Background: Pyk2 is abundantly expressed in platelets.
Results: Pyk2 regulates thromboxane A2 generation induced by both 2-methylthio-ADP and AYPGKF.
Conclusion: Pyk2 is an important functional tyrosine kinase that is activated by both G12/13 and integrin αIIbβ3 in platelets.
Significance: Understanding the mechanism of activation of Pyk2 enhances our understanding of the platelet inside-out and outside-in signaling events.
Keywords: ADP, Integrin, Platelets, Signal Transduction, Thrombin, G12/13, Pyk2, Thromboxane
Abstract
Proline-rich tyrosine kinase 2 (Pyk2) is activated by various agonists in platelets. We evaluated the signaling mechanism and the functional role of Pyk2 in platelets by using pharmacological inhibitors and Pyk2-deficient platelets. We found that platelet aggregation and secretion in response to 2-methylthio-ADP (2-MeSADP) and AYPGKF were diminished in the presence of Pyk2 inhibitors or in Pyk2-deficient platelets, suggesting that Pyk2 plays a positive regulatory role in platelet functional responses. It has been shown that ADP-, but not thrombin-induced thromboxane (TxA2) generation depends on integrin signaling. Unlike ADP, thrombin activates G12/13 pathways, and G12/13 pathways can substitute for integrin signaling for TxA2 generation. We found that Pyk2 was activated downstream of both G12/13 and integrin-mediated pathways, and both 2-MeSADP- and AYPGKF-induced TxA2 generation was significantly diminished in Pyk2-deficient platelets. In addition, TxA2 generation induced by co-stimulation of Gi and Gz pathways, which is dependent on integrin signaling, was inhibited by blocking Pyk2. Furthermore, inhibition of 2-MeSADP-induced TxA2 generation by fibrinogen receptor antagonist was not rescued by co-stimulation of G12/13 pathways in the presence of Pyk2 inhibitor. We conclude that Pyk2 is a common signaling effector downstream of both G12/13 and integrin αIIbβ3 signaling, which contributes to thromboxane generation.
Introduction
Platelet activation plays an important role in hemostasis and thrombosis (1). When platelets are stimulated with agonists, platelets change their shape, aggregate, release their granule contents, and generate thromboxane A2 (TxA2),2 leading to the activation of platelets. ADP induces platelet activation by signaling through Gq-coupled P2Y1 and Gi-coupled P2Y12 receptors. Unlike ADP, a number of receptors can couple to G12/13 including thrombin and TxA2, and platelet shape change induced by these agonists is mediated by both calcium-dependent and -independent mechanisms that occurs through Gq and G12/13 pathways, respectively (2). G12/13 has been shown to regulate the Rho-dependent response (3), and the RhoA-p160ROCK pathway plays an important role in G12/13-mediated platelet shape change (4). It has also been shown that G12/13 pathways regulate dense granule secretion through RhoA-p160ROCK pathways (5) and contribute to the tyrosine phosphorylation of PKCδ on the Tyr-311 residue (6) that regulates thromboxane generation (7). Recent studies have shown that G12/13 pathways could be activated directly through integrin outside-in signaling events (8).
The non-receptor, proline-rich protein tyrosine kinase Pyk2 is a member of focal adhesion protein tyrosine kinase (FAK) family. Both Pyk2 and FAK have a molecular mass between 110 and 125 kDa and are closely related in their overall structure. These two kinases lack SH2 and SH3 domains but, in their C-terminal region, possess two proline-rich domains (9). Pyk2 contains an N-terminal FERM domain, a central kinase domain, three proline-rich motifs, and a C-terminal focal adhesion targeting domain. Tyr-402 phosphorylation serves as a binding site for Src with subsequent phosphorylation of activation loop residues Tyr-579, Tyr-580, and Tyr-881 in the focal adhesion targeting domain, which promotes binding of the adapter protein Grb2 (10). Recently, Pyk2 knock-out mice have been generated and the role of Pyk2 in macrophages (11) and osteoclasts (12) have been studied.
Pyk2 is abundantly expressed in both megakaryocytes and platelets, in addition to brain tissues and epithelial cells (13, 14). Treatment of platelets with several agonists induced the tyrosine phosphorylation of Pyk2 through integrin-dependent and integrin-independent mechanisms (15). They have also shown that Pyk2 tyrosine phosphorylation is regulated by calcium and is mediated through PKC pathways. However, it has been also reported that PKC activation, but not calcium mobilization, is involved in Pyk2 phosphorylation in thrombin-activated platelets (16). Stimulation of human platelets with von Willebrand factor also induces the rapid phosphorylation of Pyk2, which is not affected by either calcium chelation or PKC inhibition (17). In addition, Pyk2 phosphorylation has been shown to be mostly dependent on integrin αIIbβ3 and PKC in human platelets (18). Moreover, PI 3-kinase activity has been shown to be involved in Pyk2 tyrosine phosphorylation in low-dose thrombin-stimulated platelets (19). In contrast, it has been shown that the Pyk2 activity is not affected by blocking PI 3-kinase (20). Thus, the signaling mechanism of Pyk2 activation in platelets is complex and controversial and the functional role of Pyk2 in platelet activation has not been fully understood.
TxA2 is generated from its precursor arachidonic acid through cycloxygenase pathways, acts as a positive feedback mediator, and amplifies the initial platelet responses and stabilizes the hemostatic plug. Previous study has shown that ADP-induced TxA2 generation depends on outside-in signaling (21). Interestingly, it has been shown that PAR-mediated TxA2 generation occurs independently of integrin αIIbβ3-mediated outside-in signaling, and G12/13 pathways can rescue the inhibitory effect of fibrinogen receptor antagonist to induce TxA2 generation (21). These studies have raised the possibility that G12/13 pathways substitute for integrin-mediated signaling by activating similar effector molecule, but the common signaling molecule downstream of integrins and G12/13 pathways responsible for this event has not been identified.
In the present study, we demonstrate that Pyk2 is activated downstream of both G12/13 and integrin pathways, and both 2-MeSADP- and AYPGKF-induced TxA2 generation is inhibited in Pyk2-deficient platelets. We show that G12/13 pathways fail to substitute for the integrin-mediated outside-in signaling for TxA2 generation when Pyk2 is blocked. Moreover, integrin-dependent TxA2 generation induced by combined Gi and Gz stimulation is abolished by blocking Pyk2. Therefore, we conclude that Pyk2 is a common signaling effector downstream of both G12/13 and integrin αIIbβ3 signaling pathways, which plays a crucial role in thromboxane generation in platelets.
EXPERIMENTAL PROCEDURES
Materials
2-MeSADP, acetylsalicylic acid, apyrase (type VII), epinephrine, MRS-2179, sodium citrate, and bovine serum albumin (fraction V) were purchased from Sigma. YFLLRNP and AYPGKF were custom synthesized by Invitrogen. Anti-phospho-Pyk2 (Tyr-402), anti-phospho-Akt (Ser-473), anti-phospho-Src (Tyr-416), and anti-β-Actin antibodies were purchased from Cell Signaling Technology. Anti-phospho-Pyk2 (Tyr-881) antibody was from Invitrogen. Horseradish peroxidase (HRP)-labeled secondary antibody was from Santa Cruz Biotechnology. 3,5-Di-t-butyl-4-hydroxybenzylidenemalononitrile (AG17) was from EMD Millipore. SC57101 was a gift from Searle Research and Development (Skokie, IL). TAT-Pyk2-CT and TAT-GFP control were from Xiangdong Zhu, University of Chicago. YM254890 was a gift from Yamanouchi Pharmaceutical (Ibaraki, Japan). All other reagents were reagent grade, and deionized water was used throughout.
Animals
Pyk2-deficient mice were obtained from Mitsuhiko Okigaki (Kyoto Prefectural University of Medicine, Kyoto, Japan).
