Abstract
Objective
The purpose of this study was to examine the behavioral effects of four doses of psychostimulant medication, combining extended-release methylphenidate (MPH) in the morning with immediate-release MPH in the afternoon.
Method
The sample comprised 24 children (19 boys; 5 girls) who met American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV-TR) criteria for an autism spectrum disorder (ASD) on the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS), and had significant symptoms of attention-deficit/hyperactivity disorder (ADHD). This sample consisted of elementary school-age, community-based children (mean chronological age=8.8 years, SD=1.7; mean intelligence quotient [IQ]=85; SD=16.8). Effects of four dose levels of MPH on parent and teacher behavioral ratings were investigated using a within-subject, crossover, placebo-controlled design.
Results
MPH treatment was associated with significant declines in hyperactive and impulsive behavior at both home and school. Parents noted significant declines in inattentive and oppositional behavior, and improvements in social skills. No exacerbation of stereotypies was noted, and side effects were similar to those seen in typically developing children with ADHD. Dose response was primarily linear in the dose range studied.
Conclusions
The results of this study suggest that MPH formulations are efficacious and well-tolerated for children with ASD and significant ADHD symptoms.
Introduction
Children with autism spectrum disorders (ASD) are known to be at high risk for several emotional and behavioral disorders (e.g., Simonoff et al. 2008). By early adolescence, the effects of comorbid psychiatric symptomatology can have a greater impact on functional status than the core symptoms of autism (e.g., Loveland and Tunali-Kotoski 2005; Pearson et al. 2006). Indeed, 14–75% of children with ASD are reported to have symptoms of inattention, hyperactivity, and impulsivity severe enough to warrant a diagnosis of attention- deficit/hyperactivity disorder (ADHD) (Frazier et al. 2001; Goldstein and Schwebach 2004; Sturm et al. 2004; Lecavalier 2006; Leyfer et al. 2006; Pearson et al. 2006; Reiersen et al. 2007; Simonoff et al. 2008).
Findings from the titration phase of the National Institute of Mental Health (NIMH) Multimodal Treatment Study of ADHD (MTA) suggested that school-age children with ADHD (intelligence quotients [IQs] ≥80) who received higher doses of stimulant medication had generally better outcomes than those treated with lower doses (Arnold et al. 2000; MTA Cooperative Group 1999; Greenhill et al. 2001). Although such findings suggest that higher doses of carefully monitored stimulant treatment are associated with better outcomes, others (e.g., Sprague and Sleator 1977; Gan and Cantwell 1982) have reported a curvilinear response to stimulant medication: lower doses produced initial improvements relative to placebo, followed by declines at higher doses.
Recent reports have suggested that the majority of children with ASD are being treated with psychotropic medication, including 58% of children with ASD and ADHD symptoms (Frazier et al. 2011; Pringle et al. 2012). Although approximately one third of children with ASD and ADHD are treated with psychostimulants (Frazier et al. 2011), there have been surprisingly few controlled studies exploring the behavioral effects of stimulants in these children. Early studies (e.g., Campbell et al. 1972, 1975) suggested that positive effects of stimulants were outweighed by concerns such as increased irritability, aggression, and stereotypic behavior. Case study reports have also noted concerns with psychostimulant treatment in this population, for example, agitation, stereotypies, exacerbation of symptoms such as trichotillomania and dysphoria, and induction of psychotic symptoms (Sporn and Pinsker 1981; Schmidt 1982; Volkmar et al. 1985; Realmuto et al. 1989; Holttum et al. 1994). Aman (1982) suggested that findings such as these may have been attributable to stimulants further constricting an already over-focused attention in ASD.
In contrast to these early studies, a growing number of recent investigations have suggested more positive outcomes associated with stimulant treatment in elementary school-age children with ASD and symptoms of ADHD. Stimulants were associated with improved attention and declines in impulsivity and hyperactivity (e.g., Hoshino et al. 1977; Geller et al. 1981; Vitriol and Farber 1981; Strayhorn et al. 1988; Handen et al. 2000; DiMartino et al. 2004; Santosh, et al. 2006; Nickels et al. 2008). Ghuman et al. (2009) extended these positive findings to preschool children with ASD who also had symptoms of ADHD, but also noted that their response to methylphenidate (MPH) was “more subtle and variable” than that of older and more typically developing children with ADHD.
The Research Units on Pediatric Psychopharmacology (RUPP) Autism Network (RUPP Autism Network 2005) also studied MPH treatment in children with ASD. Using a double-blind, placebo-controlled, crossover design, immediate release MPH was found to decrease hyperactivity and inattention. However, fewer children with pervasive developmental disorder not otherwise specified (PDD-NOS) responded favorably to MPH than did children in the general pediatric population (48% versus 75%), and the magnitude of improvement was smaller (Scahill and Pachler 2007). Furthermore, side effects (mainly irritability) requiring discontinuation occurred in 18% of these children, compared with <4% in typically developing children in the NIMH Multimodal Treatment Study of ADHD (MTA Cooperative Group, 1999). Encouragingly, subsequent secondary reports from the RUPP study suggested that stimulant treatment was also associated with improved social communication and self-regulation (Posey et al. 2007; Jahromi 2009), the latter using a direct observational experimental task.
The emerging literature on psychostimulant medication treatment in children with ASD and ADHD has provided additional insights into the concerns noted by earlier investigators. For example, Handen et al. (2000) did not find increased irritability with stimulant treatment, whereas Quintana et al. (1995) even found a significant decline in irritability with psychostimulant treatment. Others suggested that oppositional or aggressive behavior may not be exacerbated by stimulant treatment, and, in fact, may even be significantly improved by it (Quintana et al. 1995; Aman 1996; Handen et al. 2000; DiMartino et al. 2004; Santosh et al. 2006; Posey et al. 2007). Still others have suggested that stimulant treatment may be associated with less social withdrawal (Quintana et al. 1995; DiMartino et al. 2004; RUPP Autism Network 2005), more awareness and responsiveness to others (Vitriol and Farber 1981), and being perceived as being more approachable by peers (Gringas 2000). Particularly encouraging is the evidence that treatment is not associated with significant increases in stereotypic behavior in these children (Birmaher et al. 1988; Handen et al. 2000; RUPP Autism Network 2005; Santosh et al. 2006; Posey et al. 2007; Ghuman et al. 2009).
Although this emerging literature is encouraging, stimulants have also been associated with some undesirable symptoms, including insomnia (Birmaher et al. 1988; RUPP Autism Network 2005; Santosh et al. 2006), loss of appetite (Quintana et al. 1995; RUPP Autism Network 2005), irritability (RUPP Autism Network 2005), and dysphoria (Handen et al. 2000). Although these symptoms are similar to those seen in the general pediatric ADHD population, they sometimes outweigh improvements in ADHD symptoms in children with ASD. For example, irritability was the most common reason for discontinuing treatment in the RUPP study (18% of the sample), even though the “typical” side effects of stimulants (e.g., appetite suppression and difficulty falling asleep) were more common in the RUPP study participants (RUPP Autism Network 2005). Other investigators found that such side effect symptoms were responsive to dose reduction in children with ASD and symptoms of ADHD (Hoshino et al. 1977), and that children destined to have an unfavorable response could be identified after a single dose (DiMartino et al. 2004). Stigler and colleagues (2004) in their retrospective chart review of 195 children with ASD noted that those with Asperger's disorder had fewer side effects than children with either autism or PDD-NOS. Furthermore, stimulant medication was not associated with significant weight, pulse rate, and blood pressure change in preschoolers with ASD who also had symptoms of ADHD (Ghuman et al. 2009).
To our knowledge, there have been no reports employing the long-acting formulations of psychostimulant medication to treat symptoms of ADHD in children with ASD, despite the fact that long-acting formulations are now the current standard of practice in the field (American Academy of Child and Adolescent Psychiatry 2007). The current study builds upon the findings of the RUPP study (which used t.i.d. dosing of immediate release MPH, or IR-MPH) by employing a treatment regimen that more closely matches current clinical practice in psychostimulant treatment dosing: Extended release (ER)-MPH in the morning, and IR-MPH in the afternoon. The overall purpose of this study was to examine the effectiveness of psychostimulant treatment with ER-MPH on behavior in children with an ASD and significant symptoms of ADHD. Increasingly, ER stimulant preparations have become the standard first- time treatment for ADHD (American Academy of Child and Adolescent Psychiatry 2007). Our goals were to determine if: 1) ER-MPH was associated with improvements in parent and teacher behavioral ratings, and 2) the MPH dose-response curve was linear (i.e., higher MPH doses were associated with consistent improvements in behavioral functioning), or curvilinear (an initial behavioral improvement with MPH, followed by behavioral declines at higher doses).
