Background: The Bag6-Ubl4A-Trc35 complex is localized to the ER membrane to regulate ERAD.
Results: A disordered chaperoning domain in Bag6 forms homo-oligomer. A UBL domain recruited it to the membrane via interactions with gp78 and UbxD8.
Conclusion: Simultaneous association with ERAD factors recruits Bag6 to a retrotranslocation site to prevent protein aggregation.
Significance: The study illustrates how a chaperone interacts with the ER membranes and misfolded proteins.
Keywords: E3 Ubiquitin Ligase, Endoplasmic Reticulum Stress, Endoplasmic Reticulum(ER), ER-associated Degradation, Molecular Chaperone, Bag6/Bat3/Scythe, gp78, Holdase, Retrotranslocation
Abstract
The Bag6-Ubl4A-Trc35 complex is a multifunctional chaperone that regulates various cellular processes. The diverse functions of Bag6 are supported by its ubiquitous localization to the cytoplasm, the nucleus, and membranes of the endoplasmic reticulum (ER) in cells. In ER-associated degradation (ERAD) pathways, Bag6 can interact with the membrane-associated ubiquitin ligase gp78 via its ubiquitin-like (UBL) domain, but the relative low affinity of this interaction does not reconcile with the fact that a fraction of Bag6 is tightly bound to the membranes. Here, we demonstrate that the UBL domain of Bag6 is required for interaction with the ER membranes. We find that in addition to gp78, the Bag6 UBL domain also binds a UBL-binding motif in UbxD8, an essential component of the gp78 ubiquitinating machinery. Importantly, Bag6 contains a proline-rich (PR) domain termed PDP (Proline rich-DUF3587-Proline rich) that forms homo-oligomer, allowing the UBL domain to form multivalent interactions with gp78 and UbxD8, which are essential for recruitment of Bag6 to the ER membrane. Furthermore, the PR domain comprises largely intrinsically disordered segments, which are sufficient for interaction with an unfolded substrate. We propose that simultaneous association with multiple ERAD factors helps to anchor a disordered chaperone oligomer to the site of retrotranslocation to prevent protein aggregation in ERAD.
Introduction
Elimination of misfolded proteins from the endoplasmic reticulum (ER)2 by the ER-associated protein degradation system (ERAD) ensures that proteins entering the secretory pathway are correctly folded and properly assembled (1). This quality control mechanism operates in all eukaryotic cells to alleviate proteotoxic stress in the ER and improve cell vitality (2, 3). ERAD involves a protein translocation process termed retrotranslocation or dislocation, in which misfolded polypeptides are selectively exported from the ER before degradation by the proteasome in the cytosol (4).
The precise mechanism by which misfolded proteins are moved across the ER membrane during retrotranslocation is unclear, but it has been proposed that a few large membrane protein complexes, each assembled around a multiple-spanning transmembrane ubiquitin ligase (E3), may play crucial roles in this process (5, 6). These enzymes may participate in the formation of a putative retrotranslocon(s) using their transmembrane segments (7, 8). Their cytosolic domains contain a RING finger motif that acts in conjunction with a cognate conjugating enzyme (E2) to assemble ubiquitin chains on retrotranslocation substrates (9). Polyubiquitination serves as a pivotal signal that leads to the recruitment of the p97-Ufd1-Npl4 ATPase complex and the extraction of the retrotranslocation substrates from the membranes for subsequent proteasomal targeting (10). Moreover, the ER-associated E3 ligases can interact directly with the retrotranslocation-driving ATPase p97/VCP on the cytosolic side of the ER membrane and also communicate with ER luminal chaperones (11–14), thus coupling substrate recognition in the ER lumen to p97-mediated retrotranslocation in the cytosol.
Upon dislocation, ERAD substrates need to be efficiently channeled to the proteasome for degradation. At least for certain substrates, this process involves a recently identified chaperone complex, consisting of Bag6 and the two co-factors Ubl4A and Trc35 (15, 16). Bag6 contains a chaperone-like activity that prevents substrate aggregation. It can physically interact with the ER-associated E3 ligase gp78 as well as with the proteasome (15, 17), providing a plausible link between retrotranslocated polypeptides and the proteasome. Bag6 is an abundant cytosolic chaperone that is also involved in the biogenesis of tail-anchored (TA) ER membrane proteins as well as in quality control of mislocalized proteins and defective ribosomal products (18–21). It also participates in several cellular processes in the nucleus (22–24). These diverse functions are supported by its ubiquitous expression that localizes it to various subcellular compartments. Intriguingly, a significant fraction of Bag6 is tightly bound to the ER membrane as it requires several rounds of salt treatment to remove it (15). It is currently unclear how Bag6 interacts with the ER membrane to promote ERAD. How Bag6 recognizes unfolded substrates is also unknown.
In this study, we find that Bag6 contains an unusual disordered domain that oligomerizes to bind unfolded proteins. Oligomerization also allows its UBL domain to interact simultaneously with gp78 and its functional partner UbxD8, which localize Bag6 to the ER membrane. This ensures that protein ubiquitination, dislocation, targeting to the proteasome take place in a highly coordinated manner. Our study reveals how Bag6 interacts with the ER membrane and its substrates to promote protein homeostasis in the ER in mammalian cells.
EXPERIMENTAL PROCEDURES
Cell Lines, Plasmids, and Antibodies
The HEK293 cell line stably expressing TCRα-YFP was described previously (25). Plasmid expression gp78 was described previously (15, 26). The other mammalian expression plasmids were constructed by cloning correspond ORF into pRK vector (27). The UbxD8 shRNA construct was purchased from Origene (Rockville, MD). The targeting sequence is: CGGTTTACCTATTACACGATACTTGATAT.
Antibodies to Bag6, Ubl4A, gp78, GFP were described previously (18, 26). Other antibodies used are FLAG (M2) (Sigma), UbxD8 (Proteintech Group), p97 (Fitzgerald), and MMS1 (BIOMOL). MG132 was purchased from EMD Bioscience. Luciferase was purchased from Sigma. Hsp70, Hdj1, HOP were purchased from ENZO Life Sciences.
