Background: Nilotinib, an approved drug for leukemia, has been investigated in HCC.
Results: Nilotinib induced autophagy in HCC cell lines, including PLC5, Huh-7, and Hep3B.
Conclusion: Nilotinib-induced AMPK activation and subsequent autophagy is a major mode of action of nilotinib in HCC.
Significance: Elucidating the mechanisms by which nilotinib works on HCC is fundamental to develop the new treatment for HCC.
Keywords: AMP-activated Kinase (AMPK), Apoptosis, Autophagy, Serine Threonine Protein Phosphatase, Tyrosine Protein Kinase (Tyrosine Kinase), HCC, Nilotinib
Abstract
Hepatocellular carcinoma (HCC) is the most common liver cancer and the third-leading cause of cancer death worldwide. Nilotinib is an orally available receptor tyrosine kinase inhibitor approved for chronic myelogenous leukemia. This study investigated the effect of nilotinib on HCC. Nilotinib did not induce cellular apoptosis. Instead, staining with acridine orange and microtubule-associated protein 1 light chain 3 revealed that nilotinib induced autophagy in a dose- and time-dependent manner in HCC cell lines, including PLC5, Huh-7, and Hep3B. Moreover, nilotinib up-regulated the phosphryaltion of AMP-activated kinase (AMPK) and protein phosphatase PP2A inactivation were detected after nilotinib treatment. Up-regulating PP2A activity suppressed nilotinib-induced AMPK phosphorylation and autophagy, suggesting that PP2A mediates the effect of nilotinib on AMPK phosphorylation and autophagy. Our data indicate that nilotinib-induced AMPK activation is mediated by PP2A, and AMPK activation and subsequent autophagy might be a major mechanism of action of nilotinib. Growth of PLC5 tumor xenografts in BALB/c nude mice was inhibited by daily oral treatment with nilotinib. Western blot analysis showed both increased phospho-AMPK expression and decreased PP2A activity in vivo. Together, our results reveal that nilotinib induces autophagy, but not apoptosis in HCC, and that the autophagy-inducing activity is associated with PP2A-regulated AMPK phosphorylation.
Introduction
Hepatocellular carcinoma (HCC)2 is the fifth most common cancer and the third leading cause of cancer death worldwide (1). Advanced or recurrent HCC is frequently resistant to conventional chemotherapeutic agents and radiation, and thus remains one of the most difficult cancers to treat (2). Sorafenib, a multi-targeted receptor tyrosine kinase (RTK) inhibitor that targets the Raf kinases and other kinases such as VEGFR1–3, PDGFR-β, FLT-3, and c-kit (1, 3–4) has shown survival benefits in patients with advanced HCC and was approved for use in HCC by the United States Food and Drug Administration in 2007 (5–7). However, sorafenib only provides a modest effect, prolonging survival in patients with HCC from a median 7.9 to 10.7 months. Therefore, more effective new drugs are still urgently needed for HCC.
Autophagy, also known as type II programmed cell death (PCD), refers to an evolutionarily conserved catabolic process in which a cell degrades long-lived proteins and damaged organelles including the endoplasmic reticulum, Golgi apparatus, and mitochondria. In contrast with apoptosis, autophagy is dependent on the presence of autophagosomes and autolysosomes, as well as an intact nucleus in the cell (8). Many reports have demonstrated that autophagy is not only a survival response to either growth factor or nutrient deprivation but also an important molecular mechanism for tumor cell suicide (9). Recent studies have revealed that autophagy has an active role in cell death and is a response to various anticancer therapies in many kinds of cancer cells (6). Certain forms of cell death have also been shown to be prevented in the presence of either autophagy inhibitors or reduced expression of the ATG genes, which regulate autophagy (10–11). In HCC, autophagic cell death was found to be a major contributor to drug-induced antiprolioferation (12–13) of tumor cells. A previous study also proved that the celecoxib derivative, OSU-03012, could induce reactive oxygen species-related autophagy to inhibit HCC tumor growth (14–15).