Preparation of Human and Mouse Platelets
Human blood was obtained from a pool of healthy volunteers in a one-sixth volume of acid/citrate/dextrose. Platelet-rich plasma was prepared by centrifugation at 230 × g for 20 min at room temperature (RT). Acetylsalicylic acid was added to platelet-rich plasma to a final concentration of 1 mm, and the preparation was incubated for 45 min at 37 °C followed by centrifugation at 980 × g for 10 min at RT. In the experiments with TxB2 measurements, the treatment of platelet-rich plasma with acetylsalicylic acid was omitted.
Mouse blood was collected from anesthetized mice into syringes containing 1/10th blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100 × g for 10 min at RT. Platelet-rich plasma was recovered, and platelets were pelleted at 400 × g for 10 min. The platelet pellet was resuspended in Tyrode's buffer (pH 7.4) containing 0.05 units/ml of apyrase to a density of 2 × 108 cells/ml.
Platelet Aggregation and Secretion
Platelet aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37 °C under stirring conditions. A 0.5-ml sample of washed platelets was stimulated with different agonists, and change in light transmission was measured.
Platelet secretion was determined by measuring the release of ATP by adding luciferin-luciferase reagent. Platelet ATP release and aggregation were performed in a lumi-aggregometer at 37 °C simultaneously.
Western Blotting
Platelets were stimulated with agonists for the appropriate time, and phosphorylation events were measured as previously described (22). For outside-in signaling, washed human platelets were plated on fibrinogen-coated coverslips for 45 min at 37 °C in a CO2 incubator, and adherent cells were harvested for immunoblot analysis as described previously (23). In some experiments, platelets were stimulated in the presence of SC57101 (10 μm) to eliminate outside-in signaling.
Measurement of Thromboxane A2 Generation
Washed platelets without aspirin treatment were prepared at a concentration of 2 × 108 platelets/ml. Stimulations were performed for 3.5 min and the reaction was stopped by snap freezing. Levels of TxB2 were determined in duplicate using a Correlate-EIA thromboxane B2 enzyme immunoassay kit (Assay Designs, Inc., Ann Arbor, MI), according to the manufacturer's instructions.
Statistical Analysis
All statistical tests were carried out using Prism software (version 3.0). Data are presented as mean ± S.E. Statistical significance was determined by Student's t test and analysis of variance. p < 0.05 was considered statistically significant.
RESULTS
Time- and Concentration-dependent Phosphorylation of Pyk2 in Platelets
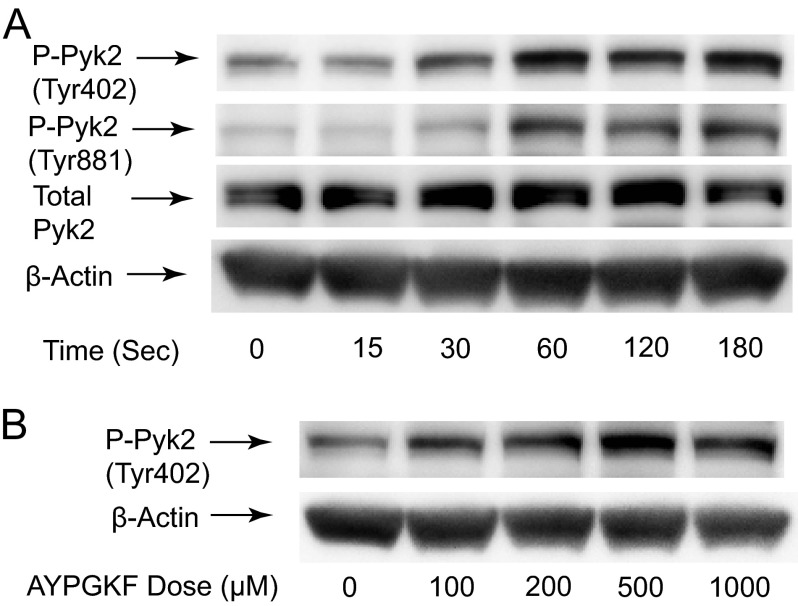
It has been shown that treatment of platelets with various agonists including thrombin induces phosphorylation of Pyk2 in platelets. To determine the kinetics of Pyk2 phosphorylation, Tyr-402 and Tyr-881 phosphorylation in response to PAR4-activating peptide AYPGKF were monitored over a time range of 0.5–2 min. Fig. 1A shows a time-dependent increase in Pyk2 phosphorylation in which a rapid increase in Pyk2 phosphorylation in response to AYPGKF was detectable as early as 30 s after stimulation. We also exposed platelets to different concentrations of AYPGKF, and Tyr-402 phosphorylation was measured at 2 min after the addition of agonist. Fig. 1B shows a concentration-dependent increase in Pyk2 phosphorylation. An increase in Tyr402 phosphorylation was detectable at concentrations above 100 μm AYPGKF, and higher concentrations induced further phosphorylation that peaked at concentrations above 500 μm AYPGKF. A similar pattern of time- and concentration-dependent phosphorylation of Tyr-402 in response to 2-MeSADP, SFLLRN, and thrombin was also detected (data not shown).
FIGURE 1.
Time- and dose-dependent phosphorylation of Pyk2 in response to AYPGKF. A, washed human platelets were stimulated at 37 °C for the time points indicated with AYPGKF (500 μm). B, washed platelets were stimulated with different concentrations of AYPGKF for 2 min at 37 °C. The reaction was stopped by the addition of 3× SDS sample buffer. Samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-Pyk2 (Tyr-402 or Tyr-881), anti-Pyk2, or anti-β-actin (lane loading controls) antibodies. The data shown are representative of three experiments.
Characterization of the Activation of Pyk2 Downstream of G12/13 and Integrin αIIbβ3-dependent Outside-in Pathways in Platelets
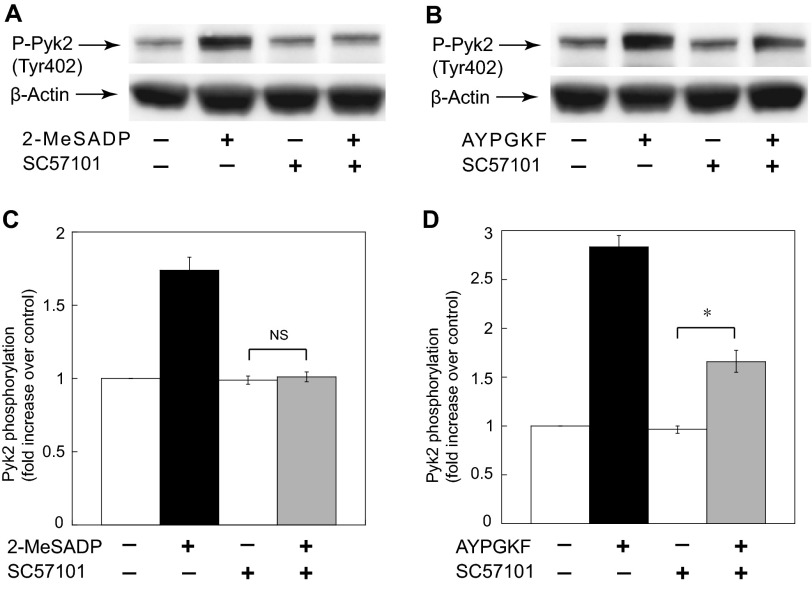
Previous studies have shown that Pyk2 is regulated by various cell type-dependent mechanisms. To investigate the signaling mechanism of Pyk2 activation in platelets, we first evaluated the role of integrin αIIbβ3 in Pyk2 activation. As shown in Fig. 2A, Pyk2 was phosphorylated downstream of ADP receptors, which was completely blocked by fibrinogen receptor antagonist SC-57101. Similarly, 2-MeSADP failed to induce Pyk2 phosphorylation under non-stirring conditions (data not shown). ADP stimulates both Gq and Gi pathways to induce fibrinogen receptor activation. Thus, these results suggest that Pyk2 phosphorylation by ADP occurs in an integrin-dependent manner, and either Gq or Gi pathways cannot directly activate Pyk2. Interestingly, AYPGKF-induced Pyk2 phosphorylation was significantly but not completely inhibited in the presence of SC-57101 (Fig. 2B), indicating that AYPGKF-induced Pyk2 phosphorylation occurs through both integrin-dependent and -independent pathways. Unlike ADP, it has been shown that PAR agonists can stimulate G12/13 pathways. Thus, it has raised the possibility that G12/13 pathways can induce Pyk2 phosphorylation in the presence of SC57101 through the integrin-independent pathways.