Immunoblotting, Immunoprecipitation, and Pulldown Assays
Cells were lysed in the Nonidet P-40 lysis buffer containing 50 mm Tris-HCl pH 7.4, 150 mm sodium chloride, 2 mm magnesium chloride, 0.5% Nonidet P-40, and a protease inhibitor mixture. Cell extracts were subject to centrifugation to remove insoluble materials. For most experiments, the supernatant fractions were analyzed. Where indicated in the figure legends, the Nonidet P-40 insoluble pellet fractions were resolubilized by the Laemmli buffer for immunoblotting. Immunoblotting was performed according to the standard protocol. Fluorescence-labeled secondary antibodies (Rockland) were used for detection. The fluorescent bands were imaged and quantified on a LI-COR Odyssey infrared imager using the software provided by the manufacturer. For immunoprecipitation, the whole cell extract was incubated with FLAG-agarose beads (Sigma) or protein A-Sepharose CL-4B (GE Healthcare) bound with antibodies against specific proteins. After incubating, the beads were washed two times by Nonidet P-40 wash buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm sodium chloride, 2 mm magnesium chloride, 0.1% Nonidet P-40. The proteins on beads were assayed by immunoblotting.
Analytical Size Exclusion Chromatography
FLAG-tagged Bag6 was purified by affinity chromatography using FLAG-agarose beads (Sigma) as described previously. To analyze purified Bag6 by size exclusion chromatography, the protein was applied on a Superdex 200 10/300 GL size exclusion column (GE Healthcare) pre-equilibrated with the PB buffer and resolved at a flow rate of 0.4 ml/min on an AKTA (GE Healthcare) automated liquid chromatography system. To analyze endogenous Bag6, 293T cells were collected from an 80% confluent 15-cm dish and lysed in 600 μl of Nonidet P-40 lysis buffer containing a protease inhibitor mixture. The whole cell extract (WCE) was filtered through a 0.22-μm filter and applied onto a Superose 6 10/300 GL size exclusion column (GE Healthcare) pre-equilibrated with the PB buffer. Fractions of 0.4 ml were collected and analyzed by immunoblotting.
Gene Knockdown and ERAD Assay
To knock down UbxD8 and SGTA, 0.5 × 106 293T cells were seeded on Day 0 and transfected with shRNA constructs using Lipofectamine 2000 on Day 1 and Day 2. 72h post the first transfection, cells were harvested in a buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm sodium chloride, 2 mm magnesium chloride, 0.5% Nonidet P-40, and a protease inhibitor mixture. SDS and DTT were added to 1% and 5 mm, respectively and the samples were heated at 65 °C for 15 min before being diluted 10-fold with the lysis buffer. TCRα-YFP was immunoprecipitated using a GFP antibody followed by immunoblotting.
Membrane Binding Assays
To analyze Bag6 membrane interaction, purified Bag6 (1.5 μg) was incubated at 4 °C with purified cow liver microsomes (CLM) that were pretreated with buffer F (50 mm HEPES 7.3, 1.15 m potassium acetate, 10 mm magnesium acetate, 1 mm DTT) containing a protease inhibitor mixture. The reaction was layered on top of 1 m sucrose in the same buffer (180 μl). The samples were subject to centrifugation at 35,000rpm (43,000 × g) in a Beckman TLA100.1 rotor for 15 min. The membrane pellet and the supernatant fractions were analyzed by immunoblotting. To make gp78- or UbxD8-depleted proteolipisomes, salt-treated CLMs were solubilized in a lysis buffer containing 1% DeoxyBigCHAP, 30 mm Tris 7.4, 150 mm potassium acetate, 4 mm magnesium acetate, 1 mm DTT and a protease inhibitor mixture. The extracts were then incubated with protein A beads immobilized with either UbxD8 or gp78 antibodies. After two rounds of depletion, the extracts (750 μl) were incubated with 180 mg Bio-Beads SM2 at 4 °C overnight with gentle shaking. The samples were centrifuged at 3,000 × g for 2 min to remove the beads. The supernatant fractions containing the reformed proteolipisome vesicles were then incubated with purified Bag6 to examine its membrane association as mentioned above. As a negative control, lysis buffer treated with Bio-Beads was incubated with Bag6 to monitor background binding to residual Bio-Beads in the reactions.
