Background: Estrogen supplementation enhances voluntary alcohol consumption in ovariectomized rodents. The effects of the enhanced alcohol consumption on post-infarct myocardial repair are unknown.
Results: Ethanol-mediated suppression of endothelial progenitor cells produces diminished post-ischemic left ventricular function.
Conclusion: Estrogen-induced increases in alcohol consumption negatively compete with the cardioprotective effects of estrogen.
Significance: Alcohol consumption during estrogen replacement therapy must be observed closely.
Keywords: Alcohol, Cardiovascular, Estrogen, Ischemia, Signal Transduction
Abstract
We have shown previously that estrogen (estradiol, E2) supplementation enhances voluntary alcohol consumption in ovariectomized female rodents and that increased alcohol consumption impairs ischemic hind limb vascular repair. However, the effect of E2-induced alcohol consumption on post-infarct myocardial repair and on the phenotypic/functional properties of endothelial progenitor cells (EPCs) is not known. Additionally, the molecular signaling of alcohol-estrogen interactions remains to be elucidated. This study examined the effect of E2-induced increases in ethanol consumption on post-infarct myocardial function/repair. Ovariectomized female mice, implanted with 17β-E2 or placebo pellets were given access to alcohol for 6 weeks and subjected to acute myocardial infarction. Left ventricular functions were consistently depressed in mice consuming ethanol compared with those receiving only E2. Alcohol-consuming mice also displayed significantly increased infarct size and reduced capillary density. Ethanol consumption also reduced E2-induced mobilization and homing of EPCs to injured myocardium compared with the E2-alone group. In vitro, exposure of EPCs to ethanol suppressed E2-induced proliferation, survival, and migration and markedly altered E2-induced estrogen receptor-dependent cell survival signaling and gene expression. Furthermore, ethanol-mediated suppression of EPC biology was endothelial nitric oxide synthase-dependent because endothelial nitric oxide synthase-null mice displayed an exaggerated response to post-acute myocardial infarction left ventricular functions. These data suggest that E2 modulation of alcohol consumption, and the ensuing EPC dysfunction, may negatively compete with the beneficial effects of estrogen on post-infarct myocardial repair.
Introduction
Epidemiological and cohort studies have long suggested that estrogen (E2)2 replacement therapy in postmenopausal women might be beneficial in the prevention of adverse cardiovascular events (1, 2). A large number of animal studies have also supported this notion (3, 4). However, data from large randomized clinical trials of primary Women's Health Initiative (WHI) or secondary Heart and Estrogen-Progestin Replacement Study (HERS) prevention have failed to confirm cardioprotection with hormone replacement therapy (5, 6). Patient-to-patient variation in hormone replacement therapy variables, such as time of treatment initiation, duration of therapy, and the selection of the optimal hormone replacement therapy regimen, may all help to explain some of the inconsistencies observed between the large clinical studies and the cardioprotective benefits of E2 reported in observational and animal studies (3, 7–9). However, many other mitigating factors that may mask the beneficial or protective effects of E2 on cardiovascular outcome need to be considered. Animal studies (10, 11), including our own study (12), indicate that estrogen supplementation increases the consumption of alcohol. Moreover, research has shown that females are more susceptible to alcohol-mediated pathophysiologies, including cardiac dysfunction (13). Although ethanol is one of the oldest known substances of abuse, the mechanism(s) whereby this compound causes cardiac dysfunction, especially in regard to the repression of the cardioprotective effects of estrogen, is not understood. Increasing evidence from many studies suggests that E2 supplementation enhances the recovery of ischemic tissues, including myocardium, partly by mobilizing bone marrow-derived hematopoietic stem cells/endothelial progenitor cells (EPCs), to participate in neo-vascularization and tissue repair (4, 14–16). The participation of bone marrow-derived progenitor cells in myocardial neo-vascularization has recently been indicated further by a number of clinical trials showing promising results (17–19). Despite this encouraging data, a great deal remains unknown regarding how extracellular stressors like alcoholism impact the functional properties of EPCs during angiogenesis/vasculogenesis and tissue repair. Although the effect of alcohol on the repression of both differentiation and the functional properties of various stem/progenitor cells have been reported recently (20, 21), the information on the effect of alcohol on the function of EPCs is lacking. Because the beneficial effects of E2 on endothelial cells and EPCs generally mirror the effects on cardiovascular events overall, improvement in EPC function is an important mechanism by which estrogen replacement therapy could provide cardioprotection. Accordingly, no information exists in the literature regarding the cross-action of alcohol and E2 on EPC function. Here we report that enhanced alcohol consumption occurring after E2 supplementation suppresses E2-mediated benefits on post-AMI cardiac functions, especially those mediated by EPCs, and that alcohol exposure to EPCs alters estrogen receptor-mediated cell survival signaling cascades. The impact of alcohol consumption on E2-mediated EPC function and biology and the mechanisms by which alcohol may interfere with E2 signaling following acute myocardial ischemia are presented here.
EXPERIMENTAL PROCEDURES
Ovariectomy, E2 Supplementation, and Ethanol Feeding
Mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and all surgical procedures and animal care protocols were approved by the Northwestern University Animal Care and Use Committee. Procedures related to OVX, pellet implantation, and chronic ethanol feeding can be found in previous publications (12, 16). Briefly, 32 (n = 32) C57BL/6J female mice underwent OVX and, 1 week later, were implanted with either a 90-day-release E2 pellet (1.7 mg of E2, release rate of ∼188 pg/day) (Innovative Research of America, Sarasota, FL) or a placebo pellet, implanted subcutaneously under the skin between the shoulder blades. Mice implanted with E2 pellets were randomized to receive ethanol (10% in water) or water alone (n = 16 each) for 6 weeks. Following OVX and pellet implantation, animals were divided into three groups as follows: E2 pellet with no ethanol access (E2/water), E2 pellet and 10% ethanol solution in water (E2/ethanol), and placebo pellet 10% ethanol solution in water (placebo/water). One group of mice implanted with either placebo or E2 pellets was given immediate access to 10% ethanol for 6 weeks, and consumption was recorded every 24 h. The body weight of each mouse in the two groups receiving ethanol was recorded twice per week. Each mouse was housed in separate cages under a 12-h light/dark cycle. Bottles were weighed before and after a 24-h drinking cycle to calculate the grams of ethanol consumed in the preceding 24 h. Control water bottles were also weighed for measuring water consumption, which did not differ significantly between groups. Following the chronic ethanol feeding cycle, all mice underwent surgery to induce acute myocardial infarction as described in our previous publications (22, 23). Circulating blood ethanol levels were monitored weekly in each animal via assessment of the blood obtained from a tail vein using a commercially available ethanol detection kit (Sigma). eNOS-null mice were purchased from The Jackson Laboratory and underwent procedures similar to wild-type animals.
Induction of Acute Myocardial Infarction
AMI was induced as described previously (22, 23). Briefly, mice were anesthetized, intubated orally, the chest was shaved, and under a dissecting microscope, a left thoracotomy was performed in the fourth intercostal space. An 8–0 monofilament nylon suture needle was passed under the left anterior descending coronary artery 2 mm below the left atrium and tied off permanently. The chest layers were then closed sequentially by suturing. A 22-gauge syringe was used to reestablish negative pressure within the chest cavity prior to extubation. Animals received postsurgical pain and inflammatory management with buprenorphine and meloxicam, respectively. Animals were recovered until freely mobile and then placed into a clean cage.
Bone Marrow Transplant (BMT) and Identification of Transgenic EPCs in Myocardium
BMTs were conducted as reported previously (15, 24). Briefly, recipient C57BL/6 mice were lethally irradiated with a 9.0-gray dose followed by a BMT from a transgenic donor mouse expressing β-gal encoded by the lacZ gene under the transcriptional regulation of an endothelial-specific promoter Tie-2 (Tie2-lacZ mice) via an intravenous injection of 2 × 106 donor BM cells. Reconstitution of the transplanted transgenic BM yielded mice in which expression of lacZ was restricted to the BM-derived cells expressing Tie-2. At 6 weeks after BMT, recipient mice were subjected to OVX, pellet implantation, chronic ethanol feeding, and AMI as described above. At day 28 post-AMI, AMI-induced EPC mobilization to the heart was determined. Post-AMI day 28, cardiac tissue sections were permeabilized and stained with anti-β-gal antibodies followed by incubation with respective secondary antibodies. Nuclei were counterstained with DAPI (1:5000, Sigma Aldrich), and sections were examined with a fluorescent microscope (Nikon Eclipse TE200). EPC engraftment was assessed in 10 randomly selected high-power visual fields in the border zone of the infarcted myocardium.
