Abstract
Cellular transplantation using neural stem cells and progenitors is a promising therapeutic strategy that has the potential to replace lost cells, modulate the injury environment, and create a permissive environment for the regeneration of injured host axons. Our research has focused on the use of human glial restricted progenitors (hGRP) and derived astrocytes. In the current study, we examined the morphological and phenotypic properties of hGRP prepared from the fetal central nervous system by clinically-approved protocols, compared with astrocytes derived from hGRP prepared by treatment with ciliary neurotrophic factor or bone morphogenetic protein 4. These differentiation protocols generated astrocytes that showed morphological differences and could be classified along an immature to mature spectrum, respectively. Despite these differences, the cells retained morphological and phenotypic plasticity upon a challenge with an alternate differentiation protocol. Importantly, when hGRP and derived astrocytes were transplanted acutely into a cervical dorsal column lesion, they survived and promoted regeneration of long ascending host sensory axons into the graft/lesion site, with no differences among the groups. Further, hGRP taken directly from frozen stocks behaved similarly and also supported regeneration of host axons into the lesion. Our results underscore the dynamic and permissive properties of human fetal astrocytes to promote axonal regeneration. They also suggest that a time-consuming process of pre-differentiation may not be necessary for therapeutic efficacy, and that the banking of large quantities of readily available hGRP can be an appropriate source of permissive cells for transplantation.
Key words: astrocyte transplantation, axon regeneration, glial restricted progenitors, human astrocytes, spinal cord injury
Introduction
Traumatic injury to the spinal cord results in the immediate death of a variety of central nervous system (CNS) cell subtypes with concomitant interruption of ascending and descending axonal tracts, disruption of the normal architecture of the cord, and a cascade of secondary events involving inflammation and post-traumatic damage triggered by the initial injury. Cellular transplantation is a promising therapeutic strategy for spinal cord injury (SCI) that has the potential to replace lost and damaged cells, prevent ongoing secondary injury, mitigate the inflammatory lesion microenvironment, and promote the regeneration of damaged axons with the goal of recreating structural and electro-physiologically–active synapses between regenerating axons and functional targets. Although many studies have focused on the therapeutic potential of genetically modified fibroblasts (which fail to replace lost astrocytes and myelinating oligodendrocytes, or neural stem cells), far fewer have addressed the phenotypic diversity or the therapeutic application of glial restricted progenitors (GRP), particularly those derived from human fetal tissue.
Following lineage restriction in the CNS, GRP—the earliest progenitor cell type with the potential to give rise exclusively to glial phenotypes—can be isolated via cell surface expression of the A2B5 marker.1–4 Initial studies using GRP isolated from E13.5 rat spinal cord demonstrated that these cells can give rise to astrocytes and oligodendrocytes in vitro3,5 and in vivo.6,7 Further, acute transplantation of rodent GRP into SCI models demonstrated their ability not only to survive and differentiate into astrocytes and oligodendrocytes but also their capability to provide significant neuroprotection, modify the local microenvironment, alter the glial scar and deposition of inhibitory molecules, and promote axonal growth,8 underscoring the therapeutic potential of these cells.
The initial isolation and characterization of GRP from human tissues has been performed only recently and has demonstrated the potential of these cells to give rise to astrocytes and oligodendrocytes in vitro,1,9–11 similarly to studies performed with rodent GRP. In addition, human GRP (hGRP) have been transplanted into the injured spinal cord and have demonstrated cell survival and differentiation into astrocytes and oligodendrocytes, as well as an ability to limit post-injury scar formation,12 promote motor and sensory recovery,13 limit hyperactive autonomic responses,12 and preserve electrophysiological function.14
Despite the potential benefits of rodent and human GRP described by numerous studies, disagreement remains regarding the therapeutic application of GRP. Davies and colleagues have reported that only specific subsets of rodent- and hGRP-derived astrocytes, generated through a process of pre-differentiation into astrocytes with bone morphogenetic protein 4 (BMP-4), are capable of eliciting neuroprotection, suppressing host scar formation, promoting regeneration of host axons, and facilitating significant functional recovery.15–17 In contrast, rodent or human GRP, or GRP pre-differentiated into an alternate astrocyte subpopulation with ciliary neurotrophic factor (CNTF), failed to achieve such therapeutic benefit and instead resulted in mechanical allodynia and thermal hyperalgesia.16,17 However, our recent studies have shown that despite the morphological and phenotypic differences between GRP and astrocytes derived from GRP by BMP-4 or CNTF differentiation, they all have permissive properties, highlighting the general therapeutic potential of embryonic astrocytes for SCI.18,19 In our studies, GRP and GRP-derived astrocytes showed equivalent abilities to support axonal growth and regeneration, without neuropathic pain.12,19
The various glial phenotypes resulting from the process of limited GRP pre-differentiation3,15–17,19 underscores the specific properties and functions that these cells serve in the developing and adult CNS—neuronal development, neuronal survival, axonal growth, synapse formation, blood–brain barrier maintenance, and metabolic and trophic support.10,20–23 Indeed, there is growing evidence for a diverse population of astrocytes,20,23–28 particularly in humans,29,30 capable of performing functions that are temporally, regionally, and contextually specific.10,23,24,31,32 The generation of different astrocyte subtypes, each possessing distinct properties that manifest during embryonic development and in the adult CNS, is determined by the interaction of the intrinsic expression program with external soluble or cell–cell signals.20,33 This interaction with the external microenvironment allows for a significant degree of morphological, phenotypic, physiological, and functional plasticity, particularly following injury.22,23,34 Similarly, limited pre-differentiation of GRP into immature, embryonic astrocytes still allows for significant morphological and phenotypic plasticity upon exposure to an alternate environment,19 suggesting that the therapeutic potential of naïve GRP and embryonic astrocytes following SCI stems from their relatively immature state,8,19 similar to the therapeutic potential demonstrated by grafts of immature astrocytes.35–40
In this study, we used hGRP and astrocytes derived from hGRP for a detailed morphological and phenotypic analysis. Our aim was to meticulously characterize the phenotypic diversity and examine the plasticity of hGRP prepared by a Good Manufacturing Practice (GMP) production standard for possible clinical application. In addition, hGRP and hGRP-derived astrocytes were grafted acutely into a dorsal column hemisection model of SCI to analyze their phenotypic fate and ability to support axonal growth and regeneration. We found that hGRP and hGRP-derived astrocytes showed excellent survival, expressed astrocyte markers, and supported axonal growth and regeneration into the graft/lesion site. Further, hGRP taken directly from frozen stocks and acutely grafted into the lesion demonstrated similar phenotypic and permissive properties, suggesting that a lengthy process of pre-differentiation may not be necessary for therapeutic application.
Methods
Ethics statement
All animal procedures and care were performed according to the guidelines established by the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee, an Association for Assessment and Accreditation of Laboratory Animal Care accredited site.
Preparation of hGRP
Stocks of hGRP cells (Batch AE2 080606, isolated June 6, 2008, 20-week-old fetal cadaveric brain tissue and Batch AE2 060530-01, isolated May 30, 2008, 21-week-old fetal cadaveric brain tissue) were obtained from Q-Therapeutics (Salt Lake City, Utah). Tissue was procured by specialists employed by Advanced Bioscience Resources, (Alameda, CA, FEIN 3005208435) following donor ID/informed consent standard operating procedures and donor medical record review procedures. All protocols and procedures for hGRP isolation were reviewed by the Western Institutional Review Board (IRB), which deemed that any further IRB oversight was unnecessary.9,11 Single-cell hGRP were purified by Miltenyi immunomagnetic columns as previously described.9,11 Positively-selected cells were cultured for 20 days (two passages) in DMEM/F12 supplemented with 0.01% human serum albumin, penicillin/streptomycin/Fungizone, 1×N2 supplement, 20 ng/mL basic fibroblast growth factor (bFGF), 10 ng/mL PDGF-AA, and 5 ng/mL NT-3. hGRP were harvested with TrypLE (Invitrogen, Grand Island, NY), cryopreserved in ProFreeze 15% DMSO, and stored in vapor phase liquid nitrogen.
Glial differentiation
For differentiation into astrocytes, hGRP (Batch AE2 080606) were plated on PolyL-Lysine (PLL, Sigma, St. Louis, MO) 100 ng/mL-coated coverslip at 8000 cell/cm2. After 24 h, Q-basal medium (DMEM-F12 [Invitrogen, BSA [1 mg/mL; Sigma-Aldrich, St. Louis, MO]/laminin, penicillin/streptomycin [50 IU/mL; Invitrogen], N2 [10 μL/mL; Invitrogen]) supplemented with 20 ng/mL bFGF, 5 ng/mL NT3, and 10 ng/mL PDGF-AA was removed and replaced by (i) Q-basal medium + 10% fetal bovine serum (FBS), (ii) Q-basal medium + 10 ng/mL BMP-4, (iii) Q-basal medium + 10 ng/mL CNTF, or (iv) Q-basal medium + 20 ng/mL bFGF + 5 ng/mL NT3 +10 ng/mL PDGF-AA (control). Cells were incubated for three, six, and 10 days, with the medium changed every other day. Phase contrast images to document cell morphology were taken on days 0, 3, 6, and 10. To evaluate the stability of astrocyte cultures, hGRP that had been differentiated for 10 days with BMP-4 or CNTF, were challenged for an additional four days with an alternative factor. Phase contrast images to document cell morphology were taken on day 14. For comparison and reproducibility between cell batches, hGRP (Batch AE2 080530-01) were differentiated using the same protocols as above.
For injection into SCI, hGRP were plated on PLL (15 μg/mL)/laminin (15 μg/mL)-coated 75cm2 flasks and differentiated for 10 days using the differentiation protocols described above. Phase contrast images to document cell morphology were taken on days 0, 3, 6, and 10. On the morning of transplantation, cells were dissociated from flasks with 0.05% Trypsin-EDTA (Cellgro, Mediatech, Manassas, VA), and cell viability was assessed using trypan-blue. Cells were suspended at 200,000 cells/μL in 50% PureColl/50% Q-basal medium (pH, 7.5) and were kept on ice until all surgeries were completed.
Alternatively, hGRP were prepared directly from frozen stocks. In this paradigm, hGRP were washed twice with 1×Hank's Buffered Salt Solution (HBSS) and suspended as above.
Immunofluorescent staining
At 0, 3, 6, 10, and 14 days hGRP (Batch AE2 080606) were fixed using 4% paraformaldehyde (Electron Microscopy Services, Hatfield, PA) and immunostained with markers for progenitor cells (A2B5, nestin), astrocytes (glial fibrillary acidic protein [GFAP]), and proliferating cells (Ki67); see Table 1 for antibody sources and dilutions. For comparison and reproducibility between cell batches, hGRP (Batch AE2 080530-01) were differentiated and stained as described above. Immunofluorescent staining was performed as previously described,19,41 followed by incubation with 4′ 6′ diamidino-2-phenyindole (Dapi; Sigma Aldrich, St. Louis, MO). Coverslips were mounted on glass slides using FluoroSave Reagent (Calbiochem, San Diego, CA). Staining with antibodies against A2B5 surface antigen, with appropriate secondary antibody was performed on live cells for 35 min each at 37°C, prior to fixation and incubation with other primary and secondary antibodies. Cell immunofluorescent staining was visualized using a Leica DM5500B fluorescent microscope (Leica Microsystems, Buffalo Grove, IL) with a Retiga-SRV camera (QImaging, Surrey, British Columbia). Selected images were captured using Slidebook Software, version 4.2 (Olympus, Center Valley, PA).
Table 1.