FIGURE 2.
The effect of integrin αIIbβ3 inhibition on Pyk2 phosphorylation induced by 2-MeSADP and AYPGKF. Platelets were stimulated with 100 nm 2-MeSADP (A) or 500 μm AYPGKF (B) at 37 °C for 2 min in the presence and absence of 10 μm SC57101. Equal amounts of proteins were separated by SDS-PAGE, Western blotted, and probed for anti-phospho-Pyk2 (Tyr-402) or anti-β-actin (lane loading control) antibodies. The blot shown is representative of three independent experiments. C and D, densitometric measurement of phospho-Pyk2, expressed as fold-increase over control. Data are mean ± S.E. (n = 3). *, p < 0.05.
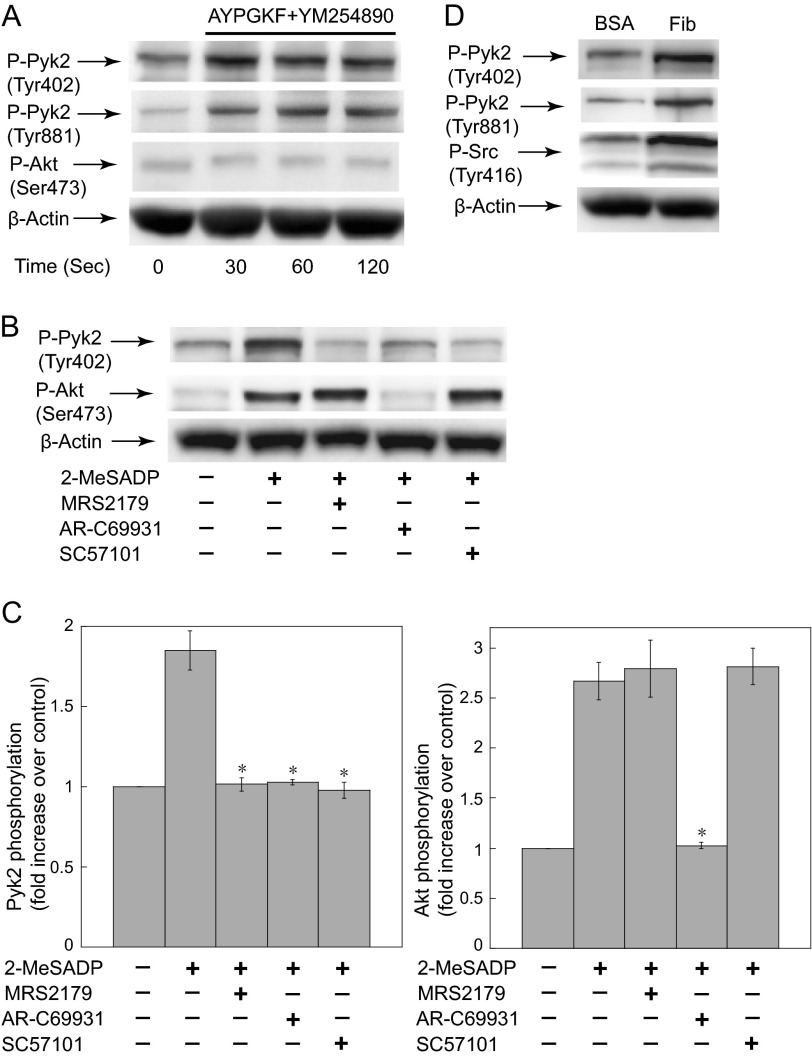
To confirm the contribution of G12/13 and integrin αIIbβ3-mediated outside-in signaling to Pyk2 phosphorylation, we have investigated whether selective activation of G12/13 pathways and integrin αIIbβ3-mediated outside-in signaling can activate Pyk2. We have previously shown that YM-254890 selectively inhibits Gq signaling in platelets, in which AYPGKF caused G12/13-induced platelet shape change in the presence of YM-254890 that was further abolished by the addition of Rho kinase inhibitor Y-27632 (22). Selective activation of G12/13 pathways by AYPGKF in the presence of Gq selective inhibitor YM254890 resulted in Pyk2 phosphorylation (Fig. 3A). It appears that G12/13-mediated Pyk2 phosphorylation plateaus faster in the absence of integrin-mediated signaling because Pyk2 phosphorylation at later time points is mainly mediated by integrin-mediated signaling. Akt phosphorylation was measured to verify the selective activation of G12/13 pathways because we have shown that G12/13 pathways alone cannot induce Akt phosphorylation (22). Selective activation of Gq pathways (2-MeSADP + AR-C69931MX) or Gi pathways (2-MeSADP + MRS2179) failed to induce Pyk2 phosphorylation (Fig. 3, B and C), confirming that Gq and Gi alone cannot cause Pyk2 activation. We have measured Akt phosphorylation to verify the selective blockade of Gq, Gi, or integrin pathways because we and others have shown that ADP-induced Akt phosphorylation is only dependent on Gi pathways (24, 25). In addition, platelet adhesion to immobilized fibrinogen resulted in an increase in the phosphorylation of Pyk2 (Fig. 3D), confirming the role of integrin αIIbβ3-mediated signaling in Pyk2 phosphorylation. Platelet adhesion to fibrinogen also caused an increase in the phosphorylation of Src Tyr-416, which has been identified as a prominent signaling complex downstream of integrin αIIbβ3 (23). Thus, these results confirm that Pyk2 is activated by both G12/13 pathways and integrin αIIbβ3-mediated outside-in signaling in platelets.
FIGURE 3.
Activation of Pyk2 downstream of G12/13 and outside-in signaling. A, washed human platelets were stimulated in the presence of 100 nm YM254890 with 500 μm AYPGKF for various time points and probed with anti-phospho-Pyk2 (Tyr-402 and Tyr-881), anti-phospho-Akt (Ser-473), or anti-β-actin (lane loading control) antibodies by Western blotting. B, platelets were stimulated with 100 nm 2-MeSADP for 2 min in the presence and absence of 100 μm MRS2179, 100 nm AR-C69931MX, or 10 μm SC57101 and probed with anti-phospho-Pyk2 (Tyr-402), anti-phospho-Akt (Ser-473), or anti-β-actin (lane loading control) antibodies by Western blotting. C, densitometric measurement of phospho-Pyk2 and phospho-Akt, expressed as fold-increase over control. Data are mean ± S.E. (n = 3). *, p < 0.005 compared with agonist. D, lysates from non-adherent (BSA) and fibrinogen-adherent (Fib) platelets were probed with anti-phospho-Pyk2 (Tyr-402 and Tyr-881), anti-phospho-Src (Tyr-416), or anti-β-actin (lane loading control) antibodies. The blot shown is representative of three independent experiments.
