results establish that electrical stimulation of embryonic neurons for 1 h immediately after transplantation increases neuron, motoneuron, and myelinated axon counts, resulting in long-term improvements in muscle reinnervation and function. Electrical stimulation is a practical option for neuroprotection, particularly given that young embryonic neurons adapted readily in peripheral nerve after just one only 1 h of treatment.
Electrical stimulation increased motoneuron survival and axon growth
Immediately after transplantation, cell death arises from a disturbed ionic homeostasis and extracellular matrix, hypoxia/ischemia, inflammation, and/or trophic factor deprivation.35,60 These conditions initiate commitments to cell death that occur over several hours.64,65 Therefore, our acute stimulation must counteract some of these early checks on neuron viability and axon growth, which is consistent with in vitro data that show that depolarization improves survival of motoneurons.44,66 One hour of electrical stimulation may change cytoplasmic Ca2+ through release of intracellular Ca2+ stores, Ca2+ influx, and/or movement of Ca2+ into the nucleus.67 Elevated Ca2+ can trigger activation of cAMP-response element-dependent gene expression (CREB) and at least 30 min of electrical stimulation is needed to reach maximal levels of CREB phosphorylation, an upstream regulator of brain-derived neurotrophic factor gene transcription.68 Therefore, some of the effects of electrical stimulation may arise from endogenous synthesis of neurotrophic factors.56,58
Physical stress at the time of cell dissociation and neural transplantation causes shear and compressive forces that remove axons and dendrites, disrupt membrane integrity, and induce Ca2+ influx leading to death.35,60,69–72 Thus, membrane depolarization may have long-lasting effects on neuron survival and axon growth because it rapidly initiates re-sealing of the cell membrane72,73 and growth cone creation, a process also dependent on cellular levels of Ca2+.74,75 The higher axon count reported here with acute stimulation of the transplanted cells supports this idea (Fig. 1). Any axons that immediately grow from the transplanted neurons are also likely to explore the local microenvironment and gain access to adhesive substrates and neurotrophic support.
Comparison of the current results in which the treatment involved electrical stimulation but no factors to our published studies in which glial cell-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1) were added to the cell transplant42 show that each intervention increased ChAT-positive neurons similarly. However, inclusion of neurotrophic factors induced larger increases in myelinated axons, all LG muscles functioned, and the EMG evoked in LG was larger than with the acute electrical stimulation intervention (Fig. 3). Future studies that combine stimulation with factors could distinguish whether these treatments have independent effects in support of earlier studies on retinal ganglion cells that show that neuron survival and axon regeneration depend upon both activity-dependent and activity-independent processes.43 Similarly, the current protocol could be optimized to determine whether pulse frequency, number, pattern, or duration of stimulation improve outcomes further.
Stimulation-induced increases in motoneuron survival and axon growth improve muscle reinnervation
In addition to neural effects, acute electrical stimulation of the transplant promoted functional reinnervation of both LG and MG muscles at 10 weeks (Figs. 2 and 3). More functional muscles may arise from greater motoneuron survival rather than embryonic neurons establishing a motoneuron identity in a peripheral nerve. A combination of actions, including appropriate growth factors, morphogens, and regulatory and spatial gene expression patterns may be needed to generate motoneurons capable of innervating muscle.76 It is of equal importance that reinnervation from embryonic neurons also modified muscle. Excitability was restored, atrophy was reduced, and more muscle fibers were reinnervated with acute stimulation of the transplant (Fig. 2). Despite these positive effects, some muscle fibers remained denervated. Retrograde signals from muscles influence motoneuron excitability, survival, and differentiation,77–82 and maturation of the neuromuscular junction;83–85 therefore, both incomplete differentiation of motoneurons in nerves and lack of muscle activity likely produced the immature neuromuscular junctions that we commonly observed (Fig. 2K), limiting reinnervation and function.86