Bone Marrow Cell Isolation and EPC Culture
EPC isolation, ex vivo expansion, and culture of EPCs was performed as described previously (24). In brief, bone marrow mononuclear cells were isolated from mice by density gradient centrifugation with Histopaque-1083 (Sigma) and macrophage-depleted by allowing isolated cells to attach to uncoated plates. After 2 h of culture, the unattached cells were removed and plated on culture dishes coated with 5 μg/ml human fibronectin (Sigma) and cultured in phenol red-free endothelial cell basal medium 2 (EBM-2, Clonetics) supplemented with the EG-MV2 bullet kit. Cells were maintained at 37 °C with 5% CO2 in a humidified atmosphere. After 4 days in culture, non-adherent cells were removed by washing with PBS, and new medium was then applied. The culture was maintained through day 7, at which point EPCs were recognized as attached spindle-shaped cells.
Physiological Assessments of Left Ventricular Function
Transthoracic two-dimensional echocardiographic measurements were performed with a commercially available high-resolution echocardiographic system (VEVO 770TM, VisualSonics Inc., Toronto, Canada) equipped with a 30-MHz transducer. Echocardiographic analysis was performed before AMI (base line) and at 7, 14, and 28 days post-AMI on all mice anesthetized with a mixture of 1.5% isoflurane and oxygen (1 liter/min). End-systolic and end-diastolic left ventricular areas were determined by M mode in long-axis configuration, and fractional shortening was measured at the midventricular level. The left ventricular chamber volumes in diastole and systole were derived from their respective measured two-dimensional areas using an LV volume algorithm within the Vevo770 echo software. Cardiac ejection fraction was determined offline by the following equation: [ejection fraction = (diastolic volume − systolic volume/diastolic volume) × 100].
Harvest of Cardiac Tissue, Histology, and Immunofluorescent Assessments
All hearts were harvested after the 28-day echocardiographic analysis as described previously (25, 26), embedded in paraffin, and cut into sections for immunohistochemical staining as described previously (22–24, 27). Specimens were fixed in 10% (v/v) buffered formaldehyde, dehydrated with graded ethanol series, and embedded in paraffin. Serial transverse sections of 5 μm were cut across the long axis of the heart and subsequently mounted on slides.
Infarct Size Determination
All fixed hearts were sectioned starting from the height of the ligating suture and then sequentially at 250-μm distances below the suture as far as effective sectioning would permit. Infarct size was evaluated on Masson's trichrome-stained heart sections cut 500 μm below the ligation point with ImageJ (National Institutes of Health), and the transmural, fibrotic infarct perimeter was then assessed as a percentage of the entire LV chamber perimeter.
Capillary Density Analysis
Capillaries were identified by injecting mice with BS-1 lectin (Vector Laboratories, Burlingame, CA) 10 min prior to sacrifice. Subsequent staining of sections included a goat anti-lectin primary antibody (Vector Laboratories) and FITC-conjugated donkey anti-goat IgG secondary antibody. Slides were imaged using fluorescent microscopy (Zeiss), and capillary density was evaluated by counting positively stained tubular structures within the infarct border zone in sections 500 μm below the ligation point in all hearts. Three high-power visual fields (×20) were analyzed from three independent sections/mouse.
FACS Analysis to Assess AMI-Induced EPC Mobilization
Freshly isolated mononuclear cells were collected from peripheral blood (via the tail vein) and separated by Histopaque-1083. Isolated cells were then stained with FITC-conjugated anti-mouse stem cell antigen 1 (Sca-1) and phycoerythrin-conjugated anti-mouse fetal liver kinase 1 (Flk1/VEGFR2) antibodies and/or phycoerythrin-conjugated CD31antibodies (BD Biosciences) in 0.1% PBS/BSA. Isotype-matched IgG served as negative control. Quantitative fluorescence analyses were performed with an LSR II flow cytometer (BD Biosciences) and Flow-Jo software (Tree Star, Inc.). 50,000 events were counted per sample. All groups were studied at least in triplicate.
Matrigel Tube Formation Assay
Isolated EPCs were starved overnight prior to the Matrigel assay in EBM-2 medium containing 0.1% BSA. Starved EPCs were then seeded in 4-well glass slides coated with growth factor-reduced Matrigel (BD Biosciences). Tube formation was examined by phase-contrast microscopy 5 h later.
Migration Assay
EPC migration assays were performed in a 24-well transwell chamber (8.0-μm pore size, polycarbonate membrane, Corning Costar, Corning Inc. Life Sciences, Acton, MA) as described previously (15).
EPC Proliferation Assays
Cell proliferation was assayed by BrdU uptake assays. Cells were starved overnight in low serum (0.2%) followed by replenishment of complete medium containing indicated doses of ethanol ± E2 and then cultured for 48–72 h. BrdU was added for the last 18 h of culture. Cells were then fixed and stained with anti-BrdU antibodies followed by microscopy.
Caspase Activation Assays
Caspase 3 activity was assessed by Western blotting of EPC cell lysates. Levels of pro-caspase 3/active caspase 3 were detected with specific antibodies. TNF-α was used as a positive control for apoptosis in EPCs. β-Actin was used a protein loading control for all samples.
Transient Transfections and Luciferase Activity
EPCs were transiently transfected with an ERE-luciferase reporter construct, in which luciferase is driven by estrogen-responsive elements (ERE). Transfections and determination of luciferase activity were performed as described (28).
Nuclear Run-on Assays
Nuclear run-on experiments to measure nascent RNA transcripts were performed as described before (28, 29).
RNA Isolation and Ribonuclease Protection Assay (RPA)
Cellular RNA isolation, preparation of in vitro-transcribed riboprobes and RPA assays, and mRNA half-life assays were carried out as described (28, 29).
Oligonucleotides and EMSA
EMSA utilizing consensus ERE oligonucleotides (Santa Cruz Biotechnology) and 5 μg of nuclear proteins from variously treated cells were carried out as described previously (28, 29).
Western Blot Analyses and Immunoprecipitations
Western blot analyses were performed essentially as described before (28, 30).
E2-ER Binding Assays
EPCs were treated with the indicated doses of ethanol in the presence of 1 mCi of tritiated 17b-estradiol (H3-E2, NEN Dupont). Ligand-receptor binding was also performed by using purified estrogen receptors (1 mg) and radioactive estradiol (3H-E2).
Statistical Analysis
All values are expressed as mean ± S.E., and p < 0.05 was considered statistically significant. For comparisons between two groups, significance was evaluated with an unpaired Student's t test. Comparisons among three or four groups were assessed by a one-factor analysis of variance followed by a Tukey post hoc test when p < 0.05. Comparisons for measurements taken at multiple time points were assessed by a two-way repeated measures analysis of variance followed by a Tukey post-hoc test.
RESULTS
Ethanol Consumption Blunts E2-mediated Improvements in LV Function Post-AMI
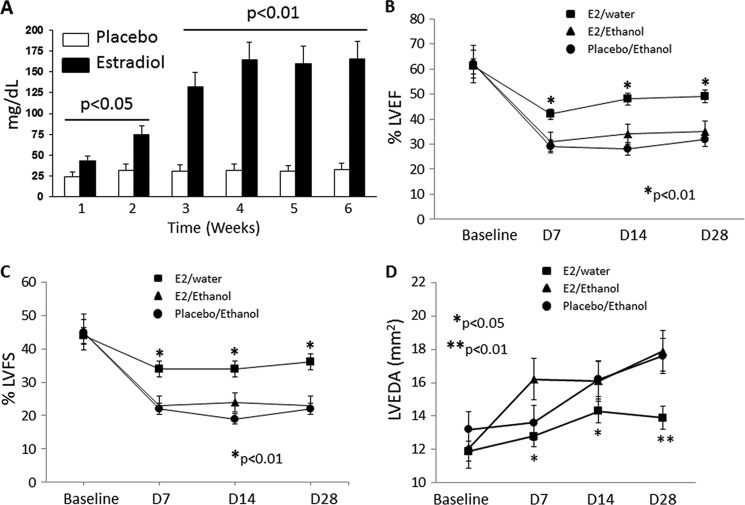
Eight-week-old female mice were subjected to bilateral OVX and the subsequent implantation of either E2- or placebo-secreting pellets. Following implantation, mice were then selected randomly for a feeding regimen that allowed free consumption of ethanol (10% v/v in water) or water over a 6-week period. Blood alcohol levels, assessed as an index of ethanol consumption, were measured over the feeding period and were found to be increased significantly in only the E2-supplemented animals compared with placebo (Fig. 1A) with no change in body weights (data not shown). Animals receiving placebo pellets consumed very little ethanol and showed no difference from animals receiving the placebo and no access to ethanol (i.e. water alone) when subjected to ischemia. We reported similar findings in our previous study (12). Therefore, for the purpose of this study, only animals receiving placebo pellet and access to ethanol were followed up further as a control group. Immediately following the ethanol feeding protocol, mice were subjected to AMI. Serial echocardiographic measurements, including ejection fraction and fractional shortening, were used to assess left ventricular dimensions and function prior to AMI and at 1, 2, and 4 weeks post-AMI. As compared with presurgical basal measurements, both ejection fraction and fractional shortening were depressed consistently in mice receiving placebo/ethanol and E2/ethanol (Fig. 1, B and C). However, supplementation of E2 alone improved fractional shortening at all time points significantly, indicating that ethanol counteracts the positive benefit that E2 imparts on cardiac function post-AMI (Fig. 1, B and C; p < 0.01, E2-water versus E2-ethanol). Assessment of the LV end diastolic area revealed that both animal groups subjected to ethanol feeding (i.e. placebo/ethanol and E2/ethanol) exhibited dilated chamber areas as compared with E2/water-treated mice, indicating that ethanol reverses the cardioprotective effect of E2 following AMI (Fig. 1D, ■, day 28 post AMI, p < 0.01, comparison between E2/placebo versus E2/ethanol). These data indicate that moderate ethanol consumption suppresses E2-mediated cardioprotective effects observed on LV dimension and cardiac function following AMI.