Effect of Pyk2 Inhibition on Human Platelet Aggregation and Secretion
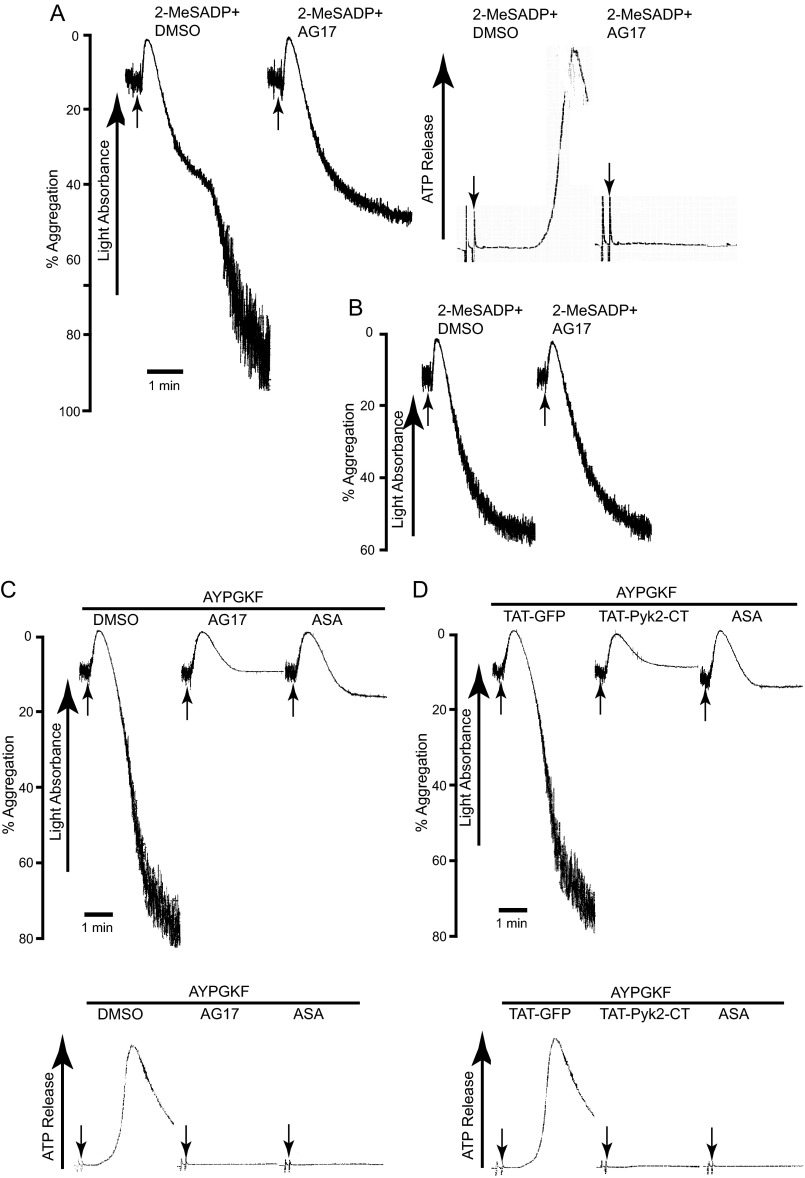
To determine the functional role of Pyk2 in platelets, we first examined the effect of the Pyk2 selective inhibitor AG17 on 2-MeSADP-induced aggregation and dense granule secretion. As shown in Fig. 4A, 2-MeSADP-induced secondary aggregation and dense granule secretion in washed non-aspirin-treated human platelets were inhibited in the presence of AG17. Thromboxane production in response to ADP in non-aspirin-treated platelets results in dense granule secretion and subsequent secondary aggregation. Thus, it raised the possibility that Pyk2 plays a role in ADP-induced platelet responses through the regulation of TxA2 generation. To rule out the secondary effects of TxA2 on 2-MeSADP-induced aggregation, we evaluated the effect of AG17 on aspirin-treated platelets. There was no significant difference in 2-MeSADP-induced platelet aggregation in the presence and absence of AG17 (Fig. 4B), indicating the role of Pyk2 in regulating TxA2 generation.
FIGURE 4.
The effect of Pyk2 inhibition on agonist-induced platelet aggregation and secretion. A, non-aspirin-treated and B, aspirin-treated washed human platelets were pre-treated with Pyk2 inhibitor AG17 (1 μm) at 37 °C for 5 min following stimulation with 50 nm 2-MeSADP under stirring conditions. Non-aspirin-treated and aspirin-treated (ASA) washed human platelets were preincubated with Pyk2 inhibitors (C) AG17 (1 μm) or (D) TAT-Pyk2-CT (2 μm) or TAT-GFP (control) at 37 °C for 3.5 min and stimulated with 60 μm AYPGKF. Platelet aggregation and ATP secretion were measured by aggregometry. Arrow indicates when agonist is added. Tracings are representative of experiments performed using platelets from at least three different donors.
We also observed that platelet aggregation and dense granule release induced by AYPGKF were inhibited in the presence of the Pyk2 inhibitor AG17 (Fig. 4C). A highly selective TAT-mediated protein transduction of dominant-negative C-terminal Pyk2 (TAT-Pyk2-CT), a fusion protein in which TAT peptide was fused to the C-terminal Pyk2 (amino acid residues 680–1009), has been recently developed to block the activation of Pyk2 (26). Consistent with the result in Fig. 4C, AYPGKF-induced platelet aggregation and secretion were inhibited in the presence of TAT-Pyk2-CT, whereas TAT-GFP control had no effect (Fig. 4D). We also compared the effect of Pyk2 inhibition and aspirin treatment in response to AYPGKF, and aspirin treatment had a similar inhibitory effect on AYPGKF-induced platelet aggregation and secretion compared with AG17, confirming that Pyk2 inhibits platelet aggregation through the regulation of TxA2 generation. These agonist-induced platelet aggregation and secretions were diminished upon blockade of Pyk2 over a wide range of agonist concentrations. Platelet response was more significantly diminished at lower concentrations of agonist, and differences became minor at higher doses of agonist. These results show that inhibition of Pyk2 in platelets was found to be defective in their function ex vivo, strongly indicating that Pyk2 plays an important role in regulation of platelet function.