Functional implications
Another important question is whether this neuron transplantation strategy is of potential clinical use. Injury to the human spinal cord and motoneuron diseases commonly cause death of spinal motoneurons, which denervates skeletal muscles. For example, one in six people with cervical spinal cord injury (SCI) have complete, bilateral denervation of hand muscles.3 Both regenerative distance and time are important factors that limit reinnervation of human muscles.20 Even though muscle control is missing in our model, reinnervation of muscle after weeks, versus months, is critical to the success of any rehabilitation. With reinnervation, low intensity patterned electrical stimulation can be used to selectively activate different muscles to generate useful behaviors, such as pinch and key grips in people with high cervical SCI.87
Acknowledgments
The authors thank Drs. Michelle Rudinsky and Hilary Bennett for help with experiments. This research was funded by United States Public Health Service grant NS-39098, the State of Florida, a Lois Pope Summer Fellowship, and The Miami Project to Cure Paralysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bradley W.G. Good P. Rasool C.G. Adelman L.S. Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann. Neurol. 1983;14:267–277. doi: 10.1002/ana.410140304. [DOI] [PubMed] [Google Scholar]
- 2.Kim W.K. Liu X. Sandner J. Pasmantier M. Andrews J. Rowland L.P. Mitsumoto H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology. 2009;73:1686–1692. doi: 10.1212/WNL.0b013e3181c1dea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas C.K. Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve. 2006;33:21–41. doi: 10.1002/mus.20400. [DOI] [PubMed] [Google Scholar]
- 4.Tovar Y. Romo L.B. Santa–Cruz L.D. Tapia R. Experimental models for the study of neurodegeneration in amyotrophic lateral sclerosis. Mol. Neurodegener. 2009;20:31. doi: 10.1186/1750-1326-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Damme P. Robberecht W. Recent advances in motor neuron disease. Curr. Opin. Neurol. 2009;22:486–492. doi: 10.1097/WCO.0b013e32832ffbe3. [DOI] [PubMed] [Google Scholar]
- 6.Kühnlein P. Gdynia H.J. Sperfeld A.D. Lindner–Pfleghar B. Ludolph A.C. Prosiegel M. Riecker A. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat. Clin. Pract. Neurol. 2008;4:366–374. doi: 10.1038/ncpneuro0853. [DOI] [PubMed] [Google Scholar]
- 7.Rowland L.P. Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 8.Bunge R.P. Puckett W.R. Hiester E.D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 1997;72:305–315. [PubMed] [Google Scholar]
- 9.Carlstedt T. Root repair review: basic science background and clinical outcome. Restorative Neurol. Neurosci. 2008;26:225–241. [PubMed] [Google Scholar]
- 10.Collazos–Castro J.E. Soto V.M. Gutierrez–Davila M. Nieto–Sampedro M. Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J. Neurotrauma. 2005;22:544–558. doi: 10.1089/neu.2005.22.544. [DOI] [PubMed] [Google Scholar]
- 11.Dietz V. Curt A. Neurological aspects of spinal-cord repair: promises and challenges. Lancet Neurol. 2006;5:688–694. doi: 10.1016/S1474-4422(06)70522-1. [DOI] [PubMed] [Google Scholar]
- 12.Grumbles R.M. Brunschwig J.P. Thomas C.K. Motoneuron death after human spinal cord injury. J. Neurotrauma. 1999;16:997. doi: 10.1089/neu.2015.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milhorat T.H. Capocelli A.L., Jr. Anzil A.P. Kotzen R.M. Milhorat R.H. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J. Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 14.Thomas C.K. Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve. 1997;20:788–799. doi: 10.1002/(sici)1097-4598(199707)20:7<788::aid-mus2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Blesch A. Tuszynski M.H. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Gowing G. Svendsen C.N. Stem cell transplantation for motor neuron disease: current approaches and future perspectives. Neurotherapeutics. 2011;8:591–606. doi: 10.1007/s13311-011-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindvall O. Barker R.A. Brustle O. Isacson O. Svendsen C.N. Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell. 2012;10:151–155. doi: 10.1016/j.stem.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu P. Wang Y. Graham L. McHale K. Gao M. Wu D. Brock J. Blesch A. Rosenzweig E.S. Havton L.A. Zheng B. Conner J.M. Marsala M. Tuszynski M.H. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan C.H. Functional results of primary nerve repair. Hand Clin. 2000;16:67–72. [PubMed] [Google Scholar]