FIGURE 1.
Ethanol negates the positive benefit of E2 on cardiac function following AMI. A, average consumption of ethanol per week for mice implanted with either placebo or estradiol pellets. Statistically significant increases in ethanol consumption are seen at all time points for mice implanted with E2 pellets. Prior moderate ethanol consumption ablates the significant E2-mediated improvement in cardiac function following AMI in terms of ejection fraction (EF) (B) and fractional shortening (FS) (C) at 1, 2, and 4 weeks post-infarction. D, day. D, E2-mediated cardioprotection of left ventricular end diastolic area (LVEDA) is also lost in ethanol-consuming mice despite E2 supplementation (n = 8 mice/group).
Ethanol Ablates E2-mediated Improvement in LV Fibrosis and Border Zone Capillary Density
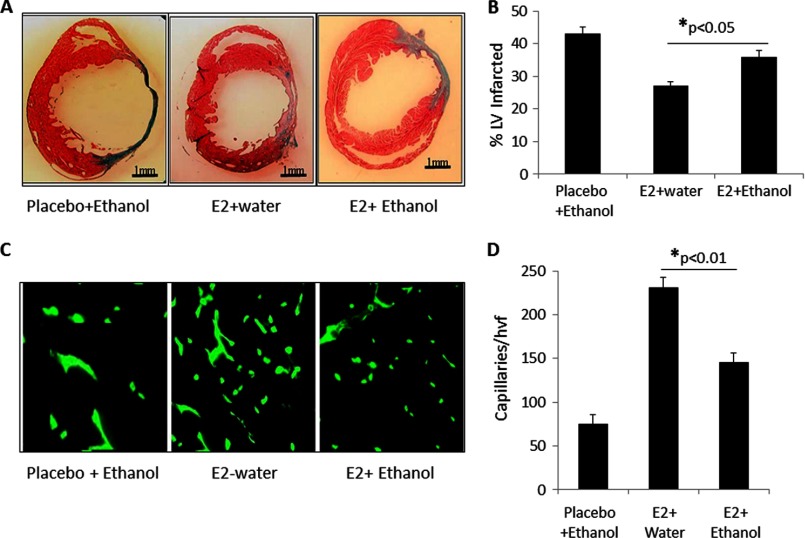
The negative effect of ethanol on E2-mediated preservation of LV function was corroborated further by histological evaluation of hearts 4 weeks after AMI. Using Masson's trichrome staining, hearts from mice receiving E2/ethanol showed significantly larger infarct sizes than mice receiving E2/water (Fig. 2, A and B). Furthermore, cardiac tissue sections were also stained with FITC-labeled BS-1 lectin to determine the capillary density in the border zone of infarcted myocardium. As seen in Fig. 2C and quantified in D, a significant reduction in the capillary density within this region was observed in mice receiving E2/ethanol as compared with those receiving E2/water. Together, these findings suggest that ethanol consumption suppresses E2-mediated anatomical repair, producing increased infarcts and reducing capillary density.
FIGURE 2.
Reduced infarct size and increased border zone capillary density in hearts of mice receiving E2 is lost when ethanol is consumed. A, representative cross-sectional Masson's trichrome-stained histological sections depicting the infarct zone in mice receiving either placebo/ethanol, E2/water, or E2/ethanol (n = 8 mice/group). B, graph depicting the quantification of the infarct length relative to the entire left ventricular circumference, which is improved significantly in animals receiving E2/water but lost when ethanol is consumed. C, representative immunofluorescence images taken within the infarct border zone of mice treated with placebo/water, E2/water, or E2/ethanol. Capillaries were stained with isolectin B4 (green). D, graph depicting the quantification of border zone capillary number across treatments presented as the number of isolectin B4-positive capillaries/high-power visual field (hvf) (n = 8 mice/group).
Ethanol Inhibits E2-induced Mobilization and Ischemic Myocardial Homing of BM-EPC
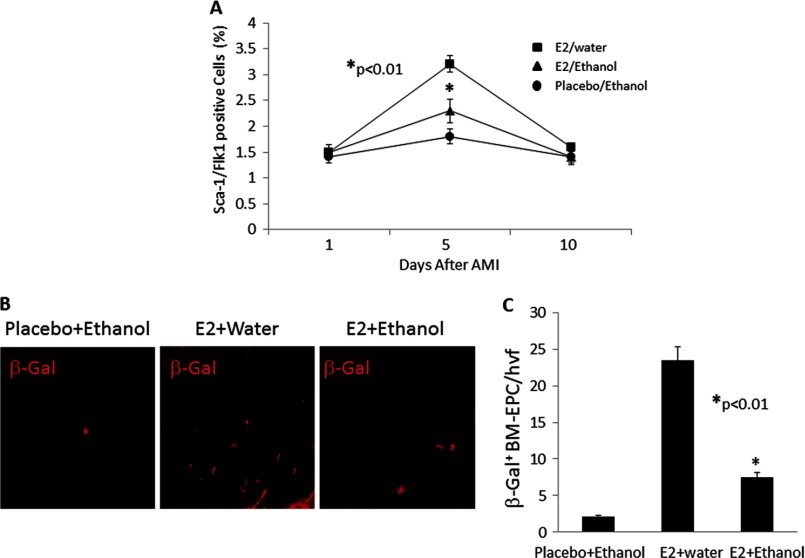
Because E2-mediated vascular repair in post-infarct myocardium is mediated partly by E2-induced mobilization of bone marrow-derived EPCs (15), we determined whether reduced capillary density in the E2/ethanol group reflects a defect in EPC mobilization. As determined by FACS analysis of circulating Sca1/Flk1 double-positive EPCs, the number of EPCs peaked on day 5 after AMI in all groups. However, the number of circulating EPCs in the E2/water group was significantly higher compared with that from animals receiving E2/ethanol or placebo/ethanol (Fig. 3A, p < 0.01), suggesting that alcohol consumption blunts E2-induced mobilization of EPCs after injury. The effect of ethanol on E2-induced homing of bone marrow-derived EPCs in the infarcted myocardium was evaluated using Tie2-LacZ BMT mice where wild-type female mice received marrow from Tie2-LacZ transgenic donor mice after lethal irradiation. After BMT, mice received either E2/water or E2/ethanol (n = 8 for each group) for 6 weeks prior to AMI surgery. Mice receiving placebo/ethanol served as controls (n = 8). Following AMI, homing of BM-EPCs was ascertained by staining the myocardial sections for β-gal. As shown in Fig. 3B, a significant number of BM-EPCs were recruited to the myocardium in mice receiving E2 alone, whereas the number of β-gal+ cells present in the myocardium of mice receiving E2/ethanol or placebo/ethanol was reduced significantly (Fig. 3C, p < 0.01).
FIGURE 3.
Ethanol consumption reduces E2-induced mobilization and homing of bone marrow-derived EPCs. A, FACs analysis was used to determine that mobilization of Sca1+ and Flk1+ cells in peripheral blood was augmented in all three treatment groups on days 1, 5, and 10 after AMI (n = 8 mice/group). E2-stimulated enhancements in EPC mobilization at day 5 were diminished significantly when ethanol was consumed. B, representative immunofluorescence images taken within the infarct border zone of Tie2-LacZ bone marrow transplant recipient mice treated with placebo/ethanol, E2/water, or E2/ethanol and then subjected to AMI. Staining for β-gal indicates the number of bone marrow-derived cells homing to the ischemic myocardium following AMI. C, graph depicting the quantification of β-gal + EPCs/high-power visual fields (hvf) in the E2/water and E2/ethanol groups, indicating a significant reduction in the homing of EPCs to ischemic cardiac tissue in mice consuming ethanol (n = 8 mice/group).