Methods
Participants
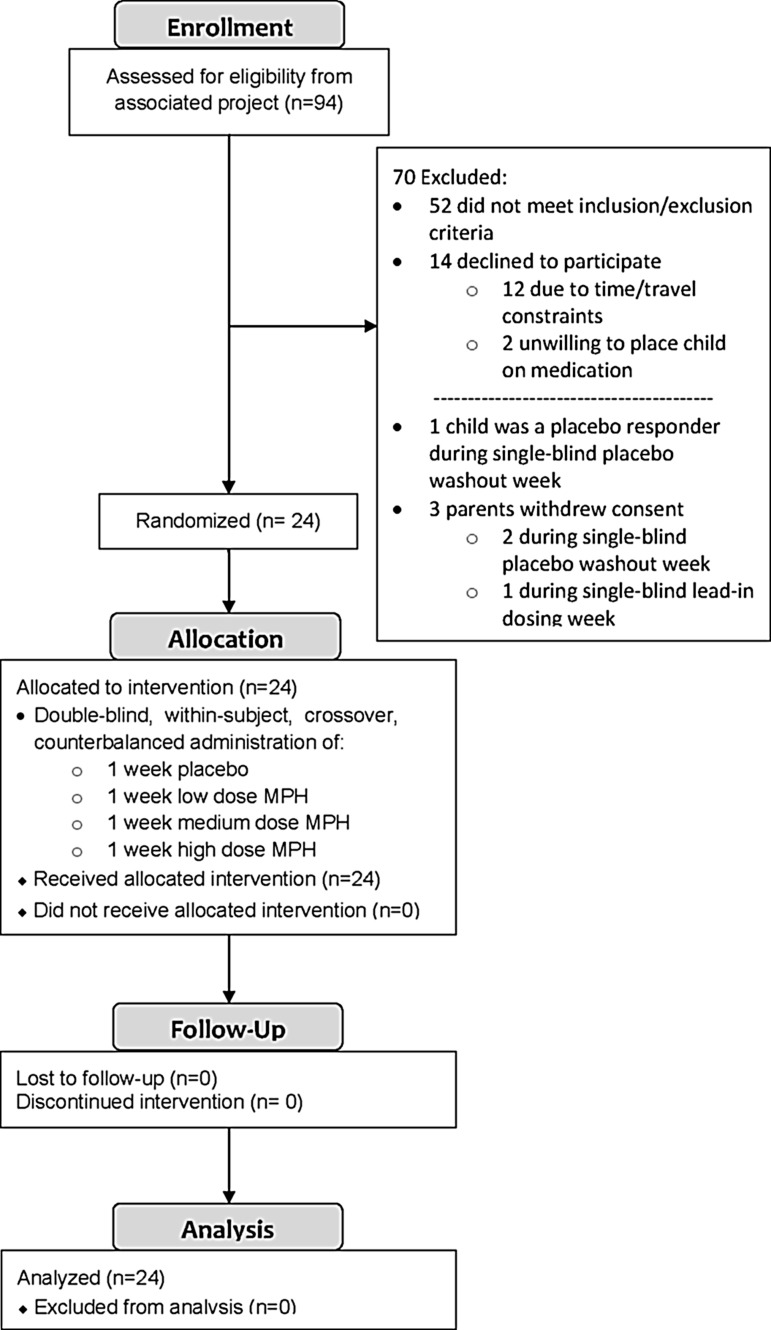
As can be seen in Figure 1, 24 children (19 boys and 5 girls) with ASD and symptoms of ADHD participated. As indicated in Table 1, the mean chronological age of the sample was 8.8 years (SD=1.6), and the mean full scale IQ (Stanford-Binet 5th ed.; Roid 2003) was 85.0 (SD=16.8). The ethnic breakdown of these children was: 13 Caucasian, 5 Hispanic, 4 African-American, 1 Asian, and 1 multiple races. Their mean Hollingshead four-factor social class was 1.7 (SD=0.9; Hollingshead 1975). The mean education level was 15.8 (SD=2.3) years for mothers and 17.2 (SD=3.1) years for fathers. These children were assessed using the Autism Diagnostic Interview-R (ADI-R; Rutter et al. 2003), the Autism Diagnostic Observation Schedule (ADOS; Lord et al.1999), a clinical interview, clinic observation, and record review by two licensed psychologists (DAP and KAL) who are both highly experienced in the assessment and diagnosis of ASDs, and who are certified as meeting research reliability on both the ADI-R/ADOS. Nineteen (79%) children met American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) criteria for autistic disorder, three (13%) had Asperger's disorder, and two (8%) had PDD-NOS (American Psychiatric Association 2000).
FIG. 1.
Study recruitment and retention.
Table 1.
Sample Characteristics
| Variable | Mean | SD | Range |
|---|---|---|---|
| Chronological age (years) | 8.8 | 1.7 | 7.1–12.7 |
| Stanford-Binet Intelligence Scale, 5th ed. | |||
| Verbal IQ | 80.1 | 19.7 | 48–115 |
| Nonverbal IQ | 90.8 | 15.2 | 50–118 |
| Full scale IQ | 85.0 | 16.8 | 46–112 |
| Verbal age equivalent (months) | 79.7 | 25.1 | 43–143 |
| Nonverbal age equivalent (months) | 89.8 | 23.2 | 64–142 |
| Full scale age equivalent (months) | 84.0 | 23.5 | 52–145 |
| Vineland Adaptive Behavior Scales-II | |||
| Communication Domain | 77.6 | 7.2 | 62–94 |
| Daily Living Skills Domain | 80.3 | 8.0 | 61–97 |
| Socialization Domain | 76.7 | 6.8 | 64–89 |
| Vineland Composite | 76.4 | 6.1 | 61–89 |
| Social Communication Questionnaire | 23.4 | 5.2 | 14–35 |
| Hollingshead 4 Factor Social Class | 1.7 | 0.9 | 1–4 |
| Hollingshead 4 Factor SES Score | 52.3 | 10.8 | 29–66 |
| Parent educational level (no. years) | |||
| Father | 17.2 | 3.1 | 12–25 |
| Mother | 15.8 | 2.3 | 12–21 |
IQ, intelligence quotient; SES, socioeconomic status
As ascertained by interviewing parents with the Diagnostic Interview for Children and Adolescents-V (DICA-IV; (Reich et al. 1997), 19 children met DSM-IV-TR criteria for ADHD-combined type, and 5 met criteria for ADHD-predominantly inattentive type (ignoring the ASD exclusion). Severity of ADHD symptomatology was assessed using the ADHD Index from Conners Parent Rating Scale-Revised (CPRS-R; Conners 1997; mean CPRS-R ADHD Index T-score=76.1, SD=6.7) and the Conners Teacher Rating Scale-Revised (CTRS-R; Conners 1997; mean CTRS-R ADHD Index T-score=67.2, SD=8.7). In addition, the DICA-IV (Reich et al. 1997) revealed that five children also met DSM-IV criteria for oppositional defiant disorder, two for obsessive compulsive disorder, and one for separation anxiety. Exclusion criteria included serious neurological disorders (e.g., stroke, seizures), Down syndrome, Fragile X syndrome, Tourette syndrome, psychosis, and mood disorders. Thirteen children had previously taken stimulant medication, which was discontinued ≥1 week prior to entry into the trial (mean discontinuation before trial=63 days, range: 7–547 days). Seven children were stable on long-term (>3 months) medications that they continued (at a constant dose) during the trial: risperidone (n=3), aripiprazole (n=1), sertraline (n=1), bupropion (n=1), and trazodone (n=1). All children were community based and lived at home; they were recruited from special education classrooms of a large metropolitan public school district. The study was approved by the University of Texas Health Science Center Institutional Review Board (IRB).
Design
We used a within-subject, crossover, placebo-controlled design. Prior to starting the drug trial, all children received a single-blind week of placebo (study personnel were unblinded), during which the medication-taking regimen was established both at home and at school. As an extra safety precaution following the single-blind placebo week, each child received a single blind week of “lead-in dosing” during which they were given 2 days each of low, medium, and high MPH doses that they would receive in the double-blind phase of the study, in ascending order. The children were seen by the study physician (CWS) and the study psychologist (DAP) at the end of this lead-in dosing week to insure that they tolerated the three doses well; all 24 children were cleared to take all three doses of the medication in the double-blind phase. Although all 24 children completed the trial, 5 of the 24 children discontinued the afternoon IR-MPH dose because of behavior concerns in late afternoon/evening. All five of these children experienced irritability; in addition, the following symptoms were seen in two or more children: decreased sleep (two), increased stereotypic behaviors (two).
During the actual medication trial, each child received 1 week each of the four MPH dosing regimens (placebo, low dose MPH, medium dose MPH, and high dose MPH). The order of dosage administration was counterbalanced across children using a digram-balanced Latin squares procedure that controls for both order and sequence (Wagenarr 1969). Dosing was based on body weight; doses were similar to those used in the MTA and the RUPP MPH trial. As can be seen in Table 2, the children received Ritalin LA (ER-MPH) at breakfast and IR-MPH in the afternoon. In an attempt to minimize side effects, no child received a dose greater than the equivalent of an IR-MPH dose of 0.6 mg/kg, and no child's total daily dose exceeded the equivalent of an IR-MPH b.i.d. dose of 50 mg. As seen in Table 2, the mean IR-MPH per dose equivalents of the Ritalin LA (given in the morning) were 0.21 mg/kg MPH in the low dose, 0.35 mg/kg in medium dose, and 0.48 mg/kg in the high dose. The IR-MPH dose (given in the afternoon) was sculpted to be approximately half of each single-dose equivalent of the morning's Ritalin LA. Ritalin LA was selected as the ER-MPH formulation because its pharmacokinetics mirrored the b.i.d. dosing of IR-MPH used in the morning/noon dosing of the RUPP study, its ER formulation improved ease of administration and hence compliance, and its beaded technology allowed for sprinkling the beads on applesauce for children with oral apraxias (eight study children did this). The study medication was prepared by the University of Texas Psychiatry Research Pharmacy: the Ritalin LA beads were mixed with (inert) placebo beads and placed in two opaque gelatin capsules, and the white generic IR-MPH was crushed and mixed with cornstarch and placed in two size 1 gelatin capsules. All study personnel with patient contact were blind with respect to dosages given during the drug trial.
Table 2.
Extended Release Methylphenidate and Immediate Release Methylphenidate (IR-MPH) Dosing Levels, by Child's Body Weight
| Methylphenidate (MPH) dose regimen | Morning dose: Ritalin LA | Afternoon dose: IR-MPH |
|---|---|---|
| Lower body weight group (20–24 kg) | ||
| Low dose | 10 mg Ritalin LA | 2.5 mg IR-MPH |
| Medium dose | 15 mg Ritalin LA | 5 mg IR-MPH |
| High dose | 20 mg Ritalin LA | 5 mg IR-MPH |
| Medium body weight group (25–33 kg) | ||
| Low dose | 10 mg Ritalin LA | 5 mg IR-MPH |
| Medium dose | 20 mg Ritalin LA | 5 mg IR-MPH |
| High dose | 30 mg Ritalin LA | 10 mg IR-MPH |
| Larger body weight group (34–59 kg) | ||
| Low dose | 20 mg Ritalin LA | 5 mg IR-MPH |
| Medium dose | 30 mg Ritalin LA | 10 mg IR-MPH |
| High dose | 40 mg Ritalin LA | 10 mg IR-MPH |
Mean MPH Dosing Levels, in mg/kg IR-MPH Dose Equivalents, Across All Body Weight Groups
| MPH dosing regimen | Morning Ritalin LA: mg/kg equivalent in each of the two IR-MPH doses | Afternoon IR-MPH dose |
|---|---|---|
| Low dose | 0.21 mg/kg | 0.14 mg/kg |
| Medium dose | 0.35 mg/kg | 0.24 mg/kg |
| High dose | 0.48 mg/kg | 0.27 mg/kg |
Procedure
Participants were recruited to the medication treatment trial after completing assessment that included a standardized neuropsychological test battery (including Stanford-Binet) and psychiatric interview. Participants were given a physical examination by the study physician (CWS) to confirm medical eligibility to take MPH. The children were seen at the end of the (single-blind) placebo baseline week, the end of the lead-in dosing week, and at the end of each week of the drug trial for both a medication check with the study physician (CWS) and for an interview with the study psychologist (DAP). Parents completed behavioral questionnaires each week, and were urged to base their ratings on weekend behaviors (when they would have seen their children under the influence of the full dose of medication), and to focus on their child's morning behavior (i.e., the Ritalin LA dose), to create comparability with the teacher ratings (who would only see the children on the Ritalin LA). Parents also completed a medication side effects questionnaire each week referring to common side effects associated with MPH treatment (Physician's Desk Reference; Thompson Health Care 2009). Similar questionnaires and side effects lists were also collected from the teachers each week. Parent ratings were available for all 24 children, whereas teacher ratings were only available for 18 children (6 were assessed in summer when school was in recess).
