Background: Accumulation of epidermal hyaluronan is an early event in UVB-induced squamous cell carcinomas.
Results: UVB increases keratinocyte hyaluronan synthesis by up-regulating hyaluronan synthases Has1 and -2 via p38 and Has3 via Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling.
Conclusion: UVB triggers deposition of epidermal hyaluronan through at least two signaling pathways.
Significance: Increased hyaluronan synthesis is an inherent feature of keratinocyte adaptation to radiation injury.
Keywords: CaMKII, Epidermis, Hyaluronate, Keratinocytes, p38, UVB, Hyaluronan Synthase (HAS)
Abstract
Hyaluronan, a major epidermal extracellular matrix component, responds strongly to different kinds of injuries. This also occurs by UV radiation, but the mechanisms involved are poorly understood. The effects of a single ultraviolet B (UVB) exposure on hyaluronan content and molecular mass, and expression of genes involved in hyaluronan metabolism were defined in monolayer and differentiated, organotypic three-dimensional cultures of rat epidermal keratinocytes. The signals regulating the response were characterized using specific inhibitors and Western blotting. In monolayer cultures, UVB increased hyaluronan synthase Has1 mRNA already 4 h postexposure, with a return to control level by 24 h. In contrast, Has2 and Has3 were persistently elevated from 8 h onward. Silencing of Has2 and especially Has3 decreased the UVB-induced accumulation of hyaluronan. p38 and Ca2+/calmodulin-dependent protein kinase II pathways were found to be involved in the UVB-induced up-regulation of Has2 and Has3 expression, respectively, and their inhibition reduced hyaluronan deposition. However, the expressions of the hyaluronan-degrading enzymes Hyal1 and Hyal2 and the hyaluronan receptor Cd44 were also up-regulated by UVB. In organotypic cultures, UVB treatment also resulted in increased expression of both Has and Hyal genes and shifted hyaluronan toward a smaller size range. Histochemical stainings indicated localized losses of hyaluronan in the epidermis. The data show that exposure of keratinocytes to acute, low dose UVB increases hyaluronan synthesis via up-regulation of Has2 and Has3. The simultaneously enhanced catabolism of hyaluronan demonstrates the complexity of the UVB-induced changes. Nevertheless, enhanced hyaluronan metabolism is an important part of the adaptation of keratinocytes to radiation injury.
Introduction
Proper responsiveness to ultraviolet (UV) radiation and other external stimuli is crucial for the structural integrity and function of the skin, which protects us from physical, biological, and chemical hazards. UVB2 (280–315 nm) is an especially potent inducer of biochemically and genetically significant changes in the epidermis and its main cell type, keratinocyte. Elucidating the effects and mechanisms of action of UVB is increasingly significant as the incidence of skin carcinomas and melanomas continues to rise (1). However, UVB may also have beneficial effects on barrier regulation and natural immunity (2).
Like all epithelia, epidermis is characterized by a scanty extracellular matrix. However, in the extracellular matrix of dividing and differentiating basal and spinous cell layers, hyaluronan reaches high concentrations (3). Hyaluronan is clearly one of the main extracellular matrix molecules of epidermal keratinocytes as well as of the skin as whole (4, 5). Hyaluronan is a high molecular weight, non-sulfated, free glycosaminoglycan consisting of a linear polymer of repeating disaccharide units of N-acetyl-d-glucosamine (GlcNAc) and d-glucuronic acid (GlcUA). In mammals, it is synthesized by three hyaluronan synthases (HAS1–3) on the inner surface of the plasma membrane and extruded directly into the extracellular space (6, 7). In the pericellular matrix, it is organized into diffuse coats often supported by distinct membrane protrusions (8–10) or longer, cable-like structures that are induced by inflammation or cell stress (11, 12). The most prominent hyaluronan receptor on the keratinocyte surface is the transmembrane protein CD44 (13), which is also crucial for mediating hyaluronan signaling (14).
Hyaluronan is very hygroscopic and a good space filler. Moreover, it is needed by various cell types for attachment, migration, proliferation, and signaling, and its roles in embryonic development (15, 16), inflammation (17, 18), and cancer initiation and progression are widely recognized (19, 20). In the adult skin, hyaluronan participates in hydration (5), wound healing (21, 22), and maintenance of the balance between cell division and differentiation in the epidermis (23).
Hyaluronan content and HAS transcript levels change in response to UV irradiation in cultured keratinocytes and fibroblasts as well as in intact skin. Both up-regulation (24–28) and down-regulation (29–31) have been reported. The response undoubtedly depends on the cellular background, radiation type (narrowband versus broadband) and wavelength, dosage, exposure scheme (single, repeated, or chronic), and the time point of observation (32–34). Release of KGF and IL-1β has been implicated in the hyaluronan response seen in UV-treated keratinocyte cultures (25). In fibroblasts, collagen fragments produced by UVB-activated matrix metalloproteinases down-regulate HAS2 expression by altering pERK-pELK1 signaling (31). It has also been shown that HAS3 is up-regulated via p38 signaling in human skin treated with simulated solar radiation (26). Despite these advances, our understanding of the specific signaling pathways leading to the observed changes in hyaluronan synthesis is essentially lacking.
In general, alterations in cellular metabolism in response to UVB involve the interplay of several factors. The effects of high energy radiation range from direct DNA damage to generating reactive oxygen species (ROS) that can modify other biomolecules and trigger signaling cascades. Activated plasma membrane receptors, their phosphorylated effector kinases, and transcription factors downstream are often central to the responses (35–38). For example, a key role has been demonstrated for epidermal growth factor receptor (EGFR), a prominent receptor tyrosine kinase in keratinocytes (39), in relaying UVB-induced responses (40). It is also important to distinguish between immediate and delayed effects (41). This versatility in responses and the multitude of pathways potentially activated could account for the dynamic temporal patterns previously observed in hyaluronan secretion and HAS expression.
In this work, we wanted to characterize the mechanisms of hyaluronan response to acute, low dose UVB. We used both monolayer and organotypic cultures of rat epidermal keratinocytes, which provide a useful platform for modeling epidermal biology and hyaluronan metabolism (42–46). We found a rapid increase of hyaluronan production induced by UVB exposure via up-regulation of hyaluronan synthases (Has1–3) through the p38 and Ca2+/calmodulin-dependent protein kinase II (CaMKII) pathways. Displaying the complexity of the UVB response, the expression of enzymes involved in hyaluronan catabolism was also up-regulated. Experiments using organotypic three-dimensional cultures suggested UVB-induced fragmentation of hyaluronan, in addition to enhanced synthesis.
EXPERIMENTAL PROCEDURES
Keratinocyte Cultures
A continuous cell line of rat epidermal keratinocytes (REKs) originally isolated by Baden and Kubilus (47) was cultured in minimum essential medium (MEM; Invitrogen) supplemented with 10% FBS (HyClone, Thermo Fisher Scientific Inc.), 4 mm l-glutamine (Euroclone, Pavia, Italy), and penicillin/streptomycin (50 μg/ml streptomycin, 50 units/ml penicillin; Euroclone). REKs were passaged 2–3 times a week using 0.05% trypsin, 0.02% EDTA (w/v) in PBS (Biochrom AG, Berlin, Germany) and plated at a ratio of 1:6–1:12.
For the organotypic cultures, REKs were cultured on semipermeable inserts coated with type I collagen as described by Pasonen-Seppänen et al. (48). The three-dimensional cultures were maintained for 2 weeks in DMEM (Invitrogen) supplemented with 10% FBS (HyClone), 4 mm l-glutamine (Euroclone), and penicillin/streptomycin (50 μg/ml streptomycin, 50 units/ml penicillin; Euroclone). Fresh medium was changed every 1–2 days. l-Ascorbic acid (40 μg/ml; Sigma-Aldrich) was added from 1 day after lifting the cultures onto the air-liquid interface (culture day 4) to facilitate normal differentiation but left out 1 day before the UVB exposures.
UVB Irradiation
REKs were seeded on 6-well plates at 150,000 cells/well in 2 ml of growth medium and grown for 24 h prior to UVB exposure. The light source was a portable UV lamp (UVM-57; UVP, Upland, CA) that emits midrange UV at a nominal wavelength of 302 nm and emission maximum at 312 nm (supplemental Fig. 1). The characteristics of the lamp were verified by spectroradiometry (Macam SR9910; Macam Photometrics Ltd., Livingston, Scotland, UK), and the exposure distance and time were set to obtain effective doses of 2.5, 5, 10, 15, 20, 30, and 40 mJ/cm2.
For monolayer exposure, the culture medium was removed, and 0.5 ml of Dulbecco's PBS (Euroclone) per well was added. The UVB and sham treatments (UV light off) were performed with the plate lid removed. After the UVB or sham treatment, Dulbecco's PBS was removed, and 1 ml of fresh medium was added. For the experiments on dose dependence, the plates were further incubated for 24 h, and the culture medium was quantified for hyaluronan. To measure temporal changes after a 10-mJ/cm2 dose, hyaluronan was assayed 12, 24, 36, and 48 h after exposure.
UVB exposure of the organotypic cultures was done 13–15 days after starting the cultures. For this, the medium beneath each insert was briefly replaced with 0.8–1.5 ml of Dulbecco's PBS to wash the culture. Subsequently, 800 μl of Dulbecco's PBS was added for the duration of the UVB or sham treatment. Afterward, 1.0–1.5 ml of fresh medium was added, and the cultures were incubated for 24 h and collected for histology and/or biochemical analyses. In a subset of experiments, the organotypic cultures were maintained in a base medium (DMEM; Invitrogen) without phenol red and irradiated directly in 1.5 ml of the medium with lower serum (5% FBS). The cultures were exposed to 10–40 mJ/cm2 for the histological examinations and 20–30 mJ/cm2 for the metabolic labeling of newly synthesized glycosaminoglycans.
Treatments with Inhibitors of Signaling Pathways
To investigate the intracellular signaling pathways involved, REKs were seeded on 6-well plates at 150,000–175,000 cells/well in 2 ml of monolayer growth medium, grown for 24 h, and treated for 2 h with inhibitors targeting EGFR, MEK1/2, PI3K, Akt1/2, p38, JNK, STAT3, and CaMKII before exposure to 10 mJ/cm2 of UVB. Following irradiation, the cells were grown for either 8 and 36 h (for gene expression analyses) or 24 h (for hyaluronan assay) in fresh medium containing the respective inhibitors (supplemental Table 1).
Quantification of Hyaluronan
Hyaluronan concentration of the media was quantified using a sandwich-type enzyme-linked sorbent assay as described previously (49). Briefly, 96-well plates were coated with hyaluronan-binding complex (HABC; 0.5 or 1.0 μg/ml, prepared in-house as described previously (50)), blocked with 1% BSA in PBS, and incubated with 0–50 ng/ml hyaluronan standards (Provisc; Algon Laboratories, Fort Worth, TX) or samples diluted with 1% BSA in PBS. Biotinylated HABC (prepared in-house) was added to detect the bound hyaluronan. Visualization was performed by sequential additions of horseradish peroxidase, 3,3′,5,5′-tetramethylbenzidine, H2O2, and 50 μl of 2 m H2SO4 for absorbance at 450 nm. Standards and samples were run as duplicates or triplicates.