Ethanol Exposure Diminishes the E2-mediated Angiogenic Capacity and Survival of EPCs
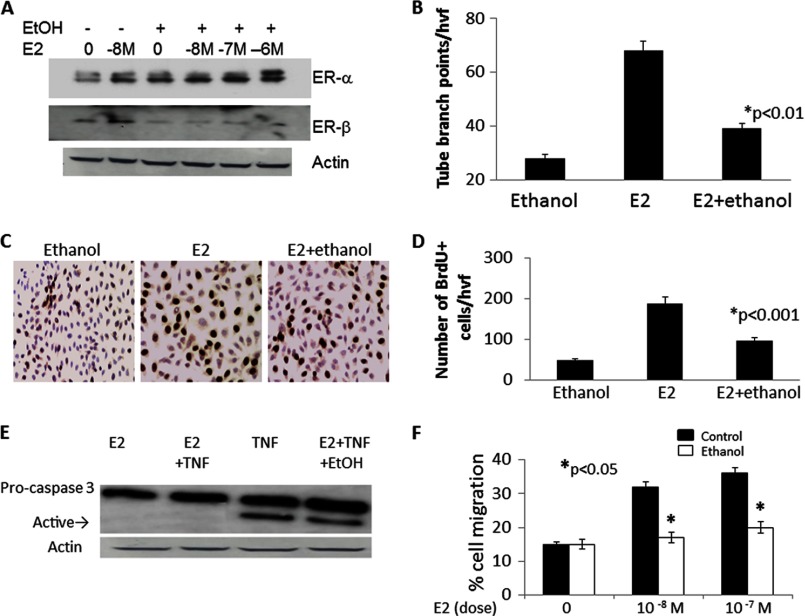
Following from the observations that ethanol-fed animals fail to exhibit E2-mediated cardioprotection against the architectural and functional deficits associated with AMI and that AMI-induced mobilization of EPCs is vastly reduced in ethanol-fed mice, we chose to further evaluate the phenotype of EPCs in response to E2 ± ethanol treatments using in vitro EPC functional assays. BM-EPCs were in vitro culture-expanded before being exposed to E2 (10−8 m) and/or ethanol (25 mm for 48 h). The in vitro doses of E2 and ethanol were determined according to our published data (12). EPCs express both the α and β isoforms of the ER, which are the main receptors that transduce estrogen signaling in these cells. Because ethanol consumption reduced capillary density and LV functions in vivo, we determined the effect of in vitro ethanol exposure on the expression of ERs in EPCs. Cells were incubated with 25 mm ethanol for 48 h, a dose and time that have been shown previously to mimic the effects of long-term chronic alcohol consumption (29, 31) in the presence or absence of E2 (10−8 m). E2 treatment increased the protein expression of both ERα and ERβ (Fig. 4A). Interestingly, ethanol treatment had no discernible effect on the E2-induced expression of ERα. However, it reduced ERβ protein expression substantially (Fig. 4A). Following E2/ethanol treatments, EPCs were cultured further on 4-well glass slides coated with cytokine-reduced Matrigel matrix. Tube formation activity was assessed 5 h after plating using light microscopy. As shown in Fig. 4B, tube forming abilities (measured as number of tube-branching points/high visual field) were diminished significantly using EPCs pretreated with E2/ethanol compared with E2 alone (p < 0.01). Similarly, BrdU incorporation studies revealed that cotreatment of EPCs with E2/ethanol reduced the number of proliferating cells compared with E2 treatment alone (Fig. 4, C and D; p < 0.001). Moreover, E2-mediated inhibition of TNF-induced caspase 3 activation (an indicator of apoptosis) was repressed by cotreatment of EPCs with ethanol (Fig. 4E). Migration of isolated EPCs was also assessed using the modified Boyden chamber (3). Analysis revealed that the migratory activity of EPCs toward E2 (10−8 m) was reduced significantly in cells pretreated with ethanol compared with non-ethanol-treated control EPCs (Fig. 4F; p < 0.05). Together, these data suggest that ethanol counteracts the E2-induced enhancement of EPC functions in vitro.
FIGURE 4.
Ethanol exposure reduces the E2-mediated angiogenic capacity and survival of EPCs. A, protein expression of ERα and ERβ in cultured day 7 EPCs showing regulation of expression by various in vitro treatments of E2 or ethanol. Actin was used as a loading control. B, tube-forming capacity in in vitro culture expanded BM-EPCs exposed to E2 (10−8 m) or E2 + ethanol (25 mm) for 48 h. hvf, high-power visual field. C, representative immunohistochemical images of BrdU incorporation in EPCs treated as above in B and quantified in D, indicating reduced proliferation of cells exposed to ethanol. E, representative Western blot analysis showing E2-mediated inhibition of TNF-induced caspase 3 activation (indicated by the arrow designating the active form of caspase) that is repressed by cotreatment of EPCs with ethanol. F, E2-stimulated migration of isolated EPCs in a modified Boyden chamber is significantly reduced in cells pretreated with ethanol as compared with control EPCs. All experiments were conducted at least three times.
Ethanol Blunts E2 Binding to ERα and Inhibits eNOS Expression by Transcriptional and Posttranscriptional Mechanisms
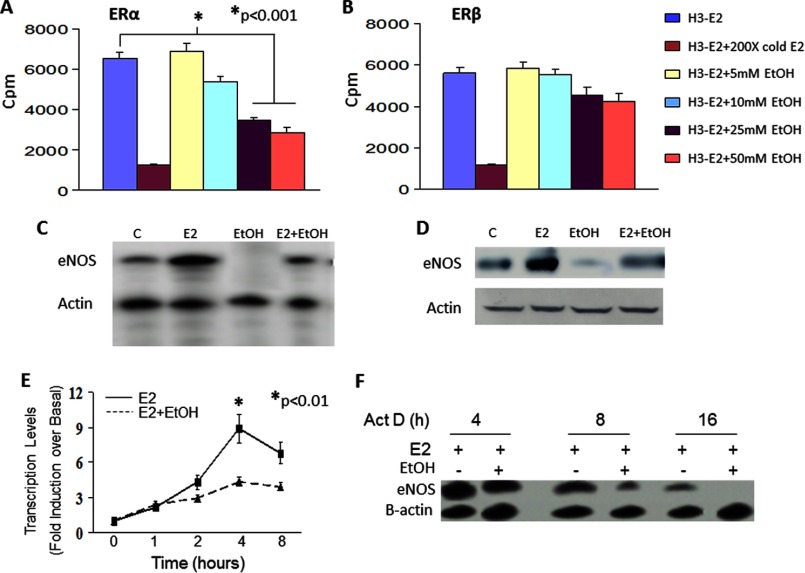
We next determined the involvement of individual ERs in alcohol-induced modulation of E2 function in EPCs. Because downstream effects of E2 are largely mediated by ERα and because ethanol had no effect on ERα expression, we determined the effect of ethanol on E2 binding to purified recombinant ERα and ERβ proteins. 3H-E2 specifically bound to both the recombinant receptors, and the binding could be competed off with 200× unlabeled cold E2 added 1 h prior to the addition of radioactive E2. Ethanol dose-dependently significantly inhibited the binding to ERα at ethanol doses of 25 mm and higher (Fig. 5A), although ethanol at all dose had no significant effect on 3H-E2 binding to ERβ (B).
FIGURE 5.
Ethanol impairs E2-ER binding and represses E2-induced eNOS expression. A, binding assays were also performed using purified ERα and ERβ. Ethanol dose-dependently inhibited the binding of E2 to ERα, with low doses having no effect but doses of 25 mm and higher significantly reducing the binding. B, ethanol at any dose had no significant effect on E2 binding to ERβ. C, RNA isolated from EPCs with the indicated treatments was assessed for eNOS mRNA expression by RPA. A representative autoradiograph of three similar experiments is shown. D, protein extracted from a subset of EPCs was also assessed for eNOS protein expression in Western blot analyses. In panels C and D, lane C indicates control untreated cells. E, ethanol significantly reduced the rate of de novo eNOS mRNA transcription. Presented is the average nuclear run-on data obtained from three similar experiments. F, EPCs treated with E2 or E2 + ethanol were cultured in the presence or absence of 5 μg/ml actinomycin (Act D). RNA isolated at the indicated times was assessed for eNOS mRNA half-life by RPA. A representative autoradiograph of three similar experiments is shown.
In the next series of experiments, we explored the molecular signaling behind the E2-ethanol interactions observed in our in vitro EPC experiments. eNOS is the major isoform of nitric oxide synthase expressed in endothelial linage cells, including EPCs, and is known to be induced and activated in response to E2. To evaluate the effect of ethanol on E2-inducible gene expression, we determined the expression of eNOS mRNA and protein in EPCs cultured in the presence of E2 ± ethanol. mRNA expression was determined by RPA using an in vitro-transcribed eNOS riboprobe (Ambion). Treatment of cells with E2 (10−8 m) for 16 h substantially up-regulated eNOS mRNA expression, which was completely abrogated in cells also exposed to 25 mm ethanol (Fig. 5C). Reduction in eNOS mRNA expression by ethanol was also evident by the similar repression of eNOS protein (Fig. 5D). To gain insight into the molecular mechanism(s) by which ethanol might reduce E2-induced eNOS expression, nuclear run-on (to measure the rate of de novo mRNA synthesis) and actinomycin D chase (to measure mRNA half-life) experiments were conducted. Ethanol treatment diminished the rate of de novo eNOS transcription (Fig. 5E) and also substantially reduced eNOS mRNA stability (eNOS t½ = ∼15 h in E2-treated cells compared with < 8 h in E2 + ethanol-treated cells, F). These results indicate that ethanol mediates transcriptional as well as posttranscriptional repression of E2-induced eNOS mRNA expression.