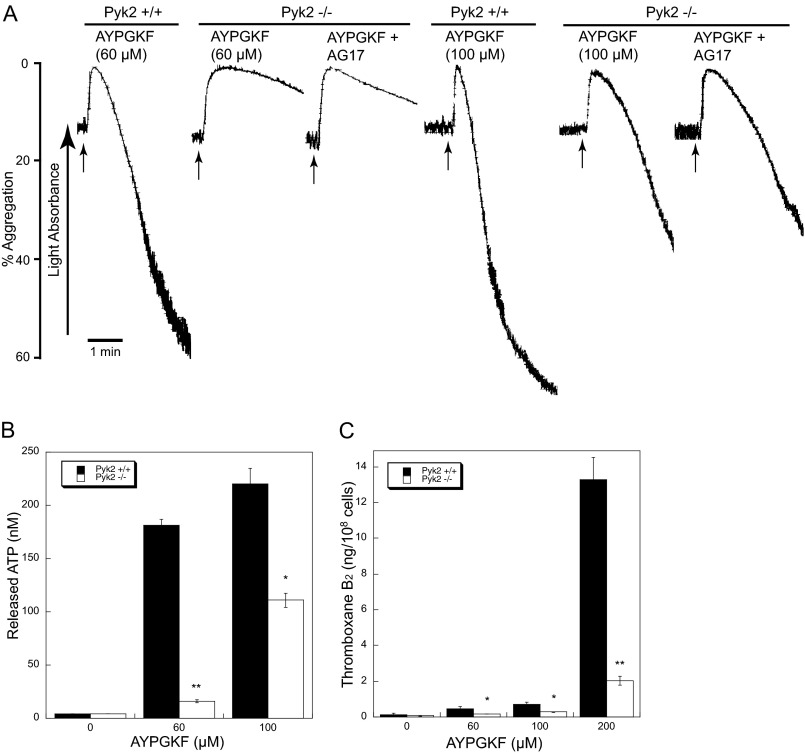
Role of Pyk2 in AYPGKF-induced Platelet Aggregation, Secretion, and TxA2 Generation in Pyk2-deficient Platelets
As pharmacological inhibitors are known to have off-target and broad-spectrum effects, we have examined platelets from Pyk2−/− mice to ascertain the role of Pyk2 in platelet function. Consistent with the results obtained with Pyk2 inhibitors, platelet aggregation (Fig. 5A) and ATP secretion (Fig. 5B) were diminished in Pyk2-deficient platelets compared with the wild type platelets. In addition, the Pyk2 inhibitor AG17 did not show an additional inhibitory effect on AYPGKF-induced platelet aggregation in Pyk2-deficient platelets, indicating that the effects of AG17 are likely mediated by Pyk2. Similarly, the concentration-response curves for AYPGKF-induced TxA2 generation were shifted to the right in Pyk2-deficient platelets as the level of TxB2 generation in Pyk2-deficient platelets in response to AYPGKF was significantly decreased compared with WT platelets (Fig. 5C), suggesting that Pyk2 positively regulates TxA2 generation. Because TxA2 has a short half-life and is rapidly converted to stable product TxB2, TxB2 was measured as TxA2 by ELISA. It has been shown that PAR agonists cause TxA2 generation independently of integrin signaling, and we have observed that Pyk2 is activated by both G12/13 pathways and integrin signaling (Fig. 3). Thus, our results have raised the possibility that Pyk2 is the common signaling molecule downstream of both G12/13 pathways and integrin signaling, which plays an essential role in AYPGKF-induced TxA2 generation.
FIGURE 5.
AYPGKF-induced platelet aggregation, secretion, and TxA2 generation in Pyk2-deficient platelets. Non-aspirin-treated washed platelets from Pyk2−/− mice and Pyk2+/+ littermates were stimulated with different concentrations of AYPGKF at 37 °C for 3.5 min, and platelet aggregation (A), ATP secretion (B), and TxA2 generation (C) were measured as described under “Experimental Procedures.” The arrow indicates when agonist is added. In some experiments, platelets were preincubated with 1 μm AG17 prior to platelet stimulation as noted. All data shown are representative of three independent experiments. Data are mean ± S.E. (n = 3). *, p < 0.05; **, p < 0.005 compared with wild-type.
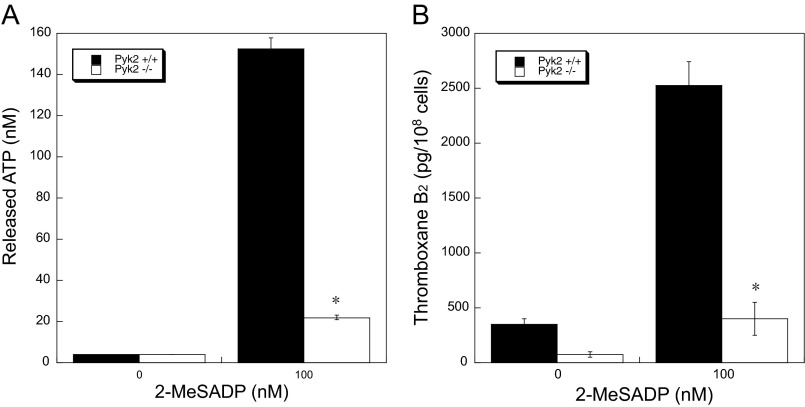
Role of Pyk2 in 2-MeSADP-induced Secretion and TxA2 Generation in Pyk2-deficient Platelets
It has been shown that ADP-induced TxA2 generation and subsequent secretion is dependent on integrin activation. To confirm the role of Pyk2 downstream of integrins, we next measured TxA2 generation and ATP secretion in response to 2-MeSADP in Pyk2-deficient platelets. Consistent with the result in Fig. 4A, 2-MeSADP-induced ATP secretion (Fig. 6A) and TxA2 generation (Fig. 6B) were completely inhibited in Pyk2-deficient platelets, confirming the contribution of Pyk2 to TxA2 generation downstream of integrins.
FIGURE 6.
2-MeSADP-induced secretion and TxA2 generation in Pyk2-deficient platelets. Non-aspirin-treated washed platelets from Pyk2−/− mice and Pyk2+/+ littermates were stimulated with 100 nm 2-MeSADP for 3.5 min, and ATP secretion (A) and TxA2 generation (B) were measured. The values are representative of three independent experiments. Data are mean ± S.E. (n = 3). *, p < 0.005.
Effect of Pyk2 on Regulation of TxA2 Generation Downstream of Integrin-dependent Outside-in and G12/13 Pathways
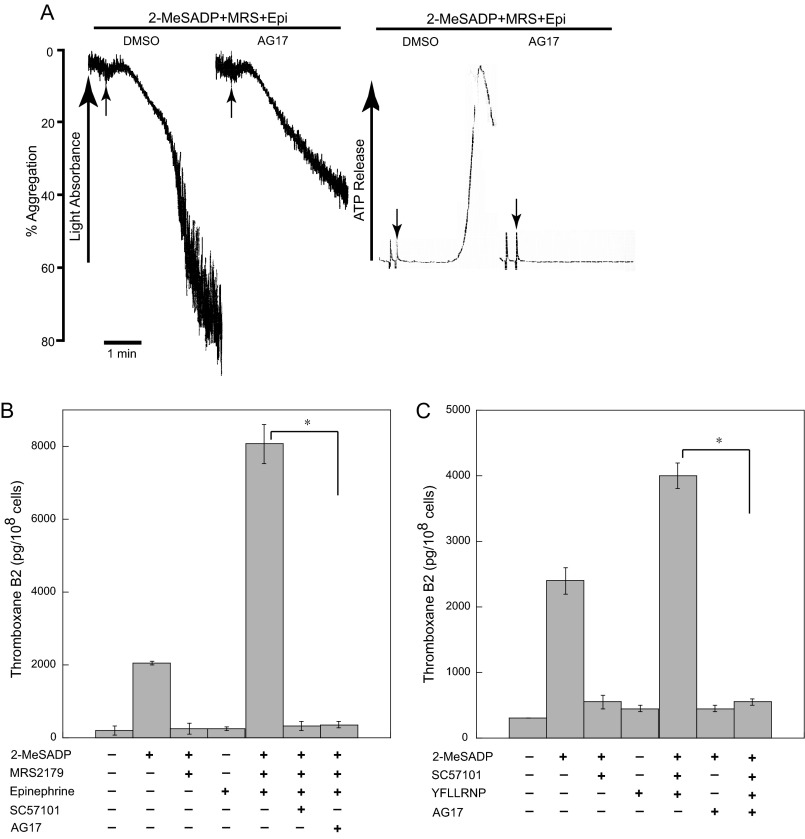
We have previously shown that the combined stimulation of Gi and Gz pathways causes platelet aggregation and thromboxane generation, which is dependent on integrin-mediated outside-in signaling (27). To verify the role of Pyk2 in outside-in signaling, we first tested the effect of Pyk2 inhibition on platelet aggregation under these conditions. As shown in Fig. 7A, a combination of Gi stimulation (2-MeSADP plus P2Y1 antagonist MRS2179) with Gz pathways (epinephrine) caused robust aggregation and dense granule secretion in non-aspirin-treated human platelets. However, pre-treating the platelets with AG17 caused an inhibition in secondary platelet aggregation and dense granule secretion. Because secondary platelet aggregation and dense granule secretion in this condition is mediated by TxA2 generation, which is dependent on outside-in signaling, we then measured the effect of Pyk2 inhibition on TxA2 generation induced by co-stimulation of Gi and Gz pathways. Selective stimulation of Gi (2-MeSADP plus MRS2179) or Gz pathways (epinephrine) alone failed to cause TxA2 generation (Fig. 7B). However, co-stimulation of Gi and Gz pathways caused a significant increase in TxA2 generation as previously described (27). When platelets were pre-treated with SC57101 or AG17, co-stimulation of Gi and Gz pathways failed to induce TxA2 generation, indicating that Pyk2 is necessary for thromboxane generation mediated by outside-in signaling.
