Abstract
Macromolecular crowding (MMC) has been shown to have a beneficial effect on production and maturation of extracellular matrices in a monolayer cell culture model. To explore its potential in tissue engineering, a mixture of Ficoll 70 and Ficoll 400 is used to examine its MMC effect on cartilaginous matrix production of monolayer cultured porcine chondrocytes as well as on in vitro engineered cartilage formation using porcine chondrocytes and polyglycolic acid-unwoven fibers. The results showed that the production of total collagens and glycosaminoglycans production was enhanced by MMC in monolayer cultured cells (two-dimensional model), but the matrix production and tissue formation were significantly inhibited in the in vitro engineered cartilage (three-dimensional model) by the macromolecules. Further mechanism study on this phenomenon will be important for MMC applications in regenerative medicine.
Introduction
Tissue engineering employs seed cells and scaffolds to generate live tissue based on the concept that extracellular matrices secreted by seed cells can be assembled and remodeled into tissue structure with the gradual degradation of scaffold materials.1,2 As reported by the early literatures, concept proof studies were exclusively performed with in vivo animal models due to the necessity of an in vivo environment for engineered tissue formation.3–5
In recent years, with the urge of developing engineered tissue products, an in vitro tissue-engineering approach has been developed to meet this unmet need, such as in vitro engineered skin,6,7 tendon,8,9 and blood vessel.10 Despite the reported success, it is obviously noted that in vitro engineered tissue could never be as mature as in vivo engineered tissue, and the maturation of engineered tissue has to be carried out in vivo, suggesting that an in vitro culture system lacks proper environmental factors that are needed for tissue maturation in vivo.
Recent studies revealed that macromolecular crowding (MMC) is one of the factors that constitute an in vivo niche, but has been neglected in an in vitro culture system. In 2007, Lareu et al. reported an interesting phenomenon that adding the macromolecules of negatively charged dextran sulfate to a culture medium could enhance collagen matrix deposition and crosslinking via an artificially created crowding effect, suggesting its potential application in tissue engineering.11,12
Cartilage is characterized by its dense and abundant extracellular matrices, and MMC might be important for engineered cartilage formation and maturation, as significant difference in tissue structure and matrix deposition was observed between in vitro and in vivo engineered cartilage.13 Employing previously used models, the MMC effect was investigated in both a monolayer cell culture model (two-dimensional [2D]) and an in vitro cartilage-engineering model (three-dimensional [3D]).
Materials and Methods
Experimental animals
Newborn hybrid pigs were purchased from the Shanghai Chuansha Experimental Animal Raising Farm (Shanghai, China). All the experimental protocols were approved by the Animal Care and Experiment Committee of the Shanghai Jiao Tong University School of Medicine.
Cell isolation and culture
Chondrocytes were isolated and cultured as previously described.14 Briefly, fresh articular cartilage was harvested from newborn hybrid pig knee joints of hind legs immediately after euthanasia. Cartilage was cut from the femoral condyles and minced into small fragments (1 mm3) using a razor blade, followed by enzyme digestion with 0.25% trypsin plus 0.02% EDTA (Sigma) in phosphate-buffered saline (PBS) at 37°C for 30 min. Then, the fragments were further digested with 0.1% collagenase II (SERVA, GER) in the serum-free Dulbecco's modified Eagle's medium (DMEM; Hyclone) at 37°C for 12–16 h. The cell suspension was harvested and filtered through a 70-μm cell strainer (BD Falcon) to remove tissue residues. The filtrate was centrifuged, and cell pellets were resuspended in the DMEM plus 10% fetal bovine serum (FBS; Hyclone). Chondrocytes were counted and seeded onto 10-cm culture dishes at a cell density of 2×104/cm and cultured in the DMEM plus 10% FBS. Passage 1 cells were used for experiments.
Immunofluorescent staining
For a 2D model study, chondrocytes were cultured on cover slides in a regular culture medium first. On the following day, the regular medium was replaced with the culture medium with or without macromolecules. The employed macromolecules were a mixture of Fc 70 and Fc 400 at the concentration of 1×(37.5 mg/mL Fc70 and 25 mg/mL Fc 400), 2×(75 mg/mL Fc70 and 50 mg/mL Fc 400), and 4×(150 mg/mL Fc70 and 100 mg/mL Fc 400). After 3 days, cultured chondrocytes were fixed with 4% paraformaldehyde in PBS (Sigma–Aldrich) followed by collagen II staining. Briefly, the cover slides were first blocked in PBS containing 0.5% bovine serum albumin (BSA) for 30 min and then incubated with a primary antibody at 37°C for 2 h. The primary antibody was mouse anti-human type II collagen monoclonal antibody (1:200 in PBS containing 1% BSA; Abcam). After two washes with PBS, the slides were incubated with a fluorescence-conjugated secondary antibody (goat anti-mouse IgG, 1:500 in PBS containing 1% BSA, Alexa Fluor 488; Invitrogen) in dark for 1 h at 37°C. After two washes, nuclei were counterstained with propidium iodide (PI, 1:1000 in PBS; Invitrogen), and the stained cells were observed under a fluorescent microscope (Nikon). The experiment was repeated with three batches of cells derived from three animals.
