Background: HMGN5 is a nucleosome-binding protein that affects chromatin structure and function, and Lap2a is a lamin-binding protein that can also bind to DNA.
Results: HMGN5 and LAP2α interact and reciprocally affect each other's genome-wide distribution.
Conclusion: Nucleosome-binding proteins and lamin-binding proteins interact functionally.
Significance: We report a novel type of link between the chromatin fiber and the nuclear lamin network.
Keywords: Cell Biology, Chromatin, Chromosomes/Non-histone Chromosomal Proteins, Lamin, Nuclear Organization, HMG Proteins, LAP2Alpha
Abstract
The interactions of nuclear lamins with the chromatin fiber play an important role in regulating nuclear architecture and chromatin function; however, the full spectrum of these interactions is not known. We report that the N-terminal domain of the nucleosome-binding protein HMGN5 interacts with the C-terminal domain of the lamin-binding protein LAP2α and that these proteins reciprocally alter their interaction with chromatin. Chromatin immunoprecipitation analysis of cells lacking either HMGN5 or LAP2α reveals that loss of either protein affects the genome-wide distribution of the remaining partner. Our study identifies a new functional link between chromatin-binding and lamin-binding proteins.
Introduction
The structure and activity of chromatin are regulated by the dynamic binding of numerous nuclear proteins to their nucleosomal targets. Many of these proteins are targeted to specific sites by unique DNA sequence motifs or specific histone modifications. On the other hand, architectural proteins such as the linker histone H1 (1, 2) and high mobility group (HMG)4 proteins (3–5) bind to chromatin without any obvious specificity for DNA sequence or histone modification. The mechanisms regulating their interactions with chromatin are not fully understood. Members of the high mobility group N (HMGN) protein family bind specifically to the nucleosomes (6) throughout the genome (7). However, these interactions are not random; genome-wide analysis revealed that the HMGN1 variant preferentially localizes to regulatory regions such as enhancers and promoters (7). Elucidation of the factors that affect the interaction of HMGNs with chromatin is important because these proteins affect chromatin compaction, the levels of histone modification, and the fidelity of transcription.
HMGN proteins have a disordered primary structure and associate with numerous nuclear proteins (8, 9). These associations could conceivably affect the chromatin interactions and function of HMGNs. Here we report that the human HMGN5 variant (10) interacts with the lamina-associated polypeptide 2α (LAP2α). LAP2α is a LEM domain protein that interacts with A-type lamins (11). However, unlike most other LEM domain proteins, LAP2α lacks a transmembrane domain and localizes throughout the nucleus, where it interacts with the nucleoplasmic pool of A-type lamins (12) and also binds DNA, either directly or through an association with the chromatin-binding protein barrier to autointegration factor (BAF) (13).
Given that both HMGN5 and LAP2α bind to chromatin and because their binding partners could potentially affect these interactions, we tested whether changes in the levels of either HMGN5 or LAP2α affect the nuclear dynamics and chromatin interactions of their partner. By fluorescence recovery after photobleaching (FRAP) and chromatin immunoprecipitation analysis (ChIP), we find that indeed, the proteins mutually affect their interaction with chromatin.
The nuclear lamina is in direct contact with the chromatin fiber, and changes in these contacts or alterations in the nuclear lamin network can affect chromatin structure and function and influence the cellular phenotype (13–15). Our studies reveal a functional connection between nucleosome-binding and lamin-binding proteins and suggest an additional link between the chromatin fiber and the nuclear lamin network.
EXPERIMENTAL PROCEDURES
Cell Lines
The HEK-293T and HeLa cells were maintained in DMEM containing GlutaMAX and supplemented with 10% FBS, 37 °C, 5% CO2, H2O-saturated. HeLa cells stably expressing Lap2αshRNA or control shRNA cells were previously described (16).
Plasmids and Transfection
The HaloTag-HMGN5 was constructed using the pHTC Halo-Tag CMV (Promega). The pEGFP-HMGN5 and pEGFP-LAP2 plasmids were constructed by subcloning the respective cDNA into pEGFP-N1 vectors. For siRNA-mediated down-regulation of HMGN5, HeLa cells were transfected with specific or control siRNA ON-TARGETplus SMARTpool from Dharmacon using the DharmaFECT 4 transfection reagent.