Instruments
Behavioral ratings of medication response were obtained using parent/teacher questionnaires and clinician ratings of global impressions of severity and improvement. Parents and teachers were asked to rate the children's behavior during the previous week (i.e., from the start of the new medication dose). Multiple behavioral questionnaires were administered: the Conners because it measured core ADHD behavior and closely comorbid behavior, the Swanson, Nolan, and Pelham Questionnaire, Revised for DSM-IV (SNAP-IV) because it measures DSM-IV based symptomatology, the ADD-H Comprehensive Teacher Rating Scale (ACTeRS) because of its emphasis on attention and also social skills, and the Aberrant Behavior Checklist (ABC) because it covers behavioral domains associated with developmental disabilities. Based on our previous work (Pearson et al. 2003), the primary outcome measure for this study was the CTRS-R. Although other instruments were used to assess specific aspects of MPH response in this study, they should be interpreted in the context of being secondary outcome measures.
CPRS-R and CTRS-R
These widely used questionnaires assess ADHD symptomatology and comorbid behaviors commonly associated with ADHD, including both externalizing and internalizing symptoms. They are sensitive to medication treatment response in children with ADHD in the general school-age population (Conners 1997), and in children with ASD and symptoms of ADHD (e.g., Handen et al. 2000; Ghuman et al. 2009). The short forms of the CPRS-R (CPRS-R:S–27 items) and CTRS-R (CTRS-R:S–28 items) were used, as well as the parent and teacher forms of the Conners Global Index (CGI-P, 10 items; CGI-T, 10 items). The CPRS-R:S includes four subscales: Oppositional, Cognitive Problems/Inattention, Hyperactivity, and the ADHD Index. The CGI-P yields scores on two subscales (CGI: Restless-Impulsive and CGI: Emotional Lability) as well as an overall score, CGI: Total (previously known as the “Hyperactivity Index”). These instruments are normed for children and adolescents 3–17 years old. Estimates of symptom severity were obtained using T-scores (mean=50, SD=10), with higher T-scores reflecting greater psychopathology.
SNAP-IV
The SNAP-IV (Swanson et al. 2001) quantifies the DSM-IV criteria for ADHD, using a 0–3 Likert scale. In this study, we used the 18 item version, which contains the Inattention subscale (9 items) and the Hyperactivity-Impulsivity subscale (9 items). Parents and teachers completed the SNAP-IV, which has been found to be sensitive to MPH treatment in children with PDD and symptoms of ADHD (Posey et al. 2007).
ACTeRS, Parent and Teacher Forms
The ACTeRS (Ullmann et al. 2000) questionnaires measure core ADHD symptoms of attention and hyperactivity, and the highly comorbid concerns of social skills and oppositional behavior. Whereas higher scores on hyperactivity and oppositional behavior are associated with worse symptomatology, higher scores on attention and social skills are associated with better behavior. The ACTeRS is sensitive to stimulant effects in children with and without developmental disabilities (Waterhouse et al. 1996; Pearson et al., 2003), and is normed for children 5–12 years old.
ABC
The ABC (Aman et al. 1985; Aman & Singh 1994) is a behavior questionnaire developed to rate symptoms of hyperactivity, irritability, social withdrawal, stereotypic behavior, and inappropriate speech in individuals with developmental disabilities. It is sensitive to MPH treatment in children with autism and symptoms of ADHD (e.g., Handen et al. 2000; RUPP Autism Network 2005). Because some studies have shown stimulant-related increases in stereotypies in some children with autism, it was important to monitor these symptoms.
Visual Analog Scale (VAS; Wewers & Lowe 1990)
Parents were asked to first describe the most troublesome symptom that their child displayed (e.g., inattentiveness, hyperactivity), and then to indicate the severity along a 100 mm line from “very mild” to “extremely severe.” The dependent measure was the number of mm from the left (very mild) side of the horizontal line to the parent's mark; higher VAS scores were indicative of more problematic behavioral concerns. This measure has been shown to be sensitive to medication treatment in autism (e.g., Aman et al. 2002).
Clinician measures
Clinical Global Impressions (CGI)
The CGI has two key domains: The Severity and Improvement scales. Using the modifications described by Arnold et al. (2000) for children with ASD, we obtained CGI-Severity (CGI-S) and CGI-Improvement (CGI-I) ratings after each study visit. Both the CGI-S and CGI-I are scaled from 0 to 7, and were used to document overall severity (e.g., ADHD, autistic) and improvements relative to the baseline week of the trial. Two blinded clinicians (DAP and CWS) completed these measures, after achieving reliability on training vignettes provided by MGA. All sources of information were taken into account including data from behavioral questionnaires, interviews with the parents and children, and observations by the study staff.
Side effects questionnaire
The parents and teachers completed a brief checklist based on the most common side effects of MPH listed in the Physician's Desk Reference (Thompson Health Care 2009). In addition to the symptoms listed in the checklist, there was an open-ended question at the end about other atypical behaviors that parents/teachers noticed during the week.
Compliance
Compliance was assessed by having the parents complete a medication administration form, documenting dates and times that doses were dispensed. Parents were also asked about any missed or late doses during their weekly clinic interview (with DAP and RM); teachers were also queried by a study coordinator (RM) during weekly school visits to collect and dispense teacher questionnaires. The number of pills remaining in the returned home and school vials was counted and verified against the medication administration forms. Families were asked for additional information about discrepancies. If a question was left unanswered on a behavioral questionnaire, the parent or teacher was asked for additional information.
Data analysis
The data were analyzed using an SPSS-PC (Version 19.0) repeated measures analysis of variance procedure (ANOVA), with MPH dosage as the within-subjects variable. Because preliminary analyses revealed no significant effects of sex or dose order, these factors were dropped from subsequent analyses. For significant effects, follow-up trend analyses were performed to determine if dose response was primarily linear, or if there were other components of trend such as a curvilinear response (e.g., an initial improvement in behavior from placebo to a low or medium dose, followed by behavioral declines). Finally, for measures that demonstrated significant MPH dosage effects, a sequential Bonferroni post-hoc analysis (Holm 1979) was used to determine which MPH doses were significantly different from one another (p≥0.05).
Results
Parent behavioral ratings are summarized in Table 3 and illustrated in Figure 2, teacher ratings in Table 4 and Figure 3, and clinician ratings are summarized in Table 5. For instruments that have both raw scores (which measure absolute level of symptomatology) and standard scores (that measure symptomatology in comparison with the child's peer group), both sets of scores were analyzed; both produced identical patterns of results. Although raw scores are presented for most instruments in Tables 3 and 4, the T-scores for the Conners scales are presented for ease of interpretation.
Table 3.
Effect of Methylphenidate (MPH) Treatment on Parent Behavioral Ratings
| |
MPH dose level |
Effect of MPH dose level |
|
|||||
|---|---|---|---|---|---|---|---|---|
| Parent behavioral instrument (Means/SD) | Placebo | Low dose MPH | Medium dose MPH | High dose MPH | ANOVA p-value | Linear trend p-value | Quadratic trend p-value | Source of significance |
| Conners Parent Rating Scale-Revised (CPRS-R) | ||||||||
| CGI: Restless-Impulsive | 69.5 | 63.9 | 60.9 | 58.2 | 0.000 | 0.000 | 0.429 | P:M, P:H. L:H |
| (12.6) | (10.3) | (9.8) | (9.8) | |||||
| CGI: Emotional Lability | 57.3 | 54.2 | 54.5 | 49.3 | 0.089 | 0.020 | 0.573 | (n/a) |
| (15.8) | (14.0) | (13.6) | (10.8) | |||||
| CGI: Total | 66.8 | 61.9 | 59.9 | 55.8 | 0.001 | 0.000 | 0.818 | P:M, P:H. L:H |
| (12.7) | (11.7) | (9.8) | (9.3) | |||||
| Oppositional | 59.2 | 53.1 | 53.8 | 49.8 | 0.021 | 0.010 | 0.620 | None pairwise |
| (16.0) | (15.3) | (12.4) | (11.4) | |||||
| Cognitive problems/Inattention | 70.3 | 67.7 | 63.9 | 60.0 | 0.001 | 0.000 | 0.818 | P:M, P:H. L:H |
| (12.8) | (11.6) | (12.5) | (11.7) | |||||
| Hyperactivity | 70.8 | 62.6 | 62.1 | 58.3 | 0.000 | 0.000 | 0.335 | P:M, P:H |
| (15.2) | (12.2) | (12.0) | (10.2) | |||||
| ADHD Index | 70.1 | 64.9 | 62.3 | 59.9 | 0.000 | 0.000 | 0.454 | P:M, P:H |
| (11.9) | (9.3) | (10.0) | (10.5) | |||||
| SNAP-IV-Parent | ||||||||
| Inattentive | 17.8 | 15.0 | 14.0 | 12.7 | 0.000 | 0.000 | 0.463 | P:M, P:H |
| (5.8) | (6.1) | (4.8) | (6.0) | |||||
| Hyperactive | 13.8 | 11.0 | 8.8 | 8.9 | 0.001 | 0.000 | 0.146 | P:M, P:H |
| (6.6) | (6.5) | (5.8) | (5.3) | |||||
| Combined | 31.6 | 26.0 | 22.8 | 21.6 | 0.000 | 0.000 | 0.245 | P:M, P:H |
| (11.0) | (11.1) | (9.6) | (9.6) | |||||
| ACTeRS Parent | ||||||||
| Attention | 10.9 | 12.4 | 13.4 | 14.0 | 0.068 | 0.001 | 0.665 | P:H |
| (4.7) | (5.3) | (5.0) | (4.2) | |||||
| Hyperactivity | 15.7 | 13.3 | 12.0 | 12.0 | 0.000 | 0.000 | 0.069 | P:M, P:H |
| (4.5) | (4.5) | (4.0) | (4.3) | |||||
| Social Skills | 12.8 | 13.3 | 12.0 | 14.0 | 0.048 | 0.288 | 0.235 | M:H |
| (4.5) | (4.4) | (3.7) | (4.3) | |||||
| Oppositional | 8.4 | 7.8 | 6.8 | 6.6 | 0.035 | 0.021 | 0.596 | P:M |
| (5.3) | (5.6) | (4.0) | (2.6) | |||||
| Aberrant Behavior Checklist | ||||||||
| Irritability | 12.6 | 10.0 | 8.2 | 7.2 | 0.004 | 0.002 | 0.489 | P:H |
| (10.4) | (9.2) | (8.1) | (6.9) | |||||
| Lethargy/Social withdrawal | 9.3 | 7.3 | 8.1 | 8.5 | 0.424 | 0.678 | 0.180 | (n/a) |
| (8.1) | (5.6) | (5.9) | (6.6) | |||||
| Stereotypy | 4.9 | 4.3 | 4.0 | 3.5 | 0.302 | 0.073 | 0.907 | (n/a) |
| (5.4) | (4.5) | (3.8) | (3.8) | |||||
| Hyperactivity | 24.1 | 18.1 | 14.5 | 14.5 | 0.000 | 0.000 | 0.082 | P:M, P:H |
| (13.0) | (10.5) | (7.7) | (9.2) | |||||
| Inappropriate speech | 5.2 | 4.3 | 4.0 | 3.9 | 0.010 | 0.003 | 0.214 | P:M, P:H |
| (3.1) | (3.2) | (3.1) | (3.1) | |||||
| Visual Analogue Scale(VAS) | ||||||||
| VAS Measure (in mm) | 95.6 | 80.9 | 79.6 | 69.0 | 0.006 | 0.005 | 0.666 | P:H |
| (20.2) | (25.2) | (29.1) | (32.1) | |||||
| Social Communication Questionnaire(SCQ) | ||||||||
| SCQ Score | 15.5 | 14.2 | 15.2 | 13.4 | 0.046 | 0.026 | 646 | None pairwise |
| (6.1) | (6.6) | (6.2) | (6.2) | |||||
Bold indicates that there was a statistically significant effect of MPH dose (i.e., p < .05).