- 20.Hoke A. Brushart T. Introduction to special issue: challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2010;223:1–4. doi: 10.1016/j.expneurol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terzis J.K. Vekris M.D. Soucacos P.N. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast. Reconstr. Surg. 1999;104:1221–1240. doi: 10.1097/00006534-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Young J.Z. The functional repair of nervous tissue. Physiol. Rev. 1942;22:318–374. [Google Scholar]
- 23.Roytta M. Salonen V. Long-term endoneurial changes after nerve transection. Acta Neuropathol. 1988;76:35–45. doi: 10.1007/BF00687678. [DOI] [PubMed] [Google Scholar]
- 24.Li H. Terenghi G. Hall S.M. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997;20:333–347. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Richardson P.M. Peripheral nerve regeneration: an overview. In: Squire L.R., editor. Encyclopedia Neuroscience. Academic Press; New York: 2009. pp. 557–560. [Google Scholar]
- 26.Weinberg H.J. Spencer P.S. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978;7:555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- 27.Gutmann E. Zelena J. Morphological changes in the denervated muscle. In: Gutmann E., editor. The Denervated Muscle. Publishing House of the Czechoslovak Academy of Sciences; Prague: 1962. pp. 57–102. [Google Scholar]
- 28.Lu D.X. Huang S.K. Carlson B.M. Electron microscopic study of long-term denervated rat skeletal muscle. Anat. Rec. 1997;248:355–365. doi: 10.1002/(SICI)1097-0185(199707)248:3<355::AID-AR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Schmalbruch H. Al Amood W.S. Lewis D.M. Morphology of long-term denervated rat soleus muscle and the effect of chronic electrical stimulation. J. Physiol. 1991;441:233–241. doi: 10.1113/jphysiol.1991.sp018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kern H. Boncompagni S. Rossini K. Mayr W. Fano G. Zanin M.E. Podhorska–Okolow M. Protasi F. Carraro U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol. Exp. Neurol. 2004;63:919–931. doi: 10.1093/jnen/63.9.919. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer J.T. Motor prostheses. In: Brookhart J.M., editor; Brooks V.B., editor; Geiger S.R., editor; Mountcastle V.B., editor. Handbook of Physiology–A critical, comprehensive presentation of physiological knowledge and concepts. American Physiological Society; Bethesda, MD: 1981. pp. 155–187. [Google Scholar]
- 32.Erb D.E. Mora R.J. Bunge R.P. Reinnervation of adult rat gastrocnemius muscle by embryonic motoneurons transplanted into the axotomized tibial nerve. Exp. Neurol. 1993;124:372–376. doi: 10.1006/exnr.1993.1208. [DOI] [PubMed] [Google Scholar]
- 33.Thomas C.K. Erb D.E. Grumbles R.M. Bunge R.P. Embryonic cord transplants in peripheral nerve restore skeletal muscle function. J. Neurophysiol. 2000;84:591–595. doi: 10.1152/jn.2000.84.1.591. [DOI] [PubMed] [Google Scholar]
- 34.Thomas C.K. Sesodia S. Erb D.E. Grumbles R.M. Properties of medial gastrocnemius motor units and muscle fibers reinnervated by embryonic ventral spinal cord cells. Exp. Neurol. 2003;180:25–31. doi: 10.1016/s0014-4886(02)00024-9. [DOI] [PubMed] [Google Scholar]
- 35.Brundin P. Karlsson J. Emgard M. Schierle G.S. Hansson O. Petersen A. Castilho R.F. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- 36.Grumbles R.M. Casella G.T.B. Rudinsky M.J. Godfrey S. Wood P.M. Thomas C.K. The immunophilin ligand FK506, but not the P38 kinase inhibitor SB203580, improves function of adult rat muscle reinnervated from transplants of embryonic neurons. Neuroscience. 2005;130:619–630. doi: 10.1016/j.neuroscience.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Hu Z. Ulfendahl M. Olivius N.P. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Kakinohana O. Cizkova D. Tomori Z. Hedlund E. Marsala S. Isacson O. Marsala M. Region-specific cell grafting into cervical and lumbar spinal cord in rat: a qualitative and quantitative stereological study. Exp. Neurol. 2004;190:122–132. doi: 10.1016/j.expneurol.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Lowry N. Goderie S.K. Adamo M. Lederman P. Charniga C. Gill J. Silver J. Temple S. Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp. Neurol. 2008;209:510–522. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Webber D.J. Bradbury E.J. McMahon S.B. Minger S.L. Transplanted neural progenitor cells survive and differentiate but achieve limited functional recovery in the lesioned adult rat spinal cord. Regen. Med. 2007;2:929–945. doi: 10.2217/17460751.2.6.929. [DOI] [PubMed] [Google Scholar]