FRAP
HeLa cells were transiently transfected, as described above, with Lap2α-Cherry- and HMGN5-YFP-expressing plasmid, incubated for 24 h, and analyzed as described (17). Each experiment was repeated at least three times.
Antibodies
Affinity-purified rabbit anti-hHMGN5 (HPA000511) and anti-actin (A5316) were from Sigma, anti-Halo was from Promega (catalog number G928A), anti-Lap2α was from Millipore, and secondary antibodies were from Jackson ImmunoResearch Laboratories; HRP-conjugated secondary antibodies used in Western blots were from Millipore.
HaloTag and GST Pulldown
For HaloTag pulldown, cells expressing HaloTag fusion proteins were lysed in mammalian lysis buffer (Promega) and processed according to the manufacturer's instructions. Protein samples were analyzed by either mass spectroscopy or Western blot. [35S]LAP2α recombinant proteins were prepared with a TnT T7 quick-coupled transcription/translation system (Promega), according to the manufacturer's instructions. For the in vitro GST pulldown assays, GST fusion HMGN5 proteins were incubated with [35S]LAP2α recombinant protein for 1 h at room temperature and then adsorbed on glutathione-Sepharose beads (Amersham Biosciences) for 2 h at 4 °C, washed three times with PBS, resolved by 10% SDS-PAGE, and autoradiographed.
ChIP/Re-ChIP and ChIP-seq Assays
HeLa cells were grown on a 150-mm culture dish to approximate 80% confluence fixed with 1% formaldehyde (Thermo Scientific) for 10 min at room temperature. Cross-linked chromatin was then sheared by sonication to 200-700-bp fragments with Misonix sonicator 3000. First immunoprecipitation was performed with the Dynabeads protein G immunoprecipitation Kit (life Technologies) using Lap2α antibody. Immunoprecipitated DNA-protein complex was eluted in 100 μl of elution buffer. 10 μl of chromatin samples were used as control for the first ChIP reaction. The remaining chromatin was diluted to 2 ml with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl, pH 8.0, 167 mm NaCl, and protease inhibitor freshly added). Each 1-ml aliquot was incubated with either HMGN5 antibody or normal rabbit IgG. DNA was extracted with MiniElute purification Kit (Qiagen) after reverse cross-linking and subjected to PCR analysis. Primers used in PCR are available upon request. The ChIP-seq library was prepared following the manufacturer's instructions (Illumina, Inc.).
Preliminary ChIP-seq Analysis
Reads were aligned to the hg19 build of the human genome using Bowtie (18) with the following parameters: -n 2 mode; -a -m 10 –best –strata. Score profiles for visualization on the University of California Santa Cruz (UCSC) Genome Browser (19) were generated as described previously (20).
Peak Finding
Regions of enrichment (“peaks”) for each ChIP-seq library were identified using SICER (21) with the parameters: redundancy threshold = 1, window = 200, fragment size = 200, gap size = 200, false discovery rate = 0.01.
Scatter Plots
Scatter plots for HMNG5 and LAP2α (see Fig. 3, A and B) were constructed by first obtaining the union set of peaks for HMGN5 and LAP2α. Read counts for both libraries were summed for each peak in the union set, and the log of the counts on each peak was plotted. The union peak set was classified into “Promoter” peaks that were within 1 kb of an annotated UCSC gene transcription start site and peak sets that were classified as “Distal” peaks otherwise. Promoter peaks were further classified as corresponding to expressed or non-expressed based on FPKM values of the corresponding genes. Expressed genes were determined as detected (FPKM > 0) in two replicate experiments. A more conservative threshold to determine detected genes (FPKM > 1) was also tried, but this did not alter the results.
FIGURE 3.