CGI, Clinical Global Impressions; ADHD, attention-deficit/hyperactivity disorder; SNAP-IV, Swanson, Nolan, and Pelham Questionnaire, Revised for American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV); ACTeRS, ADD-H Comprehensive Teacher Rating Scale; P, placebo; L, low dose MPH; M, medium dose MPH; H, high dose MPH.
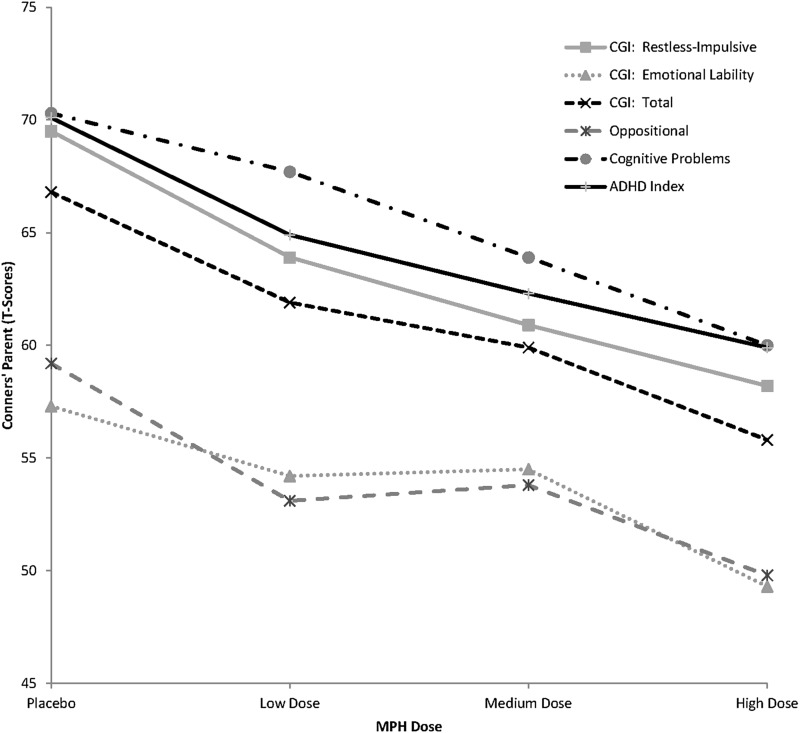
FIG. 2.
Parent behavioral ratings on the Conners' Parent Rating Scale-Revised (CPRS-R) as a function of methylphenidate dose in children with autism spectrum disorders (ASD) and symptoms of attention-deficit/hyperactivity disorder (ADHD).
Table 4.
Effect of Methylphenidate (MPH) Treatment on Teacher Behavioral Ratings
| |
MPH dose level |
Effect of MPH dose level |
|
|||||
|---|---|---|---|---|---|---|---|---|
| Teacher behavioral instrument (Means/SD) | Placebo | Low dose MPH | Medium dose MPH | High dose MPH | ANOVA p-value | Linear trend p-value | Quadratic trend p-value | Source of significance |
| Conners Teacher Rating Scale-Revised (CTRS-R) | ||||||||
| CGI: Restless-Impulsive | 76.0 | 63.6 | 62.3 | 61.2 | 0.000 | 0.000 | 0.058 | P:L, P:M, P:H |
| (10.9) | (10.8) | (10.8) | (13.9) | |||||
| CGI: Emotional Lability | 66.3 | 59.1 | 59.4 | 53.2 | 0.043 | 0.022 | 0.874 | None pairwise |
| (18.8) | (16.8) | (14.8) | (9.5) | |||||
| CGI: Total | 75.6 | 63.4 | 62.4 | 59.3 | 0.000 | 0.000 | 0.144 | P:L, P:M, P:H |
| (11.5) | (12.8) | (12.5) | (12.7) | |||||
| Oppositional | 65.1 | 56.6 | 58.1 | 55.1 | 0.111 | 0.097 | 0.367 | (N/A) |
| (19.5) | (13.2) | (15.4) | (13.5) | |||||
| Cognitive problems/Inattention | 63.0 | 58.3 | 57.8 | 59.3 | 0.044 | 0.104 | 0.023 | P:L, P:M, P:H |
| (11.2) | (10.7) | (9.5) | (11.1) | |||||
| Hyperactivity | 70.3 | 59.9 | 59.9 | 57.7 | 0.000 | 0.000 | 0.107 | P:L, P:M, P:H |
| (13.5) | (13.6) | (11.4) | (11.3) | |||||
| ADHD Index | 72.8 | 63.1 | 63.6 | 61.5 | 0.001 | 0.000 | 0.127 | P:L, P:M, P:H |
| (12.0) | (11.2) | (10.4) | (13.0) | |||||
| SNAP-IV-Teacher | ||||||||
| Inattentive | 18.2 | 15.2 | 13.9 | 14.0 | 0.106 | 0.070 | 0.258 | (N/A) |
| (6.0) | (6.1) | (5.7) | (7.7) | |||||
| Hyperactive | 14.4 | 9.5 | 9.7 | 7.8 | 0.003 | 0.004 | 0.244 | P:H |
| (8.3) | (6.9) | (7.2) | (5.7) | |||||
| Combined | 32.7 | 24.7 | 23.7 | 21.8 | 0.012 | 0.010 | 0.235 | P:H |
| (12.4) | (10.9) | (11.1) | (11.6) | |||||
| ACTeRS Teacher | ||||||||
| Attention | 11.3 | 14.3 | 13.7 | 15.9 | 0.072 | 0.029 | 0.714 | (N/A) |
| (4.0) | (6.2) | (5.5) | (8.5) | |||||
| Hyperactivity | 18.7 | 11.8 | 12.8 | 11.2 | 0.000 | 0.000 | 0.048 | P:L, P:M, P:H |
| (5.2) | (5.6) | (5.5) | (5.0) | |||||
| Social skills | 15.8 | 19.2 | 16.7 | 18.5 | 0.080 | 0.196 | 0.389 | (N/A) |
| (3.6) | (4.2) | (4.0) | (5.8) | |||||
| Oppositional | 10.6 | 7.6 | 9.2 | 7.1 | 0.008 | 0.015 | 0.601 | P:H |
| (5.8) | (3.5) | (5.5) | (2.1) | |||||
Bold indicates that there was a statistically significant effect of MPH dose (i.e., p < .05).
CGI, Clinical Global Impressions; ADHD, attention-deficit/hyperactivity disorder; SNAP-IV, Swanson, Nolan, and Pelham Questionnaire, Revised for American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV); ACTeRS, ADD-H Comprehensive Teacher Rating Scale; P, placebo; L, low dose MPH; M, medium dose MPH; H, high dose MPH.
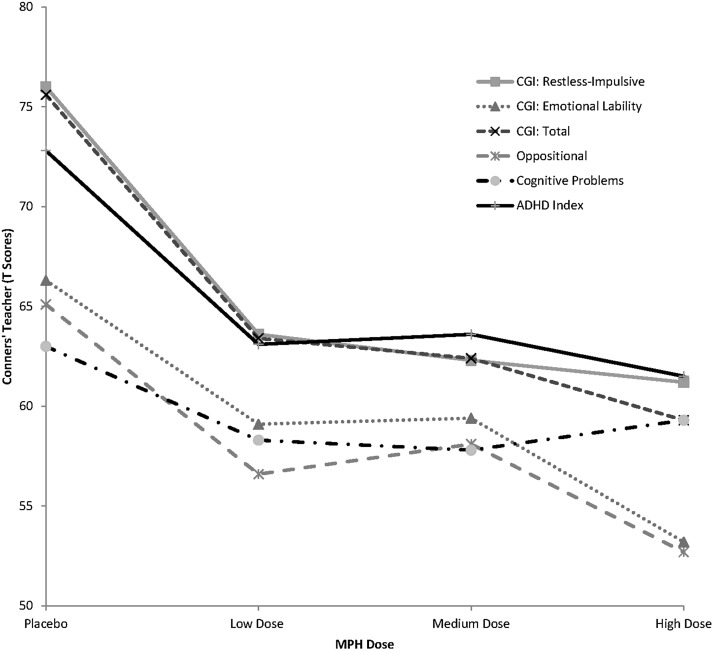
FIG. 3.
Teacher behavioral ratings on the Conners' Teacher Rating Scale-Revised (CTRS-R) as a function of methylphenidate dose in children with autism spectrum disorders (ASD) and symptoms of attention-deficit/hyperactivity disorder (ADHD).
Table 5.