Nilotinib is a second generation tyrosine kinase inhibitor (TKI) that functions via ATP-competitive inhibition. In the treatment of drug-resistant chronic myeloid leukemia (CML), nilotinib possesses an in vitro Bcr-Abl binding potency 30 times higher than imatinib in imatinib-resistant cells and a 5–7 times higher potency in imatinib-sensitive leukemic cells (8). In addition to Bcr-Abl inactivation, nilotinib also inhibits kinases including KIT, DDR, MAPK, ZAK, and PDGFR with less potency (6). Its broad spectrum of kinase-suppression activity makes nilotinib further applicable to the treatment of other types of cancers such as gastrointestinal stromal tumors (GIST), breast cancer, and melanoma. Nilotinib was demonstrated to have significant clinical activity in imatinib- and sunitinib-resistant GIST (10–11). Nilotinib also exerted antiproliferative effects in an estrogen-deprived breast cancer cell line MCF-7 (15). Metastatic melanoma cells expressing c-Abl/Arg kinase activity are also susceptible to nilotinib-mediated cell growth inhibition (13). In this study, we explored whether nilotinib exerts any antitumor activity against HCC. Our data show that nilotinib is an impressive killer of HCC cells. Surprisingly, this cell death was mediated by activation of autophagy, rather than apoptosis. We validated nilotinib induction of autophagic cell death through deactivating phosphatase PP2A and subsequently increasing AMPK phosphorylation. The antitumor activity exerted by nilotinib-mediated autophagy was further confirmed in an in vivo nude mouse model. In light of the identification of the PP2A-AMPK axis as a novel target of nilotinib-induced autophagy, further studies are warranted to assess nilotinib as an anti-HCC treatment.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Nilotinib (Tasigna) was kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). For in vitro studies, nilotinib at various concentrations was dissolved in DMSO and then added to cells in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS). The final DMSO concentration was 0.1% after addition to medium. Okadaic acid (OA), forskolin, and 3-methyladenine (3-MA) were purchased from Cayman Chemical (Ann Arbor, MI). Hydroxychloroquine (HCQ) was from Sigma-Aldrich (Seelze, Germany). Antibodies for immunoblotting as anti-LC3, -P-Akt (Ser473), -4EBP1, -P-4EBP1, -P-mTOR, -mTOR, -P-S6K, -S6K, anti-S6, -P-S6, -ATG3, -ATG5, -ATG7, and -Beclin 1 were from Cell Signaling (Danvers, MA).
Cell Culture and Western Blot Analysis
The PLC5 and Hep3B cell lines were obtained from American Type Culture Collection (Manassas, VA). The Huh-7 HCC cell line was obtained from the Health Science Research Resources Bank (Osaka, Japan; JCRB0403). Cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml, penicillin G, 100 μg/ml streptomycin sulfate, and 25 μg/ml amphotericin B in a 37 °C humidified incubator in an atmosphere of 5% CO2 in air. Western blot analysis was performed as previously reported (16).
Flow Cytometry for Apoptosis Analysis
Apoptotic cells (sub-G1) were analyzed by flow cytometry as described previously (16).
Autophagy Analysis
Drug-induced autophagy was assessed by several assays including: 1) Western blot analysis of microtubule-associated protein 1 light chain 3 (LC3-II); 2) immunofluorescence of LC3-II. Briefly, cells were seeded in a 6-cm dish. After being washed with PBS, cells were treated with nilotinib at 10 μm for 24 h, and fixed with ice-cold 4% paraformaldehyde. The fixed cells were subsequently incubated with the primary antibody rabbit anti-LC3II (1:200, #3868, Cell Signaling Technology, Danvers, MA), in blocking solution (1% bovine serum albumin in TBST) for 1 h at room temperature and then stained with anti-rabbit IgG (H+L), F(ab′)2 Fragment (Alexa Fluor 488 Conjugate, #4412, Cell Signaling) and DAPI. Cells were examined under a Leica DM2500 fluorescence microscope.
HCC Cells with Constitutively Active PP2A-C
PP2A-C cDNA was purchased from Origene (RC219918; Rockville, MD). Briefly, following transfection, cells were incubated in the presence of G418 (0.78 mg/ml). After 8 weeks of selection, surviving colonies, i.e. those arising from stably transfected cells were selected and individually amplified. HCC cells with stable expression of CIP2A-myc were then treated with drugs, harvested, and processed for Western blot analysis (16).