RESULTS
The UBL Domain Is Required for Membrane and Substrate Binding
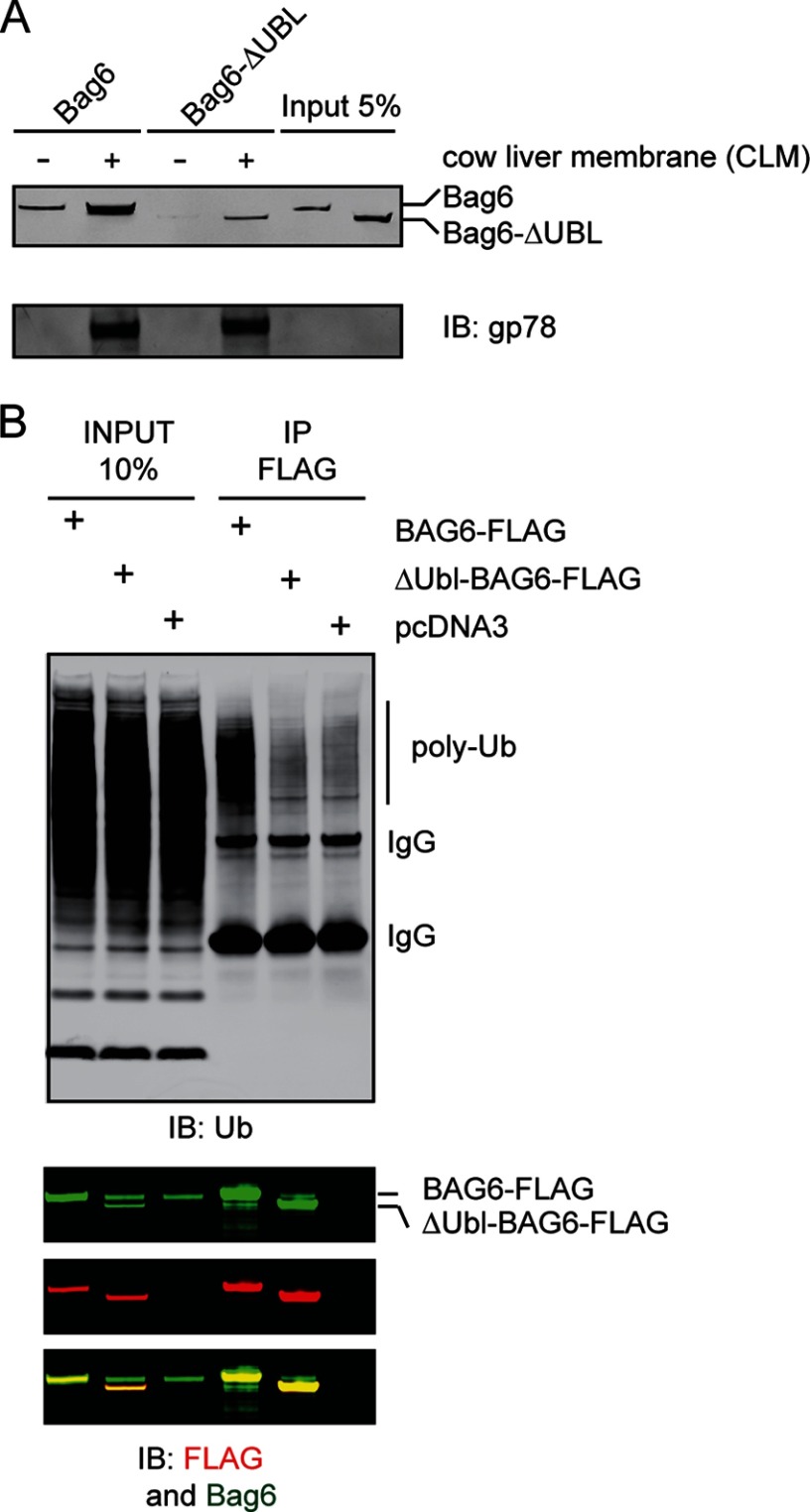
To test whether the Bag6 UBL domain is required for membrane association, we developed an in vitro membrane binding assay. We incubated purified Bag6 or a Bag6 variant lacking the UBL domain with ER-enriched CLM that had been pre-treated with a high salt-containing buffer. The sample was layered on top of a sucrose cushion and subjected to centrifugation. The high salt treatment removed most endogenous Bag6 from the membrane, vacating the binding sites for exogenously added Bag6. The association of recombinant Bag6, which contains a FLAG tag was then examined by immunoblotting using FLAG antibodies. The results showed that wild type Bag6 efficiently bound the ER membranes whereas the Bag6ΔUBL mutant bound the membranes much more weakly (Fig. 1A). Thus, the UBL domain of Bag6 promotes its recruitment to the ER membrane.
FIGURE 1.
The Bag6 UBL domain is required for membrane interaction. A, purified Bag6 or the Bag6ΔUBL mutant was incubated with salt-treated cow liver microsome membranes (CLM). The samples were layered on top of 1 m sucrose cushion and subject to centrifugation at 100,000 × g for 20 min. A fraction of the purified proteins and the proteins bound to the membrane were analyzed by immunoblotting (IB). Note that a small amount of Bag6 was present in the pellet fraction in the absent of CLM, likely due to oligomerization as described below. B, UBL domain is required for association with ubiquitinated proteins in cells. Cells transfected with the indicated DNA were lysed in a Nonidet P-40-containing buffer. A fraction of the extracts were analyzed directly by immunoblotting (input). The remaining samples were subject to immunoprecipitation with anti-FLAG antibodies. Bag6 and associated proteins were analyzed by immunoblotting.
Since substrates undertaking retrotranslocation are often ubiquitinated, we asked whether the association of Bag6 with ubiquitinated proteins required UBL. We expressed wild type Bag6 or Bag6ΔUBL in HEK293 cells and subjected the cell lysates to immunoprecipitation by FLAG antibodies. Immunoblotting showed that in control as well as Bag6ΔUBL-expressing cells, few ubiquitinated proteins could be precipitated by FLAG antibodies. By contrast, an interaction between ubiquitinated proteins and wild type Bag6 was readily detected (Fig. 1B). Collectively, we conclude that the UBL domain of Bag6 recruits it to the ER membrane for interaction with ubiquitinated proteins.
Bag6 Interacts with UbxD8 through a UBL-UBA Interaction
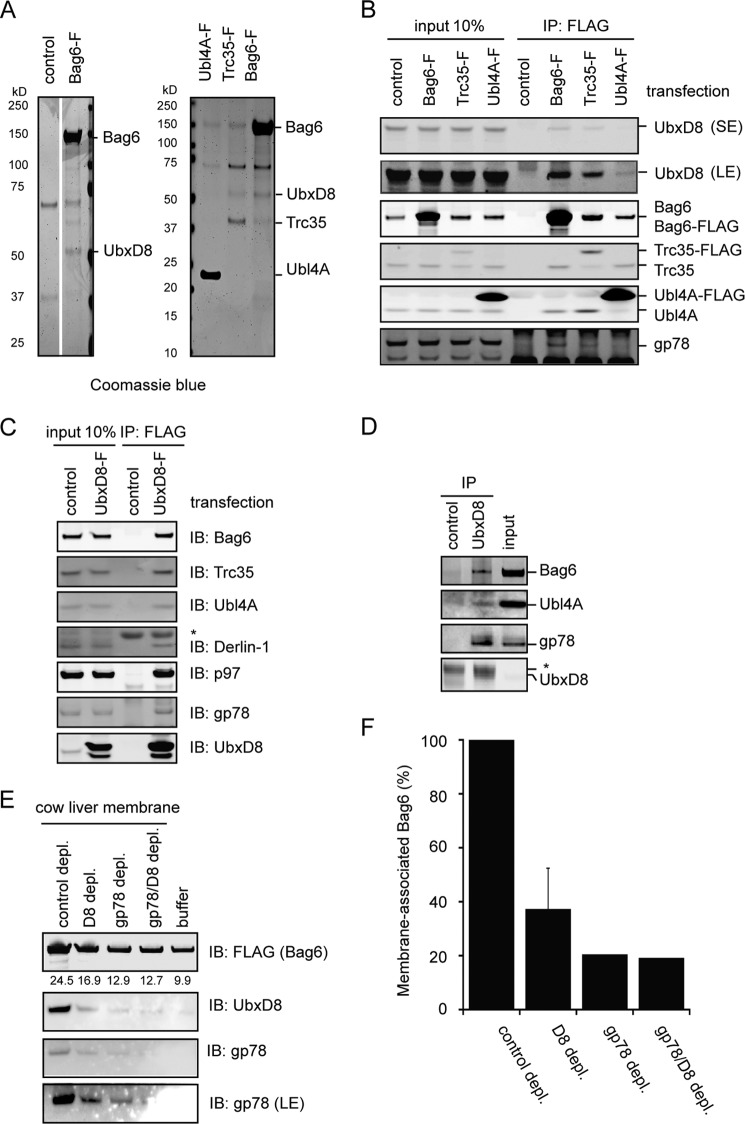
The Bag6 UBL domain binds the CUE domain of gp78 with a relatively low affinity with fast off-rate (Kd = ∼5 μm).3 This weak interaction cannot be the only link that recruits Bag6 to the ER membrane. We therefore suspected that the Bag6 complex might bind other factors in the gp78 complex as well. To identify the additional Bag6 interactor(s), we expressed each component of the Bag6 complex in HEK293 cells as a FLAG-tagged protein and purified them from cell extracts under high salt conditions. Coomassie staining showed that a protein of ∼53kD was readily co-purified with Bag6 despite high salt treatment, and to a lesser extent with Trc35, but not with Ubl4A (Fig. 2A). Mass spectrometry and immunoblotting identified it as UbxD8 (Fig. 2B and data not shown), an ER membrane-bound protein previously known to interact with gp78 and p97 (28). The levels of UbxD8 in the purified samples correlated with Bag6, suggesting that Bag6 might directly interact with UbxD8. Reciprocal immunoprecipitation showed that overexpressed UbxD8 also bound the endogenous Bag6 complex in addition to its known interactors gp78, Derlin-1, and p97 (Fig. 2C). An interaction between the endogenous Bag6 complex and UbxD8 could also be detected when UbxD8 immunoprecipitated from an extract of untransfected cells was subject to immunoblotting by antibodies to either Bag6 or Ubl4A (Fig. 2D). These results identify UbxD8 as a new interacting partner of the Bag6 complex.