FIGURE 7.
Effect of Pyk2 to TxA2 generation downstream of integrins and G12/13 pathways. A, non-aspirin-treated washed human platelets were pre-treated with AG17 (1 μm) at 37 °C for 5 min following co-stimulation with 100 nm 2-MeSADP and 10 μm epinephrine in the presence of 100 μm MRS2179 for 3.5 min under stirring conditions. Platelet aggregation and ATP secretion were measured by aggregometry. The arrow indicates when agonist is added. Tracings are representative of three independent experiments. B, the effect of Pyk2 inhibition on combined Gi and Gz stimulation on TxA2 generation. 10 μm SC57101 was added 1 min prior to the addition of agonists where noted. C, the effect of Pyk2 inhibition on G12/13-dependent TxA2 generation. Non-aspirin-treated washed human platelets were stimulated with 100 nm 2-MeSADP, 60 μm YFLLRNP, or 100 nm 2-MeSADP + 60 μm YFLLRNP as indicated for 3.5 min, and the effect of AG17 on TxA2 generation was measured. Data are mean ± S.E. (n = 3). *, p < 0.005.
The study from our group has also shown that inhibition of ADP-induced thromboxane generation by a fibrinogen receptor antagonist was rescued by co-stimulation of G12/13 pathways by YFLLRNP (21), suggesting that G12/13 pathways can substitute integrin-mediated signaling by probably activating common signaling effectors downstream of both integrins and G12/13 pathways. To confirm the role of Pyk2 downstream of G12/13 pathways in TxA2 generation, we co-stimulated platelets with 2-MeSADP and YFLLRNP in the presence of the fibrinogen receptor antagonist SC57101 and then compared the effect of Pyk2 inhibitor AG17 on TxA2 generation. As shown in Fig. 7C, 2-MeSADP-induced TxA2 generation was completely blocked in the presence of SC57101 or AG17, confirming the role of Pyk2 in ADP-induced TxA2 generation, which depends on integrin-mediated signaling. Selective activation of G12/13 pathways with YFLLRNP failed to cause TxA2 generation, but co-stimulation of platelets with 2-MeSADP and YFLLRNP in the presence of SC57101 caused a significant increase in TxA2 generation, indicating that G12/13 pathways substitute for integrin signaling for TxA2 generation. However, TxA2 generation under these conditions was completely blocked by AG17, confirming that G12/13 pathways rescue integrin-mediated outside-in signaling for TxA2 generation through Pyk2.
DISCUSSION
It has been shown that Pyk2 is abundantly expressed in platelets and is activated by various agonists including thrombin, collagen, or von Willebrand factor in platelets (15). However, the signaling mechanism of Pyk2 activation in platelets is complex and controversial and the functional role of Pyk2 in platelet activation has not been fully understood. Therefore, we have used pharmacological inhibitors of Pyk2 and Pyk2-deficient platelets to identify the signaling pathways of Pyk2 and its role in platelet function.
Pyk2 has been shown to be phosphorylated through integrin-mediated pathways, intracellular Ca2+ mobilization, and PKC activation in several cells, including human B cells (28), neurons (29), CMK megakaryocytic cells (30, 31), and PC12 cells (14, 32). However, it has been shown that Pyk2 activation mediated by thrombin is dependent on intracellular calcium but not dependent on PKC, Src, or PI 3-kinase in human endothelium (33). It has also been shown that several platelet agonists induce the tyrosine phosphorylation of Pyk2 through integrin-dependent and integrin-independent mechanisms (15). Thus, there appears to be a cell type-dependent mechanism regulating Pyk2 activation. We have investigated whether selective activation of G12/13 pathways, Gq pathways, Gi pathways, and integrin-mediated outside-in signaling can activate Pyk2. We found that 2-MeSADP-induced Pyk2 phosphorylation was induced by integrin-dependent pathways, whereas PAR agonists-induced Pyk2 phosphorylation was dependent on both integrin-dependent and -independent mechanism in platelets. PAR agonists can couple to G12/13 pathways, and we observed that selective activation of G12/13 pathways resulted in Pyk2 phosphorylation. We also observed that 2-MeSADP failed to induce Pyk2 phosphorylation in the presence of P2Y1 or P2Y12 receptor antagonists indicating that Gq or Gi pathways alone cannot directly activate Pyk2. Thus it appears that some of inhibitory effects of other signaling molecules on Pyk2 activation in previous studies might not be due to their direct inhibitory effect on Pyk2 but might be related to their inhibitory effect on platelet aggregation and the subsequent outside-in signaling.
Only a few selective inhibitors of Pyk2 have been identified. As pharmacological inhibitors are known to have off-target and broad-spectrum effects, the effect of Pyk2 in platelets has not been completely determined. Salicylate has been shown to inhibit Pyk2 phosphorylation, but it also exhibits an inhibitory effect on c-Src (34). It has recently been shown that the Pyk2 inhibitor AG17 reduces neutrophil adhesion to adherent platelets (35). A highly selective TAT-mediated protein transduction of dominant-negative C-terminal Pyk2 (TAT-Pyk2-CT) has been recently developed and used in several studies to selectively inhibit Pyk2 activity (26, 36–38). We have demonstrated the efficacy of these inhibitors on Pyk2 phosphorylation induced by AYPGKF. Importantly, Pyk2 knock-out mice have been generated and the role of Pyk2 in macrophages (11) and osteoclasts (12) have been studied. We found that platelet aggregation and dense granule release induced by various agonists including 2-MeSADP and AYPGKF were inhibited in the presence of Pyk2 inhibitors AG-17 and TAT-Pyk2-CT in human platelets suggesting that Pyk2 positively regulates platelet function. Consistently, platelet aggregation and secretion induced by 2-MeSADP and AYPGKF were diminished in Pyk2-deficient platelets compared with WT platelets.