PP2A Phosphatase Activity
The protein phosphatase activity in total cellular lysate was determined by measuring the generation of free phosphate from threonine phosphopeptide using the malachite green-phosphate complex assay as described by the manufacturer (Upstate Biotechnology, Lake Placid, NY). Cell lysates were prepared in a low-detergent lysis buffer (1% Nonidet P-40, 10 mm HEPES, 150 mm NaCl, 10% glycerol, 1 mm PMSF, 5 mm benzamidine, and 10 g/ml leupeptin). The phosphatase assay was performed in a PP2A-specific reaction buffer (Upstate) containing 750 μm phosphopeptide substrate. After 10 min of incubation at 30 °C, the malachite dye was added, and free phosphate was measured by optical density at 650 nm. To avoid variability due to differences in the amounts of immunoprecipitated protein between samples, the phosphatase activities were normalized to the amount of PP2A immunoprecipitated, as detected and quantified by immunoblot analysis for each treatment group (17).
Co-immunoprecipitation Assay
Cells were harvested and lysed on ice for 30 min in lysis buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 0.5% Nonidet P-40, 50 mm NaF, 1 mm Na3VO4, 5 mm sodium pyrophosphate, and a protease inhibitor tablet). The cell lysates were centrifuged at 14,000 × g for 15 min, and the supernatants were recovered. Supernatants containing equal amounts of proteins were incubated with 2.5 mg of primary antibodies overnight at 4 °C. The immunoprecipitates were harvested using protein G PLUS-agarose beads (Santa Cruz Biotechnology) that were washed once with regular washing buffer (50 mm Tris-HCl, 100 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40), twice with high salt washing buffer (50 mm Tris-HCl, 500 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40), and another time with regular washing buffer. Immunoprecipitates were then eluted by boiling the beads for 5 min in SDS/PAGE sample buffer and characterized by Western blotting with appropriate antibodies (17).
Xenograft Tumor Growth
Male NCr athymic nude mice (5–7 weeks of age) were obtained from the National Laboratory Animal Center (Taipei, Taiwan). The mice were housed in groups and maintained under standard laboratory conditions on a 12-h light-dark cycle. They were given access to sterilized food and water ad libitum. All experimental procedures using these mice were performed in accordance with protocols approved by the Institutional Laboratory Animal Care and Use Committee of National Taiwan University. Each mouse was inoculated s.c. in the dorsal flank with 1 × 106 PLC5 or Huh-7 cells suspended in 0.1 ml of serum-free medium containing 50% Matrigel (BD Biosciences, Bedford, MA). When tumors reached 50–100 mm3, mice of PLC5 received nilotinib (10 mg/kg/day or 20 mg/kg/day) orally for the duration of treatment. Mice of Huh-7 were divided randomly into four groups and subjected to vehicle, HCQ (50 mg/kg/day, intraperitoneally), nilotinib (10 mg/kg/day, orally), or the combination of nilotinib (10 mg/kg/day) and HCQ (50 mg/kg/day) for the duration of treatment. Controls received vehicle (16).
Statistical Analysis
Tumor growth data points are reported as mean tumor volume ± S.E. Comparisons of mean values were performed using the independent samples t test in SPSS for Windows 11.5 software (SPSS, Inc., Chicago, IL).