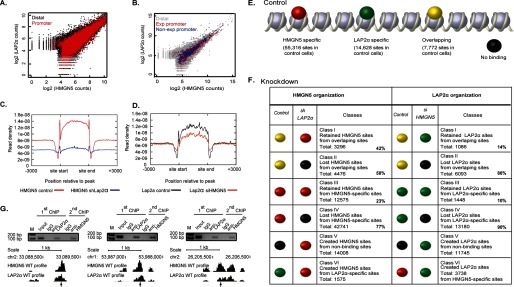
Reciprocal effects of HMGN5 and LAP2α on their genome-wide distribution. A and B, LAP2α and HMGN5 preferentially co-localize at promoters. C, loss of LAP2α reduces the binding of HMGN5 across the genome. D, loss of HMGN5 slightly reduces the binding of LAP2α across the genome. E, chromatin binding sites are indicated as follows: red, HMGN5-specific binding sites; green, LAP2α-specific binding sites; yellow, binding sites for both HMGN5 and LAP2α; black, chromosomal regions with no HMGN5 or LAP2α binding. F, left panel, genome-wide distribution of HMGN5 before and after LAP2α knockdown. Right panel, genome-wide distribution of LAP2α before and after HMGN5 knockdown; the change of protein bindings is color-coded as indicated in E. The percentage of sites retained or lost upon deletion of either HMGN5 or Lap2α is indicated for each class of binding sites. G, re-ChIP validation of genomic regions associated with both LAP2α and HMGN5. Chromatin was first immunoprecipitated with LAP2α antibody followed by a second round of ChIP with HMGN5 antibody. The protein profile at the genomic location analyzed is indicated. The positions amplified for Q-PCR analysis are indicated by arrows. M indicates molecular mass markers. chr1 and chr2, chromosomes 1 and 2.
Alignment Profiles
Alignment profiles (see Fig. 3, C and D) were constructed as described previously (20). FASTQ files for HeLa cell RNA-seq data were downloaded from the ENCODE project at UCSC. FPKM values were obtained with TopHat (22) and Cufflinks (23).
MNase Digestion Assay
MNase digestion assay was performed using EZ nucleosomal DNA prep kit (Zymo Research) according to the manufacturer's instructions.
RESULTS AND DISCUSSION
Specific Interaction between HMGN5 and LAP2α
To identify potential HMGN5 binding partners, we stably transfected HEK-293T cells with vectors expressing either HMGN5-Halo or unconjugated Halo tag and used HaloLink affinity chromatography of extracts from these cells to search for proteins that specifically bound the HMGN5 protein. Fractionation of the affinity-purified fractions on SDS-PAGE gradient gels revealed two bands that specifically interacted with Halo-HMGN5 (Fig. 1A). Mass spectrometry analysis of the bands identified several putative HMGN5-interacting proteins including the lamin-binding protein LAP2α.
FIGURE 1.
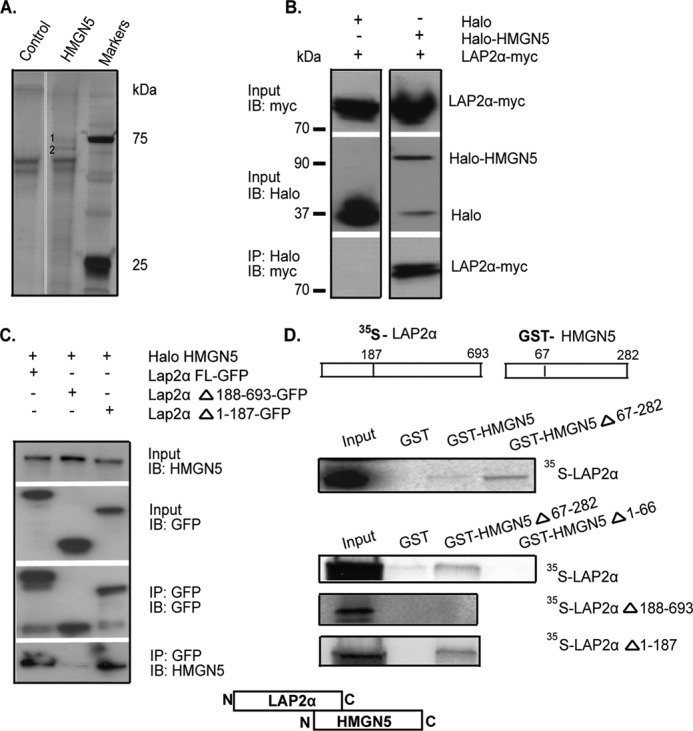
HMGN5 and LAP2α interact in vivo and in vitro. A, silver-stained SDS-PAGE gel of proteins recovered from HaloLink resin. Specific bands are marked as 1 and 2. B, immunoprecipitation of LAP2α-Myc by Halo-HMGN5 from HeLa cell extracts. C, HMGN5 interacts with the C-terminal region of LAP2α. Shown are Western blots (IB) of input and immunoprecipitated proteins from HeLa cells expressing Halo-HMGN5 and LAP2α-GFP constructs. D, GST pulldown assay indicates that the N terminal region of HMGN5 interacts with the C terminus of LAP2α. 35S-labeled LAP2α or its deletion mutants was incubated with purified GST-HMGN5 or its deletion mutants, and the complex was purified on glutathione-Sepharose, fractionated by SDS-PAGE, and visualized by autoradiography. The scheme visualizes that the C-terminal region of LAP2α interacts with the N-terminal region of HMGN5.