Effect of Methylphenidate (MPH) Treatment on Clinical Global Impressions (CGI) Ratings
| |
MPH dose level |
Effect of MPH dose level |
|
|||||
|---|---|---|---|---|---|---|---|---|
| CGI measure: Mean/SD (Clinician) | Placebo | Low dose MPH | Medium dose MPH | High dose MPH | ANOVA p-value | Linear trend p-value | Quadratic trend p-value | Source of significance |
| CGI-Severity (DAP) | 4.8 | 4.0 | 3.8 | 3.8 | 0.000 | 0.001 | 0.018 | P:L, P:M, P:H |
| (0.61) | (0.81) | (0.82) | (0.74) | |||||
| CGI-Improvement (DAP) | 4.0 | 2.8 | 2.4 | 2.1 | 0.000 | 0.000 | 0.126 | P:L, P:M, P:H |
| (0.81) | (1.3) | (1.3) | (1.2) | |||||
| CGI-Severity (CWS) | 4.7 | 4.0 | 4.0 | 3.9 | 0.000 | 0.001 | 0.086 | P:L, P:M, P:H |
| (0.76) | (0.72) | (0.62) | (0.74) | |||||
| CGI-Improvement (CWS) | 4.1 | 2.8 | 2.6 | 2.0 | 0.000 | 0.000 | 0.218 | P:L, P:M, P:H |
| (0.95) | (1.4) | (1.3) | (1.0) | |||||
Bold indicates that there was a statistically significant effect of MPH dose (i.e., p < .05).
P, placebo; L, low dose MPH; M, medium dose MPH; H, high dose MPH.
Parent measures
MPH treatment was associated with consistent improvements in parent ratings in core symptoms of ADHD, as well as in symptoms closely associated with ADHD. Significant dose-related improvements in attention were noted on the Conners F(3,69)=8.11, p<0.001 and the SNAP-IV, F(3,69)=5.92, p=0.001. Although attentional improvements were also noted on the ACTeRS, they were not quite significant, F(3,69)=2.67, p=0.068. Similar reductions in symptoms of hyperactivity were also noted on four parent instruments: the ABC, F(3,69)=10.38, p<0.001; the Conners, F(3,69)=7.19, p<0.001; the ACTeRS, F(3,69)=7.15, p<0.001; and the SNAP-IV, F(3,69)=7.51, p<0.001. MPH treatment was also associated with substantial declines in symptoms of impulsivity on the Conners, F(3,69)=8.62, p<0.001. MPH response was linear (see Table 3): higher doses of MPH were associated with successive declines in core ADHD symptoms in the dose range studied in this project. Inspection of the mean scores, for example, on the Conners, suggest that MPH treatment resulted in declines from the clinically significant range (e.g., T-scores of ∼70) to the nonclinical range (T-scores of ≤59).
In addition to symptoms of ADHD, MPH treatment was also associated with reductions in oppositional behavior on both the Conners, F(3,69)=3.46 p<0.021 and the ACTeRS, F(3,69)=3.03 p=0.035. Interestingly, MPH treatment in this higher-functioning sample of children with ASD was associated with decreases in irritability, F(3,69)=4.92, p=0.004, and in inappropriate speech, F(3,69)=4.05, p=0.01 on the ABC. MPH treatment was associated with improvements in social skills on the ACTeRS, F(3,69)=2.76, p=0.048. Although decreases in social withdrawal/lethargy and stereotypic behavior were also seen at higher MPH doses on the ABC; neither of these declines was significant. It is important to note that MPH did not exacerbate stereotypies in this population, and actually reduced symptoms of irritability. Finally, as indicated by their weekly VAS ratings, parents also noted significant declines with MPH treatment in the symptom that they found to be the most troublesome in their child, F(3,69)=4.57 p=0.006.
Post-hoc analyses of the parent behavior ratings revealed that the most effective reductions in hyperactive, impulsive, and inattentive behaviors, relative to placebo, occurred with the highest MPH dose condition. However, significant improvements (relative to placebo) were also detected by parents at the medium MPH dose level, and the only significant improvement for MPH in oppositional behavior (relative to placebo) was noted at this medium dose. Interestingly, parents did not detect significant behavioral improvements between the placebo and the low MPH dose in any behavioral domain, suggesting that the low dose was insufficient to produce a detectable behavioral response in the home (most children were observable for the full day during weekends at home). In addition to these improvements in inappropriate behaviors, parents also noted a significant gain in social skills at the highest MPH dose, along with a significant decline in irritability. Therefore, the highest MPH dose produced both reductions in inappropriate behaviors and improvements in prosocial behavior.
Parents also noted significant improvements on their VAS ratings of their child's most problematic symptoms. Although their ratings declined successively with ascending MPH doses, it was only at the highest MPH dose that a significant improvement relative to placebo was noted on the VAS.
Teacher measures
Teachers noted dose-related declines in symptoms of hyperactivity (Conners: F[3,51]=8.25, p<0.001; SNAP-IV: F[3,51]=5.26, p<0.003; ACTeRS: F[3,51]=9.67, p<0.001) and impulsivity (Conners: F[3,45]=9.69, p<0.001). Although teachers reported declines in oppositional behavior at higher MPH doses; only one measure reached statistical significance (ACTeRS: F[3,51]=4.35, p=0.008), whereas the other did not (Conners: F[3,51]=2.11, p=0.11). Teachers also noted a decline in emotional lability (Conners: F[3,45]=2.94, p=0.04) in the school setting. Declines in inattention were not as dramatic (Conners: F[3,51]=2.90, p=0.04) as the declines in hyperactivity and impulsivity. Two other teacher ratings of attention were not associated with significant MPH-related improvements (ACTeRS: F[3,51]=2.48, p=0.07; SNAP-IV: F[3,51]=2.14, p=0.11). These findings are consistent with the RUPP (2005) finding of less MPH-related improvement in inattention, relative to improvement in hyperactivity, in children with ASD and symptoms of ADHD.
Post-hoc analyses revealed that teachers—like parents—detected the greatest gains in behavior relative to placebo at the highest MPH dose. They were also able to detect significant behavioral gains at all three MPH dose levels, relative to placebo. Unlike parents in the home setting, teachers were able to discern improvements in inattentive, hyperactive, and impulsive behaviors at the lowest MPH dose. Like parents, they also found MPH to be effective in treating oppositional symptoms, but only at the highest dose level on the ACTeRS. Although teachers did not complete an instrument measuring irritability, it is interesting to note that their ratings of emotional lability in the classroom declined from the very clinically significant range (T=76) to the nonclinical range (T=61) on the Conners.
Finally, trend analyses of parent and teacher behavioral ratings generally revealed that MPH dose response was linear, that is, higher doses of MPH were associated with successive improvements in behavior. In fact, only two subscales (ACTeRS-Teacher: Hyperactivity and CTRS-R: Cognitive Problems/Inattention) showed significant quadratic dose-response trends. Inspection of these two dose-response curves revealed a leveling off or declines in hyperactivity on the ACTeRS-T (the linear trend was also significant), but some deterioration in attention on the Conners was noted by teachers at the highest dose.
Clinician measures
As noted in Table 5, significant effects of MPH treatment on Clinical Global Ratings (CGI's) of both current severity and improvement (relative to the single-blind placebo run-in week) were found by both the psychiatrist (CWS) (CGI-S: F[3,69]=7.62, p<0.001; CGI-I: F[3,69]=15.49, p<0.001) and psychologist (DAP) (CGI-S: F[3,69]=12.46, p<0.001; CGI-I: F[3,69]=12.62, p<0.001). Although CWS and DAP completed their ratings independently, their ratings were quite similar (kappas for these two raters on the various CGI ratings ranged from 0.496 to 0.721, all p<0.001), and both noted very substantial improvements relative to placebo for all three MPH doses. The most dramatic improvement occurred at the highest dose, in which both clinician ratings were ∼2.0 (“much improved”), relative to placebo ratings of 4.1 (no change). According to the ratings done by CWS, 67% of the sample achieved a CGI-I score of 1 or 2 at one of the MPH doses; according to ratings done by DAP, 79% of the sample did. As noted in Table 6, these doses were not associated with significant elevations in blood pressure or pulse rate or with significant weight loss during this brief treatment trial (all tests were nonsignificant).
Table 6.
Weight, Blood Pressure, and Pulse at Baseline and at each Methylphenidate (MPH) Dosing Level: Means and Standard Deviations
| Variablea | Baseline | Placebo | Low dose MPH | Medium dose MPH | High dose MPH |
|---|---|---|---|---|---|
| Weight in kg | 32.49 | 33.03 | 32.90 | 32.47 | 32.39 |
| (8.3) | (8.6) | (8.8) | (8.8) | (8.5) | |
| Blood pressure: Systolic | 105.5 | 105.7 | 106.4 | 103.6 | 109.0 |
| (8.4) | (10.3) | (10.1) | (9.4) | (8.6) | |
| Blood pressure: Diastolic | 71.17 | 70.48 | 70.43 | 70.17 | 73.04 |
| (8.1) | (8.3) | (8.4) | (7.5) | (7.6) | |
| Pulse | 91.22 | 89.57 | 94.17 | 97.43 | 94.83 |
| (14.4) | (17.3) | (13.3) | (15.8) | (14.9) |
There was no significant effect of MPH dose on any of these variables.
Ratings of side effects
Parent and teacher ratings of side effects are summarized in Table 7. There were no significant effects of medication dose on any of the teacher ratings. Parents reported significant loss of appetite (F[3,69]=6.14, p=0.001) and sleeping problems (F[3,69]=2.81, p=0.05) at higher doses of methylphenidate. Nine of 24 parents (38%) reported insomnia at the high dose, compared to five (21%) while taking placebo; 9 parents reported loss of appetite at the high dose, compared to only one during placebo.
Table 7.