Cytotoxicity assay
For a 2D model study, passage-1 chondrocytes were seeded onto 96-well cell culture plates with 3000 cells per well in 200 μL culture medium. After adherence for 24 h, the regular culture medium was replaced with the culture medium containing negative-charged dextran (1×, 70 mg/mL, molecular weight 70 kDa; Sigma-Aldrich), neutral 70 kDa Ficoll™ (1×, 37.5 mg/mL, Fc; Sigma-Aldrich), neutral 400-kDa Ficoll (1×, 25 mg/mL, Fc; Sigma-Aldrich), or a mixture of neutral 70- and 400-kDa Ficoll at the concentration of 1×(37.5 mg/mL Fc70 and 25 mg/mL Fc 400), 2×(75 mg/mL Fc70 and 50 mg/mL Fc 400), and 4×(150 mg/mL Fc70 and 100 mg/mL Fc 400), respectively, as experimental groups, and with the regular culture medium as a control. The cells were cultured continuously until day 11 with a medium change every 3 days. The cell survival rate was analyzed with Cell Counting Kit-8 (CCK-8; Dojindo) as reported previously.15 Briefly, at days 1, 3, 5, 7, 9, and 11, the culture medium was first removed, and then CCK-8 was added in 20 μL per well and incubated for 3 h at 37°C in 5% CO2. The absorbance of the samples was measured with a microplate reader (Thermo Scientific Varioskan Flash) at 490-nm wavelength, and the value was normalized to a blank control. The assay was triplicated with three repeats.
Preparation of cell–scaffold constructs
For 3D model study, the scaffolds in a cylinder shape and composed with polyglycolic acid (PGA) fibers coated with 1.0% polylactic acid (PLA) were prepared in the same way as previously described.4 Briefly, 14 mg of PGA unwoven fibers (JuRui) was compressed into a cylinder shape 10 mm in diameter and 1.5 mm in thickness. A solution of 1.0% PLA (Sigma) in dichloromethane was evenly dripped onto the PGA scaffolds and then dried in room temperature. Chondrocytes were resuspended in a culture medium to a concentration of 60×106/mL, and an aliquot of 150 μL of cell suspension was added onto each scaffold. The cell–scaffold constructs were then incubated in an incubator at 37°C for 4 h to allow for complete adhesion of the cells to the scaffolds. Afterward, a regular culture medium was added to cover the cell–scaffold constructs. All the constructs were cultured under the same conditions. Totally, 4 samples were included in each tested group.
MMC effect on chondrocyte pellet formation
For a 3D model study of pellet culture, 0.5×106 cells were centrifuged at 1,080 rpm for 5 min to form a cell pellet in a 15-mL centrifuge tube. After 3 days, the regular culture medium was replaced with the culture medium containing Fc70+400 at the concentration of 1× as the experimental group, and with the regular culture medium as a control. The cell pellets were cultured continuously until week 4 with a medium change every 3 days. At 4 weeks, cell pellets of all groups were harvested and measured for their wet weight and tissue volume and analyzed with histochemical, histological, and immunohistochemical examinations as well. The nontreatment group served as the control. Totally, five samples were included in each tested group.
Experimental design
For a 3D model study, cell–scaffold constructs were cultured for total 4 weeks before histological and quantitative evaluation. To examine the effect of MMC as well as the effect of drug concentration and treatment times on cartilage formation, the drugs were added, respectively, at day 2, 1 week, and 2 weeks post-cell seeding with respective concentrations of 1× or 2× or 4×, and the constructs were cultured till the end of 4 weeks. To further examine the MMC effect on long-term cultured cartilage, 1×Fc70+Fc400 was added at 4 weeks and 6 weeks post-cell seeding, respectively, and engineered tissues were harvested at 8 weeks for the same analyses. As a control, the cell–scaffold constructs were cultured in a regular culture medium without drug treatment. Totally, four samples were included in each tested group.
Quantitative evaluation of in vitro engineered cartilage
At 4 weeks or 8 weeks post-cell seeding, tissues of all groups were harvested and measured for their wet weight and tissue volume, and were quantitatively analyzed for total collagen and glycosaminoglycan (GAG) contents as previously described.13 Total four samples were included in each tested group.
Total GAG quantification assay
For both 2D and 3D model studies, the total GAGs derived either form cultured chondrocytes or from in vitro engineered cartilage were precipitated by a guanidinium chloride (Gncl) solution as previously described.13 After dissolving the GAG precipitate, the optical density (OD) was measured at 600 nm. A standard curve was established using chondroitin-4-sulfate, and the total GAG amounts were determined from the OD value that correlated to the corresponding GAG amount in the standard curve. Totally, four samples were included in each tested group.
Total collagen quantification assay
Total collagen contents of either cultured chondrocytes (2D) or in vitro engineered cartilage (3D) were quantified by a hydroxyproline assay as previously described.13 Briefly, the same amount of cells or the same weight of tissues was subjected to alkaline hydrolysis in boiling water for 20 min. The pH value of hydrolyzed samples was adjusted to 6.0–6.8, and then the active carbon was added to absorb color. After oxidation for 25 min at room temperature, the chromophore was then developed with the addition of dimethylaminobenzaldehyde. Finally, the absorbance of the reddish purple liquid was measured at 550 nm using a spectrophotometer. Absorbance values were plotted against the concentration of standard hydroxyproline, and the presence of hydroxyproline content in cell or tissue extracts was determined from the standard curve. The hydroxyproline content was finally converted to total collagen content by multiplying 7.35. Totally, four samples were included in each tested group.
Histology, histochemistry, and immunohistochemistry
For a 3D model study, constructed tissues were subjected to histological, histochemical, and immunohistochemical examinations. All specimens were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and then sectioned into 5-μm sections. These sections were stained with hematoxylin and eosin (HE) to evaluate the structure and with safranin O to evaluate the structure and cartilage extracellular matrix deposition. The expression of type II collagen was detected by a mouse anti-human type II collagen monoclonal antibody (1:200 in PBS containing 1% BSA; Abcam), followed by the incubation with a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:200 in PBS containing 1% BSA; Santa Cruz), and color development with diaminobenzidine tetrahydrochloride (DAB; Santa Cruz) as previously described.16 Total four samples were included in each tested group.