Co-immunoprecipitation experiments with extracts from cells co-expressing LAP2α-Myc and either Halo-HMGN5 or unconjugated Halo verified that HMGN5 and Lap2α interact in living cells (Fig. 1B). Furthermore, co-immunoprecipitation with extracts from cells coexpressing Halo-HMGN5 and LAP2α-GFP, its C-terminal deletion mutant LAP2αΔ188-693-GFP, or its N-terminal deletion mutant LAP2αΔ1-187-GFP indicated that HMGN5 interacts with the C-terminal region of LAP2α (Fig. 1C). In vitro co-immunoprecipitation experiments of 35S-labeled LAP2α, or its C-terminal and N-terminal deletion mutants, with purified GST-labeled HMGN5, HMGN5Δ67-282, or HMGN5Δ1-66 revealed that the two proteins interact directly and that this interaction involves the N-terminal region of HMGN5 and the C-terminal region of LAP2α (Fig. 1D).
LAP2α Affects the Nuclear Dynamics of HMGN5
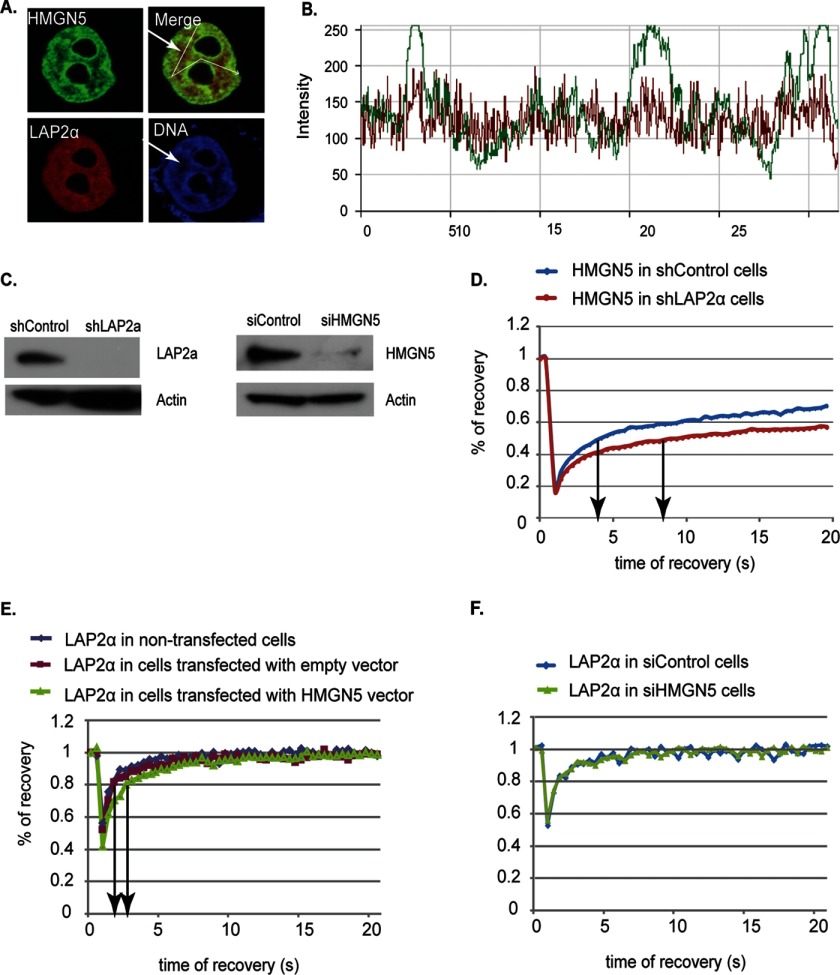
Given that both HMGN5 and LAP2α are nuclear proteins and interact in living cells, we examined their organization in the nuclei of cells expressing both LAP2α-Cherry and HMGN5-YFP. We find that both LAP2α and HMGN5 are distributed throughout the nucleus, but HMGN5 appears to be enriched in heterochromatin, as reported previously (10). The merged images and the corresponding localization profile suggest extensive colocalization throughout the nucleus; however, the relative amounts of HMGN5 to LAP2α are higher in the condensed heterochromatin regions and lower in regions depleted of condensed chromatin, which appear to be enriched in LAP2α (Fig. 2, A and B).