Number of Children Experiencing Side Effect Symptoms, by Methylphenidate (MPH) Dose Condition and Rater
| |
Parent Ratings |
Teacher Ratings |
||||||
|---|---|---|---|---|---|---|---|---|
| Symptom | Placebo | Low dose MPH | Medium dose MPH | High dose MPH | Placebo | Low dose MPH | Medium dose MPH | High dose MPH |
| Loss of appetitea | 1 | 7 | 9 | 9 | 0 | 0 | 1 | 2 |
| (4%) | (29%) | (38%) | (38%) | (0%) | (0%) | (4%) | (8%) | |
| Trouble sleepingb | 5 | 7 | 12 | 9 | 1 | 0 | 1 | 1 |
| (21%) | (29%) | (50%) | (38%) | (4%) | (0%) | (4%) | (4%) | |
| Anxiety | 4 | 4 | 6 | 2 | 4 | 5 | 6 | 5 |
| (17%) | (17%) | (25%) | (8%) | (17%) | (21%) | (25%) | (21%) | |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | |
| Drowsiness | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 2 |
| (4%) | (4%) | (4%) | (8%) | (8%) | (0%) | (0%) | (8%) | |
| Dry mouth | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 1 |
| (0%) | (4%) | (8%) | (0%) | (0%) | (4%) | (0%) | (4%) | |
| Euphoria | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| (4%) | (0%) | (0%) | (0%) | (4%) | (0%) | (0%) | (0%) | |
| Facial or body tics | 0 | 1 | 1 | 2 | 3 | 2 | 1 | 1 |
| (0%) | (4%) | (4%) | (8%) | (13%) | (8%) | (4%) | (4%) | |
| Fever | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (4%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | |
| Hair or Skin Pulling | 2 | 1 | 1 | 2 | 1 | 0 | 2 | 1 |
| (8%) | (4%) | (4%) | (8%) | (4%) | (0%) | (8%) | (4%) | |
| Headache | 2 | 2 | 4 | 1 | 0 | 1 | 0 | 1 |
| (8%) | (8%) | (17%) | (4%) | (0%) | (4%) | (0%) | (4%) | |
| Irritability | 11 | 8 | 8 | 7 | 5 | 4 | 5 | 2 |
| (46%) | (33%) | (33%) | (29%) | (21%) | (17%) | (21%) | (8%) | |
| Nausea | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 |
| (4%) | (0%) | (4%) | (4%) | (0%) | (0%) | (8%) | (0%) | |
| Racing heart | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| (0%) | (0%) | (4%) | (0%) | (0%) | (0%) | (4%) | (0%) | |
| Repetitive behavior | 12 | 11 | 12 | 9 | 8 | 9 | 9 | 6 |
| (50%) | (46%) | (50%) | (75%) | (33%) | (38%) | (38%) | (25%) | |
| Repetitive language | 12 | 9 | 12 | 9 | 7 | 4 | 6 | 5 |
| (50%) | (75%) | (50%) | (75%) | (29%) | (17%) | (25%) | (21%) | |
| Sadness | 3 | 2 | 4 | 1 | 5 | 4 | 3 | 3 |
| (13%) | (8%) | (17%) | (4%) | (21%) | (17%) | (13%) | (13%) | |
| Skin rash | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| (0%) | (4%) | (4%) | (4%) | (0%) | (0%) | (0%) | (0%) | |
| Staring | 4 | 0 | 2 | 1 | 4 | 5 | 5 | 7 |
| (17%) | (0%) | (8%) | (4%) | (17%) | (21%) | (21%) | (29%) | |
| Stomachache | 1 | 1 | 3 | 2 | 0 | 0 | 0 | 1 |
| (4%) | (4%) | (13%) | (8%) | (0%) | (0%) | (0%) | (4%) | |
| Unusual blinking | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| (0%) | (4%) | (4%) | (0%) | (4%) | (4%) | (4%) | (4%) | |
Parents noted significantly higher rate of loss of appetite at higher MPH doses, p=0.001
Parents noted significantly higher rate of sleeping problems at higher MPH doses, p=0.05
There were no other significant effects of MPH does on any other symptoms.
Adherence
Medication adherence was quite high, with less than one dose being missed each week overall. Percent adherence for the morning dose (Ritalin LA) was 99.4% in the placebo week, 100% in the low and medium dose weeks, and 98% in the high dose week. Adherence for the afternoon dose (IR-MPH) was 100% in the placebo condition, 95% in the low dose, 93% in the medium dose, and 92% in the high dose.
Discussion
In this sample of children with ASD, both parents and teachers noted significant declines with MPH treatment in the three central elements of ADHD, with particularly consistent declines noted in hyperactivity and impulsivity in both the home and school settings. Improvements were also noted in oppositional behavior, irritability, and social skills. Although both parents and teachers reported the greatest improvements at the highest MPH dose level, parents and teachers also reported improvements at the medium dose, and teachers were even able to detect significant improvements at the low MPH dose. These behavioral gains were not accompanied by significant concerns in either side effects or changes in vital signs. No significant changes were noted in social withdrawal or in stereotypic behaviors (on the ABC) with MPH treatment. Therefore, the findings of this study suggest that children with ASD and symptoms of ADHD can be very effectively treated in the dose range studied with ER-MPH in the morning, and IR-MPH in the afternoon, for their ADHD symptoms, as well as oppositional symptoms, without significant risk of adverse effects.
Both parents and teachers were sensitive to MPH-related behavioral changes. This concurrence between parents and teachers stands in contrast to our previous findings of high teacher sensitivity to MPH-related behavioral improvements but relative insensitivity in parents' ability to detect MPH-related treatment change in children with intellectual disabilities and ADHD (Pearson et al. 2003). Our findings in this study are very consistent with those of the RUPP (2005) study of MPH treatment in children with ASD and symptoms of ADHD, perhaps reflecting the fact that the highest dose administered in this trial was nearly identical to that of the dose in the RUPP (0.50 mg/kg). As our group has noted (Pearson et al. 2012), it may be that parents of children with ASD share a similar perspective with teachers. Whatever the reason, it appears that clinicians can feel confident in titrating medication under circumstances in which only parent ratings are available. although parents may not be able to detect a treatment-related change at as low a dose as teachers can.
The side effects seen in our children were mild, and are very common in children with ADHD in the general population; therefore, our children with ASD and ADHD symptoms displayed similar side effects to other children with ADHD. Furthermore, symptoms that are commonly seen in the ASD population (e.g., repetitive behavior, repetitive language, irritability) did not appear to be exacerbated at higher MPH doses. Indeed, both parents and teachers reported a higher frequency of these symptoms of repetitive behavior, repetitive language, and irritability in the placebo condition than they did in the high dose condition. Therefore, our findings suggest that children with ASD and autism are not necessarily at higher risk for serious side effects of stimulant medication.
These findings should be interpreted in the context of the sample from which they were drawn. Although one third of the sample had mild intellectual disability, overall, these children represent a relatively high-functioning subgroup of community-based elementary school-age children with ASD. Previous studies have suggested that higher-functioning children with intellectual disabilities were more likely to have a favorable response to MPH treatment (Aman et al. 2003; Stigler et al. 2004). Consistent with the current estimates of psychotropic medication usage in the pediatric ASD population, approximately half this sample had taken MPH previous to their enrollment in the study (more than the ∼30% of the RUPP sample), and a few were taking stable doses of other psychoactive medications throughout the trial. Given that the majority of children with ASD have a history of psychotropic medication treatment (e.g., Pringle et al. 2012), recruiting a sample of school-age children with ASD who have significant symptoms of ADHD who were medication naive would have not been representative of the general pediatric ASD population. Furthermore, a school-age sample of stimulant-naïve children with ASD and ADHD would likely have contained only children with very mild symptoms of ADHD, and therefore, would not have been representative of children requiring treatment.
Although not a focus of this study, the overall pattern of MPH-related improvement was found in exploratory analyses to be similar in the subsample of children who were and were not stimulant naïve. Only 3/39 measures produced a significant effect of previous stimulant treatment (children with a history of previous stimulant treatment had more severe ADHD symptoms), but this effect operated similarly across MPH dosing conditions (i.e., there was no interaction with the MPH dose). This suggests that the children who previously took stimulants were not driving the pattern of treatment results found in this study, although it is possible that inclusion of children with a history of taking stimulants slightly increased the favorable clinical response to MPH.
Inclusion of children taking other medications may have improved the MPH response in this study. As Stigler and colleagues (2004) noted, concomitant medications were associated with improved response rates to MPH, perhaps because they may have, to some extent, provided protection from the adverse effects associated with psychostimulant treatment (e.g., increases in irritability, insomnia, and loss of appetite). In some cases involving cotherapy (e.g., stimulants and risperidone), it is possible that side effects cancel each other out (e.g., Farmer et al. 2011). We also conducted some exploratory analyses to examine the effect of other medication usage during the trial. The effect of taking other medication was significant for only 1/39 variables (children taking concomitant medications had more severe ADHD symptoms), and again, there was no interaction with MPH treatment. This finding is not surprising, given that concomitant medications were held constant throughout this within-subjects clinical trial. Furthermore, even with concomitant medications being used, these children still displayed sufficiently severe ADHD symptomatology that they qualified for the study.
We found no evidence of increased risk for stereotyped behaviors at higher MPH doses in this high-functioning group. Parent ratings of stereotypic behaviors actually declined steadily over higher MPH doses, albeit insignificantly so. However, this finding should be interpreted with caution, given that only one measure of this domain was assessed (i.e., by the parent ABC). Future investigations using more comprehensive measures of repetitive and stereotyped behaviors (e.g., the Repetitive Behavior Scale-Revised, Bodfish et al. 2000; Children's Yale-Brown Obsessive Compulsive Scale [CY-BOCS]-PDD, Scahill et al. 2006) across multiple settings/raters are needed to address this issue more thoroughly. Several previous controlled studies of stimulant treatment in children with ASD (Strayhorn et al. 1988; Quintana et al. 1995; Handen et al. 2000; Posey et al. 2007) also found no evidence of MPH-related increases in stereotypies in children with ASD and symptoms of ADHD. Birmaher et al. (1988) and Quintana et al. (1995) have suggested that the increase in stereotypies seen in children reported by Campbell and colleagues may have been related more to withdrawal dyskinesias associated with antipsychotic discontinuation, rather than to stimulant-induced stereotypies. Although it is difficult to draw comparisons between the high-functioning children in this sample and those reported in case studies 40 years earlier (with lower-functioning children who had more severe ASD symptoms than our sample), it is encouraging that our children did not experience the symptom of increased stereotypies with their MPH treatment.
Given the concerns noted in the early literature of stimulant treatment possibly exacerbating symptoms of ASD, we also administered a weekly screening of autistic symptomatology, the Social Communication Questionnaire (SCQ), which we reported in Table 3. Consistent with the findings of the ABC stereotypic behavioral declines, there was no evidence of an increase in autistic symptomatology with ascending MPH doses; the opposite was true, with a dose-related reduction in autistic symptoms, F(3,69)=2.81, p=0.046. Although it was not the purpose of this study to assess MPH-related changes in core autistic symptoms, these exploratory findings suggest that future investigations using more comprehensive measures of autistic symptomatology, for example, the Social Responsiveness Scale (Constantino and Gruber 2005) may be informative.