Statistical analysis
All data were expressed as mean and standard deviation. One-way analysis of variance (ANOVA) was used to analyze the difference among different groups. When the ANOVA indicated significance in the difference among data, Newman–Keuls post hoc tests were used for multiple comparisons. A p-value of<0.05 was considered statistically significant.
Results
Effect of MMC on cultured chondrocytes and engineered cartilage formation
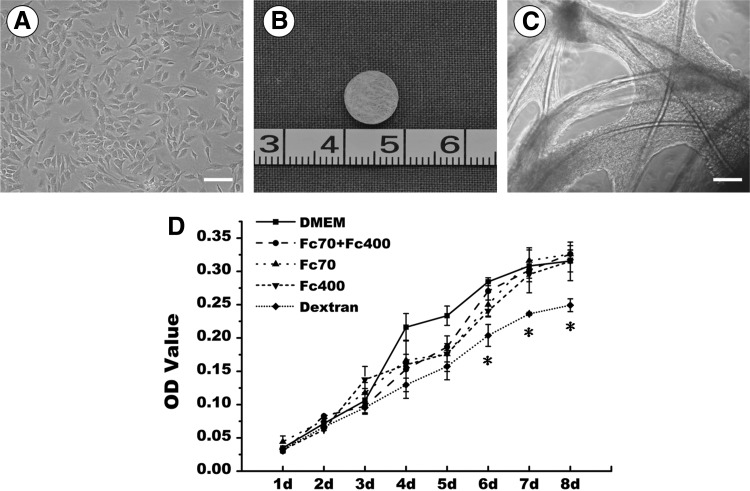
To identify which macromolecule could enhance engineered cartilage formation, Fc70 (37.5 mg/mL), Fc400 (25 mg/mL), Fc70 (37.5 mg/mL) +400 (25 mg/mL), and dextran (70 mg/mL) were tested in this study. As shown in Figure 1D, passage-1 chondrocytes grew and proliferated well in the regular medium. After being seeded onto the scaffolds and cultured in the DMEM, these chondrocytes adhered to and spread along PGA fibers within 48 h postseeding when observed with phase-contrast microscopy and produced a large quantity of extracellular matrices at 6 days of in vitro culture (Fig.1A–C), indicating the good function of cultured chondrocytes.
FIG. 1.
Preparation of cell–scaffold constructs and drug screen of monolayer cultured cells. (A) In vitro cultured porcine chondrocytes; (B) gross view of a polylactic acid-coated polyglycolic acid scaffold; (C) Phase-contrast microscopic view of a cell–scaffold construct in vitro cultured for 6 days; (D) growth curves of different groups of cells treated with different types of macromolecules. Bar=100 μm. The symbol *represents statistically significant difference from the control group (p<0.05). Means and standard deviation were derived from three independent experiments with three repeats/experiment. OD, optical density; DMEM, Dulbecco's modified Eagle's medium.
After 1 week of in vitro culture, all constructs were switched to the culture medium containing various macromolecules and cultured for another 3 weeks. Although gross view did not revealed significant difference in the size of constructed tissues, HE and safranin O staining demonstrated an obvious difference in the structure of formed tissues (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). Among all groups, the control group that received no drug treatment formed the best cartilage with a homogenous structure, whereas the drug-treated groups formed a more heterogeneous tissue structure with part of fibrotic tissue formation. Particularly, the dextran-treated group exhibited the worst tissue structure. Compared with others, the combination of Fc70 plus Fc400 formed a relatively better cartilage than the one treated with either Fc70 or Fc400 alone. We thus chose Fc70+400 as the drugs for the following tests.
Effects of Fc70+400 treatments on extracellular matrix production of cultured chondrocytes
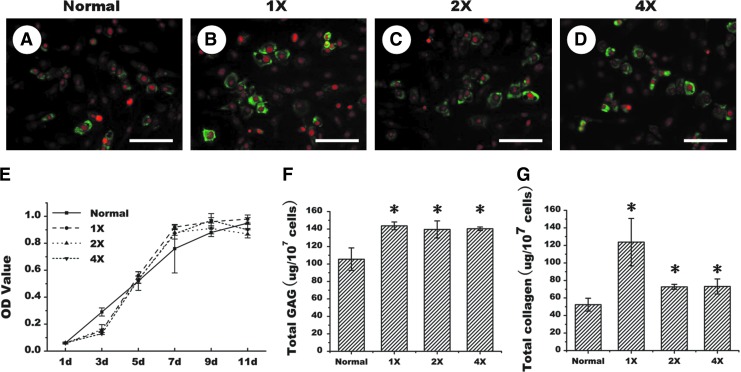
To determine an optimal concentration, various drug concentrations were tested first in a 2D cell culture model. As shown in Figure 2, immunofluorescent staining demonstrated that 1×concentration appears to promote more collagen II expression than the control cells that received no drug treatment, and other concentrations led to a similar level of collagen II expression (Fig. 2A–D). Notably, there was no significant difference in cell proliferation rate among control and various experimental groups (Fig. 2E). Additionally, quantitative analysis showed that GAG production was significantly higher in the drug-treated groups than in the untreated group (p<0.05), although no significant difference among the groups of different drug concentrations (Fig. 2F) (p>0.05). Furthermore, 1× concentration drug treatment could produce significantly more amounts of collagens than other groups (Fig. 2G) (p<0.05). All these results indicate that MMC could indeed achieve an excluded volume effect (EVE) to enhance matrix production and deposition in monolayer cultured chondrocytes.