Metabolic Labeling
In the organotypic cultures, hyaluronan synthesis was quantified using metabolic labeling as described previously (51). The organotypic cultures were irradiated as described above and incubated for 24 h in 1 ml of medium containing 1% FBS and 20 μCi/ml [3H]glucosamine and 100 μCi/ml [35S]Na2SO4 (both from PerkinElmer Life Sciences). The media were collected, and the culture inserts were rinsed with 300 μl of HBSS that was combined with the medium. The epidermis and the collagenous support layers were separated, and the three compartments (medium, epidermis, and collagen) were analyzed individually. The epidermal samples were incubated in pure acetone at 4 °C with three changes and then air-dried and weighed.
The samples were subsequently purified and quantified for hyaluronan and chondroitin sulfate as described previously (42, 51). The samples were digested overnight at 60 °C with papain (Sigma-Aldrich), boiled, and centrifuged. Standard hyaluronan (6 μg; Healon®, Advanced Medical Optics Uppsala Ab, Uppsala, Sweden) was added to the supernatant as a carrier and internal standard. The samples were precipitated with 1% cetylpyridinium chloride, and the pellets were washed with distilled H2O, dissolved in 4 m guanidium chloride, and reprecipitated with ethanol. The pellets were digested with Streptococcus hyaluronidase (1 milliunit; Seikagaku Kogyo Co., Tokyo, Japan) and chondroitinase ABC (25 milliunits; Seikagaku). Hyaluronan- and chondroitin sulfate-derived disaccharides were separated on a Superdex Peptide column (GE Healthcare) and counted for [3H]hexosamines and [35S]sulfate to quantify the newly synthesized hyaluronan and chondroitin sulfates (52). The hyaluronan disaccharide absorbance at 232 nm was used to correct for possible losses in the purification.
Molecular Mass Distribution of Hyaluronan
To analyze the size distribution of hyaluronan secreted in the medium, gel filtration on a 1 × 30-cm Sephacryl S-1000 column (HR 10/30; GE Healthcare) was done as described previously (51). 150 mm sodium acetate buffer (pH 6.8) containing 0.1% CHAPS, at a flow rate of 24 ml/h, was used for elution. For samples collected from monolayer cultures, 1-ml fractions were lyophilized and redissolved in 250 μl of 1% BSA-PBS for the determination of hyaluronan content with an enzyme-linked sorbent assay as described above. For the organotypic cultures, epidermal sheets were separated and digested with 250 μg of proteinase K in 100 mm ammonium acetate for 2 h at 60 °C. After inactivating the enzyme, supernatants were collected and analyzed with S-1000 as described above. For the organotypic specimens, no concentration step was necessary. The column was calibrated using hyaluronan standards with known molecular mass (150, 500, and 2500 kDa), purchased from Hyalose (Oklahoma City, OK).
RNA Extraction and Quantitative Real-time PCR
The transcript levels of Has1–3, Hyal1 and -2 (hyaluronidase 1 and 2), and Cd44 were measured using quantitative real-time PCR (qRT-PCR). For the monolayer cultures, cells were plated and cultured as above, exposed to 10 mJ/cm2 of UVB, and incubated for an additional 2–36 h. Cells were lysed by adding 1 ml of RNA extraction reagent/well (EuroGOLD RNAPureTM or EUROzol from Euroclone, TRI Reagent® solution from Applied Biosystems/Invitrogen, or TRIzol® reagent from Invitrogen). Total RNA was extracted with chloroform-isopropyl alcohol according to standard procedures, washed once with 75% ethanol, dissolved in nuclease-free water, and stored at −70 °C.
For the organotypic cultures, the epidermal layer was separated with fine tweezers and frozen in liquid nitrogen 24 h after the UVB exposure (30 mJ/cm2). The samples were then homogenized in Lysing Matrix D tubes (MP Biomedicals, Santa Ana, CA) with a FastPrep® homogenizer (Savant, Thermo Fisher Scientific), and total RNA was extracted with the High Pure RNA Tissue Kit (Roche Applied Science) according to the kit protocol.
For cDNA synthesis and qRT-PCR, 1 μg of total RNA was amplified with the VersoTM SYBR® Green two-step qRT-PCR kit (Abgene Ltd., Thermo Fisher Scientific) using anchored oligo(dT) primers. The cDNA was diluted 1:5, and 2 μl was added to each PCR (FastStart Universal SYBR Green Master (Rox)). The samples were amplified with a Stratagene Mx3000P real-time PCR machine (Agilent, La Jolla, CA) using 45 cycles of 20 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C, preceded by activation of the enzyme for 15 min at 95 °C. Ribosomal protein LP0 (Rplp0) was used as the reference gene. The sequences of the primers and the sizes of the amplicons are presented in supplemental Table 2.
siRNA Transfections
The specific siRNAs against Has1 and Has3 were ordered from Eurogentec (Liège, Belgium), and siRNAs against Has2 were from Ambion (Austin, TX). The control siRNA for all was from Origene (Rockville, MD). For Has1 and Has3, three different siRNAs were used in combination at a final concentration of 50 nm, whereas for Has2, a single prescreened oligonucleotide was chosen (supplemental Table 3). The transfections were performed in antibiotic-free medium (MEM with 5% FBS; Invitrogen), mixing either the control or the specific siRNAs with RNAiMAX (Invitrogen). All of the reagents were diluted in α-MEM (HyClone). RNAiMAX mixed with α-MEM served as the transfection reagent control.
The cells were transfected for 4–6 h, after which they were grown overnight in fresh, antibiotic-free medium. Thereafter, the cells were split on 6-well plates at 175,000 cells/well, grown overnight, and irradiated with 10 mJ/cm2 of UVB as described above. Total RNA was collected 8 h after and medium for hyaluronan assays 24 h after the exposure.
Western Blotting
REKs were plated on 6-cm Petri dishes. 24 h prior to UVB irradiation, the medium was changed for fresh MEM containing 1% FBS. Exposures were done as described above, followed by incubations for 15 min to 36 h before collecting the samples by a 10-min extraction on ice in one of the following buffers: 1) radioimmune precipitation assay buffer (150 mm NaCl, 50 mm Tris, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease and phosphatase inhibitors (inhibitor mixtures from Sigma-Aldrich) or 2) SDS sample buffer (62.5 mm Tris-HCl, 2% (w/v) SDS, 10% glycerol, 50 mm DTT, and 0.01% (w/v) bromphenol blue). Buffer 2 was used for the detection of activated p38, whereas radioimmune precipitation assay buffer was used for the other assays. CD44 was probed from samples in either buffer. Proteins (10–20 μg) (53) were resolved on 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Whatman Protran® BA85, Whatman Inc., Florham Park, NJ), applying a constant current of 2 mA/cm2 in a Fastblot B43 semidry blotter (Biometra GmbH, Göttingen, Germany).
The membranes were blocked in Tris-buffered saline (TBS; 10 mm Tris, 150 mm NaCl, pH 7.4) containing either 3% nonfat milk powder or 2–5% BSA on a rolling mixer for 30 min at room temperature. Primary antibodies diluted in 1–2% BSA-TBS were applied overnight on a rolling mixer at 4 °C, using the following dilutions: activated p38 (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), total p38α (1:1000; Cell Signaling Technology, Inc., Danvers, MA), pERK1/2 (1:200; Santa Cruz Biotechnology, Inc.), pJNK (1:500; Cell Signaling Technology), pSTAT3 (Tyr(P)-705, 1:1000; Cell Signaling Technology), actin (1:2000–1:6000; Sigma-Aldrich), and GAPDH (1:3000; Santa Cruz Biotechnology, Inc.). The anti-CD44 antibody used at 1:150–1:300 in Western blots was from R&D Systems (Abingdon, UK). After washes with 0.1% Tween 20-TBS, the membranes were incubated for 1 h with fluorescent secondary antibodies (Pierce/Thermo Fisher Scientific Inc.): anti-rabbit IgG (1:2000–1:5000) or anti-mouse IgG (1:2000). The membranes were scanned on an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Band intensity was analyzed using a program supplied with the instrument and normalized to that of either GAPDH (for CD44) or actin (all others).
Histochemistry and Microscopy
2-Week-old REK three-dimensional cultures were fixed in 2% paraformaldehyde, 0.5% glutaraldehyde or in Histochoice® MB (Amresco, Solon, OH) overnight at 4 °C and embedded in paraffin. Hematoxylin and eosin (H&E) stainings were performed according to standard procedures to confirm the general morphology of the cultures. Hyaluronan stainings were performed with biotinylated HABC with Mayer's hematoxylin counterstaining and mounted in DePex (BDH Laboratory Supplies, Poole, UK) as described previously (54).
For CD44 immunostaining, the sections were first incubated in target retrieval solution (Dako, Glostrup, Denmark) for 30 min at 95 °C, followed by blocking of endogenous peroxidases and nonspecific binding as described above. After an overnight incubation at 4 °C with the anti-CD44 antibody (OX-50; Chemicon/Millipore, Billerica, MA) (diluted 1:200 with 1% BSA in 0.1 m sodium phosphate buffer, pH 7.4), the sections were incubated with a biotinylated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA) (diluted 1:200 with 1% BSA in 0.1 m sodium phosphate buffer, pH 7.4) for 1 h, followed by sequential incubations with the ABC reagent and 3,3′-diaminobenzidine, counterstaining, and mounting as described above (54).
The slides were analyzed and photographed on a Nikon Microphot-FXA microscope (Nikon Corp., Tokyo, Japan) using a CoolSNAP digitizing system (Photometrics, Tucson, AZ) or on a Zeiss AxioImager M2 microscope (Carl Zeiss AG, Oberkochen, Germany) with a digital color camera (AxioCam MRc, Carl Zeiss AG).
Statistical Analyses
Statistical analyses were performed with the PASW Statistics version 18.0 software package for Mac OS X and IBM SPSS Statistics, version 19 (SPSS Inc., Chicago, IL), R for Windows version 2.7.2 (55), and GraphPad Prism version 5.03 for Windows (GraphPad Software, Inc., La Jolla, CA). Clear outliers were removed based on Grubbs' test with a significance level of p < 0.01. Mathematical transformation of variables with unequal error variances or lacking normal distribution was performed using log10 or ln transformations to correct the situation.
The data were subjected to analyses of variance using either univariate, mixed, or two-way models depending on the experimental set-up, followed by analyses of pairwise comparisons using Tukey's honestly significant difference and Bonferroni's tests or estimated marginal means (LSD), as indicated in the figure legends. In the inhibitor experiments, differences between the individual treatment groups were analyzed using the estimated marginal means, and each inhibitor-treated group was compared with control by calculating the cumulative distribution function in R for Windows (pnorm), in both cases correcting the p value (LSD) for the number of comparisons.