Primary and Secondary Antibodies Used in this Study
| Name | Type | Dilution | Source |
|---|---|---|---|
| A2B5 | Mouse IgM | 1:2 | Hybridoma |
| O4 | Mouse IgM | 1:4 | Dr. J Grinspan |
| Human Nuclear | Mouse IgG1 | 1:200 | Chemicon |
| Nestin, human | Mouse IgG | 1:1000 | Chemicon |
| Ki67 | Rabbit IgG | 1:1000 | ThermoFisher |
| Cholera Toxin B | Goat | 1:2000 | List Laboratories |
| GFAP | Mouse IgG1 | 1:1000 | Chemicon |
| Rabbit IgG | 1:2000 | Chemicon | |
| βIII tubulin | Rabbit IgG1 | 1:1000 | Covance |
| Phalloidin-RITC | 1:20 | Invitrogen | |
| goat α mouse-IgM RITC | 1:400 | Jackson | |
| goat α mouse-IgG RITC | 1:400 | Jackson | |
| goat α rabbit RITC | 1:400 | Jackson | |
| donkey α goat RITC | 1:400 | Jackson | |
| goat α rabbit FITC | 1:400 | Jackson | |
| goat α mouse-IgG FITC | 1:400 | Jackson | |
| donkey α rabbit FITC | 1:400 | Jackson |
IgM, immunoglobulin M; IgG, immunoglobulin G; GFAP, glial fibrillary acidic protein.
Cell counting
For quantification of in vitro staining, cells from at least five randomly selected fields were counted. A minimum of 250 cells per coverslip were counted for each condition with a total of three coverslips counted per condition. Each experiment was performed in triplicate on three separate occasions. Counting was performed manually using Slidebook software, ver.4.2 (Olympus, Center Valley, PA) by an observer blinded to experimental group. A2B5+, GFAP+, A2B5+GFAP+, nestin+, nestin+GFAP+, Ki67+, and Ki67+GFAP+ were presented as a percentage of total cells (DAPI+). In certain cases, double positive A2B5+GFAP+, nestin+GFAP+, or Ki67+GFAP+ cells were represented as a percentage of GFAP+ cells.
Surgery and axonal tracing
For the first set of experimental surgeries, eight adult female athymic rats (NTAC:NIH-RNU; Taconic, Cranbury, NJ) were divided into four groups (n=2/group); three groups received a different transplant of hGRP pre-differentiated with BMP-4, CNTF, or bFGF for 10 days and one group received only 50% PureColl/50% Q-basal medium as a control. Animals were anesthetized by intraperitoneal injection of an XAK cocktail: 10 mg/kg xylazine, 0.7mg/kg acepromazine maleate, and 95 mg/kg ketamine (Webster Veterinary, Devens, MA). Surgery was performed as previously described.19 Briefly, a single incision was made through the skin and muscle layers and the layers were retracted to allow access to the dorsal lamina. A laminectomy was performed at the C4-C5 vertebral level to expose the dura. The dura was incised along the rostral-caudal axis above the dorsal columns to expose the spinal cord. A 30-gauge needle was used to make a unilateral 1-mm incision into the right dorsal column to sever the tracts; a small segment of the right dorsal column at the site of the incision was excised to create a small, yet discrete cavity. Two sutures (9-0) were placed in the dura at the rostral and caudal extents of the incision but were not pulled taut. After the sutures were placed, 3 μL of cell suspension was injected directly into the cavity. Dura sutures were rapidly tightened to prevent leakage of grafted cells. Biobrane (Bertex Pharmaceutical Inc., Morgantown, WV) was placed over the exposed dura and covered by excess fat from the nuchal fat pad to prevent adhesions between the muscle and the dura. The muscles were sutured (4-0) and the skin was stapled.
For the second surgical group, nine adult female Sprague Dawley rats (Taconic) were divided into two groups (hGRP transplant, n=6; 50% PureColl/50% Q-basal media, n=3,). In this case, the hGRP transplant group consisted of hGRP taken directly from frozen vials provided by Q-therapeutics. Briefly, hGRP were washed twice with HBSS prior to suspension in Q-basal media. Cell viability was assessed via trypan blue stain and in all cases viability upon thaw was 77.5% to 85.6%. Following determination of viability, cells were suspended at a concentration of 200,000 cells/μL in 50% Q-basal media/50% PureColl. Animals were anesthetized and underwent surgery as described above. Sprague Dawley animals were maintained on 10mg/kg cyclosporine A (Novartis, New York, NY) once daily via subcutaneous injection beginning three days before transplantation and continuing for the remainder of the experiment.
Five weeks after the surgery to generate dorsal column lesions was performed, animals were re-anesthetized with an XAK cocktail and an incision was made into the upper hindquarters to expose the sciatic nerve. A 10-μL Hamilton syringe was inserted into each sciatic nerve at the junction of the fibial and tibial branches and 3 μL of cholera toxin B subunit (CTB;10 μg/μL; List Biological Laboratories, Campbell, CA) was injected to label ascending sensory axonal projections. Animals were sacrificed three days after CTB injection.
Tissue preparation and histological analysis
Animals were sacrificed with an overdose of Euthasol (Virbac Animal Health, Fort Worth, TX) and perfused transcardially with 100 mL of ice-cold 0.9% saline, followed by 500 mL of ice-cold 4% paraformaldehyde in phosphate buffer (pH 7.4). Spinal cords were removed and post-fixed in 4% paraformaldehyde for an additional 48 h followed by cryoprotection in 30% sucrose/0.1M phosphate buffer at 4°C for at least one week. Following cryoprotection, spinal cords were embedded in Shandon M-1 Embedding Matrix (Fisher Thermo Scientific, Waltham, MA) and cut sagittally in 20-μM sections. Tissue was collected on glass slides coated with gelatin, dried overnight at room temperature, and stored at 4°C until use.
For immunohistological fluorescent staining, slide-mounted tissue sections were washed three times in 1×phosphate buffered saline (PBS). Following washing, sections were blocked for one hour with 0.2% Triton X-100/10% goat serum/PBS (with the exception of tissue to be used with CTB antibody; 10% donkey serum) at room temperature. Primary antibodies (Human Nuclear Antigen [HuNu], GFAP, nestin, Ki67, Tuj1, CTB; see Table 1 for antibody sources and dilutions) were incubated overnight at room temperature in 2% serum (goat or donkey)/PBS in a humidified chamber. Following primary antibody incubation, sections were washed three times with PBS to remove unbound antibody. Appropriate conjugated secondary antibodies were added for 2 h, washed three times with PBS, coated with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA), and overlaid with a glass coverslip. Slides were visualized using a Leica DM5500B fluorescent microscope (Leica Microsystems, Buffalo Grove, IL) with a Retiga-SRV camera (QImaging, British Columbia, Canada) and selected images were captured using Slidebook software (Olympus, Center Valley, PA).
In vivo analysis of regenerating CTB-labeled axons
To determine the length of regenerating axons, double-stained HuNu and CTB in vivo sections were analyzed. CTB+ axons were traced from the caudal edge of the HuNu+ graft using Neurolucida (MBF Bioscience, Williston, VT) and subsequently analyzed for length of regeneration using Neurolucida Explorer Software (MBF Bioscience). The numbers of regenerating axons were binned according to the following lengths from the caudal edge of the HuNu+ graft: 0 to 250 μM, 250 to 500 μM, 500 to 1000 μM, and greater than 1000 μM.
Statistical analysis
To determine the overall interactions between treatment condition and time, multivariate analysis was performed. To elucidate the significance of the specific interactions, data was analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni test between each time point and treatment condition. Data were presented as group mean + standard error (SE). Significance levels were set to 0.01 for all comparisons. Data were analyzed using one-way ANOVA followed by Bonferroni test. Data were presented as group mean + SE. Significance levels were set to 0.05 for all comparisons.
Results
All hGRP used in this study were derived from stocks of cells that were prepared by GMP production standards and have previously been characterized.9,11 The hGRP were differentiated into astrocytes by distinct protocols that were previously used to obtain astrocytes with different phenotypic properties including BMP-41,10,15–17,19,42 and CNTF.16,17,19,42 Cultures grown in the presence of bFGF were used to maintain the undifferentiated, immature state of glial progenitors.1,10,11,19 Treatment with FBS3,19 was used as a morphological reference to generate a near homogenous population of fibroblast-like flat cells, compared with the two differentiation protocols. The cultures were evaluated for morphological changes and for the expression of phenotypic markers at different time points post-differentiation.
Morphological properties of hGRP and derived astrocytes
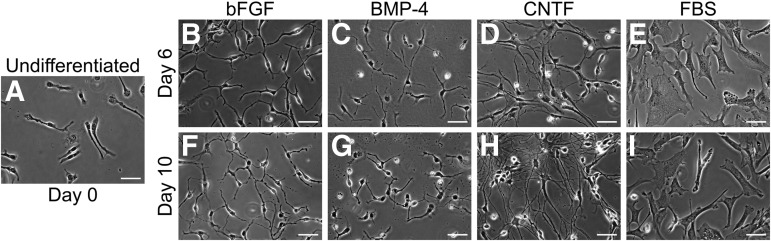
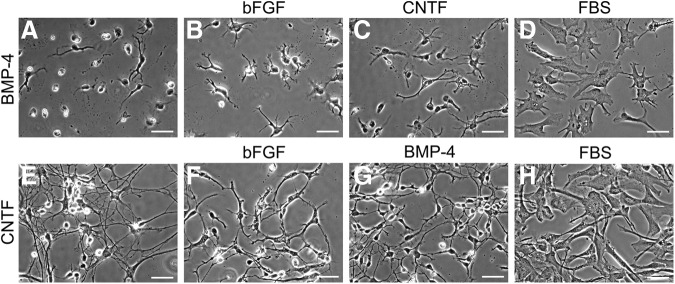
The results of the differentiation process are shown as phase contrast images taken at day 0, 6, and 10 (Fig. 1). At day 0, the majority of hGRP consisted of small unipolar or bipolar cells with short processes and small nuclei. By 6 and 10 days of differentiation, the cells exhibited striking changes in morphology that were distinct for the individual treatments. Control cultures maintained in the original growth media showed increased number of processes, becoming mostly bipolar or tripolar, yet appeared morphologically immature, with an unbranched, short-process phenotype. In contrast, differentiation of hGRP in the presence of BMP-4 or CNTF produced predominantly process-bearing cells that were distinct in terms of length and branching. BMP-4–treated cultures had short and branched processes, whereas CNTF treatment had significantly longer processes and highly refractive cell bodies. The short processes observed in BMP-4–treated hGRP rarely overlapped, while the elongate processes in CNTF-treated cultures often overlapped and created a dense network. In contrast, FBS-treated cultures had a unique flat fibroblast-like morphology with enlarged nuclei, similar to previous results19 but often included a single, short process. Similar results also were observed when cells from a different batch of the hGRP bank (AE2 080530-01) were differentiated, demonstrating the reliability of hGRP production process and the reproducibility of the differentiation protocols.
FIG. 1.
Morphological analysis of glial restricted progenitors (GRP) differentiation profiles using phase contrast microscopy. Human GRP were differentiated for six or 10 days with bone morphogenetic protein 4 (BMP-4), ciliary neurotrophic factor (CNTF), or basic fibroblast growth factor (bFGF) and evaluated by phase contrast microscopy for morphological characteristics. Treatment with fetal bovine serum (FBS) was utilized as a morphological reference. Panels B–E (day 6) and F–I (day 10) show the morphological phenotypes relative to day 0 undifferentiated control cultures (Panel A). BMP-4 and CNTF resulted in the generation of heterogeneous, albeit morphologically distinct, process-bearing cells. bFGF, in contrast, maintained cells in their original GRP state. hGRP differentiated with FBS generated a near homogenous, flat, fibroblast-like morphology, and served as a morphological reference. Scale bar=25 μm.
To confirm the analysis obtained by phase contrast microscopy and to highlight the morphologies of the hGRP and derived astrocytes, the actin cytoskeleton was stained with phalloidin (Fig. S1; see online supplementary material at http://www.liebertpub.com). The analysis confirmed that in BMP-4– or CNTF-treated cultures, the majority of cells had processes with actin staining surrounding the nuclei and present in the major processes. The staining also revealed a number of smaller, flat, fibroblast-like cells. Control hGRP, maintained in the original growth media, showed short processes, similar to the morphology visualized by phase microscopy. In contrast, hGRP treated with FBS contained flat cells with distinct stress fibers and lamellipodia.