FIG. 2.
Effect of Fc70+Fc 400 treatments on matrix production of monolayer cultured porcine chondrocytes. Immunofluorescent staining for collagen II production of the cells treated without (A) or with different concentration drugs (B–D). Growth curves (E), quantitative analyses of total glycosaminoglycan (GAG) content (F), and total collagen content (G) of the cells without or with drug treatments at various concentrations. 1×, 2×, and 4×, respectively, represent 1 time, 2 times, and 4 times of drug concentrations. d represents day. Bar=50 μm. The symbol * represents statistically significant difference from the control group (p<0.05, n=4). The red represents PI nuclear staining, whereas the green represents type II collagen staining. Color images available online at www.liebertpub.com/tec
Effect of initial drug treatment time on engineered cartilage formation in vitro
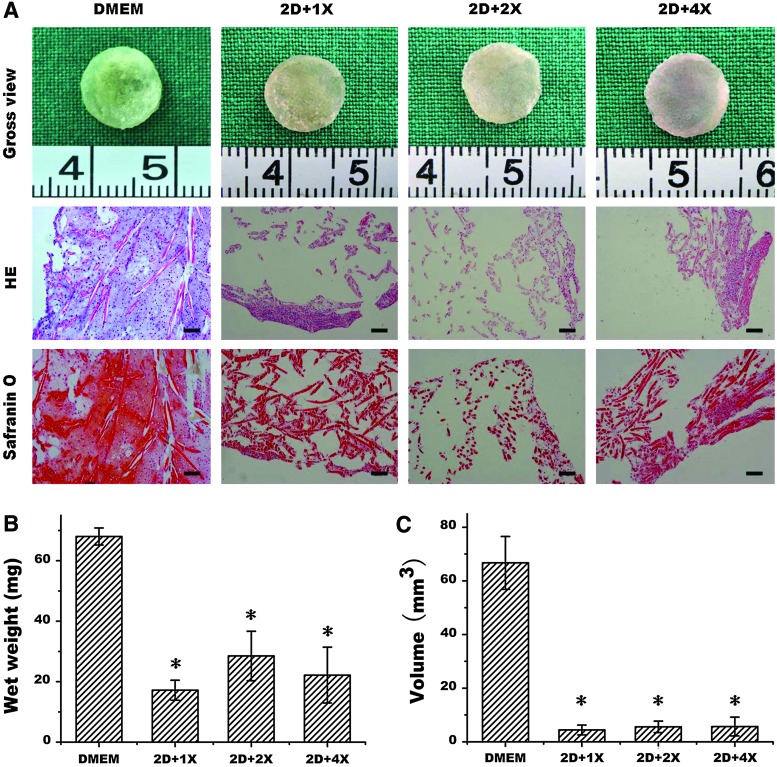
As MMC demonstrated the beneficial effect in a 2D model, but failed to enhance 3D cartilage formation, we further investigated whether initial drug treatment time and drug concentration would influence their effects. In this study, cell–scaffold constructs were switched to drug treatment 48 h post-cell seeding. At the end of 4 weeks, although gross view showed no significant difference in the construct shapes, HE examination showed that only the control group formed cartilage-like tissue, the other three groups with drug treatment only formed part of cartilage. This was also confirmed by safranin O staining, which showed that control groups produced more cartilage matrices than any other groups (Fig. 3A). Quantitative analysis also showed that the wet weight and tissue volume were significantly higher in the control group than in the drug-treated groups (Fig. 3B, C) (p<0.05). These results indicate that earlier treatment of cell–scaffold constructs with macromolecules could actually deteriorate the quality of engineered cartilage.
FIG. 3.
Effect of early drug treatment on in vitro engineered cartilage formation. Gross view, histology, and safranin O staining (A) of in vitro engineered cartilages without or with drug treatment at various drug concentrations at day-2 post-cell seeding and the quantitative analyses of wet weight (B) and tissue volume (C). 2D represents day 2, and 1×, 2×, and 4×, respectively, represent 1 time, 2 times, and 4 times of drug concentrations. Bar=100 μm. The symbol *represents statistically significant difference from the control group (p<0.05, n=4). Color images available online at www.liebertpub.com/tec