RESULTS
Nilotinib-induced Cell Death Does Not Occur via Apoptosis
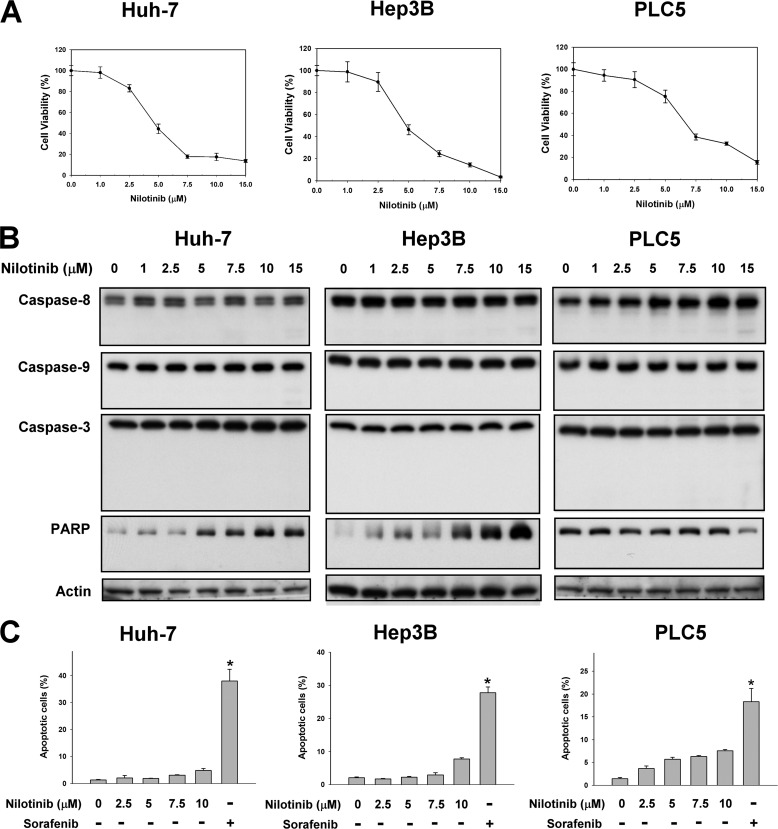
To investigate the effects of nilotinib on HCC cells, we first examined the apoptotic effects of the drug on a panel of three human HCC cell lines: Huh-7, Hep3B, and PLC5. We found that, although nilotinib treatment significantly reduced cell viability in all tested cell lines in a dose-dependent manner (Fig. 1A), the apoptotic pathway analyzed by Western blot did not seem to be activated. Our data indicate that nilotinib could not activate caspase-8, -9, and -3. Cleavage of PARP was also not observed in any of the cell lines treated with different doses of nilotinib (Fig. 1B). In addition, we analyzed nilotinib-induced apoptosis by flow cytometry and found that nilotinib did not induce significant apoptosis in our HCC cells. Notably, sorafenib, the only approved drug in HCC, induced significant apoptosis (Fig. 1C).
FIGURE 1.
Nilotinib reduced cell viability in HCC but did not induce apoptosis. A, dose-dependent effects of nilotinib on cell viability. HCC cells were treated with nilotinib at the indicated concentrations for 72 h. Cell viability was measured by MTT assay. Points, mean; bars, S.D. (n = 8). B and C, effects of nilotinib on apoptosis. B, cells were exposed to nilotinib at the indicated concentrations for 48 h. Cell lysates were assayed by Western blot to detect the activation of caspases and PARP cleavage. C, apoptotic cells (sub-G1) were analyzed by flow cytometry after cells were exposed to nilotinib at the indicated concentrations or sorafenib at 20 μm for 24 h.
Nilotinib Induces Autophagy in HCC Cells
Autophagy is the process of sequestrating cytoplasmic proteins into lytic compartments and is characterized by the formation of the autophagosome, a double membrane structure that sequesters the target organelle/protein and then fuses with endo/lysosomes where the contents and its major component, LC3, are degraded (18–19). We therefore investigated whether nilotinib could elicit autophagy in HCC cells by Western blot analysis. As shown in Fig. 2A, significantly increased expression of lipidized LC3 (LC3-II) was observed in nilotinib-treated HCC cell lines in a time-dependent manner. Next, another distinct marker of autophagy, AVO induction (20), was examined by staining with vital acridine orange. Similarly, the result showed that nilotinib promoted the accumulation of AVOs in the cytoplasm of Hep3B cells (Fig. 2B). Previous studies have proven that 3-methyladenine (3-MA), an inhibitor of phopshatidylinositol 3-kinase, can inhibit autophagy (20). Therefore, we next used 3-MA to investigate nilotinib-induced autophagy in HCC cells. As shown in Fig. 2C (top), nilotinib-induced cleavage of LC3 was attenuated by treatment with 1 mm 3-MA. hydroxychloroquine (HCQ) also inhibits autophagy by raising lysosomal pH leading to inhibition of both the fusion of the autophagosome with the lysosome, and lysosomal protein degradation. Prevention of autophagy by co-treatment with HCQ further proved that autophagy could be triggered by nilotinib (Fig. 2C, bottom). Furthermore, autophagy-related proteins including ATG3, 5, 7, 12, Beclin-1, and P62 showed constant expression levels in all tested HCC cell lines treated with nilotinib (Fig. 2D). Taken together, the data presented here indicate that nilotinib induces autophagy in HCC cells.