RESULTS
Acute UVB Exposure Stimulates Hyaluronan Synthesis in Monolayer REK Cultures
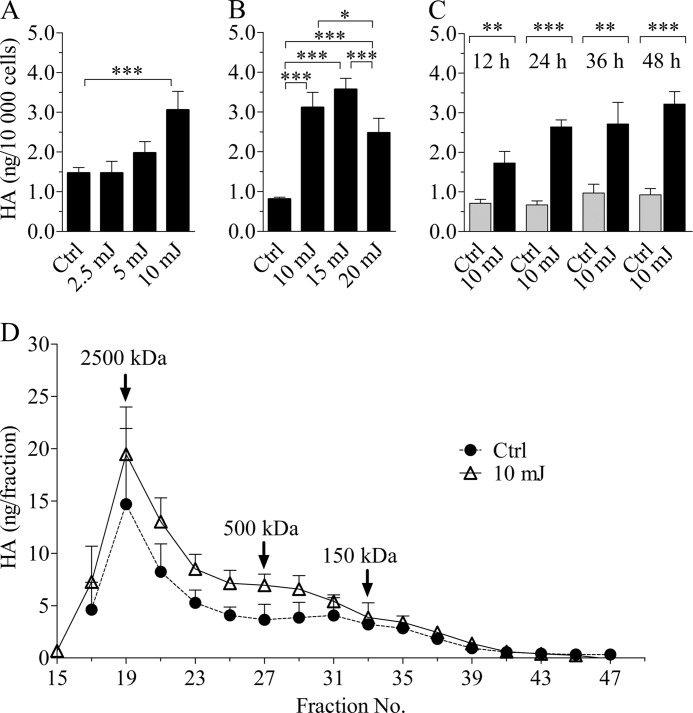
Monolayer REK cultures were subjected to a single exposure of broadband UVB, and hyaluronan secreted in the culture medium during a 24-h follow-up was analyzed. In the first experimental set-up, we used irradiation doses ranging from 2.5 to 10 mJ/cm2 (Fig. 1A). Whereas 5 mJ/cm2 of UVB caused a slight increase in the amount of extracellular hyaluronan, 10 mJ/cm2 caused a statistically significant stimulation when compared with controls.
FIGURE 1.
UVB irradiation induces hyaluronan secretion in REK monolayers in a dose-dependent manner without affecting the molecular mass distribution. A and B, REK monolayer cultures were exposed to the indicated doses of UVB, and the culture media were collected after a 24-h incubation and analyzed for hyaluronan content. The data in A and B represent means and S.E. (error bars) of three independent experiments. Statistical significance was analyzed using univariate analysis of variance. Individual means between groups were compared using Tukey's honestly significant difference (*, p < 0.05; ***, p < 0.001). C, hyaluronan secretion in the culture medium during various time intervals following a single UVB dose of 10 mJ/cm2. The overall effect of the UVB treatment was significant (p < 0.001; two-way analysis of variance). The data represent means and S.E. from three independent experiments, each containing two replicates for each time point and treatment. The results of Bonferroni's tests for individual time points are shown (**, p < 0.01; ***, p < 0.001). D, the molecular mass distribution of hyaluronan released in the culture medium during a 24-h incubation after a single UVB exposure (10 mJ/cm2) was analyzed using Sephacryl S-1000 gel chromatography. The data represent means and half-range from two independent experiments.
To study whether higher UVB doses would further increase the hyaluronan response, additional experiments were conducted, including doses up to 20 mJ/cm2 (Fig. 1B). 15 mJ/cm2 caused an effect comparable with that of 10 mJ/cm2, whereas 20 mJ/cm2 reversed the trend. Because 15 and 20 mJ/cm2 elicited up to 35% dead cells (data not shown), compared with a maximum of ∼10% seen in cultures treated with the lower doses, 10 mJ/cm2 was chosen for further experiments. With this level of irradiation, a steady, 2–3 times higher hyaluronan secretion was evident in all subsequent data sets (Figs. 1C, 3, and 4). 10 mJ/cm2 caused a transient inhibition in cell proliferation, as indicated by significantly lower cell counts at 12 h after the irradiation (supplemental Fig. 2). Thereafter, the cell numbers increased at apparently equal rates.
FIGURE 3.
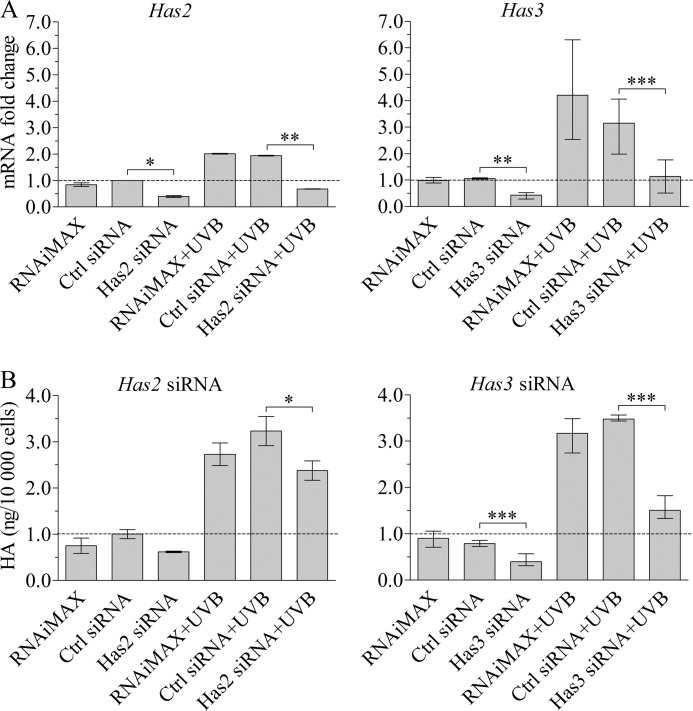
Silencing of Has2 and Has3 expression reduces hyaluronan accumulation in the culture medium after UVB. REK cells were treated either with the transfection reagent alone (RNAiMAX), control siRNA, or Has2- or Has3-specific siRNA. Two days after the transfections, the cultures were exposed to 10 mJ/cm2 of UVB. RNA was collected 8 h after and the culture media 24 h after the irradiation and analyzed for Has gene expression (A) and hyaluronan content (B). The data represent the means and range from two independent experiments for Has2 and three independent experiments for Has3, each treatment with two replicates. The statistical significances of the differences were tested using analyses of variance and estimated marginal means (LSD) for individual comparisons (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
FIGURE 4.
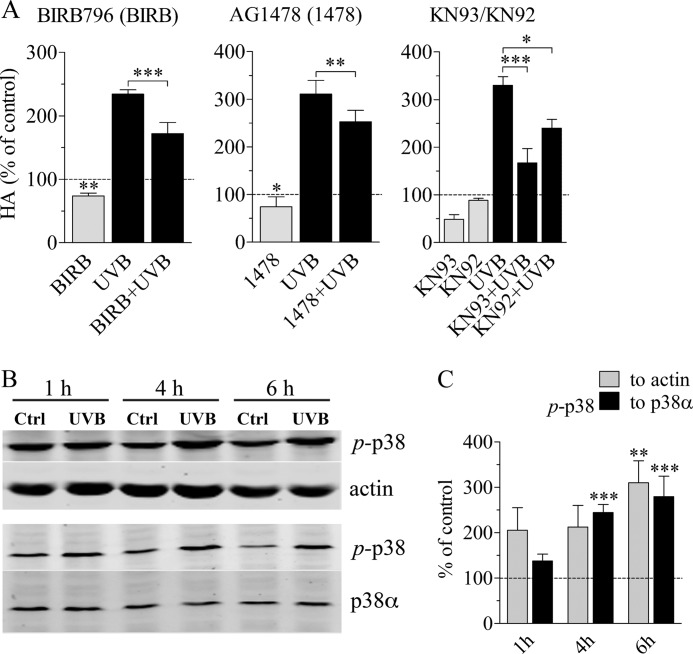
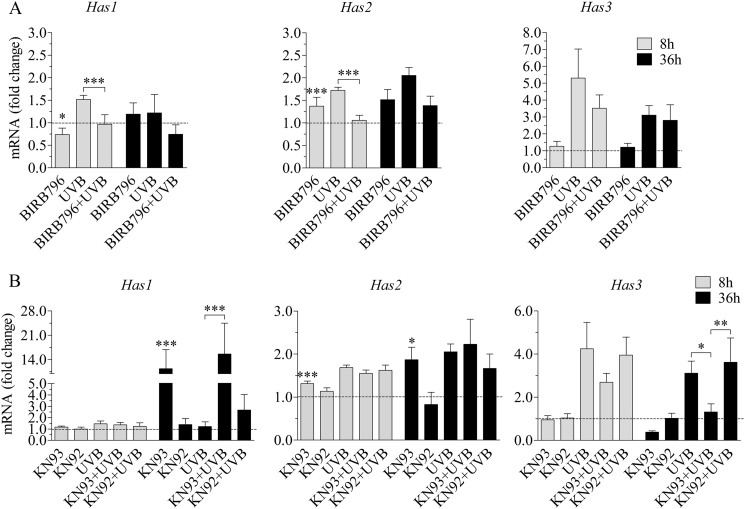
p38 activation is involved in the accumulation of hyaluronan after UVB exposure. REKs pretreated with specific inhibitors for 2 h were exposed to 10 mJ/cm2 UVB, followed by a 24-h incubation in the presence of the corresponding inhibitor, as explained in supplemental Table 1. The control cultures contained an equal concentration of DMSO, which was used to dissolve the inhibitors. A, hyaluronan accumulation with an inhibitor for p38 MAPK (BIRB796), EGFR (AG1478), and Ca2+/calmodulin-dependent protein kinase II (KN93) and its supposedly inactive control compound (KN92). The data represent means and S.E. (error bars) from three independent experiments. Statistical significance was analyzed using univariate analysis of variance, and the differences between treatments were tested using estimated marginal means (LSD) and between controls and treatments using pnorm as described under “Experimental Procedures” (*, p < 0.05; **, p < 0.01; ***, p < 0.001). B, Western blot of UVB-exposed and control specimens at different time points after a single exposure to UVB, detected with a phosphospecific antibody against active p38 (p-p38), total actin, and total p38α. Normalization against actin and total p38α, respectively, was done from the same samples, on separate blots. C, actin-normalized (gray bars) and p38α-normalized (black bars) band intensities of active p-p38 (means and S.E. from three independent experiments). Two-way analysis of variance was used to analyze the data, and the results of Bonferroni's tests for individual comparisons are shown (**, p < 0.01; ***, p < 0.001, as compared with the control at the same time point).
Temporal changes in hyaluronan secretion after a single dose (10 mJ/cm2) were examined by collecting culture media at different time points after the exposure. The content of hyaluronan in the UVB-treated cultures was 142% higher compared with the non-irradiated controls already 12 h postexposure, indicating that the response was rapid. At later time points, the difference between the UVB- and sham-treated groups was increased even further (Fig. 1C).
Because UV irradiation can break hyaluronan chains both in vivo and in vitro (56, 57), we analyzed the size distribution of the hyaluronan released into the growth medium during a 24-h period after a single UVB exposure. As reported previously (58), the majority of hyaluronan secreted by control REKs represented high molecular mass molecules (>2000 kDa; Fig. 1D). UVB treatment did not cause significant changes in this distribution.