FIGURE 2.
Bag6 associates with the ER membrane via binding the UbxD8-gp78 complex. A, purification of Bag6, Trc35, or Ubl4A was performed using HEK293 cells expressing the indicated FLAG-tagged proteins. As a negative control, cells transfected with a control empty vector were used. The purified complexes were analyzed by SDS-PAGE and Coomassie Blue staining. B, immunoblotting confirms the interaction of Bag6 with UbxD8. Cells transfected with the indicated plasmids were lysed, and proteins immunoprecipitated with FLAG beads were analyzed by immunoblotting. LE, long exposure; S.E., short exposure. C, as in B, except that cells expressing FLAG-tagged UbxD8 were used. D, interaction of the endogenous Bag6 complex with UbxD8. Whole cell extracts were subject to immunoprecipitation by the indicated antibodies. The asterisk indicates IgG. E and F, UbxD8-gp78 complex is required for membrane association of Bag6. E, CLM extracts were treated with protein A beads containing either control IgG or the indicated antibodies. After depletion, proteolipisomes were re-formed and used in binding experiments with purified Bag6. Proteins bound to the membrane pellet fractions were analyzed by immunoblotting. Where indicated, a buffer control was included to assess the levels of background binding to residual Bio-Beads present in the samples. The numbers indicate band intensity. LE, long exposure. F, graph shows the quantification of the experiment in E. Error bar indicates the mean of the two independent experiments.
To see whether the interaction of Bag6 with UbxD8 or gp78 is responsible for membrane recruitment of Bag6, we depleted UbxD8 and gp78 from solubilized cow liver ER membranes using either UbxD8 or gp78 antibodies. Depletion of UbxD8 or gp78 led to great reduction of the other protein in the extract, suggesting that most of these proteins are in complex with each other in the membranes. The control or UbxD8- and gp78-depleted extracts were then treated with Bio-Beads SM2 to remove detergent. This procedure allows reformation of vesicles with or without the UbxD8-gp78 complex. The samples were then incubated with purified Bag6 followed by centrifugation. The levels of Bag6 co-sedimented with the proteolipisomes were determined by quantitative immunoblotting. The results showed that Bag6 bound to gp78-UbxD8-containing vesicles more efficiently than those without the complex (Figs. 2, E and F). The results suggest that interactions between Bag6 UBL and the gp78-UbxD8 complex are required for ER association of Bag6.
We next determined the domain in Bag6 required for UbxD8 binding. In addition to the UBL domain, Bag6 contains two proline-rich (PR) segments flanking a domain of unknown function (DUF3587), a C-terminal BAG domain, and a zinc finger domain (Fig. 3A) (15). We expressed full-length Bag6 and three truncation mutants in HEK293 cells (Fig. 3A). Immunoprecipitation experiments showed that a Bag6 mutant lacking the C-terminal BAG domain had reduced affinity to Trc35 and Ubl4A, but its interaction with UbxD8 was maintained (Fig. 3B, lane 8 versus 7). This further suggested that the interaction of Bag6 with UbxD8 is independent of Ubl4A and Trc35. By contrast, removal of the UBL domain from Bag6 significantly impaired its binding to UbxD8, but not to Trc35 or Ubl4A (lanes 9, 10 versus lane 7).
FIGURE 3.

The UBL domain of Bag6 is required for interaction with UbxD8. A, the domain structure of Bag6. B, cells were transfected with plasmids expressing the indicated Bag6 variants. The interactions of these Bag6 variants with Trc35, Ubl4A, and UbxD8 were analyzed by immunoprecipitation and immunoblotting.