FIGURE 2.
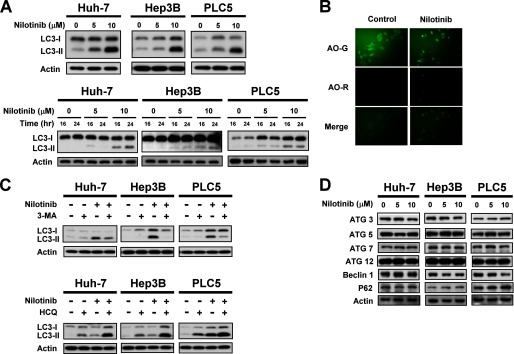
Nilotinib induces autophagy in HCC. A, top, dose-dependent effects of nilotinib-induced autophagy. HCC cells were treated with nilotinib at the indicated concentrations for 24 h. Bottom, time-dependent effects of nilotinib-induced autophagy. Cells were treated with nilotinib at the indicated concentrations for 16 or 24 h. Cell lysates were prepared for immunoblotting of microtubule-associated protein 1 light chain 3 (LC3). B, LC3-II immunofluorescence and acridine orange stain. Hep3B cells were treated with nilotinib at 10 μm for 24 h. C, top, co-treatment with autophagy inhibitor 3-MA reduced nilotinib-induced autophagy. Cells were treated with nilotinib (10 μm) and/or 3-MA (1 mm) for 24 h. Bottom, co-treatment with HCQ reduced nilotinib-induced autophagy. Cells were treated with nilotinib (10 μm) and/or HCQ (10 μm) for 24 h. D, dose-dependent analysis of autophagy-related proteins. Cells were exposed to nilotinib at the indicated concentrations for 24 h.
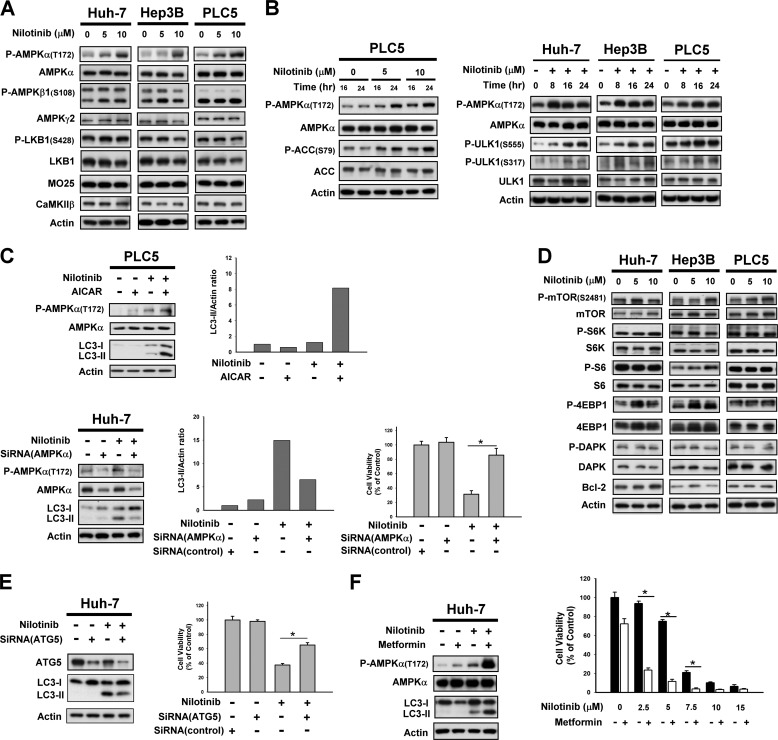
Nilotinib-induced Autophagy Relies on the AMPK Signaling Pathway
We next validated the target pathway by which nilotinib signals autophagy in HCC cells. Upstream regulation of autophagy occurs via different signaling pathways, including AMP-activated protein kinase (AMPK), which is a heterotrimeric complex consisting of a catalytic α-subunit and regulatory β- and γ-subunits (21). AMPK is activated by various conditions of stress that are known to induce autophagy. In starved cells, the LKB1/STAND/Mo25-complex phosphorylates AMPKα at Thr-172 and thus activates AMPK (22–23); while in non-starved cells, AMPK can also be phosphorylated via activation of Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β) (24). The AMPK subunits or activators were therefore examined to define their roles in HCC cells treated with nilotinib. As can be seen in Fig. 3, A and B, phosphorylation of AMPKα at Thr172, instead of the β- or γ-subunits, was increased upon nilotinib treatment in both a dose- and time-dependent manner. No significantly altered expression of either the LKB1/STAND/Mo25-complex or CaMKK were detected in nilotinib-treated HCC cells (Fig. 3A), which implies AMPK activity might be triggered by other upstream molecules. In addition, increased AMPK activity upon nilotinib treatment was further proven by analysis of the phosphorylation of the AMPK substrate acetyl-CoA carboxylase (ACC) in this set-up (Fig. 3B left). Notably, nilotinib also increased phospho-ULK1 in a time-dependent manner (Fig. 3B, right).