UVB Treatment Causes Up-regulation of All Has Isoforms
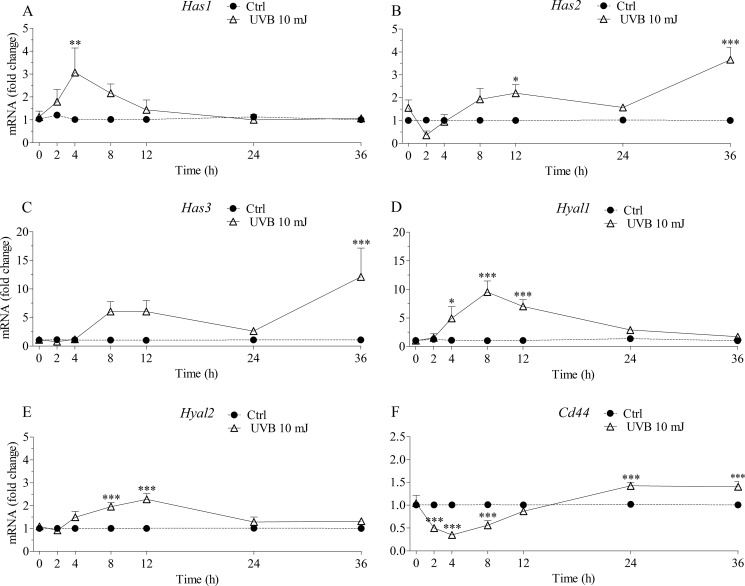
To establish the cause for the increased hyaluronan secretion, transcript levels of Has1–3 were evaluated by qRT-PCR. The mRNA levels of Has1 and Has3 were relatively low in REK monolayers as compared with Has2, with relative Ct values of about 30, 32, and 27 for Has1, Has3, and Has2, respectively (data not shown). There were clear, dynamic, and statistically significant changes in the mRNA levels of the Has enzymes after a single exposure to 10 mJ/cm2 (two-way analysis of variance; overall effect of UVB, p < 0.05 for Has1, p < 0.001 for Has2 and Has3; Fig. 2, A–C). Has1 showed the earliest induction (2-fold) after 4 h, declining thereafter back to the basal level (Fig. 2A). Has2 increased about 2-fold at 8–12 h after an initial decline (Fig. 2B). It remained up-regulated until 36 h, where the highest induction (about 3-fold) was seen. Has3 showed the highest overall induction of all Has isoforms. The up-regulation of Has3 expression started 8 h postirradiation and lasted up to 36 h (Fig. 2C).
FIGURE 2.
mRNA of Has1–3 (A–C), Hyal1 (D), Hyal2 (E), and Cd44 (F) in epidermal keratinocytes show distinct temporal patterns after a single dose of UVB. The relative expression levels were determined by qRT-PCR at the specified time points after UVB irradiation (10 mJ/cm2). All mRNA levels were normalized to Rplp0. Each time point represents means and S.E. (error bars) from 3–4 independent experiments, each done in duplicate. The differences between control and UVB-treated specimens were significant for all genes studied (p < 0.001 for Has2, Has3, CD44, Hyal1, and Hyal2 and p < 0.05 for Has1; two-way analysis of variance). The results of Bonferroni's tests for individual time points are shown (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To see whether UVB exposure influences the expression of proteins involved in the degradation of hyaluronan, we analyzed its effects on the expression of Hyal1 and Hyal2, and the hyaluronan receptor Cd44. The mRNA levels of Hyal1 appeared lower than those of Hyal2 and Cd44 in untreated cultures (relative Ct values of about 31, 26, and 24, respectively; data not shown). Hyal1 and Hyal2 transcript levels were significantly up-regulated by UVB. The two Hyal transcripts showed very similar temporal patterns after the UVB exposure, with a significant increase from 4 to 12 h. However, the -fold changes in Hyal1 transcript levels were higher than those in Hyal2 (Fig. 2, D and E). About 50% up-regulation of Cd44 was seen at the transcript level at 24–36 h after an initial 50% drop at 2–8 h post-UVB (Fig. 2F). Western blot analyses (supplemental Fig. 3) showed that REK cells express both standard (90 kDa) and several higher molecular mass isoforms similar to human keratinocytes (59). No differences between the controls and the UVB-treated cultures were detected, however.
Taken together, the data indicate that the expression of the three Has isoforms, the hyaluronan receptor Cd44, and the hyaluronan degrading enzymes Hyal1 and Hyal2 are all significantly modulated after an acute exposure to UVB. Although Has2, Has3, and CD44 transcripts follow a broadly similar pattern with a delayed and sustained up-regulation, Has1 and Hyal transcripts show a more rapid and transient increase.
Knockdown of Has3 and Has2 with siRNA Reduces the UVB-induced Accumulation of Hyaluronan
To get an idea about the contribution of the different HAS isoenzymes for the accumulation of hyaluronan in REK cultures, we used siRNAs that reduced both Has2 and Has3 mRNAs in control and UVB-irradiated samples by about 60–65% (Fig. 3A). Silencing of Has2 resulted in a modest reduction in accumulated hyaluronan both in the non-UVB-treated cultures (on average 38%) and in UVB-treated cultures (on average 26%; Fig. 3B). Silencing of Has3 expression resulted in a more substantial reduction in both the basal hyaluronan production (on average 49%) and UVB-stimulated hyaluronan production (on average 57%; Fig. 3B). Unfortunately, we were unable to effectively silence Has1 expression (data not shown).
p38 Inhibition Suppresses the UVB-induced Hyaluronan Synthesis
To find the signaling pathways involved in the UVB-induced hyaluronan response, we treated REK monolayers with inhibitors specific to several pathways previously reported to be UVB-responsive and also demonstrated to regulate hyaluronan secretion, like EGFR and its downstream effectors ERK1/2, Akt, STAT3, and the stress-responsive p38 and JNK (supplemental Table 1). The experiments showed that under the basal experimental conditions, EGFR and the MAPK p38 regulated hyaluronan production (Fig. 4A). The EGFR inhibitor AG1478 and the p38 inhibitor BIRB796 caused an ∼25% reduction in the basal level of hyaluronan secretion in REK cells, whereas the other inhibitors did not exert a significant influence (supplemental Fig. 4). Of these inhibitors, AG1478 and BIRB796 also reduced the UVB-induced increase of hyaluronan secretion (Fig. 4A). The decrease caused by the EGFR inhibitor was ∼28%, whereas the p38 inhibitor BIRB796 cut the induction by 46% (Fig. 4A).
However, inhibitors of the common downstream pathways of EGFR, UO126, and PD 98059 for MEK1/2 and wortmannin and Akt inhibitor VIII for PI3K and Akt, respectively, failed to counteract the UVB-induced hyaluronan production (supplemental Fig. 4). Similarly, the inhibitor of STAT3 (Cpd188), a downstream target of the EGFR and JAK pathways, exerted no effect on hyaluronan release (supplemental Fig. 4), not even with a higher dose (10 μm; data not shown). Furthermore, inhibition of stress-related signaling pathways by targeting JNK with SP600125 had no influence on hyaluronan secretion (supplemental Fig. 4). In conclusion, of the signals previously known to stimulate hyaluronan synthesis, p38 was clearly associated with the UVB-induced response, whereas the contribution of EGFR was more modest.
Because UV radiation has been shown to activate CaMKII by regulating intracellular calcium levels in immune cells and fibroblasts (60, 61), and CaMKII can control various signaling pathways, including MAPKs, we tested the effect of a specific CaMKII inhibitor, KN93, on hyaluronan synthesis in REK cells (Fig. 4A). This inhibitor exerted a strong inhibitory activity both on the basal hyaluronan level (−51%) and the UVB-induced increase in hyaluronan accumulation (−71%; Fig. 4A). However, KN92, the generally used inactive control compound for KN93, also decreased the UVB-induced response by 39%, whereas its effect on the basal secretion was small (−11%; Fig. 4A).
Regulation of UVB-induced Has Expression Is Associated with p38 and CaMKII
In order to see if the observed changes in hyaluronan synthesis and Has expression correlated with activation of the corresponding signaling pathways, we performed Western blots with phosphospecific antibodies (Fig. 4, B and C, and supplemental Fig. 5). Phosphorylated MAPK p38 showed a constant 2–3-fold increase compared with the control specimens collected at the same time point (Fig. 4, B and C), whereas JNK had a lower level of activation (supplemental Fig. 5), and ERK1/2 showed no activation at all (supplemental Fig. 5). STAT3 phosphorylation was down-regulated (although not statistically significantly) at 4 h postexposure and returned back to the control level at 6 h post-UVB (supplemental Fig. 5), a pattern similar to the one previously suggested by Kim et al. (62).
Because the effects of inhibitors on hyaluronan synthesis suggested involvement of p38, EGFR, and CaMKII in the UVB-induced hyaluronan response, we wanted to see if inhibitors of these signaling routes influence Has expression. The 8 h time point was checked first, because all Has isoenzymes were up-regulated then (Fig. 2, A–C). The p38 inhibitor BIRB796 effectively cut the UVB-induced Has1 and Has2 expression down to the level of the control cultures (Fig. 5A), although it also affected the basal expression of both Has isoforms. In contrast, the EGFR inhibitor AG1478 had no effect on the mRNA levels of Has2 and Has3, whereas there was actually an increase in Has1 (supplemental Fig. 6). At 8 h postirradiation, the CaMKII inhibitor KN93 had no significant effect on Has1 and Has3 expression, whereas it slightly increased the basal expression of Has2 (Fig. 5B).
FIGURE 5.
p38 and CaMKII blocking decreases UVB-induced Has expression. The ability of the p38 inhibitor BIRB796 (A) and CaMKII inhibitor KN93 or the inactive control compound KN92 (B) to block the UVB-induced Has expression was studied using qRT-PCR of samples collected 8 and 36 h after the irradiation. Statistical significance was analyzed using analysis of variance and estimated marginal means (LSD) as described under “Experimental Procedures” (*, p < 0.05; **, p < 0.01; ***, p < 0.001). The data represent means and S.E. (error bars) from three (A and B at 36 h) or five (B at 8 h) independent experiments each done in duplicate.
Because there appeared to be a later activation of Has2 and Has3 expression at the 36 h time point (Fig. 2, A–C), we also tested whether p38 and CaMKII were involved in those up-regulations. The p38 inhibitor BIRB796 tended to reduce the UVB-induced Has2 response also at this time point but had no effect on the induction of Has3 (Fig. 5A). At 36 h, the CaMKII inhibitor KN93 up-regulated the basal expression of Has1 and Has2, whereas the UVB-induced Has2 response was not affected. In contrast, the UVB response of Has3 mRNA was strongly down-regulated by KN93 at this time point, whereas the ineffective analog KN92 had no influence (Fig. 5B).
Altogether, the experiments with specific inhibitors suggest that the ERK, Akt, and STAT3 pathways have no significant role in the UVB-induced hyaluronan synthesis in rat keratinocytes. Instead, p38 increases the expression of Has2, and CaMKII increases the expression of Has3, apparently accounting for the hyaluronan response.