Limitations
As noted, some of the children in this sample were not stimulant naïve, and the children seen in this study were high-functioning relative to the broad spectrum of functional status in ASD. Our findings should be interpreted in the context of its community-based elementary school age sample. However, we believe that these are precisely the children who are most likely to be seen by pediatricians and child psychiatrists in general clinical practice (as opposed to specialized developmental pediatrics clinics). Another potential limitation is the brevity of this clinical trial. However, stimulant response is rapid, and as DiMartino et al. (2004) noted, children who did not show adverse effects of MPH in a single dose also did not demonstrate adverse effects in a 3 month trial. Furthermore, children who completed the initial 4 weeks of the RUPP MPH trial with a favorable response continued responding well (e.g., maintaining improvements in symptoms of hyperactivity) in their optional 2 month open-label follow-up period. Although findings such as these suggest that a favorable MPH response in a brief trial may be maintained at longer intervals, this question cannot be answered by this study. It is interesting to note that initial ADHD treatment response has not been found to predict long-term follow-up in either preschool children with ADHD after 6 years (Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study [PATS]; Riddle et al. 2013) or in school-age children with ADHD in the MTA after 3 years (Jensen et al. 2007) or after 8 years (Molina et al. 2009).
Our sample size was small, making it difficult to examine the results for predictors or moderators/mediators of response. Although ours was a relatively small study, it is only the second randomized controlled trial (RCT) to study the effect of MPH treatment in children with ASD and ADHD with a sample size >20 (Siegel and Beaulieu, 2012). Finally, our dosing range only extended to a “high” dose of ∼0.48 mg/kg (IR-MPH equivalent), similar to the RUPP study's highest dose (0.50 mg/kg). Investigators in the future may wish to explore the possibility that higher MPH doses might be associated with even greater behavioral improvements in some children with ASD, with appropriate vigilant monitoring of side effect symptoms.
Clinical Significance
Psychostimulant treatment—using a dosing regimen featuring ER-MPH (in the morning) and IR-MPH (in the afternoon)—in children with ASD and symptoms of ADHD, is associated with significant improvement in ADHD symptomatology, particularly in symptoms of hyperactivity and impulsivity. Dose-response was linear in the dose range studied, suggesting that higher doses were associated with successive improvements in behavioral functioning at home and school.
Conclusions
We concur with the recommendation of Santosh et al. (2006) in initiating stimulant treatment at a low dose and increasing that dose slowly while carefully monitoring side effects. Although parents and teachers are generally consistent with each other in their behavioral ratings (see Pearson et al. 2012), clinicians may find that teachers report effective response to treatment before parents are able to detect improvement in the home. Therefore, it is ideal for clinicians to assess behavioral response in both the home and school settings when titrating MPH treatment in children with ASD and significant ADHD symptomatology. Although our findings do not suggest a high risk of side effects (e.g., increases in stereotypies or irritability), it is important to monitor each child for these concerns and to make appropriate adjustments such as discontinuing the afternoon boosting dose, or even discontinuing treatment entirely.
Despite the fact that many children with ASD and symptoms of ADHD are being treated with stimulant medication, there are only a handful of well-controlled clinical trials assessing the efficacy of this medication in ASD. Furthermore, to our knowledge, this is the only trial examining the response of children with ASD and ADHD to the current standard of ER-MPH. We are truly only beginning to understand the role that stimulants play in the treatment of children with ASD who have significant symptoms of ADHD. In particular, there is a need to study dose-related changes in cognitive response to MPH in children with ASD and ADHD. As Aman et al. (2005) and Nicolson and Castellanos (2000) have noted, there is a pressing need for more studies of cognition in this group, particularly in light of the observation of Posey and McDougle (2000) that educational and behavioral interventions are unlikely to reach their optimal efficacy in children with ASD and ADHD symptoms without adequate control of symptoms of inattention and hyperactivity. Given the advent of the American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) recognition of ADHD as a legitimate comorbid diagnosis of ASD, and given the growing prevalence of ASD in the school-age population, further investigation into this area is clearly warranted.
Acknowledgments
The authors express their appreciation to the children, parents, and teachers who participated in this study, and to Cara Miekka, TuongVy Dang, Jessica Bell, and Brittney Hunter for their editorial assistance.
Disclosures
Dr. Pearson and Ms. Mansour have received travel reimbursement and research support from the Forest Research Institute and Curemark LLC; Dr. Pearson has also served as a consultant to Curemark LLC and United BioSource Corporation (now Bracket). Dr. Santos has received research support from Curemark LLC. Dr. Aman has received research contracts from, consulted with, or served on advisory boards of BioMarin Pharmaceuticals, Bristol-Myers Squibb, Confluence Pharmaceutica, Forest Research, Hoffman LaRoche, Johnson & Johnson, and Supernus Pharmaceutica. Dr. Arnold has received research funding from Curemark, Lilly, and Shire; has consulted to Organon, Sigma Tau, and Targacept; has served on advisory boards for AstraZeneca, BioMarin, Novartis, Noven, and Seaside Therapeutics; and has received travel support from Noven. Dr. Casat has received research funding from Eli Lilly, Novartis, and Abbott Laboratories. Dr. Bukstein has served as a consultant to Ezra Innovations and PRIME CME; he has also received book royalties from the Routledge Press. Dr. Jerger has served as a consultant to Pearson Assessments/Psychological Corporation. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- Aman MG. Stimulant drug effects in developmental disorders and hyperactivity: Toward a resolution of disparate findings. J Autism Dev Disord. 1982;12:385–398. doi: 10.1007/BF01538326. [DOI] [PubMed] [Google Scholar]
- Aman MG. Stimulant drugs in the developmental disabilities revisited. J Dev Physical Disabilities. 1996;8:347–365. [Google Scholar]
- Aman MG. Buican B. Arnold LE. Methylphenidate treatment in children with borderline IQ and mental retardation: Analysis of three aggregated studies. J Child Adolesc Psychopharmacol. 2003;13:29–40. doi: 10.1089/104454603321666171. [DOI] [PubMed] [Google Scholar]
- Aman MG. DeSmedt G. Derivan A. Lyons B. Findling RL. Hagerman R. Handen B. Hellings J. Lesem M. Pahl J. Pearson DA. Rieser M. Singh NN. Swanson J. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- Aman MG. Lam KSL. Van Bourgondien ME. Medication patterns in patients with autism: Temporal, regional, and demographic influences. J Child Adolesc Psychopharmacol. 2005;15:116–126. doi: 10.1089/cap.2005.15.116. [DOI] [PubMed] [Google Scholar]
- Aman MG. Singh NN. Aberrant Behavior Checklist: Community Supplementary Manual. East Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- Aman MG. Singh NN. Stewart AW. Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–491. [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry (AACAP) Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic, and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Arnold LE. Aman MG. Martin A. Collier–Crespin A. Vitiello B. Tierney E. Asarnow R. Bell-Bradshaw F. Freeman BJ. Gates-Ulanet P. Klin A. McCracken JT. McDougle CJ. McGough JJ. Posey DJ. Scahill L. Swiezy NB. Ritz L. Volkmar F. Assessment in multisite randomized clinical trials of patients with autistic disorders: The Autism RUPP network. J Autism Dev Disord. 2000;30:99–111. doi: 10.1023/a:1005451304303. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Quintana H. Greenhill LL. Methylphenidate treatment of hyperactive autistic children. J Am Acad Child Adolesc Psychiatry. 1988;27:248–251. doi: 10.1097/00004583-198803000-00020. [DOI] [PubMed] [Google Scholar]
- Bodfish JW. Symons FJ. Parker DE. Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Campbell M. Pharmacotherapy in early infantile autism. Biol Psychiatry. 1975;10:399–423. [PubMed] [Google Scholar]
- Campbell M. Fish B. David R. Shapiro T. Collins P. Koh C. Response to tri-iodothyronine and dextroamphetamine: A study of preschool schizophrenic children. J Autism Child Schizophr. 1972;2:343–358. doi: 10.1007/BF01538168. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners Rating Scales–Revised. North Tonawanda, NY: Multi-Health Systems, One; 1997. [Google Scholar]
- Constantino JN. Gruber CT. Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- DiMartino A. Melis G. Cianchetti C. Zuddas A. Methylphenidate for pervasive developmental disorders: safety and efficacy of acute single dose test and ongoing therapy: An open-pilot study. J Child Adolesc Psychopharmacol. 2004;14:207–218. doi: 10.1089/1044546041649011. [DOI] [PubMed] [Google Scholar]
- Farmer CA. Arnold LE. Bukstein OG. Findling RL. Gadow KD. Li X. Butter EM. Aman MG. The treatment of severe child aggression (TOSCA) study: Design challenges. Child Adoles Psychiatry and Mental Health. 2011;5:36. doi: 10.1186/1753-2000-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA. Biederman J. Bellordre CA. Garfield SB. Geller DA. Coffey BJ. Faraone SV. Should the diagnosis of attention-deficit/hyperactivity disorder be considered in children with pervasive developmental disorder? J Attention Disord. 2001;4:203–211. [Google Scholar]
- Frazier TW. Shattuck PT. Narendorf SC. Cooper BP. Wagner M. Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/disorder. J Child Adolesc Psychopharmacol. 2011;21:571–579. doi: 10.1089/cap.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J. Cantwell DP. Dosage effects of methylphenidate on paired associate learning: positive/negative placebo responders. J Am Acad Child Adolesc Psychiatry. 1982;21:237–242. doi: 10.1016/s0002-7138(09)60876-1. [DOI] [PubMed] [Google Scholar]
- Geller B. Guttmacher LB. Bleeg M. Coexistence of childhood onset pervasive developmental disorder and attention deficit disorder with hyperactivity. Am J Psychiatry. 1981;138:388–389. doi: 10.1176/ajp.138.3.388. [DOI] [PubMed] [Google Scholar]