Phenotypic analysis of hGRP and hGRP-derived astrocytes
A2B5 and GFAP
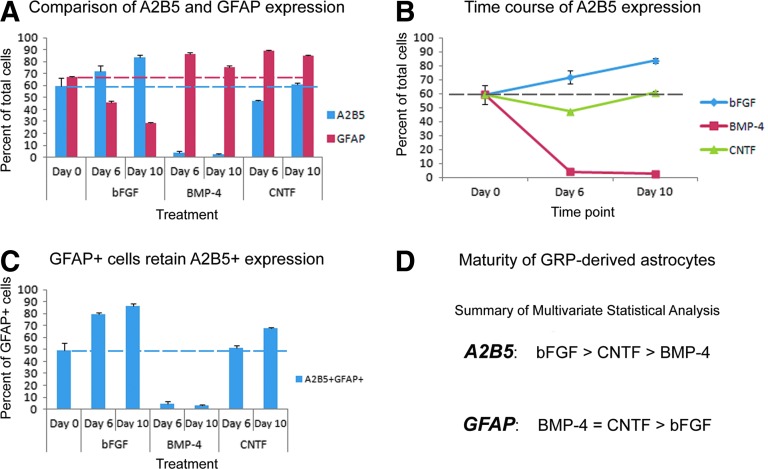
To begin the phenotypic characterization of hGRP and hGRP-derived astrocytes, a detailed time course phenotypic analysis was carried out with antibodies against A2B5, a marker of immature GRP, and GFAP, a marker of astrocytes as previously described1,9,11,17 (see Fig. 2 and Fig. 3). Analysis of undifferentiated control cells at day 0 (the starting conditions for all cultures) revealed that 59.2% of the cells were A2B5+ and 61.48% were GFAP+ (Fig. 3A), similarly to previous studies that characterized these cells.1,9,11 To evaluate the relative maturity of GFAP+ cells, the percentage of GFAP+ cells that retained the immature A2B5 marker was assessed. The results demonstrated that about half (49.5%) of the GFAP+ cells also were labeled with A2B5, underscoring the immature nature of these cells (Fig. 3C). After six days in differentiation medium, the percentage of A2B5+ cells dramatically decreased in BMP-4–treated cultures (4.1%), slightly decreased in CNTF-treated cultures (47.3%), and increased in bFGF-treated control cultures (71.7%). Consistent with this maturation profile, BMP-4– and CNTF-treated cultures displayed a significant increase in GFAP+ cells (86.6% and 89.1%, respectively), whereas control cultures maintained in bFGF had slightly decreased numbers of GFAP+ cells (46.0%). Analysis of BMP-4–treated cultures revealed that only 4.5% of the GFAP+cells were co-labeled with the A2B5 marker, demonstrating the maturity of the astrocyte population. In contrast, bFGF- and CNTF-treated cultures had higher levels of GFAP+ that were also A2B5+ (79.7% and 51.5%, respectively), suggesting that at day 6 of treatment, a large proportion of cells that were labeled with GFAP retained immature properties.
FIG. 2.

Phenotypic analysis of glial restricted progenitors (GRP) differentiation profiles using A2B5 and glial fibrillary acidic protein (GFAP) markers. Human GRP were differentiated for six or 10 days with bone morphogenetic protein 4 (BMP-4), ciliary neurotrophic factor (CNTF), and basic fibroblast growth factor (bFGF) and double stained with A2B5 and GFAP antibodies for evaluation of immature and mature phenotypes, respectively. Panels B–D (day 6) and E–G (day10) show the staining patterns and indicate that BMP-4 treatments reduced the level of the immature A2B5 and increased the astrocytic GFAP markers. In contrast, both CNTF and bFGF maintained high levels of immature cells that resembled the original GRP state. Scale bar=50 μm.
FIG. 3.
Quantitative and statistical analysis of A2B5 and glial fibrillary acidic protein (GFAP). The percentages of A2B5- and GFAP-positive cells for days 6 and 10 were calculated relative to day 0 undifferentiated controls. The quantitative analysis in panel A shows the percentage of cells staining positively for A2B5 (blue) or GFAP (red) relative to total number of cells and indicates that bone morphogenetic protein 4 (BMP-4) treatment reduced the levels of the immature marker, A2B5, and increased levels of the mature astrocytic marker GFAP. In contrast, basic fibroblast growth factor (bFGF) mitogen maintained high levels of A2B5 and exhibited reduced GFAP staining, suggestive of an overall immature state. In contrast to these two polarities, ciliary neurotrophic factor- (CNTF) treated cultures displayed high levels of both A2B5 and GFAP markers, suggestive of an intermediate phenotype. Panel B depicts the changes in the level of A2B5 expression alone. Panel C depicts the percentage of GFAP+ cells that retain markers of immaturity in each treatment group. Importantly, a greater percentage of GFAP+ cells retained the A2B5 marker in CNTF- and bFGF-treated cultures, compared with BMP-4 cultures, highlighting BMP-4 treatment as a strong maturation factor. Error bars represent 1 standard error. Dashed lines have been added to provide reference to day 0 percentages. Panel D summarizes the multivariate statistical analysis showing the significant changes among the groups. Significance was set at p<0.01 for all comparisons. Color image is available online at www.liebertpub.com/neu
By 10 days of differentiation, A2B5+ cells continued to decrease in BMP-4–treated cultures (2.7%) but were maintained at the initial day 0 levels in CNTF-treated cultures (60.8%). In contrast, A2B5+ cell numbers continued to increase in bFGF-treated control cultures (bFGF, 83.6%), reflecting the high immaturity of the GRP population. At this time point, the levels of GFAP+ cells remained high in BMP-4– or CNTF-treated cultures (75.5% and 85.0%, respectively) but continued to decrease in bFGF-treated control cultures (47.0%). Similar trends in the percentages of GFAP+ cells that express the immature A2B5 marker also were observed at day 10. The percentage of GFAP+ cells that expressed A2B5 continued to decrease in BMP-4–treated cultures (3.3%), once again highlighting the relative maturity of the astrocyte population in BMP-4–treated cultures. In contrast, cells treated with bFGF or CNTF retained high levels of GFAP+ cells that retained the immature marker A2B5 relative to BMP-4–treated cultures (86.2% and 67.6%, respectively).
A summary of phenotypic quantification is shown in Figure 3A, depicting the differences in the profile of A2B5 and GFAP expression among the treatment at days 6 and 10. Statistical analysis showed that at day 6, bFGF-treated cultures exhibited significant differences to the other treatments in terms of A2B5 expression (BMP-4, p<0.001; CNTF, p<0.001). The levels of A2B5 were also significantly different between CNTF and BMP-4 (p<0.001). The levels of GFAP expression in bFGF exhibited significant differences relative to the other treatment groups (BMP-4, p<0.001; CNTF, p<0.001), but no statistical differences were observed between BMP-4 and CNTF (p=1.0). As the process of differentiation continued (day 10), statistical analysis of A2B5+ cells revealed that bFGF again exhibited significant differences compared to the other treatment groups (BMP-4, p<0.001; CNTF, p<0.001) and that A2B5 levels were also significantly different between CNTF and BMP-4 treatments (BMP-4, p<0.001). For GFAP, bFGF displayed significant differences, compared with all other groups (BMP-4, p<0.001; CNTF, p<0.001), whereas no statistical significance was observed between the BMP-4 and CNTF treatments (p=0.093). Multivariate statistical analysis (Fig. 3D) revealed that the changes in numbers of A2B5+ cells was statistically significant between each treatment group (bFGF to BMP-4, p<0.001; bFGF to CNTF, p<0.001; BMP-4 to CNTF, p<0.001) but for GFAP+ cells only bFGF displayed significant differences, compared with BMP-4 (p<0.001) or CNTF (p<0.001), whereas no differences were observed between BMP-4 and CNTF (p=0.583). Statistical analysis of the time course of differentiation from day 0 to day 10 within a single group (Fig. 3B) revealed that treatment with bFGF resulted in a gradual but significant increase in A2B5 expression between day 0 and day 6 cultures (p=0.002) and between day 6 and day 10 cultures (p=0.004) as well as a significant decrease in GFAP expression between the day 0 and day 6 (p<0.001), but not between day 6 and day 10 (p=0.389). Treatment with BMP-4 demonstrated an early, robust process of astrocyte differentiation that plateaued at day 6 levels in both A2B5 and GFAP expression, with statistically significant differences between the day 0 and day 6 (A2B5, p<0.001; GFAP, p<0.001), but not between the day 6 and day 10 time points (A2B5, p=1.0; GFAP, p=0.015). Treatment with CNTF did not show significant differences in A2B5 expression between day 0 and day 10 (p=1.0), but displayed significant increases in GFAP expression upon comparison of these two time points (p<0.001).
Taken together, these results allowed us to classify hGRP and hGRP-derived astrocytes along a differentiation continuum. BMP-4 treatment resulted in the most mature astrocytic profile, with extremely low levels of A2B5 and high levels of GFAP. Further, the GFAP+ cell population did not label with the immature marker A2B5, suggesting that there cells were nearly exclusively GFAP+. On the other end of the differentiation spectrum, bFGF-treated cultures had the most immature phenotype. They had the highest levels of A2B5 and the lowest levels of GFAP, with nearly all of GFAP+ cells retaining A2B5 expression. In all cases, bFGF-treated cultures retained both morphological and phenotypic properties of undifferentiated hGRP. Cultures differentiated with CNTF assumed an intermediate phenotype, characterized by intermediate levels of A2B5 and high levels of GFAP. However, most GFAP+ cells retained the A2B5 marker, suggesting that these cells had undergone a more limited process of differentiation. To confirm the phenotypic profiles of hGRP differentiation, a second preparation of hGRP, AE2 080530-01, was differentiated with the same treatments and analyzed by similar morphological and phenotypic criteria. The analysis was in agreement with the differentiation profile described for the initial hGRP group (data not shown). The analyses of both preparations show the consistency of the hGRP stocks prepared by GLP methodology and highlighted the findings that BMP-4 generated the most mature astrocytes, characterized by extremely low A2B5+ and high GFAP+ cell numbers, whereas treatment with CNTF generated a less mature phenotype, which was nevertheless distinguished from the immature GRP that were maintained by bFGF.
Nestin
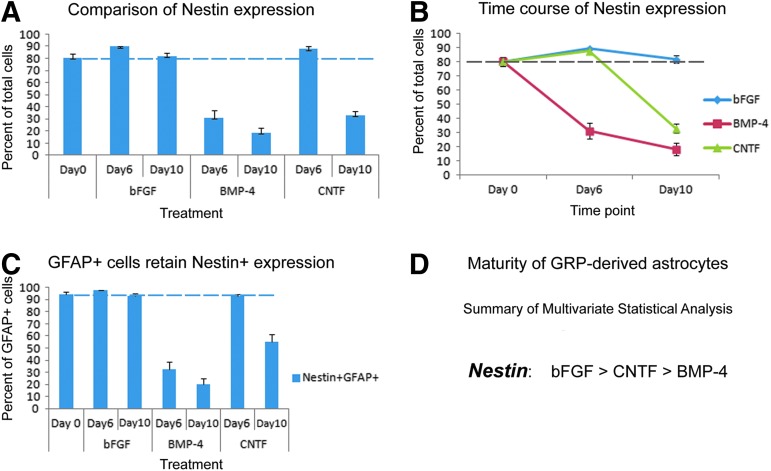
Nestin has regularly been used as a marker for immature cells in the nervous system3,4,41,43 labeling a variety of neural stem cells including embryonic GRP.19 We therefore used nestin expression to confirm the phenotypic analysis that was carried out with A2B5 using similar treatment protocols and time course (Fig. S2 [see online supplementary material at http://www.liebertpub.com] and Fig. 4). Quantitative analysis of undifferentiated control cultures at day 0 revealed that 80.4% of cells stained positively for nestin (Fig. 4A) with 94.5% of cells that expressed GFAP also labeled with nestin (Fig. 4C). After 6 days of differentiation, the percentage of nestin+ cells decreased dramatically in BMP-4–treated cultures (31.2%), but remained at high levels in bFGF- and CNTF-treated cultures (89.8% and 87.8%. respectively). In BMP-treated cultures, only a minority of GFAP+ cells were co-labeled with nestin (33.0%), whereas in CNTF- and bFGF-treated cultures the co-labeling was very high (96.7% and 92.9%, respectively). By 10 days of differentiation, nestin+ cells were further decreased in BMP-4–treated cultures (18.6%), with concomitant reductions in the proportion of GFAP+ cells that also labeled with nestin (20.2%). Taken together, the analysis of nestin expression again highlighted that BMP-4 treatment of hGRP promotes the most mature astrocytic phenotype. In contrast, nestin+cells remained nearly unchanged in cultures maintained with bFGF (82.0%), with the percentage of GFAP+ cells that retained the nestin marker also remaining high (94.86%). Interestingly, in cultures differentiated in the presence of CNTF, the percentage of GFAP+ cells that co-labeled with nestin decreased at this time point (55.4%), reflecting the gradual but limited differentiation process initiated by CNTF.