Acute UVB Exposure Activates Hyaluronan Metabolism in an Epidermal Equivalent
To study whether UVB can also regulate epidermal hyaluronan synthesis under conditions that resemble the situation in vivo, we employed organotypic three-dimensional cultures of the same REK cells. When raised to the air-liquid interface, they form a fully differentiated epidermis in 2 weeks. We exposed the differentiated cultures to slightly higher doses of UVB (20–40 mJ/cm2) than the monolayer cultures, because the radiation is filtered through an intact stratum corneum. These were biologically relevant doses, as indicated by the increased number of apoptotic cells. With 20–30 mJ/cm2 the general tissue morphology was normal, whereas 40 mJ/cm2 caused tissue necrosis.3
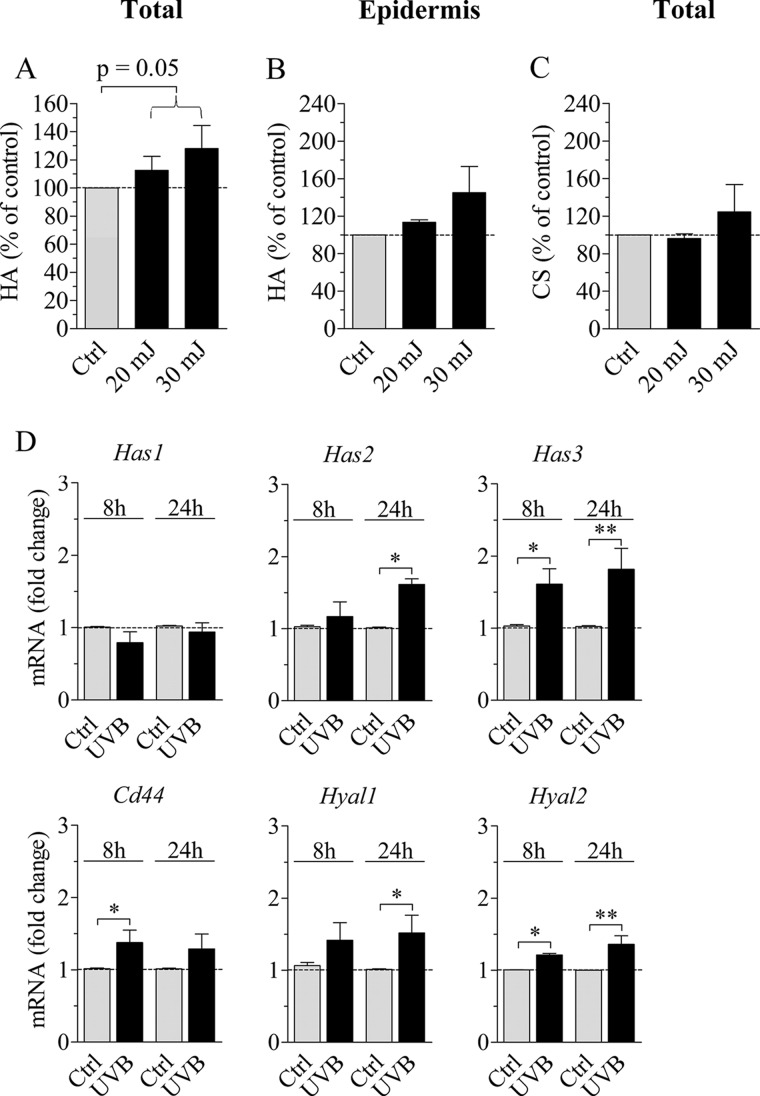
Hyaluronan synthesis was analyzed using metabolic labeling, which gives additional information on the synthesis of sulfated glycosaminoglycans. The total amount of hyaluronan synthesized during the 24-h labeling period, including that in the epidermis, the supporting collagen gel, and the culture medium, was increased after UVB (p = 0.05; Fig. 6A). A similar trend was seen in the epidermal layer alone (Fig. 6B); however, the effect was not statistically significant. This assay also indicated that the synthesis of chondroitin sulfates was not significantly stimulated by UVB irradiation (Fig. 6C).
FIGURE 6.
Synthesis of hyaluronan and chondroitin sulfates and the expression of hyaluronan synthases, hyaluronidases, and Cd44 in organotypic cultures. 2-Week-old REK organotypic cultures were exposed to UVB (20 and 30 mJ/cm2), followed by incubation in the presence of [3H]glucosamine and [35S]Na2SO4 for 24 h. Medium, collagen, and epidermis were collected, and the radioactivity in hyaluronan and sulfated glycosaminoglycans in each compartment was quantified to calculate the amount of newly synthesized glycosaminoglycans as described under “Experimental Procedures.” A, total amount of hyaluronan produced in the culture. B, hyaluronan in the epidermal compartment. C, total chondroitin sulfates synthesized in the culture. The data represent means and S.E. (error bars) from four independent experiments each done in duplicate. The data were analyzed using analysis of variance (p = 0.05 for the overall effect of UVB; 20 and 30 mJ/cm2 combined). D, mRNA levels of Has1–3, Cd44, Hyal1, and Hyal2 in organotypic cultures exposed to 30 mJ/cm2 of UVB 8 and 24 h prior to sample collection were measured with qRT-PCR and normalized against Rplp0. The data represent means and S.E. from three (8 h) and four (24 h) independent experiments, each done in duplicate. Statistical significance was evaluated by analysis of variance (*, p < 0.05; **, p < 0.01).
The transcript levels of Has1–3, Cd44, and Hyal1 and -2 were evaluated by qRT-PCR in the organotypic cultures 8 and 24 h after irradiation with 30 mJ/cm2. In the unirradiated organotypic cultures, the transcripts of Cd44 and Hyal2 appeared most abundant (Ct values around 24 and 25, respectively; data not shown), whereas Has1, Has2, Has3, and Hyal1 showed relatively lower transcript levels (Ct values ∼32, 28, 30, and 28, respectively; data not shown).
No change in Has1 mRNA level was detected in the organotypic model at either 8 or 24 h post-UVB (Fig. 6D). The mRNA level of Has2 was up-regulated by 1.6-fold at 24 h, and that of Has3 was up-regulated by 1.6- and 1.8-fold at the 8 and 24 h time points, respectively (Fig. 6D). The rise in Hyal2 and Hyal1 mRNA levels was modest although statistically significant (1.4-fold for Hyal1 at 24 h and 1.2- and 1.4-fold for Hyal2 at 8 and 24 h; Fig. 6D). Similarly, a modest, 1.4-fold increase was found in the mRNA level of Cd44 at 8 h.
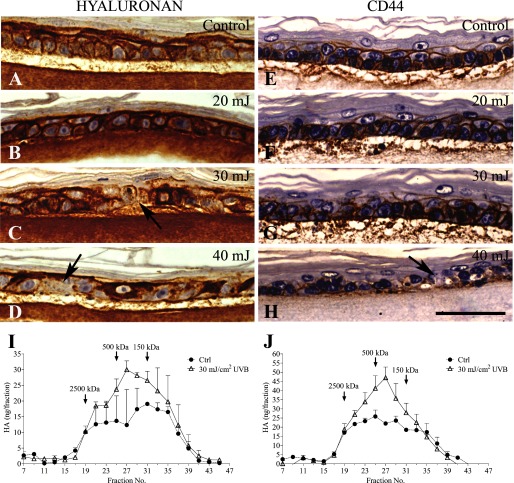
Hyaluronan staining intensity was higher in the UVB-treated epidermis than in the control cultures (Fig. 7, A–D). Although the distribution of hyaluronan staining was not affected by the lower UVB dose, increasing the dose tended to make the staining pattern irregular, with regions of increased staining interspersed with areas showing loss of hyaluronan (Fig. 7, C and D). Like hyaluronan, its receptor CD44 was localized in the basal and spinous layers (Fig. 7, E–H). With the lower UVB doses, no substantial changes in the intensity or localization of CD44 staining were detectable. However, at the highest dose (40 mJ/cm2), CD44 staining became irregular with locally reduced signals, similar to the pattern of hyaluronan (Fig. 7H).
FIGURE 7.
Epidermal hyaluronan distribution in UVB-exposed organotypic cultures. Hyaluronan was stained with biotinylated HABC (bHABC; A–D) and CD44 antibody (OX50; E–H) 24 h after a single UVB or sham exposure, as indicated in the panels. The images are from one experiment, representative of four independent experiments, each experiment containing a minimum of two replicates for each condition. The arrows indicate areas of hyaluronan and CD44 depletion in cultures treated with higher doses. The magnification bar represents 50 μm. I–J, molecular mass distribution of epidermal hyaluronan extracted 8 h (I) and 24 h (J) after a UVB (30 mJ/cm2) or sham exposure and analyzed with S1000 gel filtration. The data represent means and half-range (error bars) from two cultures.
Because the histological analyses suggested local acceleration of hyaluronan degradation in the UVB-treated samples, we assayed the molecular mass distribution of epidermal hyaluronan. The analyses showed that the UVB-treated epidermal cultures contained more hyaluronan in the lower, ∼50–500 kDa size range (Fig. 7, I and J). At 24 h, the increase of the low molecular mass hyaluronan (fractions 25–33) was 61%, as compared with 25% in the high molecular mass material (fractions 17–23; Fig. 7J). A similar trend was also seen in the size distribution of hyaluronan associated with the collagen matrix (data not shown). Because the newly synthesized hyaluronan in these cells is of high molecular mass (58) (Fig. 1D), the data suggest accelerated fragmentation of hyaluronan in the UVB-treated specimens. Thus, the organotypic epidermis, containing keratinocytes at all differentiation stages and with UVB filtered through an intact stratum corneum, also elicits a clear hyaluronan response to acute irradiation, with both the synthetic and degradative mechanisms activated after the exposure.
DISCUSSION
The present data show that keratinocytes respond to acute, low dose UVB exposure by a rapid and long lasting synthesis and deposition of hyaluronan. The increase of hyaluronan was associated with sequential, partly overlapping inductions of the Has1, Has2, and Has3 genes, the latter two mediated by the p38 and CaMKII pathways, respectively. Increased expression of Hyal1 and Hyal2 as well as Cd44 suggests that not just the synthesis, but also degradation of hyaluronan was affected. Most of these responses were reproduced in a stratified, organotypic model, supporting the in vivo relevance of the results.
Dose Dependence of the UVB-induced Changes in Hyaluronan Metabolism
To avoid overt toxicity, we used relatively low UVB doses in most of the experiments. That these doses were biologically meaningful was shown by the fact that cell proliferation temporarily slowed down. Furthermore, the results of a genome-wide gene expression array using the organotypic model indicated highly significant activation of many biological processes relevant to irradiation damage, including DNA repair and cell cycle control.3 Although an increase in hyaluronan content was first found with increasing doses of UVB, a trend toward reduction was observed at the highest dose used. A similar dose-dependent dichotomy in the acute effects of UVB irradiation on the synthesis of hyaluronan has been found in human keratinocytes (25), probably reflecting general toxicity of the high doses.
In the organotypic epidermis, UVB is filtered through stratum corneum and a part of the vital cell layers. This caused variation in the effective dose reaching the cells, and generally higher doses were required to reach effects similar to those seen in the monolayer cultures.