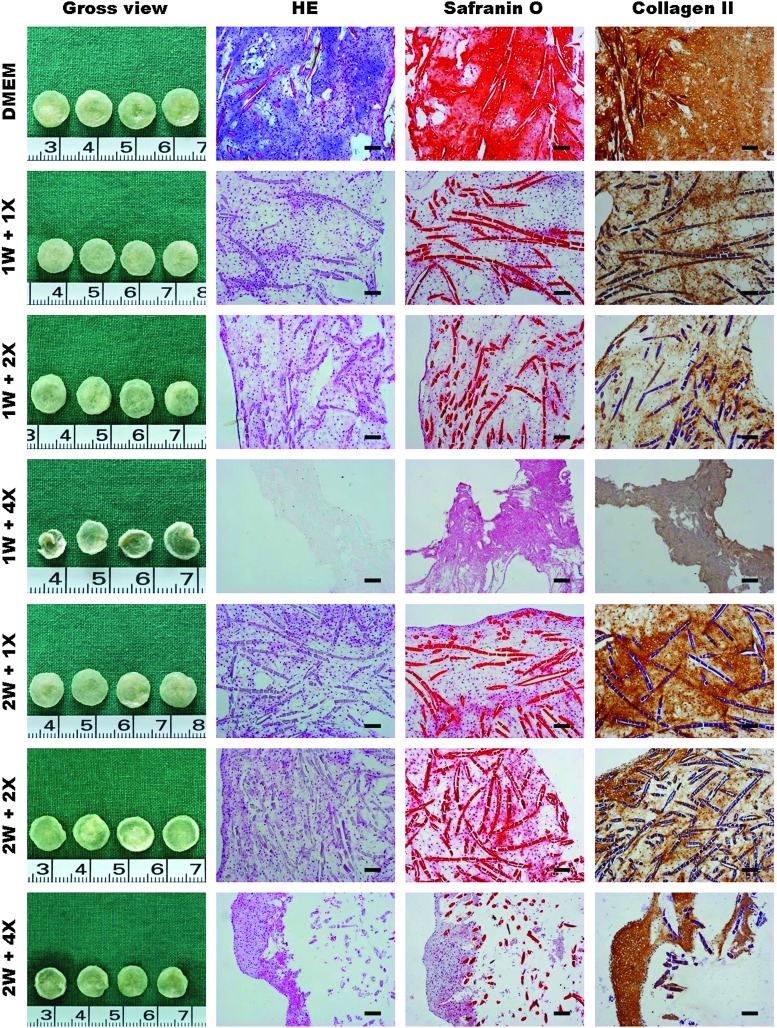
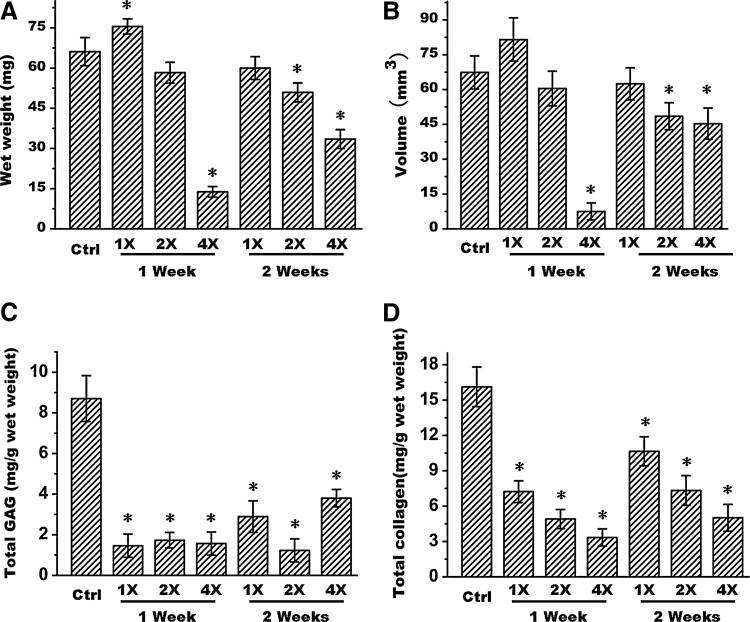
To further investigate the possible right time points, initial drug treatment time was also tested at 1 week and 2 weeks post-cell seeding. As shown in Figure 4, no matter the concentrations of 1×, 2×, or 4×, and no matter the initial drug treatment times at 1 week or 2 weeks post-cell seeding, all groups of drug treatment formed much poorer quality tissue compared to the control group, which formed a more mature and homogenous cartilage when these tissues were examined with HE staining. Safranin O and collagen II staining also demonstrated poor cartilage matrix deposition in drug-treated groups than in the nontreated group (Fig. 4). Additionally, quantitative analysis also showed much lower wet weight, tissue volume, GAG content, and total collagen content in drug-treated groups than in nontreated group (Fig. 5A–D) (p<0.05), except for the weight and volume of both 1×and 2×groups at 1 week and 1×group at 2 weeks. Particularly, macromolecules with a higher concentration (4×) could significantly inhibit engineered cartilage formation. Furthermore, drug treatment at later time points seemed to less affect tissue formation than early treatment.
FIG. 4.
Effect of late drug treatment on in vitro engineered cartilage formation. Gross view, histology, safranin O staining, and collagen II immunohistochemistry of the in vitro engineered cartilages without or with drug treatment at various drug concentrations started at either 1 week or 2 weeks post-cell seeding. 1 W and 2 W, respectively, represents post-cell seeding times at 1 week and 2 weeks, and 1×, 2×, and 4×, respectively, represent 1 time, 2 times, and 4 times of drug concentrations. Bar=100 μm. The experiment was repeated with 4 tissue samples. Color images available online at www.liebertpub.com/tec
FIG. 5.
Quantitative analyses of wet weight (A), tissue volume (B), total GAG content (C), and total collagen content (D) of in vitro engineered cartilages without or with drug treatment at various drug concentrations starting at either 1 week or 2 weeks post-cell seeding. 1×, 2×, and 4×, respectively, represent 1 time, 2 times, and 4 times of drug concentrations. The symbol *represents statistically significant difference from the control group (p<0.05, n=4).