Ethanol-mediated Repression of Post-AMI Physiological Recovery Is in Part Mediated by eNOS
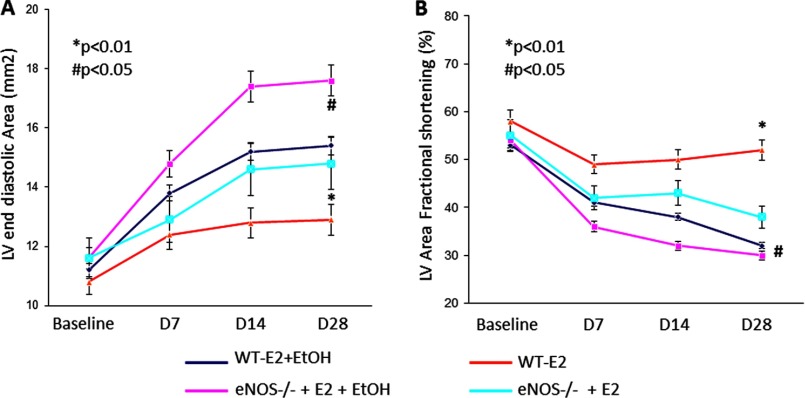
We have reported previously that E2-mediated, EPC-induced, post-AMI myocardial vascularization requires eNOS because its loss (i.e. eNOS−/− mice) correlates with ablated E2-mediated recovery of left ventricular functions in OVX mice (16). To investigate whether ethanol suppression of eNOS expression in vitro and the loss of E2 function in our AMI model involve eNOS, experiments were carried out in OVX WT and eNOS knockout mice. As shown in Fig. 6, the magnitude of ethanol-mediated repression of both left ventricular end diastolic area (Fig. 6A) and fractional shortening (B) in eNOS-null mice was much smaller although still significant (p < 0.05 eNOS-null E2 and eNOS-null E2 + ethanol compared with p < 0.01 in their WT counterparts), suggesting that loss of eNOS partly mitigates the negative effect of ethanol on post-MI LV functions.
FIGURE 6.
Loss of eNOS expression prevents E2-mediated improvements in cardiac function post-AMI. Ethanol-mediated repression of the left ventricular end diastolic area (A) and fractional shortening (B) were significantly worse in eNOS-null mice (eNOS−/−) compared with WT mice (despite E2 supplementation), indicating that ethanol consumption negatively affects post-AMI myocardial function in female eNOS knockout mice, suggesting a role for eNOS in ethanol-mediated EPC dysfunction. n = 8 mice/group.
Ethanol Inhibits ERE-DNA Binding Activity and Attenuates ERE-dependent Transcription
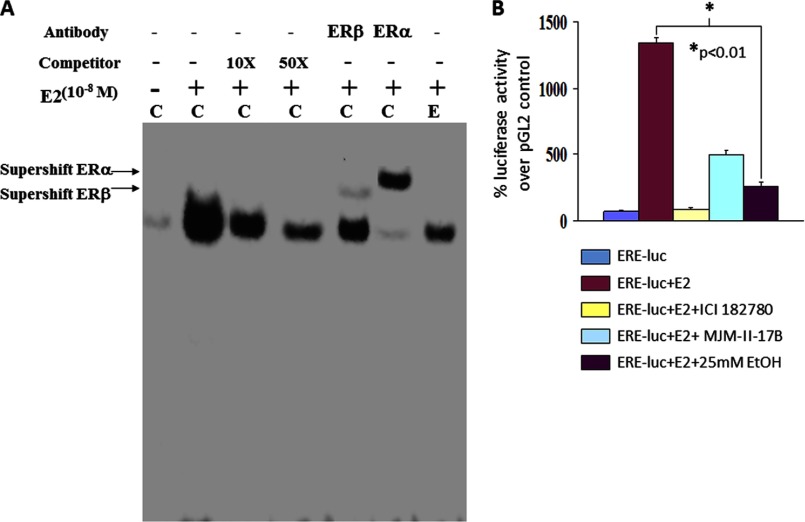
Because E2-induced cellular effects are mediated both by classical genomic functions and by non-nuclear signaling functions of ERs, we determined whether genomic ER functions (i.e. reduced eNOS transcription) are modulated by ethanol. Because E2-induced binding of ERs to EREs in the promoters of target genes initiate their transcription, we assessed the binding of nuclear proteins from ethanol-exposed, E2-treated EPCs to a consensus ERE oligonucleotide using EMSA. E2 treatment alone promoted up-regulation of specific ERE-DNA binding activity, which was supershifted by both anti-ERα and anti-ERβ antibodies, indicating that the ERE complex contains both receptors (Fig. 7A). Treatment of cells with 25 mm ethanol for 48 h dramatically attenuated E2-induced ERE binding activity, suggesting that ethanol can modulate the transcriptional inhibition of E2-induced genes by modulating ER binding to EREs in the target promoters. To show that ethanol indeed inhibits E2-induced transcription, ERE-driven transcription of a reporter luciferase gene was examined. EPCs were transiently transfected with a control pGL2-basic or the ERE-luciferase construct and were treated further with ethanol in the presence of 10−8 m E2. Fold changes in luciferase activity were calculated as a percent change in ERE-luciferase-transfected cells over control vector-transfected cells (Fig. 7B). E2 treatment significantly enhanced ERE-driven reporter gene transcription, which was blocked by the ER antagonist ICI 182780 (5 μm). Interestingly, specific inhibition of ERβ by MJM-II-17B (5 μm) also attenuated E2-induced reporter activity, suggesting that both ERα and ERβ are required for reporter gene transcription. Most interestingly, as was seen with the DNA binding activity, ERE transcriptional activity was suppressed significantly in the presence of 25 mm ethanol. Together, these data strongly suggest that ethanol interferes with the E2-mediated transcription factor function of ERs.
FIGURE 7.
Ethanol blunts E2-mediated transcriptional activity. A, representative EMSA autoradiograph showing ethanol-induced inhibition of ERE-DNA binding activity. ERE-DNA binding activity consists of both ERα and ERβ. Lane C denotes control untreated cells, and lane E denotes ethanol-treated cells. B, EPCs were transiently transfected with a control pGL2-basic plasmid or the ERE-luciferase construct and were treated further with E2 (10−8 m) ± ethanol. Fold changes in luciferase activity were calculated as a percent induction in ERE-luciferase (luc) activity over control vector-transfected cells and averaged from four similar experiments.
Ethanol Blunts E2-induced Cell Survival Signaling and Promotes Apoptotic Signaling
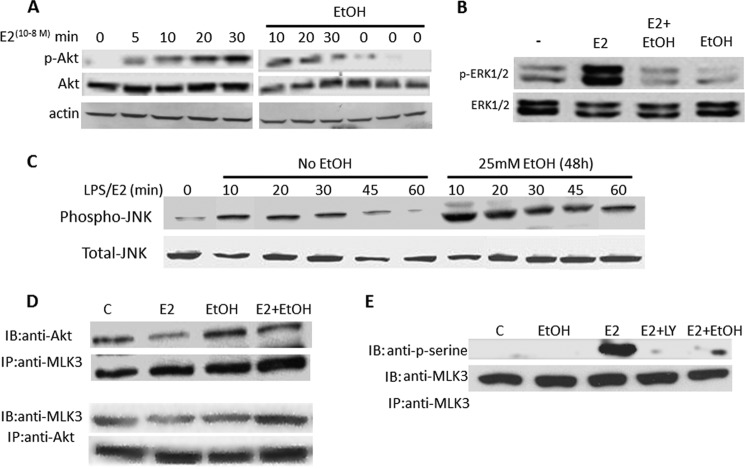
Because E2-induced Src-Akt-ERK signaling is implicated in the protective effects of E2 on ECs and because these signaling events are known to be mediated by the non-nuclear effects of ERs, we determined whether ethanol exposure influences the ability of E2 to induce activation of Akt and the ERK MAP kinases. Importantly, both proteins are downstream kinases in the PI3 kinase cell survival pathway. To examine the kinetics of Akt activation, phosphorylation of Akt was assessed in EPCs treated with 10−8 m E2 for the indicated times. Western blotting revealed a time-dependent increase in Akt phosphorylation (Fig. 8A, left panel). EPCs were exposed to 25 mm ethanol for 48 h and were further stimulated or not stimulated with 10−8 m E2 for the indicated times (Fig. 8A, right panel). Total protein extracted from these cells showed that E2 treatment led to a rapid phosphorylation of Akt, although E2-induced activation was not only substantially transient but also reduced markedly when cells were also exposed to 25 mm ethanol prior to the E2 stimulation. Similar inhibition of E2-induced activation of ERK in the presence of ethanol was also observed (Fig. 8B). These findings indicate that ethanol represses E2-induced cell survival signaling pathways, indicating a modulation of the non-genomic functions of ERs.