- Ghuman JK. Aman MG. Lecavalier L. Riddle MA. Gelenberg A. Write R. Rice S. Ghuman HS. Fort C. Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. J Child Adolesc Psychopharmacol. 2009;19:329–339. doi: 10.1089/cap.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. Schwebach AJ. The comorbidity of pervasive developmental disorder and attention deficit hyperactivity disorder: Results of a retrospective chart review. J Autism Dev Disord. 2004;34:329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Swanson JM. Vitiello B. Davies M. Clevenger W. Wu M. Arnold LE. Abikoff HB. Bukstein OG. Conners CK. Elliott GR. Hechtman L. Hinshaw SP. Hoza B. Jensen PS. Kraemer HC. March JS. Newcorn JH. Severe JB. Wells K. Wigal T. Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Gringas P. Practical paediatric psychopharmacological prescribing in autism, the potential and the pitfalls. Autism. 2000;4:229–247. [Google Scholar]
- Handen BL. Johnson CR. Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30:245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Holttum JR. Lubetsky MJ. Eastman LE. Comprehensive management of trichotillomania in a young autistic girl: Case study. J Am Acad Child Adolesc Psychiatry. 1994;33:577–581. doi: 10.1097/00004583-199405000-00016. [DOI] [PubMed] [Google Scholar]
- Hoshino Y. Kumashiro H. Kaneko M. Takahashi Y. The effects of methylphenidate on early infantile autism and its relation to serum serotonin levels. Folia Psychiatr Neurol Jpn. 1977;31:605–614. doi: 10.1111/j.1440-1819.1977.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Jahromi LB. Kasari CL. McCracken JT. Lee L. Aman MG. McDougle CJ. Scahill L. Tierney E. Arnold LE. Vitiello B. Ritz L. Witwer A. Kustan E. Ghuman J. Posey DJ. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39:395–404. doi: 10.1007/s10803-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS. Arnold LE. Swanson J. Vitiello B. Abikoff HB. Greenhill LL. Hechtman L. Hinshaw SP. Pelham WE. Wells KC. Conners CK. Elliott GR. Epstein J. Hoza B. Molina BSG. Newcorn JH. Severe JB. Wigal T. Gibbons RD. Hur K. Follow-up of the NIMH MTA study at 36 months after randomization. J Am Acad Child Adolesc Psychiatry. 2007;46:988–1001. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disorder. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Leyfer OT. Folstein SE. Bacalman S. David NO. Dinh E. Morgan J. Tager–Flusberg H. Lainhart JE. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disorder. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C. Rutter M. DiLavore PC. Risi S. Autism Diagnostic Observation Schedule (ADOS) Manual. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Loveland KA. Tunali–Kotoski B. The school-aged child with an autistic spectrum disorder. In: Volkmar F., editor; Paul R., editor; Klin A., editor; Cohen D., editor. The Handbook of Autism and Pervasive Developmental Disorders. 3rd. New York: Wiley; 2005. pp. 247–287. [Google Scholar]
- Molina BSG. Hinshaw SP. Swanson JM. Arnold LE. Vitiello B. Jensen PS. Epstein JN. Hoza B. Hechtman L. Abikoff HB. Elliott GR. Greenhill LL. Newcorn JH. Wells KC. Wigal T. Gibbons RD. Hur K. Houck PR MTA Cooperative Group. The MTA at 8 years: Prospective follow-up of children treated for combined type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Cooperative Group: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA cooperative group multimodal treatment study of children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Nickels KC. Katusic SK. Colligan RC. Weaver AL. Voight RG. Barbaresi WJ. Stimulant medication treatment of target behaviors in children with autism: A population-based study. J Dev Behav Pediatr. 2008;29:75–81. doi: 10.1097/dbp.0b013e31815f24f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R. Castellanos FX. Commentary: Considerations on the pharmacotherapy of attention deficits and hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30:461–462. doi: 10.1023/a:1005511809545. [DOI] [PubMed] [Google Scholar]
- Pearson DA. Aman MG. Arnold LE. Lane DM. Loveland KA. Santos CW. Casat CD. Mansour R. Jerger SW. Ezzell S. Factor P. Vanwoerden S. Ye E. Narain P. Cleveland LA. High concordance of parent and teacher ADHD ratings in medicated and unmedicated children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2012;22:284–291. doi: 10.1089/cap.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson DA. Loveland KA. Lachar D. Lane DM. Reddoch SL. Mansour R. Cleveland SL. A comparison of behavioral and emotional functioning in children and adolescents with Autistic Disorder and PDD-NOS. Child Neuropsychol. 2006;12:321–333. doi: 10.1080/09297040600646847. [DOI] [PubMed] [Google Scholar]
- Pearson DA. Santos CW. Loveland KA. Casat CD. Lachar D. Roache J. Faria LF. Treatment effects of methylphenidate on behavioral adjustment in children with mental retardation and ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:209–216. doi: 10.1097/00004583-200302000-00015. [DOI] [PubMed] [Google Scholar]
- Posey DJ. Aman MG. McCracken JT. Scahill L. Tierney E. Arnold LE. Vitiello B. Chuang SZ. Davies M. Ramadan Y. Witwer AN. Swiezy NB. Cronin P. Shah B. Carroll DH. Young C. Wheeler C. McDougle CJ. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: An analysis of secondary measures. Biol Psychiatry. 2007;61:538–544. doi: 10.1016/j.biopsych.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Posey DJ. McDougle CJ. The pharmacotherapy of target symptoms associated with autistic disorder and other pervasive developmental disorders. Harv Rev Psychiatry. 2000;8:45–63. [PubMed] [Google Scholar]
- Pringle BA. Colpe LJ. Blumberg SJ. Avila RM. Kogan MD. Diagnostic history, treatment of school-aged children with autism spectrum disorder, special health care needs. National Center for Health Statistics Data Brief, No. 97. May, 2012. www.cdc.gov/nchs/data/databriefs/db97.pdf. [Jul 24;2012 ]. www.cdc.gov/nchs/data/databriefs/db97.pdf [PubMed]
- Quintana H. Birmaher B. Stedge D. Lennon S. Freed J. Bridge J. Greenhill L. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25:283–294. doi: 10.1007/BF02179289. [DOI] [PubMed] [Google Scholar]
- Realmuto GM. August GJ. Garfinkel BD. Clinical effect of buspirone in autistic children. J Clin Psychopharmacol. 1989;9:122–125. doi: 10.1097/00004714-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Reich W. Welner Z. Herjanic B. Diagnostic Interview for Children and Adolescents-IV. North Tonawanda, NY: Multi-Health Systems, Inc.; 1997. [Google Scholar]
- Reiersen AM. Constantino JN. Volk HE. Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48:44–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology (RUPP) Autism Network: Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Riddle MA. Yershova K. Lazzaretto D. Pavkina N. Yenokyan G. Greenhill L. Abikoff H. Vitiello B. Wigal T. McCracken JT. Kollins SH. Murray DW. Wigal S. Kastelic E. McGough JJ. dosReis S. Bauzó–Rosario A. Stehli A. Posner K. The preschool attention–deficit/hyperactivity disorder treatment study (PATS) 6-year follow-up. J Am Acad Child Adolesc Psychiatry. 2013;52:264–278. doi: 10.1016/j.jaac.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH. Stanford–Binet Intelligence Scales. 5th. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Rutter M. Le Couteur A. Lord C. Autism Diagnostic Interview-Revised (WPS Edition) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Santosh PJ. Baird G. Pityaratstian N. Tavare E. Gringas P. Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: A retrospective and prospective effectiveness study. Child Care Health Dev. 2006;32:575–583. doi: 10.1111/j.1365-2214.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- Scahill L. McDougle CJ. Williams S. Dimitripoulos A. Aman MG. McCracken J. Tierney E. Arnold LE. Cronin P. Koenig K. Kohn A. Lam KSL. Vitiello B. The Children's Yale- Brown Obsessive-Compulsive Scale modified for pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- Scahill L. Pachler M. Treatment of hyperactivity in children with pervasive developmental disorder. J Child Adolesc Psychiatr Nurs. 2007;20:59–62. doi: 10.1111/j.1744-6171.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Schmidt K. The effect of stimulant medication in childhood-onset pervasive developmental disorder—a case report. J Dev Behav Pediatr. 1982;3:244–246. doi: 10.1097/00004703-198212000-00014. [DOI] [PubMed] [Google Scholar]
- Siegel M. Beaulieu AA. Psychotropic medications in children with autism spectrum disorders: A systematic review and synthesis for evidence-based practice. J Autism Dev Disord. 2012;42:1592–1605. doi: 10.1007/s10803-011-1399-2. [DOI] [PubMed] [Google Scholar]
- Simonoff E. Pickles A. Charmon T. Chandler S. Loucas T. Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sporn A. Pinsker H. Use of stimulant medication in treating pervasive developmental disorder. Am J Psychiatry. 1981;138:997. doi: 10.1176/ajp.138.7.997a. [DOI] [PubMed] [Google Scholar]
- Sprague RL. Sleator EK. Methylphenidate in hyperkinetic children: Differences in dose effects in learning in social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- Stigler KA. Desmond LA. Posey DJ. Wiegand RE. McDougle CJ. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2004;14:49–56. doi: 10.1089/104454604773840481. [DOI] [PubMed] [Google Scholar]
- Strayhorn JM. Rapp N. Donina W. Strain PS. Randomized trial of methylphenidate for an autistic child. J Am Acad Child Adolesc Psychiatry. 1988;27:244–247. doi: 10.1097/00004583-198803000-00019. [DOI] [PubMed] [Google Scholar]
- Sturm H. Fernell E. Gillberg C. Autism spectrum disorders in children with normal intellectual levels: Associated impairments and subgroups. Dev Med Child Neurol. 2004;46:444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Schuck S. Mann M. Carlson C. Hartman K. Sergeant J. Clevenger W. Wasdell M. McCleary R. Categorical, dimensional definitions and evaluations of symptoms of ADHD: The SNAP and SWAN ratings scales. 2001. http://www.adhd.net. [Jul 24;2012 ]. http://www.adhd.net [PMC free article] [PubMed]
- Thompson Health Care. Physician's Desk Reference. 63. Montrale, NJ: Thompson Health Care; 2009. [Google Scholar]
- Ullmann RK. Sleator EK. Sprague RL. ACTeRS Teacher and Parent Forms Manual. Champaign, IL: MetriTech, Inc.; 2000. [Google Scholar]
- Vitriol C. Farber B. Stimulant medication in certain childhood disorders [Letter to Editor] Am J Psychiatry. 1981;138:11. doi: 10.1176/ajp.138.11.1517c. [DOI] [PubMed] [Google Scholar]
- Volkmar FR. Hoder EL. Cohen DJ. Inappropriate uses of stimulant medications. Clin Pediatr. 1985;24:127–130. doi: 10.1177/000992288502400301. [DOI] [PubMed] [Google Scholar]
- Wagenarr WA. Note on the construction of digram-balanced Latin Squares. Psychol Bull. 1969;72:384–386. [Google Scholar]
- Waterhouse L. Fein D. Modahl C. Neurofunctional mechanisms in autism. Psychol Rev. 1996;103:457–489. doi: 10.1037/0033-295x.103.3.457. [DOI] [PubMed] [Google Scholar]
- Wewers ME. Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]