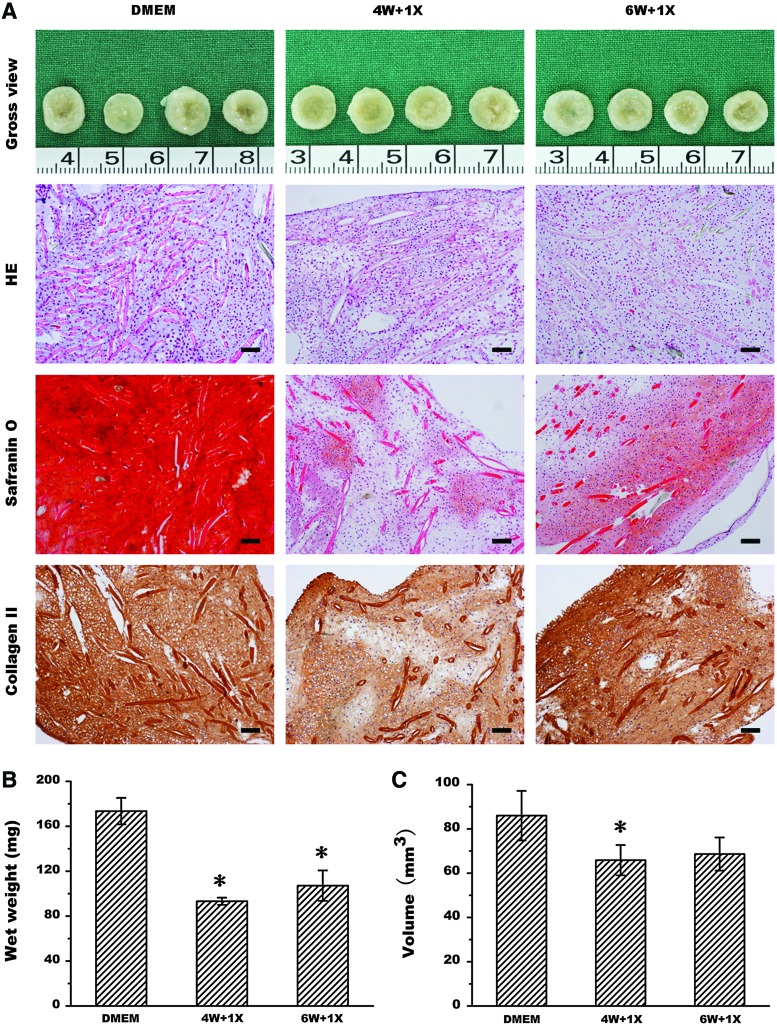
To further confirm this finding, the MMC effect on long-term cultured cartilage was also investigated. As shown in Figure 6A, even drug treatment was initiated at the late time points of 4 and 6 weeks post-cell seeding with 4 or 2 weeks of drug treatment, untreated chondrocytes formed much better cartilage tissue structure with more collagen II and GAG production than the cells received drug treatment. In addition, their wet weight (Fig. 6B) and tissue volume (Fig. 6C) were also significantly higher than those of drug-treated groups (p<0.05). Overall, these results further demonstrated that the MMC effect cannot enhance cartilage formation in an in vitro 3D model.
FIG. 6.
Effect of drug treatment on long-term cultured cartilage. Gross view, histology, safranin O staining, and collagen II immunohistochemistry (A) of the in vitro engineered cartilages without or with drug treatment at 1×concentration started at either 4 weeks or 6 weeks post-cell seeding as well as their quantitative analysis of wet weight (B) and tissue volume (C). 4 W and 6 W, respectively, represents post-cell-seeding times at 4 weeks and 6 weeks, and 1×represents 1 time of drug concentration. Bar=100 μm. The symbol * represents statistically significant difference from the control group (p<0.05, n=4). Color images available online at www.liebertpub.com/tec
Discussion
MMC has been recognized for its importance in mimicking the in vivo cell niche in which cells are bathed in an aqueous environment crowded with proteins.17 This is a truly important subject for biomedical research, because all living systems are highly crowded.18 For the interior part of a cell such as the cytoplasm, between 20% and 38% of cytoplasmic volume is attributed to large macromolecules such as proteins, carbohydrates, lipids, and nucleic acids that exist in a range of concentrations from 50 to 400 mg/mL.19–21 Protein concentrations in the extracellular space of mesenchymal stem cells range from 20.6 to 80 g/L. Enzymes and other proteins must perform their proper functions in such a highly crowded environment.17 It is generally recognized that the effects of MMC are due to the EVE, which usually leads to (1) the folding of biopolymers (e.g., proteins and nucleic acids) into native states to function optimally22; (2) the formation of stronger macromolecular transition complexes to prolong their half-lives (e.g., enzyme–substrate) for more products; (3) a buffering effect that balances adverse pH, temperature, or ionic strength of crowded environments to enhance biological functions.23
Despite the passage of more than 25 years since the initial report of EVE in cell culture by Bateman et al.,24 this concept of biomimesis has not yet been widely applied to biomedical researches. The current culture system employs supplementation with 5%–20% FBS, which results in solute concentrations of 4–16 mg/mL, much less than that of the in vivo microenvironment.17 Interestingly, our previous studies demonstrated that cartilage engineered in an in vivo crowded environment achieved robuster cartilaginous matrix deposition and maturation with stronger mechanical properties, when compared to the cartilage engineered in vitro in a noncrowded culture environment.13 We also observed a similar phenomenon in tendon engineering.9 These previous findings agree with the concept that matrix deposition, stabilization, assembly to super-molecular organization, and its remodeling must be properly managed in a crowded environment with relevant functional molecules.17
The importance of EVE in matrix production has been demonstrated in recent years, and MMC in the cell culture system has been artificially created by adding dextran or Ficoll, resulting in the beneficial effects of enhanced collagen deposition and crosslinking.11,12 These results were confirmed in later studies.25 However, all of these studies were based on a 2D cell culture system, and no results have been reported concerning the effects of EVE on tissue formation in a 3D model. Our group has recently developed techniques for in vitro engineering of various types of tissues, including tendon,8,9 cartilage,13 and blood vessel,10 and cartilage engineering was chosen as a preliminary model to evaluate the effects of EVE on tissue formation. However, the results of this study reject our hypothesis, as EVE did not enhance matrix deposition and maturation; rather, it caused deterioration in the tissue quality of in vitro engineered cartilage.