FIGURE 3.
AMPK mediated nilotinib-induced autophagy. A, target validation of CIP2A-Akt-4EBP1. A, dose-dependent analysis of AMPK-related molecules in HCC cells. Cells were exposed to nilotinib at the indicated concentrations for 24 h. B, time-dependent analysis of P-AMPKα, AMPKα, P-ACC, ACC, P-ULK1, and ULK1 in HCC cells. Cells were exposed to nilotinib at the indicated concentrations for 24 h. C, validation of AMPKα mediation of nilotinib-induced autophagy. Top, co-treatment with AICAR, an activator of AMPK, increased nilotinib-induced autophagy. Cells were treated with nilotinib (10 μm) and/or AICAR (1 mm) for 24 h. Bottom, silencing AMPKα by siRNA reduced nilotinib-induced autophagy and cell death. Huh-7cells were transfected with control or AMPKα siRNA for 48 h then treated with nilotinib (10 μm) for 24 h. Cell viability was measured by MTT assay. D, dose-dependent analysis of other autophagy-related molecules. Cells were exposed to nilotinib at the indicated concentrations for 24 h. E, silencing Atg 5 by siRNA reduced nilotinib-induced autophagy and cell death. Huh-7 cells were transfected with siRNA for 48 h then treated with nilotinib (10 μm) for 24 h. Cell viability was measured by MTT assay. F, adding metformin increased nilotinib-induced autophagy and cell death. Cells were exposed to nilotinib at 10 μm or at the indicated concentrations and/or metformin at 10 mm for 24 h.
The importance of AMPK activation in nilotinib-induced autophagy was next validated. Co-treatment with nilotinib and AICAR, an adenosine analog known to activate AMPK (25), was found to simultaneously increase phosphorylation of AMPKα and LC3 cleavage elicited by nilotinib (Fig. 3C, upper). Moreover, when AMPK was silenced by siRNA, a reduction in LC3 processing demonstrated that nilotinib-mediated autophagy was inhibited (Fig. 3C, left bottom). Notably, silencing AMPK by siRNA also reduced the effect of nilotinib on cell viability significantly (Fig. 3C, right bottom). Collectively, these results suggest that nilotinib signals autophagy in HCC cells via activation of AMPK. Moreover, analysis of the downstream regulators involved in nilotinib-induced autophagy revealed no obvious changes in the expression of mTOR, S6K, S6, 4EBP1, DAPK, or Bcl-2 (Fig. 3D). Moreover, silencing ATG5 by siRNA reduced the effect of nilotinib on autophagy and abolished the effect of nilotinib on cell death significantly (Fig. 3E). Notably, co-treatment of nilotinib and metformin, a known AMPK activator, enhanced the effect of nilotinib on autophagy and cell death (Fig. 3F).