It has been shown that ADP-induced TxA2 generation requires outside-in signaling, whereas thrombin-mediated TxA2 generation occurs independently of outside-in signaling. Platelets from patients with Glanzmann's thrombasthenia or platelets treated with fibrinogen receptor antagonist have been shown to be defective in ADP-, but not thrombin-induced TxA2 generation and secretion (39, 40). Unlike ADP, thrombin can couple to G12/13 pathways, and previous study has shown that inhibition of ADP-induced TxA2 generation by integrin αIIbβ3 blockade is rescued by co-stimulation of G12/13 pathways (21). These studies suggest that thrombin substitutes outside-in signaling through G12/13 pathways thus enables thromboxane to be generated in the absence of outside-in signaling. These observations also suggest the existence of a common signaling effector downstream of both integrins and G12/13 pathways. If Pyk2 is the common effector molecule downstream of both integrins and G12/13 pathways contributing to thromboxane generation, we anticipated that integrin-mediated TxA2 generation by 2-MeSADP and G12/13-dependent TxA2 generation by AYPGKF would be inhibited upon inhibition of Pyk2. Interestingly, we found that 2-MeSADP- and AYPGKF-induced TxA2 generation was inhibited in Pyk2−/− platelets compared with WT platelets. Our data showed that the extent of inhibition of platelet aggregation caused by Pyk2 inhibition was similar to the one caused by aspirin treatment, confirming the role of Pyk2 in TxA2 generation. In addition, we found that Pyk2 inhibition had no effect on ADP-induced platelet aggregation in aspirin-treated platelets, which is consistent with a previous study (41) showing that there was no difference in ADP-induced platelet aggregation from WT or TxA2 receptor null mice. Recent study (42) has characterized the platelets from Pyk2 knock-out mice and no significant differences are observed between WT and Pyk2 knock-out mice. In addition, arachidonic acid induces normal platelet aggregation in Pyk2-deficient platelets, and Pyk2 is linked to cPLA2 activation (42). In the experimental model adopted from a previous study (21) to confirm the role of Pyk2 downstream of G12/13 pathways in TxA2 generation, we found that G12/13 pathways failed to substitute for integrin-mediated signaling for TxA2 generation in the presence of the Pyk2 inhibitor confirming the contribution of Pyk2 downstream of G12/13 pathways. In addition, we have previously shown that combined P2Y12 receptor and α2A adrenergic receptor stimulation causes TxA2 generation with a requirement for outside-in signaling through αIIbβ3 (27). Our data showed that the Pyk2 inhibitor and αIIbβ3 antagonist completely inhibited TxA2 generation caused by costimulation of Gi and Gz signaling, further confirming an essential role of Pyk2 in TxA2 generation that is activated by outside-in signaling. Combined with our results showing that Pyk2 is activated downstream of both G12/13 and integrin-mediated outside-in pathways, these results strongly suggest that Pyk2 is the common effector molecule downstream of both G12/13 and outside-in signaling pathways, which contribute to TxA2 generation in platelets.
Recently, it has been shown that Pyk2 is activated after integrin α2β1 engagement in platelets and Pyk2 regulates PI 3-kinase β activity after integrin α2β1-mediated adhesion (43). PI 3-kinase β has been shown to play an important role in signaling downstream of αIIbβ3 (44), and our work has shown that PI 3-kinase β plays an important role in ADP-induced TxA2 generation (45). Thus, it is possible that Pyk2 mediates ADP-induced TxA2 generation by regulating PI 3-kinase β activity in platelets.
We have previously shown that ADP-induced TxA2 generation requires both P2Y1- and P2Y12-mediated signaling (46). However, the present study showed that selective activation of either P2Y1 or P2Y12 signaling failed to induce Pyk2 activation and identified an important role of Pyk2 downstream of integrins in ADP-induced TxA2 generation that is dependent on outside-in signaling. ADP fails to induce platelet aggregation when either the P2Y1 or P2Y12 receptor is blocked (47). Thus this suggests that the inhibitory effect of either P2Y1 or P2Y12 on ADP-induced TxA2 generation might be due to their inhibitory effect on platelet aggregation, which leads to the blockade of integrin-mediated signaling and Pyk2 activation.
Pyk2 is highly homologous to FAK, which plays a key role in mediating signaling downstream of integrins (48). The expression level of FAK in Pyk2 knock-out platelets has been shown to be normal. A previous study (21) has shown that FAK is activated downstream of integrins and G12/13 pathways in platelets, and 2-MeSADP-induced TxA2 generation is inhibited in the presence of the FAK inhibitor TAE-226. However, 2-MeSADP-induced TxA2 generation was not affected in Pf4-Cre/FAK-floxed mice, indicating that FAK does not contribute to TxA2 generation induced by outside-in signaling. Because TAE-226 also inhibits Pyk2 at a higher concentration (10), the inhibitory effect of TAE-226 on TxA2 generation was probably through the inhibition of Pyk2, which is consistent with our results using Pyk2 inhibitors. In conclusion, we have demonstrated that Pyk2 is an important functional tyrosine kinase that is activated by both G12/13 and integrin αIIbβ3-mediated outside-in signaling pathways and plays an important role in regulation of TxA2 generation in platelets.
This work was supported, in whole or in part, by National Institutes of Health Grants HL60683 and HL93231 (to S. P. K.) and American Heart Association Grant 12SDG8980013 (to S. K.).
- TxA2
- thromboxane A2
- Pyk2
- proline-rich tyrosine kinase 2
- PAR
- protease-activated receptor
- 2-MeSADP
- 2-methylthio-adenosine-5′-diphosphate
- AG17
- 3,5-di-t-butyl-4-hydroxybenzylidenemalononitrile
- SH2
- Src homology domain 2.
REFERENCES
- 1. Shattil S. J., Kashiwagi H., Pampori N. (1998) Integrin signaling. The platelet paradigm. Blood 91, 2645–2657 [PubMed] [Google Scholar]
- 2. Paul B. Z., Daniel J. L., Kunapuli S. P. (1999) Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J. Biol. Chem. 274, 28293–28300 [DOI] [PubMed] [Google Scholar]
- 3. Buhl A. M., Johnson N. L., Dhanasekaran N., Johnson G. L. (1995) Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 270, 24631–24634 [DOI] [PubMed] [Google Scholar]
- 4. Bauer M., Retzer M., Wilde J. I., Maschberger P., Essler M., Aepfelbacher M., Watson S. P., Siess W. (1999) Dichotomous regulation of myosin phosphorylation and shape change by Rho-kinase and calcium in intact human platelets. Blood 94, 1665–1672 [PubMed] [Google Scholar]
- 5. Jin J., Mao Y., Thomas D., Kim S., Daniel J. L., Kunapuli S. P. (2009) RhoA downstream of Gq and G12/13 pathways regulates protease-activated receptor-mediated dense granule release in platelets. Biochem. Pharmacol. 77, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murugappan S., Chari R., Palli V. M., Jin J., Kunapuli S. P. (2009) Differential regulation of threonine and tyrosine phosphorylations on protein kinase Cδ by G-protein-mediated pathways in platelets. Biochem. J. 417, 113–120 [DOI] [PubMed] [Google Scholar]
- 7. Murugappan S., Shankar H., Bhamidipati S., Dorsam R. T., Jin J., Kunapuli S. P. (2005) Molecular mechanism and functional implications of thrombin-mediated tyrosine phosphorylation of PKCδ in platelets. Blood 106, 550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gong H., Shen B., Flevaris P., Chow C., Lam S. C., Voyno-Yasenetskaya T. A., Kozasa T., Du X. (2010) G protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin “outside-in” signaling. Science 327, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Astier A., Manié S. N., Avraham H., Hirai H., Law S. F., Zhang Y., Golemis E. A., Fu Y., Druker B. J., Haghayeghi N., Freedman A. S., Avraham S. (1997) The related adhesion focal tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J. Biol. Chem. 272, 19719–19724 [DOI] [PubMed] [Google Scholar]
- 10. Lipinski C. A., Loftus J. C. (2010) Targeting Pyk2 for therapeutic intervention. Expert Opin. Ther. Targets 14, 95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. U.S.A. 100, 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gil-Henn H., Destaing O., Sims N. A., Aoki K., Alles N., Neff L., Sanjay A., Bruzzaniti A., De Camilli P., Baron R., Schlessinger J. (2007) Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J. Cell Biol. 178, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L. M., White R. A., Groopman J. E. (1995) Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 270, 27742–27751 [DOI] [PubMed] [Google Scholar]