We first screened the candidate macromolecules in 1×concentration, which have been commonly employed.11,12 As shown in Figure 1D, compared to other agents, the negatively charged molecule dextran (70 kDa) inhibited chondrocyte proliferation in a monolayer culture with a significant difference (p<0.05). This was further confirmed in a 3D model, as cartilage engineered in the dextran group was relatively poor compared with other groups (Supplementary Fig. S1). In contrast, tissue constructs treated with Ficoll 70+Ficoll 400 exhibited a rate of cell proliferation that was similar to that of the control group (Fig. 1D) with a higher quality structure than the other treatment groups (Supplementary Fig. S1). This cocktail design also was supported by Chen et al., who showed that it could substantially enhance matrix deposition.26 Therefore, this cocktail protocol was employed for further tests of EVE with different concentrations and treatment times.
In a 2D model, treatment of chondrocytes with the Fc70+400 cocktail appeared to enhance collagen II expression when assayed with immunofluorescent staining (Fig. 2A–D), although quantitative analysis is required for confirmation. Importantly, quantitative analysis did show significantly increased production of total GAG and total collagen (p<0.05) in monolayer cell culture (Fig. 2F, G). However, investigation in a 3D model showed that MMC treatment, since day-2 post-cell seeding, actually led to deteriorated matrix deposition, which became worse with the increase of drug concentration as revealed by histology (HE) and cartilaginous matrix staining (safranin O) as well as the quantitative analysis of wet weight and tissue volume (Fig. 3). Thus, MMC may exert different biological effects in 2D and 3D models in an in vitro setting.
Temporal effects were also examined by administering the cocktail treatment with different concentrations and beginning at different time points. As shown in Figure 4, regardless of drug concentration or time of treatment initiation (starting at either 1 week or 2 weeks post-cell seeding), no beneficial EVE effect was observed when compared to nontreated controls in terms of tissue structure (HE), cartilaginous matrix deposition (safranin O), and collagen II expression (immunohistochemistry). It was also noted that the inhibitory effect became stronger with increased concentrations of macromolecules. Quantitative analysis also showed that wet weight, tissue volume, total GAG content, and total collagen were lower in the tissues engineered with macromolecules than in the tissues without treatment, further confirming that no beneficial EVE on tissue formation could be observed in this 3D in vitro tissue formation model.
This finding was further confirmed by the long-term cartilage culture study that showed no beneficial EVE could be gained even when the macromolecules were added to already prematured engineered cartilage (Fig. 6). To exclude the possible influence from scaffold materials such as PGA used in this study, we further employed chondrocyte pellet culture, a scaffold-free model commonly used for studies of chondrogenesis,27 to examine the effects of EVE on chondrogenesis. As shown in Supplementary Figure S2, treatment with macromolecules led to inferior chondrogenesis when compared to nontreated controls, with significant differences in the pellet size and volume (Supplementary Fig. S2). These results indicate that no beneficial EVE could be gained in both scaffold-based and scaffold-free models of chondrogenesis.
This investigation represents a pilot study to examine the potential effect of MMC on tissue formation in a scaffold-based 3D model. The results were in opposition to our scientific hypothesis based on the 2D monolayer cell culture model, and the mechanism remains unclear. The possible reasons could be (1) when cartilage tissue initially forms in a scaffold, the cells inside the tissue already reside in a crowded microenvironment consisting of their secreted extracellular matrices, so that adding more macromolecules into the medium may not necessarily result in further increased EVE within the forming tissue; (2) the culture medium with crowded macromolecules may inhibit to a certain extent the release of the metabolism waste into culture medium, thus inhibiting tissue metabolism; (3) more crowding in addition to the already packed microenvironment may activate matrix degradation enzymes, leading to a shift from prosynthesis in a 2D environment to prodegradation in a 3D environment.28–31
Although these hypotheses require further investigation, the success of enhanced matrix deposition and assembly in the 2D environment suggests that an enhanced medium exchange for the release of metabolized molecules might be an important consideration, since the cells were exposed in a much more open space in monolayer culture for nutrient and waste exchange.11,12,13 In contrast to the in vitro tissue formation system, the in vivo microenvironment has a better system for pH adjustment and waste release due to the presence of microvasculature, which may allow MMC to better exert a biological effect in an ideal environment.
Apparently, this technique has already revealed its potential in the areas of cell therapy and matrix production by employing a 2D culture system.17 It may have potential in tissue-engineering applications that particularly utilize cell sheeting-based tissue regeneration, which mainly employs a 2D system. For tissue-engineering applications in scaffold-based 3D models, future directions may include the use of perfusion bioreactors, specifically designed structures for enhanced material exchange, and optimized buffer systems.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation (30872694, 31170937, 81271725), National ‘‘863’’ Project Grant (2012AA020507). We also thank Dr. Kara Spiller for language editing.