Time-dependent Expression of Has Isoenzymes
Whereas Has1 expression rapidly increased, Has2 was down-regulated 2–4 h after the UVB exposure, which correlates well with the previously reported decrease of Has2 transcripts at 3–6 h postirradiation in HaCaT cells (24, 63). Regardless of the fluctuations of Has expressions during the first hours, hyaluronan synthesis rose rapidly, as indicated by the 2.4-fold elevation in its content already after 12 h. Interestingly, quite a persistent up-regulation of Has2 and Has3 took place in REKs at the later time points after a single UVB exposure, causing a robust increase in hyaluronan secretion. This is also in line with the recent data of Tobiishi et al. (28), who saw in mouse epidermis a significant up-regulation of Has2 and Has3 following 1 and 2 days of acute UVB irradiation, respectively.
The Role of Different Has Isoenzymes in the UVB-induced Synthesis of Hyaluronan
Has2 and Has3 gene up-regulation generally accompanies hyaluronan accumulation in epidermal keratinocytes subjected to various growth factors or injury (22, 46, 64–66). The same was found in response to UVB in the present and previous reports (24, 25, 28). Due to their coexpression and similar reactions to many of the stimuli studied, the contribution of each individual Has to the deposition of hyaluronan in keratinocytes has been difficult. However, a drastic decline in the wounding-induced epidermal hyaluronan accumulation was observed in mice with deleted Has3 and Has1 genes, suggesting that one or both of these enzymes were responsible for the majority of hyaluronan accumulation (67). Our data using siRNA interference indicated that Has3 played a major role in UVB-induced hyaluronan synthesis in REK cells, whereas Has2 also contributed to hyaluronan accumulation, although its role was more modest. Because we were unable to silence Has1 expression, the role of Has1 remains unsettled at the moment. The less persistent up-regulation of Has1 by UVB and its dependence on high substrate levels for active hyaluronan synthesis, as compared with Has2 and Has3 (68, 69), suggest that the role of Has1 in the UVB-induced synthesis of hyaluronan in keratinocytes is less prominent than that of Has3.
UVB caused up-regulation of Has2 and Has3 expression also in the organotypic cultures. However, the magnitude of the UVB response in Has3 expression was more modest in the organotypic cultures, and no change at all was detected in Has1. Variation in the effective UVB dose received by different epidermal cell layers may contribute to the smaller response in the organotypic cultures. It is also likely that the signaling systems influencing Has expression vary between undifferentiated and differentiated cells. Likewise, release of bioactive molecules from the cornified and differentiating cells may contribute to the differential effects observed between the monolayer and organotypic models.
The Role of Hyaluronan Degradation in the UVB-induced Hyaluronan Response
While hyaluronan synthesis and content increased in the UVB-treated cultures, there was a simultaneous up-regulation of Hyal1 and Hyal2 expressions both in the monolayer and organotypic keratinocyte cultures, in line with a previous report (24). Increased hyaluronidase activity should lead to enhanced catabolism and decreased hyaluronan accumulation, counteracting the stimulated hyaluronan production by HAS activity. Although no hyaluronan fragmentation was visible by gel filtration in the REK monolayer cultures or previously in human keratinocytes (25), we observed a change in the molecular mass distribution toward smaller sizes in the organotypic model. The cause of this fragmentation, whether due to the Hyal enzymes or to non-enzymatic degradation via ROS (70) generated by the irradiation (71, 72), remains unsettled at the moment. ROS may also have an indirect effect by activating Hyal enzymes (73, 74), adding to the complex regulatory mechanisms.
However, the metabolism of epidermal hyaluronan is more efficient in organotypic cultures (51, 75) than in monolayers.4 Keratinocytes in the normal epidermis produce hyaluronan into the narrow intercellular space, where it is probably more accessible to the degradative machinery (receptors, enzymes, and ROS), compared with monolayers, where hyaluronan is mostly released into a large volume of culture medium.
In any case, the change in the molecular mass distribution and the locally reduced staining pattern of hyaluronan after the highest UVB doses suggest that acute UVB causes activation of hyaluronan catabolism in the epidermis. This, together with the lower activation of Has expression, probably explains why the surge of hyaluronan remained more modest in the organotypic cultures than in the monolayers.
p38 Activation Is Involved in Increased Hyaluronan Synthesis and Has Expression
From the multitude of possible signaling routes activated by UVB (35, 40, 41, 62, 76–78), we first checked EGFR and its downstream pathways, known to be of crucial importance in the regulation of epidermal hyaluronan synthesis (45, 46, 64, 79). However, the inhibitor of EGFR exhibited a modest effect on the UVB-induced hyaluronan response, and it failed to prevent the UVB-induced up-regulation of Has expression. Lack of UVB-induced activation of ERK1/2, STAT3, and JNK and the inability of the inhibitors of these pathways to suppress hyaluronan production excluded these pathways as mediators of the UVB-induced hyaluronan response in REK cells.
Of the MAPKs tested, only p38 showed significant activation by UVB. Accordingly, a specific inhibitor of p38 significantly suppressed the UVB-induced increase of hyaluronan. The target of p38 signaling was clearly Has expression, because p38 inhibition efficiently blocked the UVB-mediated up-regulation of Has1 and particularly Has2.
Calcium Signals Involved in the UVB-induced Hyaluronan Synthesis
UVB has been reported to increase Ca2+ flux into the cytosol, activating the production of ROS (80) and various signaling cascades (61). Our data, indicating that KN93, a specific inhibitor of CaMKII, reduced the UVB-induced hyaluronan synthesis clearly more than its inactive control compound, suggest the involvement of calcium-related pathways in the hyaluronan response. This would be in agreement with previous reports that perturbation of epidermal calcium homeostasis is associated with changes in hyaluronan synthesis (81) and that UV radiation can activate CaMKII (60, 61). There are, however, no previous reports connecting CaMKII to the regulation of Has expression.
Of the three Has isoenzymes, Has3 appeared to be the one regulated by a CaMKII-related mechanism, explaining the strong effect of KN93 on hyaluronan synthesis. The effect on Has3 expression was especially obvious at the later time points, suggesting that the up-regulation of Has3 by CaMKII probably represents an adaptive response to more immediate changes triggered by UVB. Our preliminary data suggest that G-protein-mediated mechanisms (including the P2Y2 receptor) may be involved in the induction of Has3 by UVB.5 However, further experimentation is needed to define the upstream factors evoking the CaMKII and p38 signals that lead to the up-regulation of the Has isoforms.
Importance of Hyaluronan Synthesis in UVB-induced Malignancies
The emerging link between epidermal hyaluronan and UVB is particularly noteworthy, considering the increasing incidence of basal and squamous cell carcinomas and melanomas. Squamous cell carcinomas induced by chronic UV irradiation show elevated levels of all HAS isoenzymes and accumulation of hyaluronan (27). Notably, chronic UV exposure causes hyaluronan accumulation in the epidermis already in benign lesions (27). This is in line with our present findings on acute hyaluronan response and supports the view that hyaluronan synthesis is activated early on during skin carcinogenesis. In the human skin tumors related to UV irradiation (i.e. SCC and melanoma), hyaluronan increases in early lesions, whereas it tends to be reduced in more advanced cancers6 (82). In immunostainings of human melanomas, HAS1–3 are all present, and particularly HAS1 and HAS2 are elevated in dysplastic nevi and/or melanoma in situ but decreased in metastases.6 The staining for HYAL2 is increased in both early and more advanced melanomas.6 The present findings are thus largely parallel to those in early UV-induced tumors in vivo.
Interestingly, skin tumor development appears to be dependent on p38 function (83), the same signal found here in the acute hyaluronan response to UVB. CaMKII also appears to be a key mediator in carcinomas inducing keratinocyte differentiation with Wnt5a in regressing tumors (84). Moreover, the hyaluronan-CD44 axis has been indicated to convey signals via CaMKII in head and neck squamous carcinoma cells, causing cytoskeletal rearrangements and promoting migration (85). The activation of p38, CaMKII, and hyaluronan synthesis may thus represent changes in the epidermis that facilitate cell proliferation and later, after repeated exposure, tumorigenesis.
HAS Activation as a Part of Inflammation and Wounding
p38 is activated in inflammation, and the biological significance of the acutely increased hyaluronan synthesis after UVB-irradiation may be a part of this adaptive reaction to tissue trauma. This would be similar to the p38-dependent up-regulation of Has1 and Has2 noted in synovial fibroblasts and intestinal mesenchymal cells, respectively, when subjected to mediators of inflammation (86, 87). Similarly, Has3 can be induced by proinflammatory cytokines in synovial membrane cells (88). This kind of acute increase in epidermal hyaluronan can be considered as an adaptive response to restore homeostasis, much like those seen after wounding (22) and epidermal barrier disruption (89).
Hyaluronan may also have a protective effect in itself by modulating apoptotic signals and cytokine production after UVB irradiation, like in corneal epithelial cell cultures pretreated with high molecular mass hyaluronan (90). Similarly, it was reported that exogenous hyaluronan increases the viability of UVB-exposed keratinocytes and decreases the secretion of inflammatory mediators (63). Moreover, the anti-inflammatory action of hyaluronan is supported by the finding that Has1/Has3 knock-out mice show enhanced inflammatory response after wounding (67), and overexpressing Has1 reduces leukocyte infiltration and enhances wound healing in a mouse model (91).
CONCLUSION
The present work shows for the first time that the acute up-regulation of epidermal hyaluronan synthesis by UVB radiation is mediated by p38 and CaMKII. Although Has1 and Has2 have previously been shown to be regulated through p38, our finding that Has3 is regulated by CaMKII reveals a new regulatory pathway. In addition to activating hyaluronan synthesis, UVB may also influence hyaluronan degradation, especially in the context of normal epidermal tissue architecture. The final outcome of the enhanced hyaluronan metabolism induced by UVB, whether accumulation or loss of hyaluronan, eventually depends on the balance of these opposite processes, as seen in UVB-induced skin tumors.
Supplementary Material
Acknowledgments
We are grateful to Eija Rahunen, Kari Kotikumpu, Eija Kettunen, Arja Venäläinen, Tuula Venäläinen, and Riikka Kärnä for helping with the laboratory analyses and to Dr. Timo Kumlin for calibrating the UVB lamp. Dr. Sanna Oikari, Marja-Leena Hannila, and Dr. Thomas W. Dunlop gave valuable advice on data analysis.
This work was supported by grants from Tekes (to R. T.), the Cancer Center of the University of Eastern Finland (to R. T. and M. T.), and the Juselius Foundation (to R. T. and M. T.).

This article contains supplemental Tables 1–3 and Figs. 1–6.
G. Bart, L. Hämäläinen, L. Rauhala, P. Salonen, M. Kokkonen, P. Pehkonen, T. W. Dunlop, M. Tammi, S. Pasonen-Seppanen, and R. Tammi, manuscript in preparation.
R. Tammi, unpublished observations.
G. Bart and T.A. Jokela, unpublished data.
Siiskonen, H., Poukka, M., Tyynelä-Korhonen, K., Sironen, R., and Pasonen-Seppänen, S. (2013) Inverse expression of hyaluronidase 2 and hyaluronan synthases 1–3 is associated with reduced hyaluronan content in malignant cutaneous melanoma. BMC Cancer 13, 181.
- UVB
- ultraviolet B
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- HABC
- hyaluronan binding complex
- REK
- rat epidermal keratinocyte
- ROS
- reactive oxygen species
- EGFR
- epidermal growth factor receptor
- MEM
- minimum essential medium
- qRT-PCR
- quantitative real-time PCR
- LSD
- least significant difference.