FIG. 4.
Quantitative and statistical analysis of human glial restricted progenitors (GRP) differentiation profiles using the neural progenitor marker, nestin. The percentages of nestin-positive cells for days 6 and 10 were calculated relative to day 0 undifferentiated controls. Quantitative analysis in panel A shows the percentages of cells staining positive for nestin relative to the total number of cells and indicates that bone morphogenetic protein 4 treatments reduced the levels of the progenitor marker nestin, whereas both ciliary neurotrophic factor and the basic fibroblast growth factor mitogen maintained high levels of progenitors that resembled the original, undifferentiated GRP state. Panel B represents the changes nestin expression in a time course dependent manner between the treatment groups. Panel C depicts the percentage of glial fibrillary acidic protein positive cells that retain markers of immaturity within each treatment group. The error bars represent 1 standard error. A dashed line has been added to provide reference to day 0 percentages. Panel D summarizes the statistical analysis showing the significant changes among the groups. Statistical significance was set at p<0.01 for all comparisons. Color image is available online at www.liebertpub.com/neu
A summary of the phenotypic analysis for nestin, shown in Figure 4A, indicated that at day 6, only BMP-4 exhibited significant differences relative to other culture conditions (CNTF, p<0.001; bFGF, p<0.001), with no significant differences observed between the bFGF and CNTF treatments (p=1.0). By day 10, BMP-4 again exhibited significant differences in nestin expression, compared with the other treatment groups (CNTF, p<0.001; bFGF, p<0.001) but there were also significant differences between CNTF and bFGF (p<0.001). A multivariate statistical analysis, shown in Figure 4D, indicated that the results for nestin expression are similar to A2B5, with statistically significant differences between all treatment groups (bFGF to BMP-4, p<0.001; bFGF to CNTF, p<0.001; BMP-4 to CNTF, p<0.001). Again, a second preparation of hGRP (AE2 080530-01) was differentiated along the same time course and stained with antibodies against nestin. The phenotypic analysis was in agreement with the differentiation profile described for the original hGRP group (AE2 080626). Taken together, both the A2B5 and nestin data support the classification of hGRP and astrocytes along a continuous spectrum of maturity ranging from immature GRP maintained with bFGF to the most mature phenotype of astrocytes generated by BMP-4.
In addition, the statistical differences in the expression of nestin during the time course of differentiation from day 0 to day 10 (Fig. 4B) within a single differentiation group also was analyzed to determine the progression of the differentiation process. Cultures differentiated with bFGF did not show significant differences between day 0 and day 10 cultures (p=1.0), suggesting that bFGF maintained hGRP in their immature state. BMP-4 treatment demonstrated an early, robust process of differentiation that continued through day 10, with statistically significant differences noted between the day 0 and day 6 time points (p<0.001), between the day 0 and day 10 time points (p<0.001), and between the day 6 and day 10 time points (p=0.01). CNTF exhibited a delayed process of differentiation with statistical differences noted between the day 0 and day 10 time point (p<0.001) and between the day 6 and day 10 time point (p>0.001), but not between early time points of the differentiation process, day 0 to day 6 (p=0.1).
To further explore the phenotypic properties of pre-differentiated human astrocytes and confirm their classification along the immature-mature spectrum, we performed plasticity experiments (Fig. 5), as described earlier. Quantitative analysis of BMP-4 pre-differentiated astrocytes after 14 days revealed that the percentage of nestin+ cells was low (14.7%; Fig. 6A). In contrast, BMP-4 pre-differentiated hGRP challenged with bFGF, an immature factor, displayed increases in the percentage of nestin+ cells (33.3%), which was statistically significant (p<0.001; Fig. S3 [see online supplementary material at http://www.liebertpub.com] and Fig. 6).
FIG. 5.
Morphological plasticity of differentiated glial restricted progenitors (GRP). Human GRP were differentiated for 10 days with bone morphogenetic protein 4 (BMP-4) or ciliary neurotrophic factor (CNTF), challenged for an additional four days with an alternate factor, and evaluated by phase contrast microscopy for morphological characteristics. Panels A–D (GRP pre-differentiated with BMP-4) and panels E–H (GRP pre-differentiated with CNTF), show the morphological phenotypes for each condition and indicate that BMP-4 and CNTF exhibited striking morphological plasticity when challenged with fetal bovine serum (FBS) and assume the fibroblast-like, flat-cell morphology typical of FBS treatment. These changes were less obvious when BMP-4 treated cells were challenged with CNTF and CNTF-treated cells are challenged with BMP-4. In contrast, GRP pre-differentiated with FBS displayed only minor morphological changes when switched to an alternate treatment. Scale bar=25 μm.
FIG. 6.
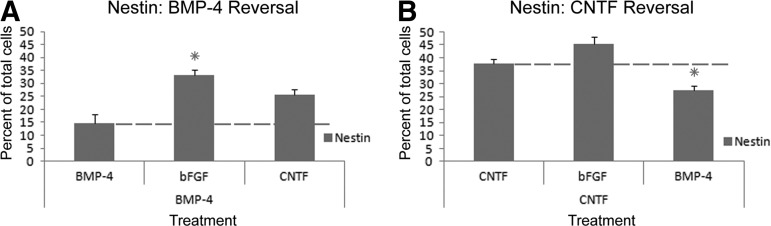
Quantitative and statistical analysis of phenotypic reversal: nestin. The percentage of nestin-positive cells at day 14 were calculated relative to cultures maintained in the original factor for 14 days. Panels A and B show the percentage of cells staining positive for nestin relative to the total number of cells. Panel A shows the quantitative analysis for glial restricted progenitors (GRP) differentiated with bone morphogenetic protein 4 (BMP-4) and indicates increased levels of the nestin marker on challenge with basic fibroblast growth factor. Panel B shows the quantitative analysis for GRP differentiated with ciliary neurotrophic factor and indicates reduced levels of the immature nestin marker on challenge with BMP-4. Error bars represent 1 standard error. Dashed lines have been added to provide reference to cultures of GRP differentiated for 14 days with an original treatment. Stars summarize the statistical analysis showing the significant changes among the groups compared to cultures grown for 14 days in the initial factor. Statistical significance was set at p<0.01 for all comparisons.
Ki67
To relate the differentiation profile of hGRP to the proliferation potential of the cells, we used the Ki67 antigen as previously described,19,42 showing and analyzing the profile in Figure S4 (see online supplementary material at http://www.liebertpub.com) and Figure 7, respectively. The analysis of undifferentiated control cultures at day 0 revealed that 25.6% of the cells were Ki67+, similar to previous studies.1,11 After six days of differentiation, Ki67+ cells dramatically decreased in BMP-4–treated cultures (8.7%). In contrast, cells cultured in the presence of either bFGF or CNTF displayed a modest increased in levels of Ki67+ (bFGF, 38.8%; CNTF, 32.3%). Statistical analysis revealed that BMP-4 levels were significantly different from other culture conditions (CNTF, p<0.001; bFGF, p<0.001), whereas no significant differences were observed between the bFGF and CNTF treatments (p=0.190). At 10 days of differentiation, the percentage of Ki67+ cells remained low in BMP-4–treated cultures (BMP-4, 13.7%), whereas CNTF-treated cultures exhibited Ki67+ cell numbers that returned to baseline, day 0 values (CNTF, 23.7%). In contrast, bFGF-treated cultures, retained elevated levels of Ki67+cells (bFGF, 34.2%), highlighting the proliferative state of the cells. Statistical analysis revealed that BMP-4 once again exhibited significant differences, compared with the other treatment groups (CNTF, p<0.001; bFGF, p<0.001) but that the percentage of Ki67+ cells were also significantly different between CNTF and bFGF (p<0.001).
FIG. 7.
Quantitative and statistical analysis of human glial restricted progenitors (GRP) differentiation profiles using the proliferation marker Ki67. The percentages of Ki67-positive cells for days 6 and 10 were calculated relative to day 0 undifferentiated controls. Quantitative analysis in panel A shows the percentages of cells staining positive for nestin relative to the total number of cells and indicates that bone morphogenetic protein 4 treatments reduced the levels of proliferating cells, whereas both CNTF and the basic fibroblast growth factor mitogen maintained higher levels of proliferation that resembled the original, undifferentiated GRP state. Panel B represents the changes Ki67 expression in a time course dependent manner between the treatment groups. The error bars represent 1 standard error. A dashed line has been added to provide reference to day 0 percentages. Panel C summarizes the statistical analysis showing the significant changes among the groups. Statistical significance was set at p<0.01 for all comparisons. Color image is available online at www.liebertpub.com/neu
A quantitative summary of the phenotypic analysis is shown, depicting the differences in Ki67 expression among the treatment profiles at days 6 and 10, relative to the undifferentiated control cultures (Fig. 7A). To determine the experimental differences in the percentages of Ki67+ cells among the treatment groups, multivariate statistical analysis was performed and is summarized in Figure 7C. The results mirrored the pattern observed for the A2B5 and nestin markers, with statistically significant difference in Ki67 expression between all treatment groups (bFGF to BMP-4, p<0.0001; bFGF to CNTF, p<0.001; BMP-4 to CNTF, p<0.001).
To confirm the classification paradigm, we once again performed plasticity experiments using the Ki67 antigen (Fig. S5 [see online supplementary material at http://www.liebertpub.com] and Fig. 8). After 14 days in BMP-4 differentiation medium, quantitative analysis revealed that the percentage of Ki67+ cells remained extremely low (8.5%). In contrast, cultures that had been pre-differentiated with BMP-4 for 10 days and subsequently challenged with bFGF for four additional days, in addition to displaying acute morphological plasticity, also exhibited striking alterations in phenotypic marker Ki67, with percentages rising to 28.6% (p<.001), reflecting the immature qualities of the bFGF differentiation program.
FIG. 8.
Quantitative and statistical analysis of phenotypic reversal: Ki67. The percentage of Ki67-positive cells at day 14 were calculated relative to cultures maintained in the original factor for 14 days. Panels A and B show the percentage of cells staining positive for Ki67 relative to the total number of cells. Panel A shows the quantitative analysis for glial restricted progenitors (GRP) differentiated with bone morphogenetic protein 4 (BMP-4) and indicates increased levels of the Ki67 marker on challenge with basic fibroblast growth factor (bFGF). Panel B shows the quantitative analysis for GRP differentiated with CNTF and indicates reduced levels of the Ki67 marker on challenge with BMP-4 and an increase upon challenge with bFGF. Error bars represent 1 standard error. Dashed lines have been added to provide reference to cultures of GRP differentiated for 14 days with an original treatment. Stars summarize the statistical analysis showing the significant changes among the groups compared to cultures grown for 14 days in the initial factor. Statistical significance was set at p<0.01 for all comparisons.
Taken together, the results observed from the quantitative and statistical analysis of the phenotypic plasticity experiments serve to reinforce the classification of hGRP and hGRP-, BMP-4–, or CNTF-derived astrocytes along the immature to mature spectrum, with bFGF or BMP-4, assuming the most immature and mature phenotypes, respectively, and CNTF assuming an intermediate phenotype. In these plasticity experiments, similarly to results from the initial phenotypic analysis, BMP-4 assumed a mature phenotype characterized by low levels of nestin and Ki67. Significant changes in these markers were not observed with CNTF, a factor that causes a limited differentiation of hGRP, reflecting the inability of an intermediate factor to alter the phenotypic repertoire of very mature astrocytic profile. In contrast, bFGF, which maintains GRP in their most immature state, significantly altered the levels of both nestin and Ki67 markers, reflecting the immature developmental program of this factor.