FIGURE 8.
Ethanol attenuates E2-induced survival signaling in EPCs. Proteins from EPCs treated as indicated were analyzed by Western blot analyses for Akt (A and B), ERK1/2 (B), and JNK1 phosphorylation (C). D, proteins from EPCs treated as indicated were assessed for Akt and MLK3 physical association by immunoprecipitation (IP) with anti-MLK3 antibodies and immunoblotting (IB) by anti-Akt antibodies (upper panel). The reciprocal experiment (lower panel) also showed enhancement of an Akt:MLK3 physical association in the presence of ethanol. E, E2 treatment induced robust MLK3 phosphorylation, which was almost completely inhibited in cells pretreated with ethanol. Interestingly, E2 failed to induce MLK3 serine phosphorylation when cells were treated simultaneously with the specific Akt inhibitor LY496002, indicating that E2-mediated MLK3 phosphorylation is PI3K/Akt-dependent. All experiments were conducted at least three times. In panels D and E, lane C denotes control untreated cells.
Because the data presented above indicate an impairment of cell survival signaling (Akt/ERK) and an increase in apoptosis of EPC following ethanol exposure (i.e. via caspase 3 activation), we sought to determine the effect of ethanol on agonist-induced JNK kinase activation. JNK is a signaling kinase upstream of caspase 3 that has a well established role in cellular apoptosis. As shown in Fig. 8C, ethanol treatment not only potentiated but also prolonged LPS-induced phosphorylation of JNK kinase. Because ethanol inhibited E2-induced Akt activation while simultaneously activating the JNK pathway, and because inhibition of the PI3K/Akt pathway and activation of JNK pathway is linked to the loss of cell survival signaling/induction of apoptosis, we examined how ethanol may induce a cross-talk between these two pathways. Several studies have shown that inhibition of the Akt pathway leads to the concomitant activation of JNK via inhibition of Akt-dependent mixed lineage kinase 3 (MLK3), the inactivation of which results in increased apoptosis (32, 33). Accordingly, we determined whether Akt and MLK3 interact physically by assessing whether MLK3 is immunoprecipitated from EPC lysates obtained from cells treated with control, E2, ethanol, or combined treatments (10−8 m E2 + 25 mm ethanol for 48 h) using specific anti-MLK3 antibodies. Immunoprecipitated proteins were resolved on SDS-PAGE gels, and protein expression of both Akt and MLK3 was examined by Western blotting. As shown in Fig. 8D, anti-MLK3 antibodies pulled down Akt from the cell lysates, thereby confirming an association between Akt and MLK3 in EPCs. Similar results were obtained in the reverse pull-down experiment (Fig. 8D, lower panel). Next, we examined the effect of E2 and ethanol on MLK3 serine phosphorylation because Akt is known to inactivate MLK3 by inducing serine phosphorylation on residue 674 (32). Cells were treated or not treated with 25 mm ethanol for 48 h and then stimulated or not stimulated with 10−8 m E2 in the presence or absence of the PI3K inhibitor LY294002 (50 μm) for an additional 30 min. Total protein lysates were immunoprecipitated with anti-MLK3 antibodies and immunoblotted with a pan-anti-phospho-serine antibody. As shown in Fig. 8E, E2-induced phosphorylation of MLK3 was almost completely inhibited in cells pretreated with ethanol. Interestingly, E2 failed to induce MLK3 serine phosphorylation when the cells were treated simultaneously with LY294002, indicating that E2-mediated MLK3 phosphorylation is PI3K/Akt-dependent.
DISCUSSION
Much of our knowledge of alcoholism and related organ dysfunctions has been gathered from studies conducted with a predominance of male subjects. Studies in the general population indicate that of the 25.1 million alcohol-abusing/dependent individuals in the United States, ∼8.6 million (nearly one-third) are women. Additionally, the interval between the onset of drinking-related problems and entry into treatment appears to be shorter for women than for men (34). Moreover, studies of female alcoholics in treatment suggest that they often experience greater physiological impairment earlier in their drinking careers despite having consumed less alcohol than men (35), possibly because of lower total body water content than men; diminished activity of alcohol dehydrogenase, the primary enzyme involved in alcohol metabolism; and the interactions of alcohol with hormones that contribute to the pathological drive to consume alcohol. The latter explanation is suggested by studies, including our own (12), showing that estrogen supplementation enhances the appetite for alcoholic beverages. Behavioral and epidemiological studies in humans, although suggestive of a hormonal role in alcohol consumption, are at best correlative (36–38). On the other hand, well controlled animal studies seeking a connection between E2 modulation of alcohol consumption are more striking and conclusive. Utilizing various models of controlled, voluntary alcohol intake, many studies have shown that in OVX female rodents, E2 supplementation consistently enhances voluntary alcohol consumption (11, 39). Furthermore, we reported the negative consequences of enhanced ethanol consumption on E2-mediated repair/angiogenesis in the hind limb ischemia model (12). Despite this compelling evidence, no information regarding the pathophysiological and mechanistic effects of E2-enhanced alcohol consumption on recovery from myocardial ischemia and EPC/stem cell dysfunction is currently available. Whether E2-induced increases in alcohol consumption compromise the benefits of E2 therapy on EPC-mediated myocardial repair and cardiovascular events in general is unknown. Our study is the first to demonstrate that prior consumption of alcohol diminishes the protective effect of estrogen on post-infarct myocardial repair, especially by diminishing EPC function and mobilization and by altering cell survival signaling.
The effects of estrogen on the survival and function of mature endothelial cells are well established (9, 40). An effect of estrogen on hematopoietic stem cells and granuloid progenitors has been noted for over two decades (41, 42). Together, these studies provide evidence of direct actions of estradiol on the bone marrow and the regulation of bone marrow-derived precursor cells. Apart from the local induction of endothelial growth factors, the underlying proposed mechanisms of E2 also include the ability of E2 to mobilize EPCs, which can incorporate into denuded carotid arteries, thereby participating in the regeneration of the neo-endothelium (3, 4). We and others (3, 14, 16) have also shown that E2-mediated enhancement in the recovery from AMI involves ischemic cardiac neovascularization, reduction in fibrosis, and enhanced expression of angiogenic growth factors in OVX mice receiving estradiol that is, at least in part, due to mechanisms involving eNOS and/or MMP9-dependent EPC mobilization, homing, and inhibition of apoptosis. In another published study, using BM-transplantation models, we have demonstrated that following E2 supplementation, EPC mobilization and EPC-mediated cardiac repair is depressed significantly in mice lacking either ERα or ERβ (15). Our data suggest that chronic ethanol intake in OVX mice receiving E2 suppresses the beneficial effect of E2 on post-infarct myocardium, and this phenomenon is in part dependent upon defective mobilization and homing of BM-EPCs. Additionally, our data also identify alcohol as an independent risk factor for altering EPC function and survival.
A growing body of evidence suggests deleterious effects of chronic alcohol abuse on the cardiovascular system (43, 44). Despite the reported cardioprotective effects of low to moderate alcohol intake, clinical, experimental, and epidemiological data over the last two decades have pointed to the relationship between heart disease and alcohol abuse (45). Several studies suggest that low alcohol intake reduces the risk of coronary artery disease (46, 47), whereas heavy chronic alcohol consumption increases the risk for cardiovascular events (48, 49). However, many of these studies yielded equivocal information because they were not primarily designed to study the role of alcohol. Moreover, most of these studies did not consider the effects of excessive and chronic alcohol consumption on the cardiovascular system. Also, given the gender differences in alcohol metabolism, it should be noted that what constitutes moderate drinking for males may constitute heavy drinking in females. Part of the complexity regarding the effect of alcohol on the cardiovascular system may be the result of contrasting influences of light versus heavy alcohol consumption on the vascular endothelium, with animal studies indicative of favorable effects on endothelial function with low-dose alcohol exposure but the induction of endothelial dysfunction with higher doses (50). However, despite compelling evidence related to alcohol-induced changes in cardiac function, the effect of chronic alcohol on vascular endothelial cell function is not studied sufficiently, and the limited available data on alcohol and EC function are inconclusive, with both negative (51, 52) and positive (albeit with low-dose ethanol) effects being reported (53, 54). We have reported previously an inhibitory effect of chronic ethanol on EC proliferation and survival (31). Risk factors for coronary artery disease were reported to be associated with a reduced number and functional activity of EPCs in the peripheral blood of patients (55). Likewise, patients with diabetes showed a lower EPC number (56). Our study, for the first time, provides direct evidence that alcohol represses EPC functional capacities that negatively influence post-infarct myocardial repair and vascularization.