- 41.Casella G.T.B. Almeida V.W. Grumbles R.M. Yang L. Thomas C.K. Neurotrophic factors improve muscle reinnervation from embryonic neurons. Muscle Nerve. 2010;42:788–797. doi: 10.1002/mus.21757. [DOI] [PubMed] [Google Scholar]
- 42.Grumbles R.M. Sesodia S. Wood P.M. Thomas C.K. Neurotrophic factors improve motoneuron survival and function of muscle reinnervated by embryonic neurons. J. Neuropathol. Exp. Neurol. 2009;68:736–746. doi: 10.1097/NEN.0b013e3181a9360f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg J.L. Espinosa J.S. Xu Y. Davidson N. Kovacs G.T. Barres B.A. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 44.Brunet N. Tarabal O. Portero–Otin M. Oppenheim R.W. Esquerda J.E. Caldero J. Survival and death of mature avian motoneurons in organotypic slice culture: trophic requirements for survival and different types of degeneration. J. Comp. Neurol. 2007;501:669–690. doi: 10.1002/cne.21157. [DOI] [PubMed] [Google Scholar]
- 45.Hegarty J.L. Kay A.R. Green S.H. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koike T. Martin D.P. Johnson E.M., Jr. Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:6421–6425. doi: 10.1073/pnas.86.16.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao Z. Bonni A. Xia F. Nadal–Vicens M. Greenberg M.E. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 48.Perez–Garcia M.J. Cena V. de Pablo Y. Llovera M. Comella J.X. Soler R.M. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol. Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- 49.Garyantes T.K. Regehr W.G. Electrical activity increases growth cone calcium but fails to inhibit neurite outgrowth from rat sympathetic neurons. J Neurosci. 1992;12:96–103. doi: 10.1523/JNEUROSCI.12-01-00096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson E.M., Jr. Koike T. Franklin J. A “calcium set-point hypothesis” of neuronal dependence on neurotrophic factor. Exp. Neurol. 1992;115:163–166. doi: 10.1016/0014-4886(92)90242-i. [DOI] [PubMed] [Google Scholar]
- 51.Kater S.B. Mills L.R. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ming G. Henley J. Tessier–Lavigne M. Song H. Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 53.Furber S. Oppenheim R.W. Prevette D. Naturally-occurring neuron death in the ciliary ganglion of the chick embryo following removal of preganglionic input: evidence for the role of afferents in ganglion cell survival. J Neurosci. 1987;7:1816–1832. doi: 10.1523/JNEUROSCI.07-06-01816.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okado N. Oppenheim R.W. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J. Neurosci. 1984;4:1639–1652. doi: 10.1523/JNEUROSCI.04-06-01639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pineda R.H. Svoboda K.R. Wright M.A. Taylor A.D. Novak A.E. Gamse J.T. Eisen J.S. Ribera A.B. Knockdown of Nav1.6a Na+ channels affects zebrafish motoneuron development. Development. 2006;133:3827–3836. doi: 10.1242/dev.02559. [DOI] [PubMed] [Google Scholar]
- 56.Flavell S.W. Greenberg M.E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spitzer N.C. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 58.Al-Majed A.A. Neumann C.M. Brushart T.M. Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grumbles R.M. Wood P. Rudinsky M. Gomez A.M. Thomas C.K. Muscle reinnervation with delayed or immediate transplant of embryonic ventral spinal cord cells into adult rat peripheral nerve. Cell Transplant. 2002;11:241–250. [PubMed] [Google Scholar]
- 60.Emgard M. Blomgren K. Brundin P. Characterisation of cell damage and death in embryonic mesencephalic tissue: a study on ultrastructure, vital stains and protease activity. Neuroscience. 2002;115:1177–1187. doi: 10.1016/s0306-4522(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 61.Mullen R.J. Buck C.R. Smith A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 62.Basgen J.M. Nicholas S.B. Mauer M. Rozen S. Nyengaard J.R. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp. Nephrol. 2006;103:e139–e148. doi: 10.1159/000092905. [DOI] [PubMed] [Google Scholar]
- 63.Grumbles R.M. Almeida V.W. Thomas C.K. Embryonic neurons transplanted into the tibial nerve reinnervate muscle and reduce atrophy but NCAM expression persists. Neurol. Res. 2008;30:183–189. doi: 10.1179/174313208X281073. [DOI] [PubMed] [Google Scholar]