REFERENCES
- 1. Diepgen T. L., Mahler V. (2002) The epidemiology of skin cancer. Br. J. Dermatol. 146, Suppl. 61, 1–6 [DOI] [PubMed] [Google Scholar]
- 2. Hong S. P., Kim M. J., Jung M. Y., Jeon H., Goo J., Ahn S. K., Lee S. H., Elias P. M., Choi E. H. (2008) Biopositive effects of low-dose UVB on epidermis. Coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J. Invest. Dermatol. 128, 2880–2887 [DOI] [PubMed] [Google Scholar]
- 3. Tammi R., Ripellino J. A., Margolis R. U., Tammi M. (1988) Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J. Invest. Dermatol. 90, 412–414 [DOI] [PubMed] [Google Scholar]
- 4. Reed R. K., Lilja K., Laurent T. C. (1988) Hyaluronan in the rat with special reference to the skin. Acta Physiol. Scand. 134, 405–411 [DOI] [PubMed] [Google Scholar]
- 5. Stern R., Maibach H. I. (2008) Hyaluronan in skin. Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 26, 106–122 [DOI] [PubMed] [Google Scholar]
- 6. Schulz T., Schumacher U., Prehm P. (2007) Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J. Biol. Chem. 282, 20999–21004 [DOI] [PubMed] [Google Scholar]
- 7. Weigel P. H., DeAngelis P. L. (2007) Hyaluronan synthases. A decade-plus of novel glycosyltransferases. J. Biol. Chem. 282, 36777–36781 [DOI] [PubMed] [Google Scholar]
- 8. Rilla K., Siiskonen H., Spicer A. P., Hyttinen J. M., Tammi M. I., Tammi R. H. (2005) Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J. Biol. Chem. 280, 31890–31897 [DOI] [PubMed] [Google Scholar]
- 9. Kultti A., Rilla K., Tiihonen R., Spicer A. P., Tammi R. H., Tammi M. I. (2006) Hyaluronan synthesis induces microvillus-like cell surface protrusions. J. Biol. Chem. 281, 15821–15828 [DOI] [PubMed] [Google Scholar]
- 10. Rilla K., Tiihonen R., Kultti A., Tammi M., Tammi R. (2008) Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J. Histochem. Cytochem. 56, 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Majors A. K., Austin R. C., de la Motte C. A., Pyeritz R. E., Hascall V. C., Kessler S. P., Sen G., Strong S. A. (2003) Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J. Biol. Chem. 278, 47223–47231 [DOI] [PubMed] [Google Scholar]
- 12. Jokela T. A., Lindgren A., Rilla K., Maytin E., Hascall V. C., Tammi R. H., Tammi M. I. (2008) Induction of hyaluronan cables and monocyte adherence in epidermal keratinocytes. Connect. Tissue Res. 49, 115–119 [DOI] [PubMed] [Google Scholar]
- 13. Pasonen-Seppänen S., Hyttinen J. M., Rilla K., Jokela T., Noble P. W., Tammi M., Tammi R. (2012) Role of CD44 in the organization of keratinocyte pericellular hyaluronan. Histochem. Cell Biol. 137, 107–120 [DOI] [PubMed] [Google Scholar]
- 14. Turley E. A., Noble P. W., Bourguignon L. Y. (2002) Signaling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 [DOI] [PubMed] [Google Scholar]
- 15. Spicer A. P., Tien J. Y. (2004) Hyaluronan and morphogenesis. Birth Defects Res. C Embryo Today 72, 89–108 [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto K., Li Y., Jakuba C., Sugiyama Y., Sayo T., Okuno M., Dealy C. N., Toole B. P., Takeda J., Yamaguchi Y., Kosher R. A. (2009) Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation, and joint formation in the developing limb. Development 136, 2825–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heldin P., Karousou E., Bernert B., Porsch H., Nishitsuka K., Skandalis S. S. (2008) Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect. Tissue Res. 49, 215–218 [DOI] [PubMed] [Google Scholar]
- 18. Oikari S., Jokela T. A., Tammi R. H., Tammi M. I. (2012) Multiple roles of hyaluronan as a target and modifier of the inflammatory response. in Extracellular Matrix: Pathobiology and Signaling (Karamanos N., ed) pp. 39–65, De Gruyter, Berlin [Google Scholar]
- 19. Auvinen P., Tammi R., Parkkinen J., Tammi M., Ågren U., Johansson R., Hirvikoski P., Eskelinen M., Kosma V. M. (2000) Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 156, 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Itano N., Kimata K. (2008) Altered hyaluronan biosynthesis in cancer progression. Semin. Cancer Biol. 18, 268–274 [DOI] [PubMed] [Google Scholar]
- 21. Chen W. Y., Abatangelo G. (1999) Functions of hyaluronan in wound repair. Wound Repair Regen. 7, 79–89 [DOI] [PubMed] [Google Scholar]
- 22. Tammi R., Pasonen-Seppänen S., Kolehmainen E., Tammi M. (2005) Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J. Invest. Dermatol. 124, 898–905 [DOI] [PubMed] [Google Scholar]
- 23. Passi A., Sadeghi P., Kawamura H., Anand S., Sato N., White L. E., Hascall V. C., Maytin E. V. (2004) Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp. Cell Res. 296, 123–134 [DOI] [PubMed] [Google Scholar]
- 24. Averbeck M., Gebhardt C. A., Voigt S., Beilharz S., Anderegg U., Termeer C. C., Sleeman J. P., Simon J. C. (2007) Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Invest. Dermatol. 127, 687–697 [DOI] [PubMed] [Google Scholar]
- 25. Kakizaki I., Itano N., Kimata K., Hanada K., Kon A., Yamaguchi M., Takahashi T., Takagaki K. (2008) Up-regulation of hyaluronan synthase genes in cultured human epidermal keratinocytes by UVB irradiation. Arch. Biochem. Biophys. 471, 85–93 [DOI] [PubMed] [Google Scholar]
- 26. Mouchet N., Adamski H., Bouvet R., Corre S., Courbebaisse Y., Watier E., Mosser J., Chesné C., Galibert M. D. (2010) In vivo identification of solar radiation-responsive gene network. Role of the p38 stress-dependent kinase. PLoS One 5, e10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siiskonen H., Törrönen K., Kumlin T., Rilla K., Tammi M. I., Tammi R. H. (2011) Chronic UVR causes increased immunostaining of CD44 and accumulation of hyaluronan in mouse epidermis. J. Histochem. Cytochem. 59, 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobiishi M., Sayo T., Yoshida H., Kusaka A., Kawabata K., Sugiyama Y., Ishikawa O., Inoue S. (2011) Changes in epidermal hyaluronan metabolism following UVB irradiation. J. Dermatol. Sci. 64, 31–38 [DOI] [PubMed] [Google Scholar]
- 29. Calikoglu E., Sorg O., Tran C., Grand D., Carraux P., Saurat J. H., Kaya G. (2006) UVA and UVB decrease the expression of CD44 and hyaluronate in mouse epidermis, which is counteracted by topical retinoids. Photochem. Photobiol. 82, 1342–1347 [DOI] [PubMed] [Google Scholar]
- 30. Dai G., Freudenberger T., Zipper P., Melchior A., Grether-Beck S., Rabausch B., de Groot J., Twarock S., Hanenberg H., Homey B., Krutmann J., Reifenberger J., Fischer J. W. (2007) Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 171, 1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Röck K., Grandoch M., Majora M., Krutmann J., Fischer J. W. (2011) Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB). New insights into mechanisms of matrix remodeling. J. Biol. Chem. 286, 18268–18276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adler V., Polotskaya A., Kim J., Dolan L., Davis R., Pincus M., Ronai Z. (1996) Dose rate and mode of exposure are key factors in JNK activation by UV irradiation. Carcinogenesis 17, 2073–2076 [DOI] [PubMed] [Google Scholar]
- 33. Brenner M., Degitz K., Besch R., Berking C. (2005) Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br. J. Dermatol. 153, 733–739 [DOI] [PubMed] [Google Scholar]
- 34. Zhong J. L., Yang L., Lü F., Xiao H., Xu R., Wang L., Zhu F., Zhang Y. (2011) UVA, UVB and UVC induce differential response signaling pathways converged on the eIF2α phosphorylation. Photochem. Photobiol. 87, 1092–1104 [DOI] [PubMed] [Google Scholar]
- 35. Peus D., Vasa R. A., Beyerle A., Meves A., Krautmacher C., Pittelkow M. R. (1999) UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J. Invest. Dermatol. 112, 751–756 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q. S., Maddock D. A., Chen J. P., Heo S., Chiu C., Lai D., Souza K., Mehta S., Wan Y. S. (2001) Cytokine-induced p38 activation feedback regulates the prolonged activation of AKT cell survival pathway initiated by reactive oxygen species in response to UV irradiation in human keratinocytes. Int. J. Oncol. 19, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 37. Xu Y., Shao Y., Zhou J., Voorhees J. J., Fisher G. J. (2009) Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J. Cell Biochem. 107, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bito T., Sumita N., Masaki T., Shirakawa T., Ueda M., Yoshiki R., Tokura Y., Nishigori C. (2010) Ultraviolet light induces Stat3 activation in human keratinocytes and fibroblasts through reactive oxygen species and DNA damage. Exp. Dermatol. 19, 654–660 [DOI] [PubMed] [Google Scholar]
- 39. Jost M., Kari C., Rodeck U. (2000) The EGF receptor. An essential regulator of multiple epidermal functions. Eur. J. Dermatol. 10, 505–510 [PubMed] [Google Scholar]
- 40. Xu Y., Voorhees J. J., Fisher G. J. (2006) Epidermal growth factor receptor is a critical mediator of ultraviolet B irradiation-induced signal transduction in immortalized human keratinocyte HaCaT cells. Am. J. Pathol. 169, 823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rezvani H. R., Dedieu S., North S., Belloc F., Rossignol R., Letellier T., de Verneuil H., Taïeb A., Mazurier F. (2007) Hypoxia-inducible factor-1α, a key factor in the keratinocyte response to UVB exposure. J. Biol. Chem. 282, 16413–16422 [DOI] [PubMed] [Google Scholar]
- 42. Tammi R., MacCallum D., Hascall V. C., Pienimäki J. P., Hyttinen M., Tammi M. (1998) Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J. Biol. Chem. 273, 28878–28888 [DOI] [PubMed] [Google Scholar]
- 43. Suhonen M.T., Pasonen-Seppänen S., Kirjavainen M., Tammi M., Tammi R., Urtti A. (2003) Epidermal cell culture model derived from rat keratinocytes with permeability characteristics comparable to human cadaver skin. Eur. J. Pharm. Sci. 20, 107–113 [DOI] [PubMed] [Google Scholar]
- 44. Ajani G., Sato N., Mack J. A., Maytin E. V. (2007) Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp. Cell Res. 313, 3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pasonen-Seppänen S. M., Maytin E. V., Törrönen K. J., Hyttinen J. M., Hascall V. C., MacCallum D. K., Kultti A. H., Jokela T. A., Tammi M. I., Tammi R. H. (2008) All-trans-retinoic acid-induced hyaluronan production and hyperplasia are partly mediated by EGFR signaling in epidermal keratinocytes. J. Invest. Dermatol. 128, 797–807 [DOI] [PubMed] [Google Scholar]