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Chung C. Burdick J.A. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y. Vacanti J.P. Paige K.T. Upton J. Vacanti C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G. Liu W. Cui L. Wang X. Liu T. Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209. doi: 10.1089/ten.2006.12.3209. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y. Chen F. Liu W. Cui L. Shang Q. Xia W. Wang J. Cui Y. Yang G. Liu D. Wu J. Xu R. Buonocore S.D. Cao Y. Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng. 2002;8:709. doi: 10.1089/107632702760240616. [DOI] [PubMed] [Google Scholar]
- 6.Hu K. Shi H. Zhu J. Deng D. Zhou G. Zhang W. Cao Y. Liu W. Compressed collagen gel as the scaffold for skin engineering. Biomed Microdevices. 2010;12:627. doi: 10.1007/s10544-010-9415-4. [DOI] [PubMed] [Google Scholar]
- 7.Paquet C. Larouche D. Bisson F. Proulx S. Simard-Bisson C. Gaudreault M. Robitaille H. Carrier P. Martel I. Duranceau L. Auger F.A. Fradette J. Guérin S.L. Germain L. Tissue engineering of skin and cornea: development of new models for in vitro studies. Ann NY Acad Sci. 2010;1197:166. doi: 10.1111/j.1749-6632.2009.05373.x. [DOI] [PubMed] [Google Scholar]
- 8.Deng D. Liu W. Xu F. Yang Y. Zhou G. Zhang W.J. Cui L. Cao Y. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30:6724. doi: 10.1016/j.biomaterials.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 9.Wang B. Liu W. Zhang Y. Jiang Y. Zhang W.J. Zhou G. Cui L. Cao Y. Engineering of extensor tendon complex by an ex vivo approach. Biomaterials. 2008;29:2954. doi: 10.1016/j.biomaterials.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z.C. Zhang W.J. Li H. Cui L. Cen L. Zhou G.D. Liu W. Cao Y. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29:1464. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Lareu R.R. Arsianti I. Subramhanya H.K. Yanxian P. Raghunath M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 2007;13:385. doi: 10.1089/ten.2006.0224. [DOI] [PubMed] [Google Scholar]
- 12.Lareu R.R. Subramhanya K.H. Peng Y. Benny P. Chen C. Wang Z. Rajagopalan R. Raghunath M. Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: the biological relevance of the excluded volume effect. FEBS Lett. 2007;581:2709. doi: 10.1016/j.febslet.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Yan D. Zhou G. Zhou X. Liu W. Zhang W.J. Luo X. Zhang L. Jiang T. Cui L. Cao Y. The impact of low levels of collagen IX and pyridinoline on the mechanical properties of in vitro engineered cartilage. Biomaterials. 2009;30:814. doi: 10.1016/j.biomaterials.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Gong Y.Y. Xue J.X. Zhang W.J. Zhou G.D. Liu W. Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 15.Huang J. Zheng D.L. Qin F.S. Cheng N. Chen H. Wan B.B. Wang Y.P. Xiao H.S. Han Z.G. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120:223. doi: 10.1172/JCI38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X. Sun H. Yan D. Zhang L. Lv X. Liu T. Zhang W. Liu W. Cao Y. Zhou G. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Chen C. Loe F. Blocki A. Peng Y. Raghunath M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev. 2011;63:277. doi: 10.1016/j.addr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fulton A.B. How crowded is the cytoplasm? Cell. 1982;30:345. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- 19.Ellis R.J. Minton A.P. Cell biology: join the crowd. Nature. 2003;425:27. doi: 10.1038/425027a. [DOI] [PubMed] [Google Scholar]
- 20.Ebel C. Zaccai G. Crowding in extremophiles: linkage between solvation and weak protein-protein interactions, stability and dynamics, provides insight into molecular adaptation. J Mol Recognit. 2004;17:382. doi: 10.1002/jmr.697. [DOI] [PubMed] [Google Scholar]
- 21.Minton A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276:10577. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 22.Cheung M.S. Klimov D. Thirumalai D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci USA. 2005;102:4753. doi: 10.1073/pnas.0409630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goobes R. Kahana N. Cohen O. Minsky A. Metabolic buffering exerted by macromolecular crowding on DNA-DNA interactions: origin and physiological significance. Biochem. 2003;42:2431. doi: 10.1021/bi026775x. [DOI] [PubMed] [Google Scholar]
- 24.Bateman J.F. Cole W.G. Pillow J.J. Ramshaw J.A. Induction of procollagen processing in fibroblast cultures by neutral polymers. J Biol Chem. 1986;261:4198. [PubMed] [Google Scholar]
- 25.Li D. Dai K. Tang T. Effects of dextran on proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2008;10:587. doi: 10.1080/14653240802238330. [DOI] [PubMed] [Google Scholar]
- 26.Chen C.Z. Peng Y.X. Wang Z.B. Fish P.V. Kaar J.L. Koepsel R.R. Russell A.J. Lareu R.R. Raghunath M. The Scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br J Pharmacol. 2009;158:1196. doi: 10.1111/j.1476-5381.2009.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estes B.T. Diekman B.O. Gimble J.M. Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastor I. Vilaseca E. Madurga S. Garces J.L. Cascante M. Mas F. Effect of crowding by dextrans on the hydrolysis of N-Succinyl-L-phenyl-Ala-p-nitroanilide catalyzed by alpha-chymotrypsin. J Phys Chem B. 2011;115:1115. doi: 10.1021/jp105296c. [DOI] [PubMed] [Google Scholar]
- 29.Garvican E.R. Vaughan-Thomas A. Redmond C. Gabriel N. Clegg P.D. MMP-mediated collagen breakdown induced by activated protein C in equine cartilage is reduced by corticosteroids. J Orthop Res. 2010;28:370. doi: 10.1002/jor.21001. [DOI] [PubMed] [Google Scholar]
- 30.Nagase H. Woessner J.F., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 31.Sternlicht M.D. Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.