FIGURE 2.
Mobility of HMGN5 and LAP2α in wild type and knock-out cells. A, localization of HMGN5 and LAP2α in HeLa cells. The arrow points to euchromatic regions. B, localization profiles along the paths depicted by the lines drawn in A. C, Western analysis reveals efficient down-regulation of either HMGN5 or LAPα levels in HeLa cells. shControl, control shRNA; shLAP2a, LAPα shRNA. D–F, FRAP recovery curves. The protein analyzed and the types of cells used are indicated on the top of each panel.
The intranuclear organization of many chromatin-binding proteins, including all the members of the HMGN protein family, is highly dynamic; the proteins continuously roam throughout the nucleus and interact transiently with their binding sites, often within a protein network in which the binding of the various members to their specific sites is interdependent (1, 24, 25). Given the extensive colocalization of HMGN5 and LAP2α throughout the nucleus, and in view of our finding that the proteins interact in living cells, we used FRAP to examine whether the proteins mutually affect their intranuclear dynamic properties. To this end, we used HeLa cells depleted of LAP2α (16) or cells depleted of HMGN5 by siRNA treatment. Immunofluorescence analysis indicates that down-regulation of one of the proteins did not affect the intranuclear distribution of the remaining partner (not shown). Western analysis of these cells verified that the level of each protein was significantly down-regulated (Fig. 2C).
We find that down-regulation of LAP2α affected the intranuclear dynamics of HMGN5. The time required to recover 50% of the HMGN5 pre-bleach fluorescence intensity was 4 s in control HeLa cells, but over 8 s in HeLa cells lacking LAP2α, a 2-fold increase in fluorescence recovery time (Fig. 2D), indicating that the mobility of HMGN5 was significantly reduced in the absence of LAP2α. Conceivably, Lap2α may affect the dissociation rate of HMGN5 from chromatin. The fluorescence recovery time of LAP2α is extremely short and was not affected by loss of HMGN5 (Fig. 2F). However, up-regulation of HMGN5 levels increased the time required to reach 80% of the LAP2α pre-bleach fluorescence intensity from 2 s in wild type HeLa cells to 3 s in cells overexpressing HMGN5 (Fig. 2E). The difference is not due to experimental manipulation because transfection with control, empty vector did not affect the FRAP recovery curves of LAP2α. Thus, alteration in the cellular levels of LAP2α affects the intranuclear dynamics of HMGN5, whereas alteration in the levels of HMGN5 has a slight effect on the mobility of LAP2α.
Reciprocal Effects of HMGN5 and LAP2α in Their Genome-wide Distribution
FRAP kinetics provide information on the apparent intranuclear mobility of the fluorescent proteins, which in part is dependent on their residence time on an immobile surface such as chromatin. For HMGN proteins, it has been directly demonstrated that point mutations that abolish the specific binding of HMGN to nucleosomes increase their intranuclear mobility (26). LAP2α interacts with nuclear lamins and with DNA, albeit with a very short residence time, as indicated by our FRAP analysis. Given that both LAP2α and HMGN5 bind to chromatin, and in view of our findings that the proteins interact and mutually affect their intranuclear mobility, we used ChIP-seq (27) analysis to examine directly their genome-wide organization and to test whether the loss of one protein affects the chromatin binding of its partner.