O4
To analyze the number of oligodendrocytes, the O4 antibody was used, as previously described.3,5,19,42,43 Analysis of undifferentiated control cultures at the day 0 time point failed to reveal any cells staining positively for O4. After 10 days in differentiation medium, a few O4+ oligodendrocytes were noted in bFGF-treated cultures, whereas no O4+ cells were observed at any time in cultures differentiated in BMP-4. Similar to cultures of rodent GRP,19 hGRP cultures treated with CNTF demonstrated an increase in O4 staining, labeling fine, lace-like processes (Fig. S6 [see online supplementary material at http://www.liebertpub.com]).
Transplantation of hGRP and hGRP-derived astrocytes
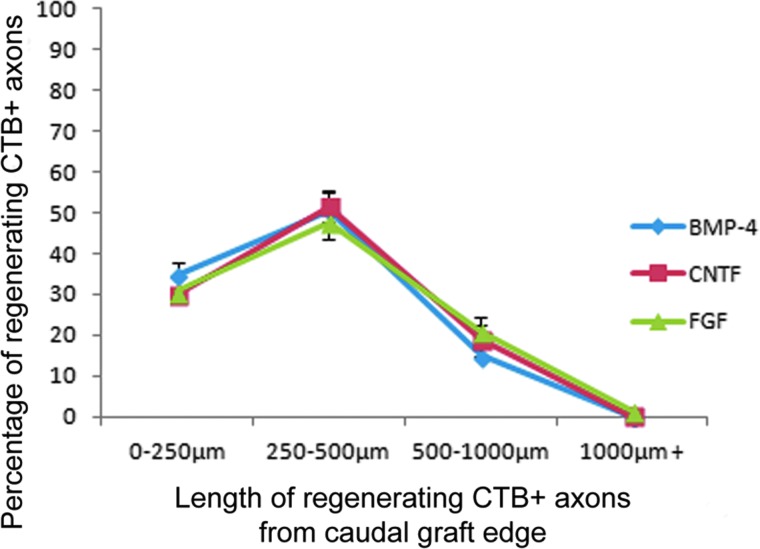
To evaluate the in vivo properties of hGRP and hGRP-derived astrocytes, hGRP that had been pre-differentiated with factors BMP-4 or CNTF were evaluated along with hGRP that had been maintained with bFGF, following engraftment into a unilateral C4/C5 dorsal column spinal cord injury model18,19 in non-immunosuppressed athymic rats, and compared with athymic rats that had received 50% PureColl/50% Q-basal media with no cell graft. Immunofluorescent microscopy for HuNu demonstrated that hGRP and hGRP-derived astrocytes survived following engraftment into the injured spinal cord, migrated extensively in the rostral and caudal directions into the adjacent white matter, and expressed a variety of phenotypic markers including GFAP (Fig. 9), nestin (Fig. 10), and Ki67 (Fig. 11). Further, each of these grafts created an environment permissive for axonal growth, supporting the growth of numerous βIII tubulin+ axons into the lesion area (Fig. 12). To determine whether hGRP and hGRP-derived astrocytes supported regeneration of long-distance, ascending sensory axons, CTB was injected into the sciatic nerve three days prior to sacrifice, five weeks after transplantation. Results from these tracing experiments revealed that the three transplant groups supported axonal regeneration into, but not out of, the graft (Fig. 13) and further, failed to reveal any differences between the groups, with the majority of axons regenerating between 250 and 500 μm (Fig. 14).
FIG. 9.
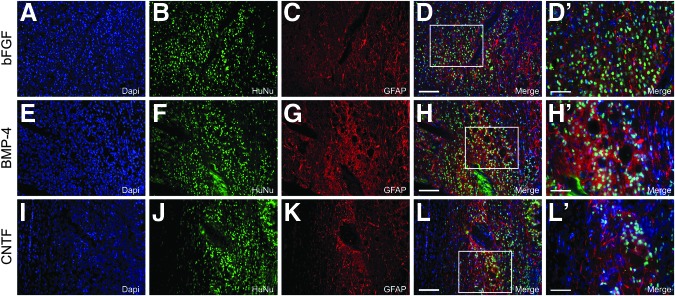
Survival and phenotypic analysis of pre-differentiated human glial restricted progenitors (hGRP) following transplantation into the injured spinal cord: glial fibrillary acidic protein (GFAP). hGRP were differentiated for 10 days with basic fibroblast growth factor (bFGF), bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor (CNTF) harvested and grafted into the injured spinal cord immediately following a C4 dorsal column hemisection. Five weeks after transplantation, spinal cord sections were analyzed by double-staining with Human Nuclear Antigen (HuNu) and GFAP antibodies. Panels A–D (bFGF), E–H (BMP-4), and I–L (CNTF) show many AP- and GFAP-positive cells. Panels D′, H′, and L′ show high magnification of the labeling for HuNu and GFAP. Scale bars A–L=100 μm; D′, H′, and L′=50 μm. Color image is available online at www.liebertpub.com/neu
FIG. 10.
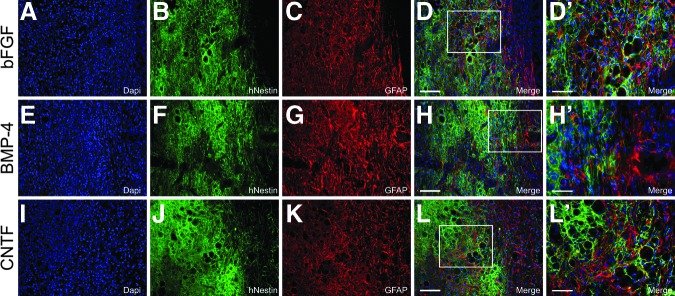
Survival and phenotypic analysis of pre-differentiated human glial restricted progenitors (hGRP) following transplantation into the injured spinal cord: nestin. hGRP were differentiated for 10 days with basic fibroblast growth factor (bFGF), bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor (CNTF), harvested, acutely grafted into a C4 dorsal column hemisection, and analyzed for their phenotypic repertoire with human-specific nestin (hNestin) and glial fibrillary acidic protein (GFAP) antibodies five weeks post-transplant. Panels A–D (bFGF), E–H (BMP-4), and I–L (CNTF) show many hNestin- and GFAP-positive cells. Panels D′, H′, and L′ show high magnification of the labeling for hNestin and GFAP. Scale bars A–L=100μm; D′, H′, and L′=50 μm.
FIG. 11.
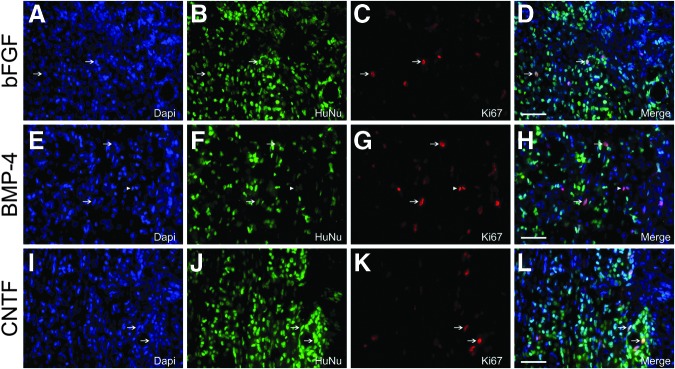
Phenotypic analysis of pre-differentiated human glial restricted progenitors (hGRP) following transplantation into the injured spinal cord: Ki67. hGRP were differentiated for 10 days with basic fibroblast growth factor (bFGF), bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor (CNTF), harvested, acutely grafted into a C4 dorsal column hemisection, and analyzed five weeks post-transplant with Human Nuclear Antigen (HuNu) and Ki67 antibodies. Panels A–D (bFGF), E–H (BMP-4), and I–L (CNTF) demonstrate the presence of a limited number of Ki67+ cells within the transplant, indicating that only a subset of transplanted cells continue to proliferate following transplantation. Arrows indicate double-labeled HuNu+Ki67+ cells, whereas arrowheads indicate cells labeling solely with the Ki67 marker. Scale bars A–L=50 μm. Color image is available online at www.liebertpub.com/neu
FIG. 12.

Transplantation of differentiated human glial restricted progenitors (hGRP) supports axonal growth into the site of spinal cord injury. hGRP were differentiated for 10 days with basic fibroblast growth factor (bFGF), bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor (CNTF), harvested, grafted into the injured spinal cord, and analyzed for their effect on axonal growth five weeks post-transplant by double-staining with Human Nuclear Antigen and βIII tubulin (Tuj1) antibodies. Panels A–D (bFGF), E–H (BMP-4), and I–L (CNTF) show the immunohistochemical stain and reveal that hGRP support the growth of axons into the transplant site, highlighting the permissive environment generated by each transplant. Scale bar=100 μm. Color image is available online at www.liebertpub.com/neu
FIG. 13.
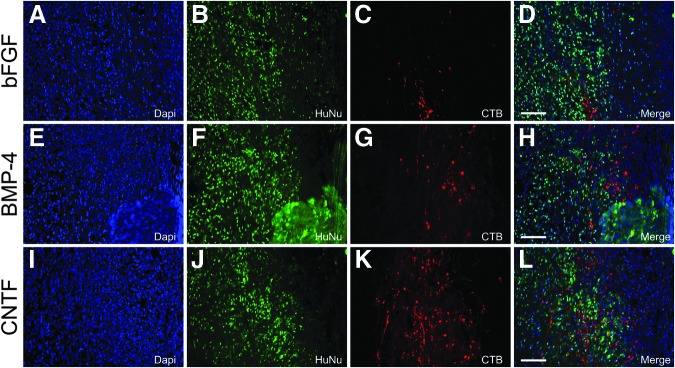
Transplantation of human glial restricted progenitors (hGRP) creates a permissive environment that supports the regeneration of injured sensory axons. hGRP were differentiated for 10 days with basic fibroblast growth factor (bFGF), bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor (CNTF), harvested and acutely grafted into the injured spinal cord. Sections were analyzed for the regeneration of sensory axons five weeks post-transplant by tracing with cholera toxin b subunit (CTB) and Human Nuclear Antigen (HuNu) antibodies. Panels A–D (bFGF), E–H (BMP-4), and I–L (CNTF) show immunohistology for HuNu and CTB and reveal that CTB-labeled regenerating axons derived from anterograde labeling of long ascending sensory axonal projections (derived from tracing of the sciatic nerve) regenerate along transplanted hGRP, highlighting the permissive nature of hGRP and embryonic astrocytes derived from hGRP. Scale bar=100 μm.
FIG. 14.
Quantitative analysis of regenerating sensory axons in the injured spinal cord. Human glial restricted progenitors (hGRP) were differentiated for 10 days with basic fibroblast growth factor, bone morphogenetic protein 4 (BMP-4), or ciliary neurotrophic factor, harvested, and grafted immediately following a C4 dorsal column hemisection injury that created a discrete cavity. Sections were analyzed for the regeneration of long ascending sensory axons five weeks post-transplant by tracing with cholera toxin b subunit (CTB) from the sciatic nerve. Quantitative analysis shows the percentages of CTB-labeled axons regenerating at 0 to 250 μm, 250 to 500 μm, and 500 to 1000 μm into the graft. No regenerating axons were observed to exit (1000+ μm) from the graft. The majority of regenerating CTB+ axons for all groups regenerated between 0 and 250 μm from the caudal edge of the graft. Error bars indicate 1 standard error. Statistical analysis failed to demonstrate any differences between the groups of pre-differentiated hGRP. Statistical significance was set at p<0.05 for all comparisons. Color image is available online at www.liebertpub.com/neu
The lack of differences between the aforementioned transplant groups, combined with the clinical usefulness of having frozen, banked stocks of cells that do not require a lengthy and time-consuming process of differentiation prior to transplantation, forced us to examine the properties of hGRP taken directly from frozen, banked stocks and immediately prepared for transplantation into a previously characterized dorsal column hemisection model of SCI.18,19 hGRP, prepared in this manner and transplanted acutely, were examined for their properties five weeks following grafting. Analysis revealed that hGRP taken from frozen, banked stocks and grafted into the injured spinal cord survived, migrated extensively in the rostral and caudal white matter, and expressed a variety of phenotypic markers, including GFAP, nestin, and Ki67 (Fig. 15). To explore the functional properties of grafted hGRP with respect to axonal growth, sections were stained with antibodies against HuNu and βIII tubulin (Tuj1; Fig. 16). Analysis revealed that grafted hGRP taken directly from frozen, banked stocks supported extensive axonal growth into the grafted area.