- 14. Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. (1995) Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376, 737–745 [DOI] [PubMed] [Google Scholar]
- 15. Raja S., Avraham S., Avraham H. (1997) Tyrosine phosphorylation of the novel protein-tyrosine kinase RAFTK during an early phase of platelet activation by an integrin glycoprotein IIb-IIIa-independent mechanism. J. Biol. Chem. 272, 10941–10947 [DOI] [PubMed] [Google Scholar]
- 16. Ohmori T., Yatomi Y., Asazuma N., Satoh K., Ozaki Y. (1999) Suppression of protein kinase C is associated with inhibition of PYK2 tyrosine phosphorylation and enhancement of PYK2 interaction with Src in thrombin-activated platelets. Thromb. Res. 93, 291–298 [DOI] [PubMed] [Google Scholar]
- 17. Canobbio I., Lova P., Sinigaglia F., Balduini C., Torti M. (2002) Proline-rich tyrosine kinase 2 and focal adhesion kinase are involved in different phases of platelet activation by vWF. Thromb. Haemost. 87, 509–517 [PubMed] [Google Scholar]
- 18. Ohmori T., Yatomi Y., Asazuma N., Satoh K., Ozaki Y. (2000) Involvement of proline-rich tyrosine kinase 2 in platelet activation. Tyrosine phosphorylation mostly dependent on αIIbβ3 integrin and protein kinase C, translocation to the cytoskeleton and association with Shc through Grb2. Biochem. J. 347, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koziak K., Kaczmarek E., Park S. Y., Fu Y., Avraham S., Avraham H. (2001) RAFTK/Pyk2 involvement in platelet activation is mediated by phosphoinositide 3-kinase. Br. J. Haematol. 114, 134–140 [DOI] [PubMed] [Google Scholar]
- 20. Sayed M. R., Sheid M. P., Stevens C. M., Duronio V. (2000) Thrombin-stimulated phosphatidylinositol 3-kinase activity in platelets is associated with activation of PYK2 tyrosine kinase. Activation of both enzymes is aggregation independent. J. Cell Physiol. 183, 314–320 [DOI] [PubMed] [Google Scholar]
- 21. Bhavaraju K., Lakhani P. R., Dorsam R. T., Jin J., Hitchcock I. S., Sanjay A., Kunapuli S. P. (2011) G12/13 signaling pathways substitute for integrin αIIbβ3-signaling for thromboxane generation in platelets. PLoS One 6, e16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim S., Jin J., Kunapuli S. P. (2006) Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood 107, 947–954 [DOI] [PubMed] [Google Scholar]
- 23. Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) Coordinate interactions of Csk, Src, and Syk kinases with [α]IIb[β]3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim S., Jin J., Kunapuli S. P. (2004) Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. 279, 4186–4195 [DOI] [PubMed] [Google Scholar]
- 25. Woulfe D., Jiang H., Morgans A., Monks R., Birnbaum M., Brass L. F. (2004) Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J. Clin. Invest. 113, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu X., Boetticher E., Wang L., Duan Y., Learoyd J., Leff A. R. (2008) Proline-rich tyrosine kinase 2 regulates spreading and migration of eosinophils after β2-integrin adhesion. Am. J. Respir. Cell Mol. Biol. 39, 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dorsam R. T., Kim S., Murugappan S., Rachoor S., Shankar H., Jin J., Kunapuli S. P. (2005) Differential requirements for calcium and Src family kinases in platelet GPIIb/IIIa activation and thromboxane generation downstream of different G-protein pathways. Blood 105, 2749–2756 [DOI] [PubMed] [Google Scholar]
- 28. Astier A., Avraham H., Manie S. N., Groopman J., Canty T., Avraham S., Freedman A. S. (1997) The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after β1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 272, 228–232 [DOI] [PubMed] [Google Scholar]
- 29. Siciliano J. C., Toutant M., Derkinderen P., Sasaki T., Girault J. A. (1996) Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase beta (PYK2/CAKβ) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J. Biol. Chem. 271, 28942–28946 [DOI] [PubMed] [Google Scholar]
- 30. Hiregowdara D., Avraham H., Fu Y., London R., Avraham S. (1997) Tyrosine phosphorylation of the related adhesion focal tyrosine kinase in megakaryocytes upon stem cell factor and phorbol myristate acetate stimulation and its association with paxillin. J. Biol. Chem. 272, 10804–10810 [DOI] [PubMed] [Google Scholar]
- 31. Li J., Avraham H., Rogers R. A., Raja S., Avraham S. (1996) Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood 88, 417–428 [PubMed] [Google Scholar]
- 32. Soltoff S. P., Avraham H., Avraham S., Cantley L. C. (1998) Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J. Biol. Chem. 273, 2653–2660 [DOI] [PubMed] [Google Scholar]
- 33. Keogh R. J., Houliston R. A., Wheeler-Jones C. P. (2002) Thrombin-stimulated Pyk2 phosphorylation in human endothelium is dependent on intracellular calcium and independent of protein kinase C and Src kinases. Biochem. Biophys. Res. Commun. 294, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 34. Wang Z., Brecher P. (2001) Salicylate inhibits phosphorylation of the nonreceptor tyrosine kinases, proline-rich tyrosine kinase 2 and c-Src. Hypertension 37, 148–153 [DOI] [PubMed] [Google Scholar]
- 35. Evangelista V., Pamuklar Z., Piccoli A., Manarini S., Dell'elba G., Pecce R., Martelli N., Federico L., Rojas M., Berton G., Lowell C. A., Totani L., Smyth S. S. (2007) Src family kinases mediate neutrophil adhesion to adherent platelets. Blood 109, 2461–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duan Y., Learoyd J., Meliton A. Y., Clay B. S., Leff A. R., Zhu X. (2010) Inhibition of Pyk2 blocks airway inflammation and hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 42, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duan Y., Learoyd J., Meliton A. Y., Leff A. R., Zhu X. (2012) Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir. Res. 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L., Learoyd J., Duan Y., Leff A. R., Zhu X. (2010) Hematopoietic Pyk2 regulates migration of differentiated HL-60 cells. J. Inflamm. 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malmsten C., Kindahl H., Samuelsson B., Levy-Toledano S., Tobelem G., Caen J. P. (1977) Thromboxane synthesis and the platelet release reaction in Bernard-Soulier syndrome, thrombasthenia Glanzmann and Hermansky-Pudlak syndrome. Br. J. Haematol. 35, 511–520 [DOI] [PubMed] [Google Scholar]
- 40. Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. (1983) A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J. Clin. Invest. 72, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas D. W., Mannon R. B., Mannon P. J., Latour A., Oliver J. A., Hoffman M., Smithies O., Koller B. H., Coffman T. M. (1998) Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Invest. 102, 1994–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Canobbio I., Cipolla L., Consonni A., Momi S., Guidetti G., Oliviero B., Falasca M., Okigaki M., Balduini C., Gresele P., Torti M. (2013) Impaired thrombin-induced platelet activation and thrombus formation in mice lacking the Ca2+-dependent tyrosine kinase Pyk2. Blood 121, 648–657 [DOI] [PubMed] [Google Scholar]
- 43. Consonni A., Cipolla L., Guidetti G., Canobbio I., Ciraolo E., Hirsch E., Falasca M., Okigaki M., Balduini C., Torti M. (2012) Role and regulation of phosphatidylinositol 3-kinase β in platelet integrin α2β1 signaling. Blood 119, 847–856 [DOI] [PubMed] [Google Scholar]
- 44. Schoenwaelder S. M., Ono A., Nesbitt W. S., Lim J., Jarman K., Jackson S. P. (2010) Phosphoinositide 3-kinase p110β regulates integrin αIIbβ3 avidity and the cellular transmission of contractile forces. J. Biol. Chem. 285, 2886–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia A., Kim S., Bhavaraju K., Schoenwaelder S. M., Kunapuli S. P. (2010) Role of phosphoinositide 3-kinase β in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem. J. 429, 369–377 [DOI] [PubMed] [Google Scholar]
- 46. Garcia A., Shankar H., Murugappan S., Kim S., Kunapuli S. P. (2007) Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets. Biochem. J. 404, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jin J., Kunapuli S. P. (1998) Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 95, 8070–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hitchcock I. S., Fox N. E., Prévost N., Sear K., Shattil S. J., Kaushansky K. (2008) Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function. Studies using a megakaryocyte lineage specific FAK knockout. Blood 111, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]