In the chromatin of control HeLa cells, we identified a total of 63,088 HMGN5 binding sites and a total of 22,400 Lap2α binding sites. Of the HMGN5 sites, 7,772 (12%) overlapped with LAP2α sites, whereas 55,316 (88%) did not. Of the LAP2α binding sites, 7,772 (35%) overlapped, whereas 14,628 (65%) did not, with HMGN5 sites (Fig. 3E). ChIP/Re-ChIP analysis of three different loci verified the concurrent occupancy by both LAP2α and HMGN5 at specific sites (Fig. 3G). Genome-wide, 23% of the overlapping sites, 16% of the HMGN5-specific sites, and 6% of the LAP2α-specific sites localized to proximal promoters with no difference between active and inactive promoters. In the overlapping sites, genomic sites where LAP2α binds strongly also demonstrate strong binding of HMGN5, with a stronger correspondence at peaks in promoter regions, regardless of expression of the corresponding gene (Fig. 3, A and B).
Down-regulation of LAP2α decreased the binding of HMGN5 (Fig. 3C), whereas loss of HMGN5 only slightly reduced the binding of LAP2α (Fig. 3D). The reciprocal effects of HMGN5 and LAP2α on their genome-wide distribution are summarized in Fig. 3, E and F. Down-regulation of either HMGN5 or LAP2α levels led to significant alterations in the interaction of both HMGN5 and LAP2α with chromatin (Fig. 3F). Down-regulation of LAP2α led to loss of HMGN5 from 77% of the HMGN5-specific sites (class IV in Fig. 3F) and from 58% of the HMGN5 sites that overlapped with LAP2α (class II). In addition, loss of LAP2α created 15,583 new HMGN5 binding sites, and of these, 14,008 sites were novel, i.e. not previously bound by either HMGN5 or LAP2α, and 1,575 sites were loci that previously contained only LAP2α (Class V and class VI sites in Fig. 3F, left).
Likewise, down-regulation of HMGN5 led to significant changes in the genome-wide organization of LAP2α. Down-regulation of HMGN5 resulted in the loss of 90% of sites occupied only by LAP2α and 86% of sites commonly occupied by both proteins, as well as the creation of 15,483 new LAP2α binding sites (Class V and VI sites, Fig. 3F, right). The total number of LAP2α binding sites in cells lacking HMGN5 was 18,610 i.e. 83% of the number of sites present in wild type cells. Thus, loss of HMGN5 changed mostly the location, rather than the number, of LAP2α binding sites.
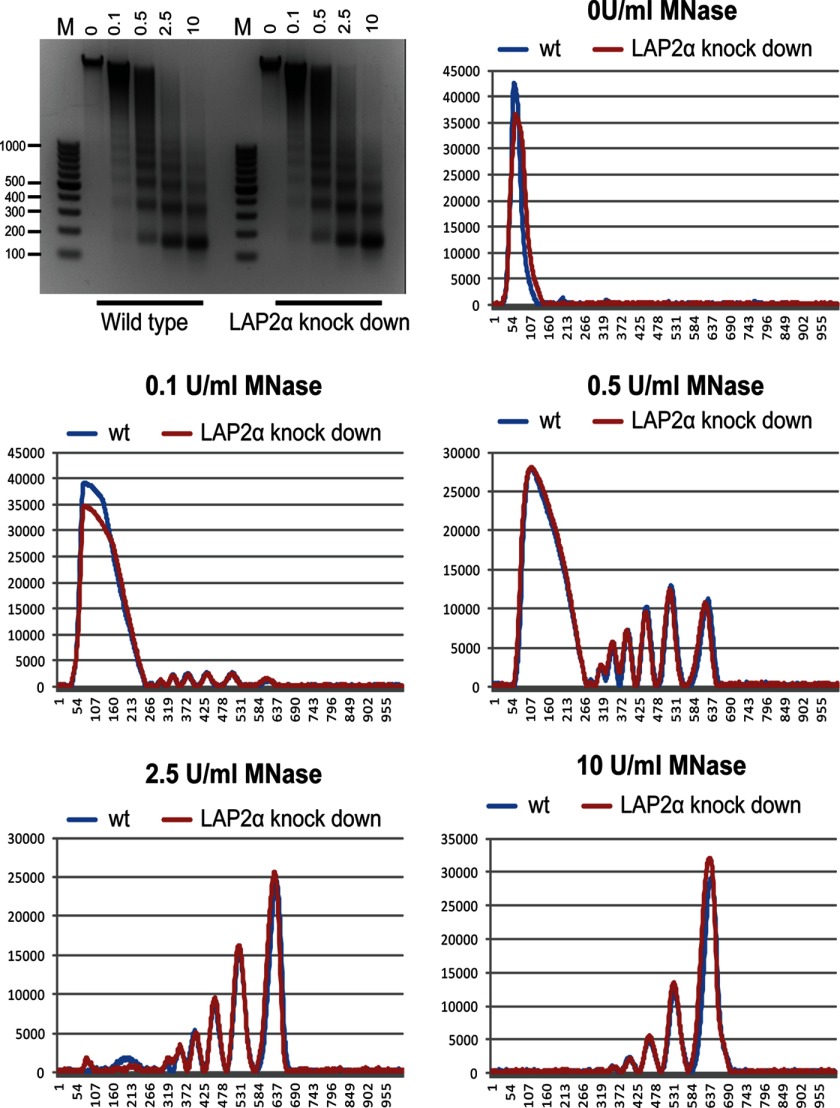