FIG. 15.
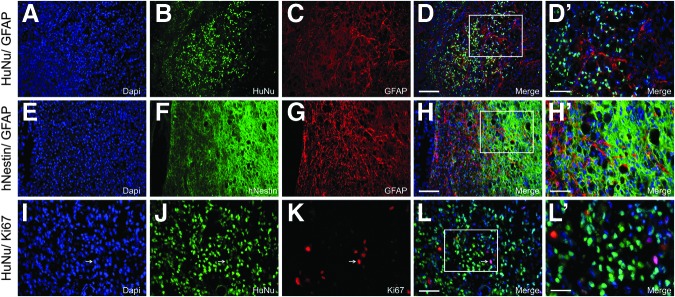
Human glial restricted progenitors (hGRP) transplanted directly from frozen banked stocks survive and differentiate following spinal cord injury. hGRP were taken directly from frozen, banked stocks and immediately grafted into an acute C4 dorsal column hemisection. Five weeks following transplantation, spinal cord sections were analyzed five weeks post-grafting by double-staining with Human Nuclear Antigen (HuNu), glial fibrillary acidic protein (GFAP), human-specific nestin (hNestin), or Ki67 antibodies for phenotypic analysis of grafted cells. Panels A–D (HuNu/ GFAP), E–H (hNestin/ GFAP), and I–L (HuNu/ Ki67) demonstrate that hGRP taken directly from frozen stocks and transplanted into the injured spinal cord survive and retain considerable phenotypic heterogeneity. Arrows indicate double labeled HuNu+Ki67+ cells. Panels D′, H′, and L′ show high magnification of immunohistological staining for greater detail. Scale bars A–H=100 μm; I–L=50 μm; D′ and H′=50 μm; L′=25 μm.
FIG. 16.
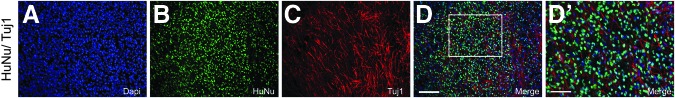
Human glial restricted progenitors (hGRP) grafted directly from frozen, banked stocks supports axonal growth in spinal cord injury. hGRP were grafted directly from frozen, banked stocks into an acute C4 dorsal column hemisection and analyzed for their effect on axonal growth five weeks post-injury by immunohistological analysis with Human Nuclear Antigen (HuNu) and βIII tubulin (Tuj1) antibodies. Panels A–D show the labeling and reveal that hGRP are capable of creating a permissive environment for the growth of axons at the site of injury. Panel D′ is a high magnification image of the HuNu and Tui1 labeling and further highlights the ability of naïve hGRP, taken directly from frozen, banked stocks to create a permissive environment for axonal growth. Scale bars A–D=100 μm; D′=50 μm. Color image is available online at www.liebertpub.com/neu
Further, to examine the effects of freshly prepared hGRP taken directly from frozen, banked stocks upon long-distance ascending sensory axonal regeneration, we once again injected CTB into the sciatic nerve three days prior to sacrifice, five weeks following grafting. Analysis revealed that hGRP prepared in this manner were capable of supporting axonal regeneration into the grafted area, with the majority of axons regenerating 250 to 500 μm into the graft (Fig. 17). Once again, however, we did not observe any regenerating axons bridging the graft or growing rostral to the graft site.
FIG. 17.
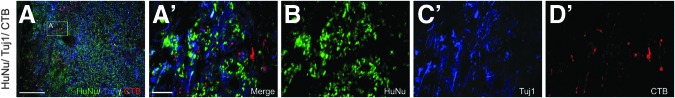
Human glial restricted progenitors (hGRP) grafted directly from frozen, banked stocks supports regeneration of ascending sensory axons. hGRP were grafted directly from frozen, banked stocks into the injured spinal cord and analyzed for their ability to support the regeneration of sensory axons by tracing five weeks post-transplant with cholera toxin b subunit (CTB) and Human Nuclear Antigen (HuNu) antibodies. Panels show the immunohistological labeling for HuNu, βIII tubulin (Tuj1), and CTB, and reveal that a subset of total axons (Tuj1), labeled with CTB, regenerate along transplanted, naïve hGRP, taken directly from frozen, banked stocks, and highlight that a process of hGRP pre-differentiation may not be required for support of axonal growth and regeneration. Panels A′–D′ show high magnification of CTB-labeled axons at various locations along the transplant site in Panel A. Scale bars A=250 μm; A′–D′=100 μm.
In summary, grafts of hGRP and hGRP-derived astrocytes survived, generated astrocytes, and supported axonal growth and regeneration to a similar extent. Further, hGRP taken directly from frozen, banked stocks and immediately grafted into the injured spinal cord, survived and also supported axonal growth and regeneration, highlighting the ability to use these permissive cells without the need for additional manipulation, such as a lengthy process of differentiation.
Discussion
Astrocytes are capable of responding to CNS injury by undergoing a process of reactive astrogliosis but there is growing appreciation that under appropriate conditions, astrocytes, particularly those derived from embryonic or neonatal tissue, also are capable of promoting neuroprotection and repair.8,13–17,19,36,40,44 Given this therapeutic potential, there has been a growing interest not only in defining astrocyte populations in terms of their phenotypic and functional properties but also in the development of protocols for efficient cell preparation and effective transplantation strategies. We therefore carried out a systematic analysis of astrocyte differentiation using clinical grade hGRP, manufactured and cryopreserved using a GMP process to meet the U. S. Food and Drug Administration (FDA) guidelines. Our aim was to study the morphological and phenotypic properties of these cells in vitro, and evaluate their behavior following transplantation into the injured spinal cord.
To analyze the morphological and phenotypic properties of hGRP and pre-differentiated astrocytes derived from hGRP, we used cells from two stocks of clinical grade hGRP (Q-therapeutics) prepared from 20- to 21-week-old fetal brain tissue, as previously described.9,11 The cells were characterized using several markers of proliferation and maturation to provide the profile of the cell population included in the hGRP stocks. Testing two separate preparations allowed us to demonstrate the consistency of the morphological and phenotypic analyses among hGRP stocks. hGRP prepared by this process express the phenotypic markers of nestin, A2B5, and GFAP, as well as the proliferation marker of Ki67—markers that are typical of glial progenitors present in the fetal brain at that age. 9,11 However, it is important to consider that the development of cellular therapies for human SCI also requires practical considerations of quality control for cell production and massive cell banking, including expansion steps of the starting material (e.g., fetal tissue, embryonic stem cells, induced pluripotent stem cells). These steps will affect the properties of GRP relative to the original progenitor population present in vivo or relative to cells isolated acutely and used without culturing or exogenous manipulation.45 Consequently, cell stocks designed for clinical applications require extensive characterization following grafting in terms of their therapeutic efficacy as well as their safety. Indeed, this work continues a series of studies testing the properties of hGRP prepared by Q-therapeutics in models of amyotrophic lateral sclerosis,46 inflammatory demyelination,14 and spinal cord contusion,12 this time focusing on their ability to promote regeneration in a model of SCI.
Distinct morphological and phenotypic properties of hGRP
Our detailed time-course in vitro analysis of astrocyte differentiation focused on the comparison between hGRP maintained in an undifferentiated state in the presence of bFGF, and astrocytes generated by BMP-4 or CNTF treatment, with FBS serving as a morphological reference. Specifically, FBS treatment consistently showed astrocytes with a near homogenous, flat, fibroblast-like morphology, whereas hGRP maintained in bFGF retained an immature bipolar or tripolar phenotype with light refractive cell bodies, in agreement with observations of previous studies that reported the response of rodent3,19 or human GRP.1,11,19 In contrast, treatment with BMP-4 or CNTF produced different astrocyte populations in a time-dependent manner characterized by short-process bearing phenotypes that rarely overlapped, or long-process bearing phenotypes that overlapped, respectively. Morphological changes occurred gradually during the 10 days of differentiation but cultures showed some heterogeneity. For example, not all of the hGRP pre-differentiated with BMP-4 or CNTF assumed a short-process or long-process phenotype, respectively.
Culture heterogeneity is dependent on the technical limitations associated with the process of isolating a pure population of GRP,1,3,4,9,11,19,47 allowing for other cells present in the culture to secrete soluble factors that affect the properties of glial phenotypes.48–50 Aside from technical limitations, culture heterogeneity also reflects the diversity and complexity of the A2B5+ progenitor populations, which include tripotential GRP and bipotential O-2A cells present in the CNS at different stages of development.1,3,51 Distinct progenitor populations also have multiple and divergent fate potentials24,52 depending on their rostrocaudal location within the CNS, as well as their dorsoventral positioning.25 As evidence of such local variation, GRP isolated from the dorsal versus ventral spinal cord exhibited divergent phenotypic characteristics following differentiation with BMP-4.5 Further, soluble factors secreted by differentiating cells themselves have the potential to alter morphological and phenotypic characteristics. For example, astrocytes differentiated in the presence of LIF, a CNTF family member, secrete BMP-4, which has been shown to have a significant effect on the morphology and phenotype of the astrocyte population.42 In addition, treatment by a single factor can activate multiple signaling pathways; treatment with BMP-4 can result in the activation of receptors BMPR1a or BMPR1b, which exert opposing effects on astrocyte hypertrophy and glial scar formation.53 It is also recognized that different signaling molecules, such as BMP-4 and CNTF, can converge and act synergistically to activate a common pathway to generate astrocytes.43,54 Despite the potential for cell heterogeneity, our results reflect the properties of hGRP prepared via a GMP process under FDA guidelines that have been used by other studies9,11,12,14,46 and that are likely to represent the gold standard for future clinical trials.
Our phenotypic analysis using the immature GRP marker A2B5 and the common astrocyte marker GFAP supported a model that defined the properties of hGRP differentiated with BMP-4, CNTF, or bFGF along a spectrum ranging from mature to immature astrocytes. Phenotypic analyses performed with markers of immature progenitors (nestin) and proliferation (Ki67) also supported the model. Differentiation of cultures with BMP-4 resulted in cells that displayed a dramatic decrease in A2B5 staining and were characterized by a mature A2B5-GFAP+ phenotype. In contrast, cultures maintained in bFGF displayed high levels of A2B5 staining and were characterized predominantly by an A2B5+/GFAP- phenotype. Treatment of hGRP with CNTF resulted in an intermediate phenotype, with the majority of cells continuing to express A2B5 and GFAP but with a time-dependent reduction in nestin staining, reflecting the gradual process of maturation while maintaining the potential for self renewal associated with progenitors. We therefore posit that hGRP prepared and pre-differentiated using CNTF or BMP reflect a continuum of astrocyte maturation consistent with other models,42,50 and that despite the observed phenotypic and biochemical differences,9,17 these cells represent embryonic astrocytes that retain their permissivity and ability to support axonal growth and synapse formation.18,19 Our results underscore the embryonic state of hGRP and permissive properties of the pre-differentiated astrocytes, which can be applied as a therapeutic tool for a variety of disorders.
Evidence for the plasticity of pre-differentiated hGRP
Astrocytes have the ability to sense extrinsic cues and modulate their morphological, phenotypic, and functional properties following a variety of physiological and pathological conditions.22–24,32 For example, following CNS injury, a sequence of signals and inflammatory mediators are released by the lesion microenvironment triggering a range of changes in the local astrocyte population. They undergo morphological alterations characterized by hypertrophy, increased GFAP expression, production of inhibitory chondroitin sulfate proteoglycan molecules—together contributing to the formation of a glial scar.32,55,56 However, this inhibitory process is accompanied by a variety of neuroprotective responses that are also linked to astrocyte activation—upregulation of glutamate scavenging channels, GLAST and GLT-1, production of laminin and fibronectin, and the secretion of various neurotrophic molecules.32,57–63 The divergent roles and properties of astrocytes are reflected in distinct patterns of gene expression of different astrocyte populations23,33,64 but they also have been associated with plasticity, suggesting the ability for transition between different states. Astrocyte plasticity has important therapeutic implications because of the potential to transform astrocytes already present at the injury site and as a factor in designing transplantation experiments using astrocytes.