To further characterize the UbxD8-Bag6 interaction, we purified a series of truncation mutants of UbxD8 from HEK293 cells (Fig. 4). Immunoprecipitation showed that the N-terminal 89 amino acids of UbxD8, which contain a ubiquitin-binding UBA domain, were required for interaction with Bag6. In comparison, the interaction of UbxD8 with p97 depended on the C-terminal UBX domain, as previously demonstrated (28), whereas the interaction of UbxD8 with gp78 relied on a TM-containing segment comprising residues 90 to 139. The interaction of UbxD8 with gp78 is highly specific as other UBX domain containing proteins did not bind gp78 with the exception of Ufd1 (Fig. 5A). These results unambiguously establish an interaction between UbxD8 and Bag6 that is independent of other ERAD factors such as gp78 and p97. Instead, it requires a canonical UBL-UBA interaction (29). In addition, these results also reveal UbxD8 as a key ERAD scaffolding coordinator as it can use distinct regions to communicate with different ERAD machinery proteins.
FIGURE 4.

The UBA domain of UbxD8 interacts with Bag6. A, schematic representation of the UbxD8 constructs used in the interaction studies. The table summarizes the co-IP results. N.A., data not available due to a protein stability issue. B, cells expressing the indicated UbxD8 variants were lysed in the Nonidet P-40 lysis buffer. Immunoprecipitation was performed using FLAG beads.
FIGURE 5.

UbxD8 is an essential co-factor of gp78. A, cells transfected with plasmids expressing the indicated UBX-containing proteins were lysed in the Nonidet P-40 lysis buffer. The UBX-containing proteins were immunopurified with FLAG beads. The purified proteins complexes were analyzed by immunoblotting with the indicated antibodies. B, cells stably expressing TCRα-YFP were transfected with either control or UbxD8-specific shRNA plasmid together with a construct expressing hemagglutinin (HA)-tagged ubiquitin. The cells were treated with either DMSO as control or with the proteasome inhibitor MG132 (10 μm, 12 h). TCRα was immunoprecipitated from the cell extracts under a denaturing condition for analysis by immunoblotting. WCE, whole cell extract.
UbxD8 Is an Integral Component of the gp78 Ligase Complex
Given its tight connection with gp78, we asked whether UbxD8 is required for the ubiquitination of the model ERAD substrate TCRα, whose degradation was strongly dependent on gp78 and Bag6 (15). Indeed, depletion of UbxD8 by shRNA-mediated gene silencing reduced the level of retrotranslocated deglycosylated TCRα. Importantly, TCRα ubiquitination was almost completely abolished (Fig. 5B, lane 4 versus 3). These results suggest that UbxD8 is an essential component of the gp78-containing E3 complex required for ubiquitination and retrotranslocation of ERAD substrates.
A Bag6 Oligomer Binds gp78 and UbxD8 Simultaneously
As Bag6 interacted directly with both gp78 and UbxD8 through its UBL domain, we asked whether Bag6 could bind to these ERAD factors simultaneously. To test this possibility, we first immunoprecipitated gp78 and subjected the eluate to another round of immunoprecipitation using a Bag6 antibody, or IgG as a negative control. The result showed that UbxD8 and gp78 were simultaneously present in a Bag6-containing complex (Fig. 6A, lane 8). This observation would only be possible if Bag6 forms an oligomer that simultaneously interacts with these factors (Fig. 6B).
FIGURE 6.
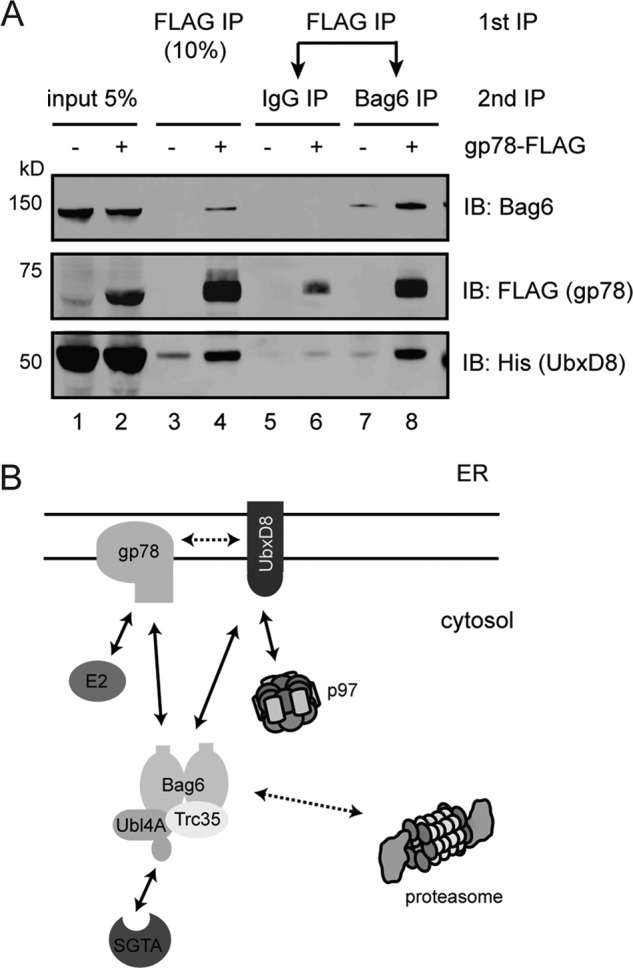
Bag6 binds gp78 and UbxD8 simultaneously. A, cells expressing Bag6, UbxD8 in the presence or absence of gp78-FLAG were lysed in the Nonidet P-40 lysis buffer. The lysates were first subject to immunoprecipitation with FLAG beads to pull down gp78-FLAG. The bound materials eluted with FLAG peptide were divided into two equal portions. One potion was incubated with control IgG whereas the other portion was incubated with anti-Bag6 antibodies to purify Bag6 and its associated proteins. The precipitated materials and a fraction of the inputs were analyzed by immunoblotting. B, diagram illustrating interactions between Bag6 and various ERAD factors. Solid arrows indicate direct interactions whereas dashed lines indicate association that may be mediated by an adaptor.