Previous studies indicated that human HMGN5 affects chromatin structure (10). Given the effect of LAP2α on the FRAP kinetics and the genome-wide organization of HMGN5, we tested whether loss of LAP2α induces major changes in chromatin organization. Comparison of the micrococcal nuclease digestion patterns of nuclei isolated from wild type and LAP2α-deficient HeLa cells revealed that neither the kinetics of digestion nor the nucleosomal repeat are affected by loss of LAP2α (Fig. 4). Thus, the altered organization of HMGN5 in LAP2α-deficient cells is not due to major changes in the nucleosomal organization of the chromatin fiber. Nevertheless, previous studies (9, 14) demonstrated that loss of just one LEM protein or HMGN variant can affect chromatin function. Our studies indicate that loss of either HMGN5 or LAP2α protein affects the dynamic binding of its protein partner to chromatin.
FIGURE 4.
Loss of LAP2α does not change overall chromatin structure. Shown is a gel depicting the kinetics of micrococcal nuclease digestion in wild type and LAP2α-deficient HeLa cells. 1.5 × 106 nuclei were treated with 0.01–1 units of micrococcal nuclease in 100 μl of digestion buffer for 10 min at room temperature. The purified nucleosomal DNA was fractionated on 2% agarose gel and quantified with ImageQuant software. The scans of corresponding lanes from the two different digests indicate that neither the kinetics of digestion nor the nucleosomal repeat are affected by loss of LAP2α. M indicates molecular mass markers.
Increasing evidence indicates that interactions between the nuclear lamin network and chromatin play an important role in nuclear architecture and gene expression. The results presented here reveal an additional link between the chromatin fiber and the lamin network formed by the interaction between a nucleosome-binding protein and a lamin-binding protein.
This work was supported, in whole or in part, by the Center for Cancer Research, Intramural Program of the NCI, National Institutes of Health (to M. B.). This work was also supported by United States-Israel Binational Science Foundation (BSF) Grants 2009236 (to M. B.) and NCI Grant K22 HL101950 (to D. E. S.) and by Grant P22043-B12 of the Austrian Research Fund (FWF) (to R. F.).
- HMG
- high mobility group
- HMGN
- high mobility group protein N
- LAP2α
- lamina-associated polypeptide 2α
- FRAP
- fluorescence recovery after photobleaching
- FPKM
- fragments per kilobase of transcript per million mapped reads
- ChIP-seq
- ChIP sequencing
- RNA-seq
- RNA sequencing.