We therefore examined whether the current protocols of hGRP differentiation resulted in stable, irreversible phenotypes or whether pre-differentiated astrocytes retained the ability of responding to alternate factors. Similarly to previous studies with rodent GRP,19 hGRP were capable of morphological and phenotypic plasticity, suggesting that the pre-differentiation protocols with BMP and CNTF did not create permanent changes associated with terminal differentiation. We considered the possibility that such phenotypic plasticity could be the result of selective survival of the pre-differentiated hGRP with subsequent expansion of distinct subpopulations of progenitors. However, the rapid and extensive phenotypic reversibility suggests a predominant mechanism of direct plasticity of astrocyte phenotype, which we confirmed by observing the morphological changes of individual cells by time lapse microscopy (data not shown). The observations imply that the regardless of whether grafts are composed of naïve hGRP or astrocytes pre-differentiated with BMP-4 or CNTF, the phenotype at the grafting site will be affected by the local environment to generate comparable astrocytes. Our work suggests that GRP and derived astrocytes, which are prepared from the embryonic CNS, retain their ability to generate a permissive environment that supports axonal growth and synapse formation consistent with their role in the developing CNS. It is, however, possible that long treatments with pro-inflammatory factors will generate a more stable state of non-permissive astrocytes similar to “activated” adult astrocytes.65
hGRP and hGRP-derived astrocytes promote axonal regeneration
The therapeutic potential of astrocytes has been demonstrated by numerous studies, highlighting that transplantation of neonatal or embryonic astrocytes exert multiple beneficial effects following experimental SCI—reduced lesion size, reduced scar, support of axonal growth and regeneration, and promotion of functional recovery.35–40 Similarly, transplantation of naïve GRP,8,19,44 which produced mostly astrocytes in vivo, generated a permissive environment and showed protective effects with respect to secondary injury. Indeed, our previous transplantation studies confirmed the permissive effects of GRP on axonal growth and regeneration in a dorsal column injury model but also demonstrated similar effects with GRP pre-differentiated with BMP-4 or CNTF.19 These studies underscored the permissive properties of astrocytes derived from embryonic CNS tissue and suggested that both the naïve GRP and the pre-differentiated GRP have therapeutic potential for SCI. An application of the permissive properties of GRP has been demonstrated in studies that produced a neuronal relay, in which grafts containing GRP not only allowed for the regeneration of host axons into the graft but also created an environment that allowed for the survival of immature neurons and facilitated the formation of structural and functional synapses between regenerating host axons and these immature neurons even in the absence of a neurotrophic gradient.18
Studies using hGRP also have demonstrated the therapeutic potential of GRP derived from human tissue on the injury environment, facilitating neuroprotection, remyelination, and recovery of function.12–14 Importantly, transplantation studies of hGRP and hGRP-derived astrocytes in a contusion model of SCI showed no major differences between naïve hGRP and hGRP pre-differentiated with BMP-4. Our current study examined the transplantation of naïve hGRP and hGRP pre-differentiated into astrocytes with BMP-4 or CNTF and demonstrated similar therapeutic capability in terms of axonal regeneration, comparable to our previous studies using rodent GRP.19 Our results highlight the fact that GRP or GRP-derived astrocytes consistently give rise to a population of embryonic astrocytes with the therapeutic potential not only to modify the injury environment but also to promote neuroprotection, remyelination, and axon regeneration. In contrast, studies by Davies and colleagues showed beneficial effects only with astrocytes produced by pre-differentiation of GRP with BMP-4 in both rodent15,16 and human cells,17 with detrimental effects reported for GRP or astrocytes pre-differentiated with CNTF.
The discrepancies in the studies of rodent GRP have been previously been analyzed19 and were related to possible variations in composition of glial progenitors, differences in transplantation and tracing protocols, and disagreements about the regenerative capacity of host axons. For example, Davies and colleagues reported robust growth beyond the injury site,15,16 while we observed growth into, but not out of, the lesion.19 Comparable differences are also apparent in the properties of human GRP reported in this study relative to a recent report by Davies and colleagues.17 Using a dorsal column injury model of athymic rats, we grafted hGRP and hGRP-derived astrocytes prepared pre-differentiation by BMP-4 or CNTF, and found that all of the grafts supported axonal growth (as evidenced by staining with βIII tubulin) and axonal regeneration into, but not out of, the graft (as evidenced by CTB staining of long ascending sensory projections from the sciatic nerve). These results are consistent not only with our in vitro findings demonstrating significant plasticity of hGRP-derived astrocytes but also with our previously published work with rodent GRP and GRP-derived astrocytes,18,19 as well as hGRP.12 Further, to examine the capability of truly naïve hGRP taken directly from frozen banked stocks to support axonal growth and regeneration, hGRP were thawed and immediately grafted into dorsal column lesion model of SCI into cyclosporine immunosuppressed Sprague Dawley animals. Naïve, un-manipulated hGRP were similarly capable of supporting axonal growth and regeneration. In contrast, the study by Davies and colleagues17 reported the permissive properties of astrocytes derived from treatment of hGRP with BMP-4 to support axonal growth but not by hGRP or hGRP pre-differentiated with CNTF.
A variety of factors could contribute to these discrepancies. The hGRP used in our study were prepared from the cortex of 20- to 21-week-old human fetal tissue samples by a GMP process as previously described and validated for a large number of preparations derived from multiple samples.9,11 Using this gestational age as a source of hGRP is related to the period of gliogenesis in humans, which has become a standard time point widely used in previous studies.1,9–12,14,19,46,66 In contrast, in their recent study, Davies and colleagues used hGRP isolated from two 9.0- to −9.5-week-old fetal tissue samples of rostral neural tube.17 This early age is puzzling since their previous studies1 and the description of the isolation method66 refer to preparation of 20-week-old human fetal tissue. It is not clear whether the departure from the common protocol represents difficulties in obtaining second trimester human fetal samples or a preference to use cells present early in development, such as the radial glia population.51 These age-, region-, and technique-dependent differences in hGRP isolation may contribute to the observed differences between the two studies related to distinct subpopulations of cells and their responsiveness to external cues. It is equally important to consider that the formation of stable BMP-4 or CNTF astrocyte phenotypes may require an extended period of in vitro pre-differentiation. It appears that the formation of stable astrocyte phenotypes may require up to five weeks of pre-differentiation when using immature NSC (Mahendra Rao, personal communication). Indeed, our results suggest that even after 10 days of pre-differentiation, hGRP derived from 20-week-old fetal tissue samples are still undergoing a process of differentiation. Thus, the seven-day treatments used in Davies study are unlikely to achieve stable astrocyte phenotypes because hGRP derived from an early developmental stage of 9 weeks old may require a much longer in vitro pre-differentiation. At the same time, however, this limited process of pre-differentiation does give rise to divergent astrocyte phenotypes and changes in gene expression, particularly in micro ribonucleic acid expression (Haas, unpublished results) that could be used as markers to identify differences between embryonic astrocytes.
Davies and colleagues17 used two individual fetuses for grafting, and presented data pertaining to pre-differentiated hGRP using BMP-4 (two samples) and CNTF (one sample), but reported wide variability in the survival of cellular grafts of BMP-4 and CNTF grafts. While the cause of limited viability of these grafts is unclear, it could be related to the separate immunosuppressive treatments that the groups received. In striking departure from evidence presented in their previous studies,15,16 their work with hGRP did not demonstrate long-distance axonal regeneration using tracing methods.17 Instead they used axonal markers, which do distinguish between sprouting and long distance regeneration. In contrast, our data demonstrate that hGRP and hGRP-derived astrocytes are able to support axonal regeneration into the graft, but not long-distance regeneration across the injury, consistent with our previous rodent data.18, 19
Although an assessment of behavioral recovery was not included in our study, previous studies using hGRP prepared by the same process demonstrated no major difference between hGRP and pre-differentiated astrocytes.12 In contrast, the behavioral data shown by Davies and colleagues demonstrated the efficacy of hGRP pre-differentiated with BMP-4 to support locomotor recovery, compared with CNTF-treated hGRP or hGRP alone.17 However, the grid-walking data presented by Davies and colleagues17 are presented as the total number of missteps rather than the percentage of correctly placed steps or the percentage of total number of steps taken,12,67–70 and therefore may exaggerate small differences between experimental groups. Further, there was a wide variability in performance on the grid-walk test observed in the control and BMP-4–treated groups,17 which may reflect differences related to issues of cell viability or immunosuppression protocols used in the study. Given the therapeutic importance of hGRP and potential for clinical translation, it is important that these results be replicated and verified by other functional tests. Although there seems to be an agreement that astrocytes derived from BMP-4 treatment of rodent or human GRP have therapeutic potential, our data indicate similar potential for GRP alone, which may significantly simplify any future clinical trial.
Conclusion
Clinical translation of cellular transplantation therapies for spinal cord injury requires the storage of large quantities of permissive cells that can be rapidly and immediately used for clinical benefit and the thorough and reproducible characterization of such cells. The transplantation of reliable and well-characterized stocks of hGRP is of particular importance due to their potential to mitigate not only traumatic injury but also multiple glial pathologies present in pediatric and adult medicine.71 Indeed, hGRP (obtained from Q-therapeutics), isolated via a GMP technique, offer an attractive option for future pre-clinical studies. Our results, using these cells, suggest that hGRP may be a promising candidate for future therapies and further confirm the permissive nature of embryonic astrocytes isolated from the fetal CNS. These results also suggest that these embryonic astrocytes alone may not be sufficient to promote functional recovery through long-distance axonal regeneration. The enhanced axonal regeneration into the graft following hGRP grafting was not accompanied by bridging axons that grow across the lesion to connect to upstream functional targets, similar to the properties observed for rodent-derived GRP.19 Effective therapeutic strategies may therefore require combinatorial strategies, coupling these permissive cells with other therapeutic interventions such as neurotrophins,72 neuronal restricted progenitors,18 cAMP,73 chondroitinase,59 exercise,74 modulation of axon growth dynamic pathways (e.g., PTEN/mTOR75) or administration of novel compounds identified through chemical screens.76 Nevertheless, hGRP prepared by a GMP production standard are a powerful in vivo tool that can be readily expanded and banked, and upon grafting into SCI, can modify the local microenvironment to make it more hospitable to neuronal survival, axonal growth, and regeneration.
Supplementary Material
Acknowledgments
We would like to thank Sonia Shah, Theresa Connors, Maryla Obrocka, and Drs. Takaya Yamagami, Joseph F. Bonner, Jed Shumsky, and James Campanelli for technical assistance and advice. hGRP were obtained from Q-therapeutics. The monoclonal antibody A2B5, developed by Z. Kaprielian, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. The antibody O4 was a generous gift from Dr. Judith Grinspan.