We therefore performed a series of experiments to determine the oligomeric state of Bag6. We first analyzed purified Bag6 by size exclusion chromatography. The result indicated that Bag6 forms a large homo-oligomeric complex of ∼1 megadalton (Fig. 7A). Similar analysis using a whole cell extract showed that endogenous Bag6 was present in a complex larger than the 600kD p97 ATPase complex (Fig. 7B). To further characterize the Bag6 oligomer, we expressed a series of FLAG-tagged Bag6 mutants in HEK293 cells (Fig. 7C). Immunoprecipitation with FLAG antibody showed that a Bag6 variant lacking the N-terminal UBL domain could be co-precipitated with endogenous Bag6 (lane 8). Deletion of the C-terminal 423 residues had no effect on Bag6 oligomerization (lane 9), but further removal of a proline-rich (PR) segment dramatically diminished the interaction (lane 10). Collectively, the data suggest that Bag6 form a homo-oligomer, and a PR domain is critical for this activity. This provides a molecular base for simultaneous association with multiple UBL-binding proteins in a core ERAD machinery complex, which increases the affinity of Bag6 to the ER membrane.
FIGURE 7.
Bag6 forms a large oligomer. A, size exclusion chromatography analysis of purified Bag6. The Coomassie Blue-stained gel shows the purified protein. B, size exclusion chromatography analysis of whole cell extract. The fractions were analyzed by immunoblotting. C, a proline-rich (PR) domain is required for Bag6 oligomerization. The scheme shows the Bag6 constructs used in the interaction studies. Cells expressing the indicated Bag6 variants were lysed in Nonidet P-40 lysis buffer. Bag6 immunoprecipitated with the FLAG beads was analyzed by immunoblotting.
A Disordered Domain in Bag6 Is Involved in Binding Unfolded Protein
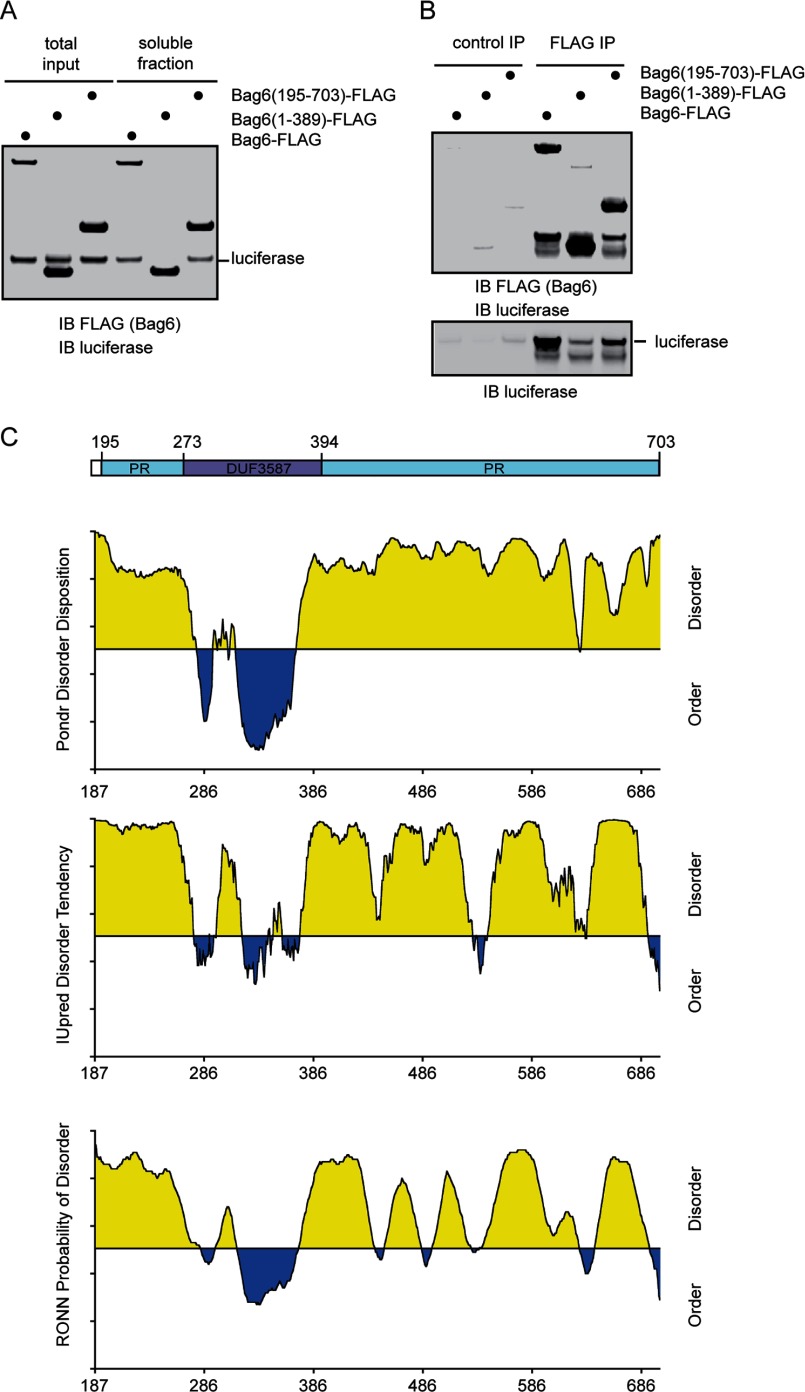
To see whether oligomerization of Bag6 is important for its function, we tested the interaction of a truncated Bag6 variant that contained residues 195 to 703 with heat-denatured luciferase. Sequence analyses predicted the presence of a domain of unknown function (DUF3587) that is sandwiched by two PR domains. We therefore named this module PDP. This Bag6 mutant was still able to form an oligomer as judged by size exclusion analyses (data not shown). We previously showed that when incubated with luciferase at 42 °C, wild type Bag6 bound denatured luciferase to stabilize it in a soluble fraction (15). Similar activity was observed with the Bag6 PDP mutant (Fig. 8A). As expected, Bag6 PDP bound heat-denatured luciferase with similar efficiency as wild type Bag6 (Fig. 8B). Interestingly, the Bag6 1–389 mutant that lacks the oligomerization-driving PR region appeared to bind denatured luciferase (Fig. 8B), but it failed to maintain the solubility of denatured luciferase, suggesting that oligomerization is required for the holdase function of Bag6. Moreover, secondary structural prediction suggests that the PDP domain of Bag6 comprises mostly disordered segments (Fig. 8C). These results establish Bag6 as a novel chaperone that may use a disordered chaperoning module in order to oligomerize and bind substrates.