Endothelial cells are recognized as important targets of estrogen. It is fairly well established that the actions of estrogen are classically mediated by their genomic effects, which includes ligand-dependent transcription factor function of the estrogen receptors ERα and ERβ. A central feature of classic ER action is ligand-dependent regulation of gene expression in target cells and tissues. Our data showing ethanol-mediated inhibition of E2-induced ERE-DNA binding activity as well as inhibition of ERE-dependent transcription of a reporter gene suggest that long-term exposure of EPCs to ethanol results in the transcriptional inhibition of ERE-dependent gene expression. Our finding that E2-induced eNOS mRNA expression is attenuated by ethanol exposure further indicates this possibility. However, it is becoming apparent that E2 also modulates the activity of intracellular second messengers, membrane-associated receptors, and signaling complexes, some of which can also enhance the classic activity of the ERs. These extranuclear mechanisms, including activation of nitric oxide, PI3 kinase, G protein-coupled receptor, and MAP kinase signaling pathways in ECs, for example, are commonly referred to as “non-nuclear” or “non-genomic” effects of estrogen. Our data showing ethanol modulation of E2-mediated PI3K/Akt and MAP kinase activation also indicate the presence of non-nuclear mechanisms in addition to genomic mechanisms regulating ethanol action on E2-induced modulations in EPCs. We are, however, aware that both genomic and non-nuclear actions of ERs in response to E2 and alcohol can lead to the modulation of a wide variety of E2-responsive target genes and signaling cascades. In addition, there is growing evidence that ERs may also have important functions in the vasculature (43). Interestingly, ethanol exposure of ECs inhibits E2-induced ERβ expression, indicating that ethanol may potentially negate beneficial effects of E2 that may be regulated specifically by ERβ. Another interesting finding of this study is the evidence of “switching” of E2-induced cell survival signaling in EPCs to cell death signaling when alcohol is present as an added stimulus.
In summary, our data provide novel mechanistic and physiologically relevant insights regarding estrogen-alcohol interactions on the biology of EPC functions and E2/EPC-mediated post-infarct myocardial repair. We hope that these data will provide a critical framework for understanding the significance of chronic alcohol consumption regarding E2-mediated benefits and EPC function. Although beyond the scope of this study, our observations could lead to a better understanding of how the putative stimulatory actions of alcohol integrate with those of estrogen to regulate EPC mobilization, homing, and their therapeutic efficacy. Additionally, this knowledge may potentially act as a novel tool/parameter in the assessment of cardioprotective benefits of estrogen replacement and stem cell-based therapies.
This work was supported, in whole or in part, by National Institute of Health Grants HL091983, HL105597, HL095874, HL053354, and HL108795 (to R. K.) and HL093439 and HL113541 (to G. Q.).
- E2
- estradiol
- EPC
- endothelial progenitor cell
- AMI
- acute myocardial infarction
- OVX
- ovariectomy
- BMT
- bone marrow transplant
- BM
- bone marrow
- LV
- left ventricle
- ERE
- estrogen-responsive element
- RPA
- ribonuclease protection assay
- ER
- estrogen receptor
- eNOS
- endothelial nitric oxide synthase.
REFERENCES
- 1. Grodstein F., Manson J. E., Colditz G. A., Willett W. C., Speizer F. E., Stampfer M. J. (2000) A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann. Intern. Med. 133, 933–941 [DOI] [PubMed] [Google Scholar]
- 2. Lindenfeld J., Ghali J. K., Krause-Steinrauf H. J., Khan S., Adams K., Goldman S., Peberdy M. A., Yancy C., Thaneemit-Chen S., Larsen R. L., Young J., Lowes B., Rosenberg Y. D. (2003) Hormone replacement therapy is associated with improved survival in women with advanced heart failure. J. Am. Coll. Cardiol. 42, 1238–1245 [DOI] [PubMed] [Google Scholar]
- 3. Iwakura A., Luedemann C., Shastry S., Hanley A., Kearney M., Aikawa R., Isner J. M., Asahara T., Losordo D. W. (2003) Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 108, 3115–3121 [DOI] [PubMed] [Google Scholar]
- 4. Strehlow K., Werner N., Berweiler J., Link A., Dirnagl U., Priller J., Laufs K., Ghaeni L., Milosevic M., Böhm M., Nickenig G. (2003) Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 107, 3059–3065 [DOI] [PubMed] [Google Scholar]
- 5. Grady D., Herrington D., Bittner V., Blumenthal R., Davidson M., Hlatky M., Hsia J., Hulley S., Herd A., Khan S., Newby L. K., Waters D., Vittinghoff E., Wenger N. (2002) Cardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and estrogen/progestin replacement study follow-up (HERS II). JAMA 288, 49–57 [DOI] [PubMed] [Google Scholar]
- 6. Rossouw J. E., Anderson G. L., Prentice R. L., LaCroix A. Z., Kooperberg C., Stefanick M. L., Jackson R. D., Beresford S. A., Howard B. V., Johnson K. C., Kotchen J. M., Ockene J. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 [DOI] [PubMed] [Google Scholar]
- 7. Xin H. B., Senbonmatsu T., Cheng D. S., Wang Y. X., Copello J. A., Ji G. J., Collier M. L., Deng K. Y., Jeyakumar L. H., Magnuson M. A., Inagami T., Kotlikoff M. I., Fleischer S. (2002) Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416, 334–338 [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y., Bian Z., Lu P., Karas R. H., Bao L., Cox D., Hodgin J., Shaul P. W., Thoren P., Smithies O., Gustafsson J. A., Mendelsohn M. E. (2002) Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295, 505–508 [DOI] [PubMed] [Google Scholar]
- 9. Krasinski K., Spyridopoulos I., Asahara T., van der Zee R., Isner J. M., Losordo D. W. (1997) Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95, 1768–1772 [DOI] [PubMed] [Google Scholar]
- 10. Ford M. M., Eldridge J. C., Samson H. H. (2004) Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin. Exp. Res. 28, 20–28 [DOI] [PubMed] [Google Scholar]
- 11. Reid M. L., Hubbell C. L., Reid L. D. (2003) A pharmacological dose of estradiol can enhance appetites for alcoholic beverages. Pharmacol. Biochem. Behav. 74, 381–388 [DOI] [PubMed] [Google Scholar]
- 12. Rajasingh J., Bord E., Qin G., Ii M., Silver M., Hamada H., Ahluwalia D., Goukassian D., Zhu Y., Losordo D. W., Kishore R. (2007) Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology 148, 3618–3624 [DOI] [PubMed] [Google Scholar]
- 13. Rubin E., Urbano-Marquez A. (1996) in Alcohol and the Cardiovascular System (Zakhari S., Wassef M., eds) pp. 127–136, National Institutes of Health, Bethesda, MD [Google Scholar]
- 14. Masuda H., Kalka C., Takahashi T., Yoshida M., Wada M., Kobori M., Itoh R., Iwaguro H., Eguchi M., Iwami Y., Tanaka R., Nakagawa Y., Sugimoto A., Ninomiya S., Hayashi S., Kato S., Asahara T. (2007) Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ. Res. 101, 598–606 [DOI] [PubMed] [Google Scholar]
- 15. Hamada H., Kim M. K., Iwakura A., Ii M., Thorne T., Qin G., Asai J., Tsutsumi Y., Sekiguchi H., Silver M., Wecker A., Bord E., Zhu Y., Kishore R., Losordo D. W. (2006) Estrogen receptors α and β mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114, 2261–2270 [DOI] [PubMed] [Google Scholar]
- 16. Iwakura A., Shastry S., Luedemann C., Hamada H., Kawamoto A., Kishore R., Zhu Y., Qin G., Silver M., Thorne T., Eaton L., Masuda H., Asahara T., Losordo D. W. (2006) Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation 113, 1605–1614 [DOI] [PubMed] [Google Scholar]
- 17. Strauer B. E., Brehm M., Zeus T., Köstering M., Hernandez A., Sorg R. V., Kögler G., Wernet P. (2002) Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 [DOI] [PubMed] [Google Scholar]
- 18. Assmus B., Schächinger V., Teupe C., Britten M., Lehmann R., Döbert N., Grünwald F., Aicher A., Urbich C., Martin H., Hoelzer D., Dimmeler S., Zeiher A. M. (2002) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 106, 3009–3017 [DOI] [PubMed] [Google Scholar]
- 19. Losordo D. W., Henry T. D., Davidson C., Sup Lee J., Costa M. A., Bass T., Mendelsohn F., Fortuin F. D., Pepine C. J., Traverse J. H., Amrani D., Ewenstein B. M., Riedel N., Story K., Barker K., Povsic T. J., Harrington R. A., Schatz R. A. (2011) Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 109, 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A. K., Gupta S., Jiang Y., Younus M., Ramzan M. (2009) In vitro neurogenesis from neural progenitor cells isolated from the hippocampus region of the brain of adult rats exposed to ethanol during early development through their alcohol-drinking mothers. Alcohol Alcohol 44, 185–198 [DOI] [PubMed] [Google Scholar]