This work was supported by the Craig H. Neilsen Foundation and National Institutes of Health grant NS055976.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dietrich J. Noble M. Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- 2.Rao M.S. Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev. Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- 3.Rao M.S. Noble M. Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y.Y. Mujtaba T. Han S.S. Fischer I. Rao M.S. Isolation of a glial-restricted tripotential cell line from embryonic spinal cord cultures. Glia. 2002;38:65–79. doi: 10.1002/glia.10049. [DOI] [PubMed] [Google Scholar]
- 5.Gregori N. Proschel C. Noble M. Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J. Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han S.S. Liu Y. Tyler-Polsz C. Rao M.S. Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- 7.Herrera J. Yang H. Zhang S.C. Proschel C. Tresco P. Duncan I.D. Luskin M. Mayer-Proschel M. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp. Neurol. 2001;171:11–21. doi: 10.1006/exnr.2001.7729. [DOI] [PubMed] [Google Scholar]
- 8.Hill C.E. Proschel C. Noble M. Mayer-Proschel M. Gensel J.C. Beattie M.S. Bresnahan J.C. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp. Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Campanelli J.T. Sandrock R.W. Wheatley W. Xue H. Zheng J. Liang F. Chesnut J.D. Zhan M. Rao M.S. Liu Y. Expression profiling of human glial precursors. BMC Dev. Biol. 2008;8:102. doi: 10.1186/1471-213X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maragakis N.J. Dietrich J. Wong V. Xue H. Mayer-Proschel M. Rao M.S. Rothstein J.D. Glutamate transporter expression and function in human glial progenitors. Glia. 2004;45:133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- 11.Sandrock R.W. Wheatley W. Levinthal C. Lawson J. Hashimoto B. Rao M. Campanelli J.T. Isolation, characterization and preclinical development of human glial-restricted progenitor cells for treatment of neurological disorders. Regen. Med. 2010;5:381–394. doi: 10.2217/rme.10.24. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y. Neuhuber B. Singh A. Bouyer J. Lepore A. Bonner J. Himes T. Campanelli J.T. Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J. Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexanian A.R. Svendsen C.N. Crowe M.J. Kurpad S.N. Transplantation of human glial-restricted neural precursors into injured spinal cord promotes functional and sensory recovery without causing allodynia. Cytotherapy. 2011;13:61–68. doi: 10.3109/14653249.2010.510504. [DOI] [PubMed] [Google Scholar]
- 14.Walczak P. All A.H. Rumpal N. Gorelik M. Kim H. Maybhate A. Agrawal G. Campanelli J.T. Gilad A.A. Kerr D.A. Bulte J.W. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59:499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies J.E. Huang C. Proschel C. Noble M. Mayer-Proschel M. Davies S.J. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies J.E. Proschel C. Zhang N. Noble M. Mayer-Proschel M. Davies S.J. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J. Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies S.J. Shih C.H. Noble M. Mayer-Proschel M. Davies J.E. Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonner J.F. Connors T.M. Silverman W.F. Kowalski D.P. Lemay M.A. Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas C. Neuhuber B. Yamagami T. Rao M. Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp. Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman M.R. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nag S. Morphology and properties of astrocytes. Methods Mol. Biol. 2011;686:69–100. doi: 10.1007/978-1-60761-938-3_3. [DOI] [PubMed] [Google Scholar]
- 22.Sofroniew M.V. Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y. Barres B.A. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Hewett J.A. Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system. J. Neurochem. 2009;110:1717–1736. doi: 10.1111/j.1471-4159.2009.06288.x. [DOI] [PubMed] [Google Scholar]
- 25.Hochstim C. Deneen B. Lukaszewicz A. Zhou Q. Anderson D.J. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller R.H. Szigeti V. Clonal analysis of astrocyte diversity in neonatal rat spinal cord cultures. Development. 1991;113:353–362. doi: 10.1242/dev.113.1.353. [DOI] [PubMed] [Google Scholar]
- 27.Miller R.H. Zhang H. Fok-Seang J. Glial cell heterogeneity in the mammalian spinal cord. Perspect. Dev. Neurobiol. 1994;2:225–231. [PubMed] [Google Scholar]
- 28.Oberheim N.A. Goldman S.A. Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberheim N.A. Takano T. Han X. He W. Lin J.H. Wang F. Xu Q. Wyatt J.D. Pilcher W. Ojemann J.G. Ransom B.R. Goldman S.A. Nedergaard M. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong V.W. Yong F.P. Olivier A. Robitaille Y. Antel J.P. Morphologic heterogeneity of human adult astrocytes in culture: correlation with HLA-DR expression. J. Neurosci. Res. 1990;27:678–688. doi: 10.1002/jnr.490270428. [DOI] [PubMed] [Google Scholar]
- 31.Matyash V. Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain. Res. Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 32.White R.E. Jakeman L.B. Don't fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor. Neurol. Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- 33.White R.E. McTigue D.M. Jakeman L.B. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J. Comp. Neurol. 2010;518:1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aloisi F. Borsellino G. Samoggia P. Testa U. Chelucci C. Russo G. Peschle C. Levi G. Astrocyte cultures from human embryonic brain: characterization and modulation of surface molecules by inflammatory cytokines. J. Neurosci. Res. 1992;32:494–506. doi: 10.1002/jnr.490320405. [DOI] [PubMed] [Google Scholar]
- 35.Joosten E.A. Veldhuis W.B. Hamers F.P. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J. Neurosci. Res. 2004;77:127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 36.Kliot M. Smith G.M. Siegal J.D. Silver J. Astrocyte-polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord. Exp. Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- 37.Smith G.M. Miller R.H. Immature type-1 astrocytes suppress glial scar formation, are motile and interact with blood vessels. Brain Res. 1991;543:111–122. doi: 10.1016/0006-8993(91)91054-5. [DOI] [PubMed] [Google Scholar]
- 38.Smith G.M. Miller R.H. Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J. Comp. Neurol. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- 39.Smith G.M. Silver J. Transplantation of immature and mature astrocytes and their effect on scar formation in the lesioned central nervous system. Prog. Brain Res. 1988;78:353–361. doi: 10.1016/s0079-6123(08)60304-0. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.J. Chuah M.I. Yew D.T. Leung P.C. Tsang D.S. Effects of astrocyte implantation into the hemisected adult rat spinal cord. Neuroscience. 1995;65:973–981. doi: 10.1016/0306-4522(94)00519-b. [DOI] [PubMed] [Google Scholar]
- 41.Mujtaba T. Han S.S. Fischer I. Sandgren E.P. Rao M.S. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Exp. Neurol. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- 42.Bonaguidi M.A. McGuire T. Hu M. Kan L. Samanta J. Kessler J.A. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- 43.Weible M.W., 2nd Chan-Ling T. Phenotypic characterization of neural stem cells from human fetal spinal cord: synergistic effect of LIF and BMP4 to generate astrocytes. Glia. 2007;55:1156–1168. doi: 10.1002/glia.20539. [DOI] [PubMed] [Google Scholar]
- 44.Lepore A.C. Rauck B. Dejea C. Pardo A.C. Rao M.S. Rothstein J.D. Maragakis N.J. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bithell A. Finch S.E. Hornby M.F. Williams B.P. Fibroblast growth factor 2 maintains the neurogenic capacity of embryonic neural progenitor cells in vitro but changes their neuronal subtype specification. Stem Cells. 2008;26:1565–1574. doi: 10.1634/stemcells.2007-0832. [DOI] [PubMed] [Google Scholar]
- 46.Lepore A.C. O'Donnell J. Kim A.S. Williams T. Tuteja A. Rao M.S. Kelley L.L. Campanelli J.T. Maragakis N.J. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One. 2011;6:e25968. doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble M. Glial Restricted Precursors. In: Rao M., editor. Neural Development and Stem Cells. Humana Press; Totowa: 2006. pp. 143–188. [Google Scholar]
- 48.Fruhbeis C. Frohlich D. Kramer-Albers E.M. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy R. Reynolds R. Neuron-oligodendroglial interactions during central nervous system development. J. Neurosci. Res. 1993;36:121–126. doi: 10.1002/jnr.490360202. [DOI] [PubMed] [Google Scholar]
- 50.Stipursky J. Spohr T.C. Sousa V.O. Gomes F.C. Neuron-astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochem. Res. 2012;37:2402–2418. doi: 10.1007/s11064-012-0798-x. [DOI] [PubMed] [Google Scholar]
- 51.Aloisi F. Giampaolo A. Russo G. Peschle C. Levi G. Developmental appearance, antigenic profile, and proliferation of glial cells of the human embryonic spinal cord: an immunocytochemical study using dissociated cultured cells. Glia. 1992;5:171–181. doi: 10.1002/glia.440050303. [DOI] [PubMed] [Google Scholar]
- 52.Kimelberg H.K. The problem of astrocyte identity. Neurochem. Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Sahni V. Mukhopadhyay A. Tysseling V. Hebert A. Birch D. McGuire T.L. Stupp S.I. Kessler J.A. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H. Grumet M. BMP and LIF signaling coordinately regulate lineage restriction of radial glia in the developing forebrain. Glia. 2007;55:24–35. doi: 10.1002/glia.20434. [DOI] [PubMed] [Google Scholar]
- 55.Fitch M.T. Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little A.R. O'Callagha J.P. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–618. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 57.Beschorner R. Simon P. Schauer N. Mittelbronn M. Schluesener H.J. Trautmann K. Dietz K. Meyermann R. Reactive astrocytes and activated microglial cells express EAAT1, but not EAAT2, reflecting a neuroprotective potential following ischaemia. Histopathology. 2007;50:897–910. doi: 10.1111/j.1365-2559.2007.02703.x. [DOI] [PubMed] [Google Scholar]
- 58.Costa S. Planchenault T. Charriere-Bertrand C. Mouchel Y. Fages C. Juliano S. Lefrancois T. Barlovatz-Meimon G. Tardy M. Astroglial permissivity for neuritic outgrowth in neuron-astrocyte cocultures depends on regulation of laminin bioavailability. Glia. 2002;37:105–113. doi: 10.1002/glia.10015. [DOI] [PubMed] [Google Scholar]
- 59.Filous A.R. Miller J.H. Coulson-Thomas Y.M. Horn K.P. Alilain W.J. Silver J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 2010;70:826–841. doi: 10.1002/dneu.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frisen J. Haegerstrand A. Risling M. Fried K. Johansson C.B. Hammarberg H. Elde R. Hokfelt T. Cullheim S. Spinal axons in central nervous system scar tissue are closely related to laminin-immunoreactive astrocytes. Neuroscience. 1995;65:293–304. doi: 10.1016/0306-4522(94)00467-j. [DOI] [PubMed] [Google Scholar]
- 61.Rothstein J.D. Dykes-Hoberg M. Pardo C.A. Bristol L.A. Jin L. Kuncl R.W. Kanai Y. Hediger M.A. Wang Y. Schielke J.P. Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 62.Tom V.J. Doller C.M. Malouf A.T. Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vera-Portocarrero L.P. Mills C.D. Ye Z. Fullwood S.D. McAdoo D.J. Hulsebosch C.E. Westlund K.N. Rapid changes in expression of glutamate transporters after spinal cord injury. Brain Res. 2002;927:104–110. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]
- 64.Hill S.J. Barbarese E. McIntosh T.K. Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J. Neuropathol. Exp. Neurol. 1996;55:1221–1229. doi: 10.1097/00005072-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Smith G.M. Rutishauser U. Silver J. Miller R.H. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev. Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- 66.Barami K. Grever W.E. Diaz F.G. Lyman W.D. An efficient method for the culturing and generation of neurons and astrocytes from second trimester human central nervous system tissue. Neurol. Res. 2001;23:321–326. doi: 10.1179/016164101101198686. [DOI] [PubMed] [Google Scholar]
- 67.Sandrow H.R. Shumsky J.S. Amin A. Houle J.D. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp. Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shumsky J.S. Tobias C.A. Tumolo M. Long W.D. Giszter S.F. Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp. Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- 69.Tom V.J. Sandrow-Feinberg H.R. Miller K. Domitrovich C. Bouyer J. Zhukareva V. Klaw M.C. Lemay M.A. Houle J.D. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp. Neurol. 2012;239C:91–100. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Z'Graggen W.J. Metz G.A. Kartje G.L. Thallmair M. Schwab M.E. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J. Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldman S.A. Nedergaard M. Windrem M.S. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338:491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao Q. Xu X.M. Devries W.H. Enzmann G.U. Ping P. Tsoulfas P. Wood P.M. Bunge M.B. Whittemore S.R. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nout Y.S. Culp E. Schmidt M.H. Tovar C.A. Proschel C. Mayer-Proschel M. Noble M.D. Beattie M.S. Bresnahan J.C. Glial restricted precursor cell transplant with cyclic adenosine monophosphate improved some autonomic functions but resulted in a reduced graft size after spinal cord contusion injury in rats. Exp. Neurol. 2011;227:159–171. doi: 10.1016/j.expneurol.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu G. Detloff M.R. Miller K.N. Santi L. Houle J.D. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp. Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu K. Lu Y. Lee J.K. Samara R. Willenberg R. Sears-Kraxberger I. Tedeschi A. Park K.K. Jin D. Cai B. Xu B. Connolly L. Steward O. Zheng B. He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Usher L.C. Johnstone A. Erturk A. Hu Y. Strikis D. Wanner I.B. Moorman S. Lee J.W. Min J. Ha H.H. Duan Y. Hoffman S. Goldberg J.L. Bradke F. Chang Y.T. Lemmon V.P. Bixby J.L. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J. Neurosci. 2010;30:4693–4706. doi: 10.1523/JNEUROSCI.0302-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.