- 64.Deshmukh M. Johnson E.M., Jr. Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 65.Festjens N. Vanden B.T. Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Perez–Garcia M.J. Gou–Fabregas M. de Pablo Y. Llovera M. Comella J.X. Soler R.M. Neuroprotection by neurotrophic factors and membrane depolarization is regulated by calmodulin kinase IV. J. Biol. Chem. 2008;283:4133–4144. doi: 10.1074/jbc.M705477200. [DOI] [PubMed] [Google Scholar]
- 67.Wiegert J.S. Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49:296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Fields R.D. Eshete F. Stevens B. Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca, cAMP-responsive element binding proteins, and mitogen–activated protein kinase signaling. J. Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emgard M. Hallin U. Karlsson J. Bahr B.A. Brundin P. Blomgren K. Both apoptosis and necrosis occur early after intracerebral grafting of ventral mesencephalic tissue: a role for protease activation. J. Neurochem. 2003;86:1223–1232. doi: 10.1046/j.1471-4159.2003.01931.x. [DOI] [PubMed] [Google Scholar]
- 70.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 71.Schlaepfer W.W. Bunge R.P. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 1973;59:456–470. doi: 10.1083/jcb.59.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie X.Y. Barrett J.N. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J. Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gitler D. Spira M.E. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron. 1998;20:1123–1135. doi: 10.1016/s0896-6273(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 74.Kamber D. Erez H. Spira M.E. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp. Neurol. 2009;219:112–125. doi: 10.1016/j.expneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Spira M.E. Oren R. Dormann A. Gitler D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 2003;457:293–312. doi: 10.1002/cne.10569. [DOI] [PubMed] [Google Scholar]
- 76.Reimer M.M. Kuscha V. Wyatt C. Sorensen I. Frank R.E. Knuwer M. Becker T. Becker C.G. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J. Neurosci. 2009;29:15,073–15,082. doi: 10.1523/JNEUROSCI.4748-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cosker K.E. Courchesne S.L. Segal R.A. Action in the axon: generation and transport of signaling endosomes. Curr. Opin. Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamburger V. Trophic interactions in neurogenesis: a personal historical account. Annu. Rev. Neurosci. 1980;3:269–278. doi: 10.1146/annurev.ne.03.030180.001413. [DOI] [PubMed] [Google Scholar]
- 79.Li X.M. Dong X.P. Luo S.W. Zhang B. Lee D.H. Ting A.K. Neiswender H. Kim C.H. Carpenter–Hyland E. Gao T.M. Xiong W.C. Mei L. Retrograde regulation of motoneuron differentiation by muscle beta–catenin. Nat. Neurosci. 2008;11:262–268. doi: 10.1038/nn2053. [DOI] [PubMed] [Google Scholar]
- 80.Pazyra–Murphy M.F. Hans A. Courchesne S.L. Karch C. Cosker K.E. Heerssen H.M. Watson F.L. Kim T. Greenberg M.E. Segal R.A. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. J Neurosci. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pratt K.G. Aizenman C.D. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J. Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puls I. Jonnakuty C. LaMonte B.H. Holzbaur E.L. Tokito M. Mann E. Floeter M.K. Bidus K. Drayna D. Oh S.J. Brown R.H., Jr. Ludlow C.L. Fischbeck K.H. Mutant dynactin in motor neuron disease. Nat. Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 83.Bernstein M. Lichtman J.W. Axonal atrophy: the retraction reaction. Curr. Opin. Neurobiol. 1999;9:364–370. doi: 10.1016/s0959-4388(99)80053-1. [DOI] [PubMed] [Google Scholar]
- 84.Shimada Y. Fischman D.A. Morphological and physiological evidence for the development of functional neuromuscular junctions in vitro. Dev. Biol. 1973;31:200–225. doi: 10.1016/0012-1606(73)90332-1. [DOI] [PubMed] [Google Scholar]
- 85.Yumoto N. Kim N. Burden S.J. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489:438–442. doi: 10.1038/nature11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grumbles R.M. Almeida V.W. Casella G.T.B. Wood P.M. Hemstapat K. Thomas C.K. Motoneuron replacement for reinnervation of skeletal muscle in adult rats. J Neuropathol. Exp. Neurol. 2012;71:921–930. doi: 10.1097/NEN.0b013e31826cf69a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mauritz K.H. Peckham H.P. Restoration of grasping functions in quadriplegic patients by Functional Electrical Stimulation (FES) Int. J. Rehabil. Res. 1987;10:57–61. [PubMed] [Google Scholar]