REFERENCES
- 1. Bustin M., Catez F., Lim J. H. (2005) The dynamics of histone H1 function in chromatin. Mol. Cell 17, 617–620 [DOI] [PubMed] [Google Scholar]
- 2. Woodcock C. L., Skoultchi A. I., Fan Y. (2006) Role of linker histone in chromatin structure and function: H1 stoichiometry and nucleosome repeat length. Chrom. Res. 14, 17–25 [DOI] [PubMed] [Google Scholar]
- 3. Hock R., Furusawa T., Ueda T., Bustin M. (2007) HMG chromosomal proteins in development and disease. Trends Cell Biol. 17, 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianchi M. E., Agresti A. (2005) HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15, 496–506 [DOI] [PubMed] [Google Scholar]
- 5. Reeves R. (2010) Nuclear functions of the HMG proteins. Biochim. Biophys. Acta 1799, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kato H., van Ingen H., Zhou B. R., Feng H., Bustin M., Kay L. E., Bai Y. (2011) Architecture of the high mobility group nucleosomal protein 2-nucleosome complex as revealed by methyl-based NMR. Proc. Natl. Acad. Sci. U.S.A. 108, 12283–12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuddapah S., Schones D. E., Cui K., Roh T. Y., Barski A., Wei G., Rochman M., Bustin M., Zhao K. (2011) Genomic profiling of HMGN1 reveals an association with chromatin at regulatory regions. Mol. Cell. Biol. 31, 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim J. H., Bustin M., Ogryzko V. V., Postnikov Y. V. (2002) Metastable macromolecular complexes containing high mobility group nucleosome-binding chromosomal proteins in HeLa nuclei. J. Biol. Chem. 277, 20774–20782 [DOI] [PubMed] [Google Scholar]
- 9. Postnikov Y., Bustin M. (2010) Regulation of chromatin structure and function by HMGN proteins. Biochim. Biophys. Acta 1799, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malicet C., Rochman M., Postnikov Y., Bustin M. (2011) Distinct properties of human HMGN5 reveal a rapidly evolving but functionally conserved nucleosome binding protein. Mol. Cell. Biol. 31, 2742–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dechat T., Korbei B., Vaughan O. A., Vlcek S., Hutchison C. J., Foisner R. (2000) Lamina-associated polypeptide 2α binds intranuclear A-type lamins. J. Cell Sci. 113, 3473–3484 [DOI] [PubMed] [Google Scholar]
- 12. Naetar N., Korbei B., Kozlov S., Kerenyi M. A., Dorner D., Kral R., Gotic I., Fuchs P., Cohen T. V., Bittner R., Stewart C. L., Foisner R. (2008) Loss of nucleoplasmic LAP2α-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat. Cell Biol. 10, 1341–1348 [DOI] [PubMed] [Google Scholar]
- 13. Wilson K. L., Foisner R. (2010) Lamin-binding Proteins. Cold Spring Harb. Perspect. Biol. 2, a000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dechat T., Adam S. A., Goldman R. D. (2009) Nuclear lamins and chromatin: when structure meets function. Adv. Enzyme Regul. 49, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dechat T., Gesson K., Foisner R. (2010) Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb. Symp. Quant. Biol. 75, 533–543 [DOI] [PubMed] [Google Scholar]
- 16. Dorner D., Vlcek S., Foeger N., Gajewski A., Makolm C., Gotzmann J., Hutchison C. J., Foisner R. (2006) Lamina-associated polypeptide 2α regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J. Cell Biol. 173, 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rochman M., Postnikov Y., Correll S., Malicet C., Wincovitch S., Karpova T. S., McNally J. G., Wu X., Bubunenko N. A., Grigoryev S., Bustin M. (2009) The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Mol. Cell 35, 642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langmead B., Trapnell C., Pop M., Salzberg S. L. (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kent W. J., Sugnet C. W., Furey T. S., Roskin K. M., Pringle T. H., Zahler A. M., Haussler D. (2002) The human genome browser at UCSC. Genome Res. 12, 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schones D. E., Zhao K. (2008) Genome-wide approaches to studying chromatin modifications. Nature reviews. Genetics 9, 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zang C., Schones D. E., Zeng C., Cui K., Zhao K., Peng W. (2009) A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25, 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trapnell C., Pachter L., Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., Salzberg S. L., Wold B. J., Pachter L. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catez F., Brown D. T., Misteli T., Bustin M. (2002) Competition between histone H1 and HMGN proteins for chromatin binding sites. EMBO Rep. 3, 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phair R. D., Scaffidi P., Elbi C., Vecerová J., Dey A., Ozato K., Brown D. T., Hager G., Bustin M., Misteli T. (2004) Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 24, 6393–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueda T., Catez F., Gerlitz G., Bustin M. (2008) Delineation of the protein module that anchors HMGN proteins to nucleosomes in the chromatin of living cells. Mol. Cell. Biol. 28, 2872–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]