FIGURE 8.
Bag6 employs a disordered domain to bind an unfolded protein. A, the PDP domain can prevent luciferase aggregation. Luciferase was incubated with excess amount of the indicated Bag6 variants at 42 °C for 20 min. A fraction of the samples were analyzed directly by immunoblotting (total input) whereas the remaining samples were subject to centrifugation to remove insoluble materials. A fraction of the soluble fractions were analyzed by immunoblotting. B, remaining samples of the soluble fractions were subject to immunoprecipitation with FLAG antibodies. The bound proteins were analyzed by immunoblotting. C, substrate binding domain of Bag6 is formed largely by disordered segments. The amino acid sequences of the Bag6 PDP domain were analyzed by the following secondary structure prediction programs, PONDR (top panel), IUPred (middle panel), and PONN (bottom panel).
DISCUSSION
Ubiquitin-like modifiers often form conjugates that encode signals distinct from ubiquitin. By contrast, many UBL domains in cells are recognized by ubiquitin binding domains (UBDs) similarly as ubiquitin (30, 31). Accordingly, many UBL domains are utilized by cells to mediate protein-protein interaction required for proteasomal degradation. A well-documented example is the proteasome adaptor Rad23 that uses a UBL domain to communicate with the proteasome, facilitating the capture of substrates by the proteasome (31). Here, we demonstrate that the UBL domain in Bag6 is required to recruit the Bag6 complex to the ER membrane. We show that the UBL domain of the Bag6 complex interacts with the CUE domain of gp78 as well as the UBA domain in the gp78 partner UbxD8. The formation of a large Bag6 oligomer enables the UBL domain to form multivalent interactions with the gp78-containing ERAD complex, which is required for the association of Bag6 with ER membrane (Fig. 6B).
gp78 is a key component of a multi-subunit complex that mediates the degradation of many misfolded ER proteins (32). Our results establish UbxD8 as an essential co-factor required for the retrotranslocation and ubiquitination functions of gp78. In vitro, purified UbxD8 does not promote gp78-mediated polyubiquitination (data not shown), but it remains to be seen whether UbxD8 may stimulate the ubiquitin ligase activity of gp78 or promote its interaction with substrates when other factors of the gp78 complex are present. Importantly, gp78 and UbxD8 each carry a p97 binding motif. Thus, these molecules can certainly provide a link between the Bag6 complex and p97, enable these factors to act in a concerted manner to guide ERAD substrates from the site of retrotranslocation to the proteasome (16).
Intriguingly, the Bag6 cofactor Ubl4A also contains a UBL domain that is structurally homologous to that of Bag6. Nonetheless, it does not bind either gp78 or UbxD8. Instead, it interacts with another Bag6 cofactor named SGTA. Thus, UBL domains containing distinct structural features can be differentially recognized by different UBL-binding domains in cells. The UBL domain in Bag6 appears to be a canonical UBL domain as it interacts with the ubiquitin binding CUE or UBA domain whereas the Ubl4A UBL is unique in a way that it utilizes many charged residues to bind its partner SGTA (29, 33).
The Bag6 complex is a multi-functional chaperone that can act in both protein biogenesis and turnover pathways (15, 18–20). In the biogenesis of ER TA proteins, the Bag6 complex associates with the ribosome, recognizes TA proteins emerging from the ribosome exiting tunnel, and escorts them to the ER membrane for insertion. In the ERAD pathways, the same complex is associated with the gp78-containing retrotranslocation machinery in the ER membrane, captures retrotranslocation substrates containing aggregation prone motifs, and targets them to the proteasome for degradation. A fraction of Bag6 is also found in the nucleus where it regulates post-translational modification of histones to influence DNA-associated events such as gene expression or DNA damage responses (22, 23, 34). The partition of the Bag6 complex between different cellular processes is likely regulated by its subcellular localization. A fraction of Bag6 is localized in the nucleus owing to a nuclear localization sequence present in its C terminus, but the interaction of Bag6 with its partner Trc35 masks this signal, retaining the majority of Bag6 in the cytoplasm (15). Our studies now establish the Bag6 UBL domain as an essential regulator of its membrane interaction. Further studies will be required to elucidate the factor(s) that mediates the interaction of the Bag6 complex with the ribosome.
Finally, our study reveals a novel chaperoning module that is formed primarily by disordered segments in Bag6. This is reminiscent of the substrate binding domain in a recently identified quantity control chaperone/ubiquitin ligase named San1 in budding yeast. San1 contains two largely disordered segments that form the substrate binding site(s). It was proposed that the disordered feature may confer plasticity, allowing San1 to accommodate substrates of various sizes and shapes (35). Mammalian cells do not contain any San1 homologues, but Bag6 may be a functional substituent for San1 in certain quality control processes. Intriguingly, in addition to binding unfolded proteins, San1 also contains ubiquitin ligase activity that promotes ubiquitination and turnover of the bound substrates. Bag6 itself does not have any ubiquitin ligase activity, but it associates with several ubiquitin ligases in cells including gp78, RNF126 and a cullin-containing ligase (24). Thus, to coordinate substrate selection and ubiquitination in quality control, yeast cells employ a simple strategy that incorporates a chaperoning activity and a ligase function in a single polypeptide. Higher eukaryotic cells appear to assign these functions to distinct polypeptides, which corporate in large protein complexes to execute a similar function.
Acknowledgments
We thank R. Deshaies (Caltech) for the plasmids for expression of the UBX-containing proteins. Harvard Taplin Mass Spectrometry Core for assistance in protein identification.
This work was supported by the Intramural Research Program of the NIDDK at the National Institutes of Health.
Y. Xu, unpublished results.
- ER
- endoplasmic reticulum
- CLM
- cow liver microsome
- ERAD
- ER-associated degradation membrane
- TA
- tail-anchored
- WCE
- whole cell extract
- UBD
- ubiquitin binding domain
- UBL
- ubiquitin-like.