- 21. Jung Y., Brown K. D., Witek R. P., Omenetti A., Yang L., Vandongen M., Milton R. J., Hines I. N., Rippe R. A., Spahr L., Rubbia-Brandt L., Diehl A. M. (2008) Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thal M. A., Krishnamurthy P., Mackie A. R., Hoxha E., Lambers E., Verma S., Ramirez V., Qin G., Losordo D. W., Kishore R. (2012) Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ. Res. 111, 180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackie A. R., Klyachko E., Thorne T., Schultz K. M., Millay M., Ito A., Kamide C. E., Liu T., Gupta R., Sahoo S., Misener S., Kishore R., Losordo D. W. (2012) Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 111, 312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnamurthy P., Thal M., Verma S., Hoxha E., Lambers E., Ramirez V., Qin G., Losordo D., Kishore R. (2011) Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ. Res. 109, 1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jujo K., Hamada H., Iwakura A., Thorne T., Sekiguchi H., Clarke T., Ito A., Misener S., Tanaka T., Klyachko E., Kobayashi K., Tongers J., Roncalli J., Tsurumi Y., Hagiwara N., Losordo D. W. (2010) CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 107, 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roncalli J., Renault M. A., Tongers J., Misener S., Thorne T., Kamide C., Jujo K., Tanaka T., Ii M., Klyachko E., Losordo D. W. (2011) Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization. J. Am. Coll. Cardiol. 57, 2444–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albrecht-Schgoer K., Schgoer W., Holfeld J., Theurl M., Wiedemann D., Steger C., Gupta R., Semsroth S., Fischer-Colbrie R., Beer A. G., Stanzl U., Huber E., Misener S., Dejaco D., Kishore R., Pachinger O., Grimm M., Bonaros N., Kirchmair R. (2012) The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 126, 2491–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kishore R., Qin G., Luedemann C., Bord E., Hanley A., Silver M., Gavin M., Yoon Y. S., Goukassian D., Goukassain D., Losordo D. W. (2005) The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-α-induced transcriptional repression of cyclin A. J. Clin. Invest. 115, 1785–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kishore R., McMullen M. R., Nagy L. E. (2001) Stabilization of tumor necrosis factor α mRNA by chronic ethanol. Role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J. Biol. Chem. 276, 41930–41937 [DOI] [PubMed] [Google Scholar]
- 30. Kishore R., Luedemann C., Bord E., Goukassian D., Losordo D. W. (2003) Tumor necrosis factor-mediated E2F1 suppression in endothelial cells. Differential requirement of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signal transduction pathways. Circ. Res. 93, 932–940 [DOI] [PubMed] [Google Scholar]
- 31. Luedemann C., Bord E., Qin G., Zhu Y., Goukassian D., Losordo D. W., Kishore R. (2005) Ethanol modulation of TNF-α biosynthesis and signaling in endothelial cells. Synergistic augmentation of TNF-α mediated endothelial cell dysfunctions by chronic ethanol. Alcohol Clin. Exp. Res. 29, 930–938 [DOI] [PubMed] [Google Scholar]
- 32. Barthwal M. K., Sathyanarayana P., Kundu C. N., Rana B., Pradeep A., Sharma C., Woodgett J. R., Rana A. (2003) Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J. Biol. Chem. 278, 3897–3902 [DOI] [PubMed] [Google Scholar]
- 33. Okubo Y., Blakesley V. A., Stannard B., Gutkind S., Le Roith D. (1998) Insulin-like growth factor-I inhibits the stress-activated protein kinase/c-Jun N-terminal kinase. J. Biol. Chem. 273, 25961–25966 [DOI] [PubMed] [Google Scholar]
- 34. Williams G. D., Grant B. F., Harford T. C., Noble B. A. (1989) Alcohol Health Res. World 13, 366–370 [Google Scholar]
- 35. Hill S. Y. (1984) in Alcohol Problems in Women. Antecedents, Consequences, and Intervention. (Wilsnack S. C., Beckman L. J., eds.) pp. 121–154, Guilford Press, New York [Google Scholar]
- 36. Chilvers C. E., Knibb R. C., Armstrong S. J., Woods K. L., Logan R. F. (2003) Post menopausal hormone replacement therapy and risk of acute myocardial infarction. A case control study of women in the East Midlands, UK. Eur. Heart J. 24, 2197–2205 [DOI] [PubMed] [Google Scholar]
- 37. Gavaler J. S., Love K., Van Thiel D., Farholt S., Gluud C., Monteiro E., Galvao-Teles A., Ortega T. C., Cuervas-Mons V. (1991) An international study of the relationship between alcohol consumption and postmenopausal estradiol levels. Alcohol Alcohol Suppl. 1, 327–330 [PubMed] [Google Scholar]
- 38. Hilakivi-Clarke L. (1996) Role of estradiol in alcohol intake and alcohol-related behaviors. J. Stud. Alcohol 57, 162–170 [DOI] [PubMed] [Google Scholar]
- 39. Ford M. M., Eldridge J. C., Samson H. H. (2002) Ethanol consumption in the female Long-Evans rat. A modulatory role of estradiol. Alcohol 26, 103–113 [DOI] [PubMed] [Google Scholar]
- 40. Mendelsohn M. E., Karas R. H. (1999) The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 340, 1801–1811 [DOI] [PubMed] [Google Scholar]
- 41. Medina K. L., Kincade P. W. (1994) Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc. Natl. Acad. Sci. U.S.A. 91, 5382–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prockop D. J. (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 [DOI] [PubMed] [Google Scholar]
- 43. Preedy V. R., Atkinson L. M., Richardson P. J., Peters T. J. (1993) Mechanisms of ethanol-induced cardiac damage. Br. Heart J. 69, 197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Regan T. J. (1990) Alcohol and the cardiovascular system. JAMA 264, 377–381 [PubMed] [Google Scholar]
- 45. Rubin E., Urbano-Marquez A. (1994) Alcoholic cardiomyopathy. Alcohol Clin. Exp. Res. 18, 111–114 [DOI] [PubMed] [Google Scholar]
- 46. Gaziano J. M., Buring J. E., Breslow J. L., Goldhaber S. Z., Rosner B., VanDenburgh M., Willett W., Hennekens C. H. (1993) Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 329, 1829–1834 [DOI] [PubMed] [Google Scholar]
- 47. Klatsky A. L. (1994) Epidemiology of coronary heart disease. Influence of alcohol. Alcohol Clin. Exp. Res. 18, 88–96 [DOI] [PubMed] [Google Scholar]
- 48. Mukamal K. J., Kronmal R. A., Mittleman M. A., O'Leary D. H., Polak J. F., Cushman M., Siscovick D. S. (2003) Alcohol consumption and carotid atherosclerosis in older adults. The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 23, 2252–2259 [DOI] [PubMed] [Google Scholar]
- 49. Schoppet M., Maisch B. (2001) Alcohol and the heart. Herz 26, 345–352 [DOI] [PubMed] [Google Scholar]
- 50. Puddey I. B., Zilkens R. R., Croft K. D., Beilin L. J. (2001) Alcohol and endothelial function. A brief review. Clin. Exp. Pharmacol. Physiol. 28, 1020–1024 [DOI] [PubMed] [Google Scholar]
- 51. Jonsson A. S., Palmblad J. E. (2001) Effects of ethanol on NF-κB activation, production of myeloid growth factors, and adhesive events in human endothelial cells. J. Infect. Dis. 184, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuhlmann C. R., Li F., Lüdders D. W., Schaefer C. A., Most A. K., Backenköhler U., Neumann T., Tillmanns H., Waldecker B., Erdogan A., Wiecha J. (2004) Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin. Exp. Res. 28, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 53. Liu J., Tian Z., Gao B., Kunos G. (2002) Dose-dependent activation of antiapoptotic and proapoptotic pathways by ethanol treatment in human vascular endothelial cells. Differential involvement of adenosine. J. Biol. Chem. 277, 20927–20933 [DOI] [PubMed] [Google Scholar]
- 54. Merritt R., Guruge B. L., Miller D. D., Chaitman B. R., Bora P. S. (1997) Moderate alcohol feeding attenuates postinjury vascular cell proliferation in rabbit angioplasty model. J. Cardiovasc. Pharmacol. 30, 19–25 [DOI] [PubMed] [Google Scholar]
- 55. Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H., Zeiher A. M., Dimmeler S. (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 89, E1–7 [DOI] [PubMed] [Google Scholar]
- 56. Ii M., Nishimura H., Iwakura A., Wecker A., Eaton E., Asahara T., Losordo D. W. (2005) Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 111, 1114–1120 [DOI] [PubMed] [Google Scholar]