- 46. Monslow J., Sato N., Mack J. A., Maytin E. V. (2009) Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding egf and activation of the EGFR. J. Invest. Dermatol. 129, 2046–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baden H. P., Kubilus J. (1983) The growth and differentiation of cultured newborn rat keratinocytes. J. Invest. Dermatol. 80, 124–130 [DOI] [PubMed] [Google Scholar]
- 48. Pasonen-Seppänen S., Suhonen T. M., Kirjavainen M., Suihko E., Urtti A., Miettinen M., Hyttinen M., Tammi M., Tammi R. (2001) Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 116, 287–297 [DOI] [PubMed] [Google Scholar]
- 49. Hiltunen E. L., Anttila M., Kultti A., Ropponen K., Penttinen J., Yliskoski M., Kuronen A. T., Juhola M., Tammi R., Tammi M., Kosma V. M. (2002) Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res. 62, 6410–6413 [PubMed] [Google Scholar]
- 50. Tammi R., Ågren U. M., Tuhkanen A. L., Tammi M. (1994) Hyaluronan metabolism in skin. Prog. Histochem. Cytochem. 29, 1–81 [DOI] [PubMed] [Google Scholar]
- 51. Tammi R. H., Tammi M. I., Hascall V. C., Hogg M., Pasonen S., MacCallum D. K. (2000) A preformed basal lamina alters the metabolism and distribution of hyaluronan in epidermal keratinocyte “organotypic” cultures grown on collagen matrices. Histochem. Cell Biol. 113, 265–277 [DOI] [PubMed] [Google Scholar]
- 52. Yanagishita M., Salustri A., Hascall V. C. (1989) Specific activity of radiolabeled hexosamines in metabolic labeling experiments. Methods Enzymol. 179, 435–445 [DOI] [PubMed] [Google Scholar]
- 53. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 54. Wang C., Tammi M., Tammi R. (1992) Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry 98, 105–112 [DOI] [PubMed] [Google Scholar]
- 55. R Development Core Team (2011) R: A Language and Environment for Statistical Computing, Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 56. Takahashi Y., Ishikawa O., Okada K., Ohnishi K., Miyachi Y. (1995) Disaccharide analysis of the skin glycosaminoglycans in chronically ultraviolet light-irradiated hairless mice. J. Dermatol. Sci. 10, 139–144 [DOI] [PubMed] [Google Scholar]
- 57. Ramamurthi A., Vesely I. (2003) Ultraviolet light-induced modification of crosslinked hyaluronan gels. J. Biomed. Mater. Res. A 66, 317–329 [DOI] [PubMed] [Google Scholar]
- 58. Tammi R., Rilla K., Pienimäki J. P., MacCallum D. K., Hogg M., Luukkonen M., Hascall V. C., Tammi M. (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 276, 35111–35122 [DOI] [PubMed] [Google Scholar]
- 59. Tuhkanen A. L., Tammi M., Tammi R. (1997) CD44 substituted with heparan sulfate and endo-β-galactosidase-sensitive oligosaccharides. A major proteoglycan in adult human epidermis. J. Invest. Dermatol. 109, 213–218 [DOI] [PubMed] [Google Scholar]
- 60. Wright S. C., Schellenberger U., Ji L., Wang H., Larrick J. W. (1997) Calmodulin-dependent protein kinase II mediates signal transduction in apoptosis. FASEB J. 11, 843–849 [DOI] [PubMed] [Google Scholar]
- 61. Hwang Y. P., Kim H. G., Han E. H., Choi J. H., Park B. H., Jung K. H., Shin Y. C., Jeong H. G. (2011) N-Acetylglucosamine suppress collagenases activation in ultraviolet B-irradiated human dermal fibroblasts. Involvement of calcium ions and mitogen-activated protein kinases. J. Dermatol. Sci. 63, 93–103 [DOI] [PubMed] [Google Scholar]
- 62. Kim D. J., Tremblay M. L., Digiovanni J. (2010) Protein tyrosine phosphatases, TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation. PLoS One 5, e10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hašová M., Crhák T., Safránková B., Dvořáková J., Muthný T., Velebný V., Kubala L. (2011) Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch. Dermatol. Res. 303, 277–284 [DOI] [PubMed] [Google Scholar]
- 64. Pienimäki J. P., Rilla K., Fulop C., Sironen R. K., Karvinen S., Pasonen S., Lammi M. J., Tammi R., Hascall V. C., Tammi M. I. (2001) Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J. Biol. Chem. 276, 20428–20435 [DOI] [PubMed] [Google Scholar]
- 65. Pasonen-Seppänen S., Karvinen S., Törrönen K., Hyttinen J. M., Jokela T., Lammi M. J., Tammi M. I., Tammi R. (2003) EGF upregulates, whereas TGF-β downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures. Correlations with epidermal proliferation and differentiation. J. Invest. Dermatol. 120, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 66. Karvinen S., Pasonen-Seppänen S., Hyttinen J. M., Pienimäki J. P., Törrönen K., Jokela T. A., Tammi M. I., Tammi R. (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J. Biol. Chem. 278, 49495–49504 [DOI] [PubMed] [Google Scholar]
- 67. Mack J. A., Feldman R. J., Itano N., Kimata K., Lauer M., Hascall V. C., Maytin E. V. (2012) Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases has1 and has3. J. Invest. Dermatol. 132, 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., Kimata K. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 69. Rilla K., Oikari S., Jokela T. A., Hyttinen J. M., Kärnä R., Tammi R. H., Tammi M. I. (2013) Hyaluronan Synthase 1 (HAS1) Requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem. 288, 5973–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ågren U. M., Tammi R. H., Tammi M. I. (1997) Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic. Biol. Med. 23, 996–1001 [DOI] [PubMed] [Google Scholar]
- 71. Heck D. E., Vetrano A. M., Mariano T. M., Laskin J. D. (2003) UVB light stimulates production of reactive oxygen species. Unexpected role for catalase. J. Biol. Chem. 278, 22432–22436 [DOI] [PubMed] [Google Scholar]
- 72. Yasui H., Hakozaki T., Date A., Yoshii T., Sakurai H. (2006) Real-time chemiluminescent imaging and detection of reactive oxygen species generated in the UVB-exposed human skin equivalent model. Biochem. Biophys. Res. Commun. 347, 83–88 [DOI] [PubMed] [Google Scholar]
- 73. Monzon M. E., Fregien N., Schmid N., Falcon N. S., Campos M., Casalino-Matsuda S. M., Forteza R. M. (2010) Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J. Biol. Chem. 285, 26126–26134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Esser P. R., Wölfle U., Dürr C., von Loewenich F. D., Schempp C. M., Freudenberg M. A., Jakob T., Martin S. F. (2012) Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One 7, e41340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tammi R., Säämänen A. M., Maibach H. I., Tammi M. (1991) Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J. Invest. Dermatol. 97, 126–130 [DOI] [PubMed] [Google Scholar]
- 76. Bickers D. R., Athar M. (2006) Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 126, 2565–2575 [DOI] [PubMed] [Google Scholar]
- 77. Li Y., Bi Z., Yan B., Wan Y. (2006) UVB radiation induces expression of HIF-1α and VEGF through the EGFR/PI3K/DEC1 pathway. Int. J. Mol. Med. 18, 713–719 [PubMed] [Google Scholar]
- 78. Van Laethem A., Garmyn M., Agostinis P. (2009) Starting and propagating apoptotic signals in UVB irradiated keratinocytes. Photochem. Photobiol. Sci. 8, 299–308 [DOI] [PubMed] [Google Scholar]
- 79. Saavalainen K., Pasonen-Seppänen S., Dunlop T. W., Tammi R., Tammi M. I., Carlberg C. (2005) The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J. Biol. Chem. 280, 14636–14644 [DOI] [PubMed] [Google Scholar]
- 80. Masaki H., Izutsu Y., Yahagi S., Okano Y. (2009) Reactive oxygen species in HaCaT keratinocytes after UVB irradiation are triggered by intracellular Ca2+ levels. J. Investig. Dermatol. Symp. Proc. 14, 50–52 [DOI] [PubMed] [Google Scholar]
- 81. Lee S. E., Jun J. E., Choi E. H., Ahn S. K., Lee S. H. (2010) Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin. Exp. Dermatol. 35, 650–657 [DOI] [PubMed] [Google Scholar]
- 82. Karvinen S., Kosma V. M., Tammi M. I., Tammi R. (2003) Hyaluronan, CD44 and versican in epidermal keratinocyte tumours. Br. J. Dermatol. 148, 86–94 [DOI] [PubMed] [Google Scholar]
- 83. Schindler E. M., Hindes A., Gribben E. L., Burns C. J., Yin Y., Lin M. H., Owen R. J., Longmore G. D., Kissling G. E., Arthur J. S., Efimova T. (2009) p38δ Mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Res. 69, 4648–4655 [DOI] [PubMed] [Google Scholar]
- 84. Nitzki F., Zibat A., König S., Wijgerde M., Rosenberger A., Brembeck F. H., Carstens P. O., Frommhold A., Uhmann A., Klingler S., Reifenberger J., Pukrop T., Aberger F., Schulz-Schaeffer W., Hahn H. (2010) Tumor stroma-derived Wnt5a induces differentiation of basal cell carcinoma of Ptch-mutant mice via CaMKII. Cancer Res. 70, 2739–2748 [DOI] [PubMed] [Google Scholar]
- 85. Bourguignon L. Y., Gilad E., Brightman A., Diedrich F., Singleton P. (2006) Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C ϵ-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J. Biol. Chem. 281, 14026–14040 [DOI] [PubMed] [Google Scholar]
- 86. Stuhlmeier K.M., Pollaschek C. (2004) Differential effect of transforming growth factor β (TGF-β) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-β-induced hyaluronan synthase 1 activation. J. Biol. Chem. 279, 8753–8760 [DOI] [PubMed] [Google Scholar]
- 87. Ducale A. E., Ward S. I., Dechert T., Yager D. R. (2005) Regulation of hyaluronan synthase-2 expression in human intestinal mesenchymal cells. Mechanisms of interleukin-1β-mediated induction. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G462–G470 [DOI] [PubMed] [Google Scholar]
- 88. Tanimoto K., Ohno S., Fujimoto K., Honda K., Ijuin C., Tanaka N., Doi T., Nakahara M., Tanne K. (2001) Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect. Tissue Res. 42, 187–195 [DOI] [PubMed] [Google Scholar]
- 89. Maytin E. V., Chung H. H., Seetharaman V. M. (2004) Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am. J. Pathol. 165, 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pauloin T., Dutot M., Joly F., Warnet J. M., Rat P. (2009) High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis. 15, 577–583 [PMC free article] [PubMed] [Google Scholar]
- 91. Caskey R. C., Allukian M., Lind R. C., Herdrich B. J., Xu J., Radu A., Mitchell M. E., Liechty K. W. (2013) Lentiviral-mediated over-expression of hyaluronan synthase-1 (HAS-1) decreases the cellular inflammatory response and results in regenerative wound repair. Cell Tissue Res. 351, 117–125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.