REFERENCES
- 1. Vembar S. S., Brodsky J. L. (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 3. Smith M. H., Ploegh H. L., Weissman J. S. (2011) Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsai B., Ye Y., Rapoport T. A. (2002) Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 3, 246–255 [DOI] [PubMed] [Google Scholar]
- 5. Sato B. K., Schulz D., Do P. H., Hampton R. Y. (2009) Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol. Cell 34, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang S., Ferrone M., Yang C., Jensen J. P., Tiwari S., Weissman A. M. (2001) The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 98, 14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hampton R. Y., Sommer T. (2012) Finding the will and the way of ERAD substrate retrotranslocation. Curr. Opin. Cell Biol. 24, 460–466 [DOI] [PubMed] [Google Scholar]
- 8. Carvalho P., Stanley A. M., Rapoport T. A. (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch C., Gauss R., Horn S. C., Neuber O., Sommer T. (2009) The ubiquitylation machinery of the endoplasmic reticulum. Nature 458, 453–460 [DOI] [PubMed] [Google Scholar]
- 10. Flierman D., Ye Y., Dai M., Chau V., Rapoport T. A. (2003) Polyubiquitin serves as a recognition signal, rather than a ratcheting molecule, during retrotranslocation of proteins across the endoplasmic reticulum membrane. J. Biol. Chem. 278, 34774–34782 [DOI] [PubMed] [Google Scholar]
- 11. Ye Y., Shibata Y., Kikkert M., van Voorden S., Wiertz E., Rapoport T. A. (2005) Inaugural Article: Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. U.S.A. 102, 14132–14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong X., Shen Y., Ballar P., Apostolou A., Agami R., Fang S. (2004) AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J. Biol. Chem. 279, 45676–45684 [DOI] [PubMed] [Google Scholar]
- 13. Gauss R., Sommer T., Jarosch E. (2006) The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment. EMBO J. 25, 1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gauss R., Jarosch E., Sommer T., Hirsch C. (2006) A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat. Cell Biol. 8, 849–854 [DOI] [PubMed] [Google Scholar]
- 15. Wang Q., Liu Y., Soetandyo N., Baek K., Hegde R., Ye Y. (2011) A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble States for proteasome degradation. Mol. Cell 42, 758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Claessen J. H., Ploegh H. L. (2011) BAT3 guides misfolded glycoproteins out of the endoplasmic reticulum. PLoS One 6, e28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minami R., Hayakawa A., Kagawa H., Yanagi Y., Yokosawa H., Kawahara H. (2010) BAG-6 is essential for selective elimination of defective proteasomal substrates. J. Cell Biol. 190, 637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mariappan M., Li X., Stefanovic S., Sharma A., Mateja A., Keenan R. J., Hegde R. S. (2010) A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 466, 1120–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leznicki P., Clancy A., Schwappach B., High S. (2010) Bat3 promotes the membrane integration of tail-anchored proteins. J. Cell Sci. 123, 2170–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hessa T., Sharma A., Mariappan M., Eshleman H. D., Gutierrez E., Hegde R. S. (2011) Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawahara H., Minami R., Yokota N. (2013) BAG6/BAT3: emerging roles in quality control for nascent polypeptides. J. Biochem. 153, 147–160 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen P., Bar-Sela G., Sun L., Bisht K. S., Cui H., Kohn E., Feinberg A. P., Gius D. (2008) BAT3 and SET1A form a complex with CTCFL/BORIS to modulate H3K4 histone dimethylation and gene expression. Mol. Cell Biol. 28, 6720–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakeman T. P., Wang Q., Feng J., Wang X. F. (2012) Bat3 facilitates H3K79 dimethylation by DOT1L and promotes DNA damage-induced 53BP1 foci at G1/G2 cell-cycle phases. EMBO J. 31, 2169–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J. G., Ye Y. (2013) Bag6/Bat3/Scythe: A novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation. Bioessays 35, 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soetandyo N., Wang Q., Ye Y., Li L. (2010) Role of intramembrane charged residues in the quality control of unassembled T-cell receptor α-chains at the endoplasmic reticulum. J. Cell Sci. 123, 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W., Tu D., Li L., Wollert T., Ghirlando R., Brunger A. T., Ye Y. (2009) Mechanistic insights into active site-associated polyubiquitination by the ubiquitin-conjugating enzyme Ube2g2. Proc. Natl. Acad. Sci. U.S.A. 106, 3722–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L., Hailey D. W., Soetandyo N., Li W., Lippincott-Schwartz J., Shu H. B., Ye Y. (2008) Localization of A20 to a lysosome-associated compartment and its role in NFκB signaling. Biochim. Biophys. Acta 1783, 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. (2008) UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell 134, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Y., Cai M., Yang Y., Huang L., Ye Y. (2012) SGTA recognizes a noncanonical ubiquitin-like domain in the Bag6-Ubl4A-Trc35 complex to promote endoplasmic reticulum-associated degradation. Cell Rep. 2, 1633–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Veen A. G., Ploegh H. L. (2012) Ubiquitin-Like Proteins. Annu. Rev. Biochem. 81, 323–357 [DOI] [PubMed] [Google Scholar]
- 31. Mueller T. D., Feigon J. (2003) Structural determinants for the binding of ubiquitin-like domains to the proteasome. EMBO J. 22, 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Z., Du S., Fang S. (2012) gp78: a Multifaceted Ubiquitin Ligase that Integrates a Unique Protein Degradation Pathway from the Endoplasmic Reticulum. Curr. Protein Pept. Sci. 13, 414–424 [DOI] [PubMed] [Google Scholar]
- 33. Chartron J. W., VanderVelde D. G., Clemons W. M., Jr. (2012) Structures of the Sgt2/SGTA dimerization domain with the Get5/UBL4A UBL domain reveal an interaction that forms a conserved dynamic interface. Cell Rep. 2, 1620–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sasaki T., Gan E. C., Wakeham A., Kornbluth S., Mak T. W., Okada H. (2007) HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev. 21, 848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenbaum J. C., Fredrickson E. K., Oeser M. L., Garrett-Engele C. M., Locke M. N., Richardson L. A., Nelson Z. W., Hetrick E. D., Milac T. I., Gottschling D. E., Gardner R. G. (2011) Disorder targets misorder in nuclear quality control degradation: a disordered ubiquitin ligase directly recognizes its misfolded substrates. Mol. Cell 41, 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]