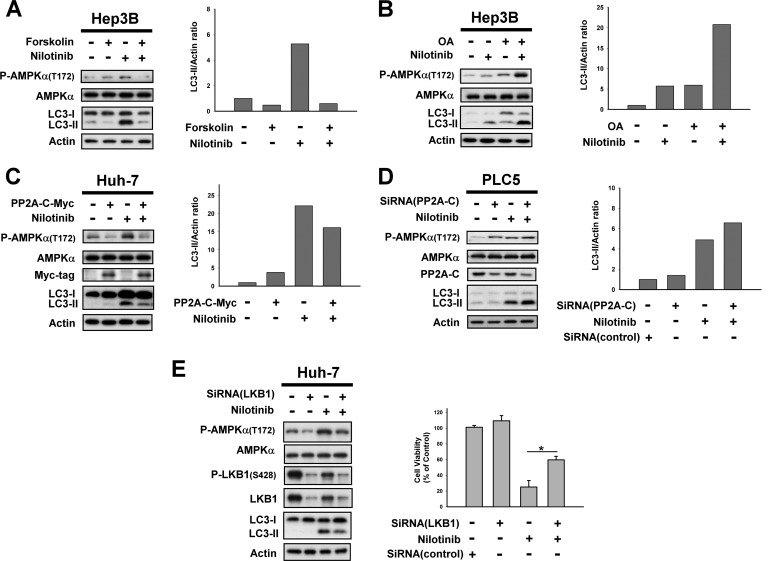
PP2A Mediates the Effect of Nilotinib on AMPK Activation and Autophagy
Protein phosphatase PP2A is an essential serine/threonine phosphatase ubiquitously expressed in eukaryotic cells that is known to mediate AMPK inhibition to induce heat shock proteins under stress (26). PP2A exists as a heterotrimeric enzyme composed of a 36-kDa catalytic subunit or C subunit, a 64-kDa scaffolding A subunit, and multiple regulatory B subunits that are thought to influence enzyme activity, substrate specificity, and subcellular localization (17). To further dissect the mechanism of AMPK phosphorylation induced by nilotinib, we inspected the effect of PP2A. Treatment of HCC cells with PP2A activator forskolin, or overexpression of PP2A reduced nilotinib-mediated phosphorylation of AMPK and autophagy (Fig. 4, A and C). In contrast, co-treatment with okadaic acid (OA), a PP2A inhibitor, or knockdown of PP2A-C enhanced the effect of nilotinib on P-AMPK and autophagy (Fig. 4, B and D). In addition, silencing LKB1 by siRNA reduced the effect of nilotinib on AMPK activation, autophagy and cell viability (Fig. 4E). These results suggest that PP2A might mediate the effect of nilotinib on the phosphorylation of AMPK and autophagy.
FIGURE 4.
PP2A mediates nilotinib-induced activation of AMPK. A, left, co-treatment with forskolin, a PP2A agonist, reverses the effect of nilotinib on P-AMPKα and autophagy. Right, immunoblots were scanned using a UVP BioSpectrum AC image system and quantitated using VisionWork LS software to determine the ratio of the level of LC3-II to actin. B, co-treatment with okadaic acid, a PP2A inhibitor, enhanced the effect of nilotinib on P-AMPKα and autophagy. C, ectopic expression of AMPKα abolishes effects of nilotinib on autophagy in Huh-7 cells. Cells were transfected with AMPKα-myc and were selected for 8 weeks by G-418. Analysis of autophagy was performed by Western blot (WB) after cells were sequentially exposed to DMSO or nilotinib (10 μm) for 24 h. D, knockdown of PP2A-C enhanced nilotinib-induced autophagy. PLC5 cells were transfected with control or AMPKα siRNA for 48 h and then treated with nilotinib (10 μm) for 24 h. E, silencing LKB1 by siRNA reduced nilotinib-induced autophagy. Huh-7 cells were transfected with control or LKB1 siRNA for 48 h then treated with nilotinib (10 μm) for 24 h. Cell viability was measured by MTT assay.
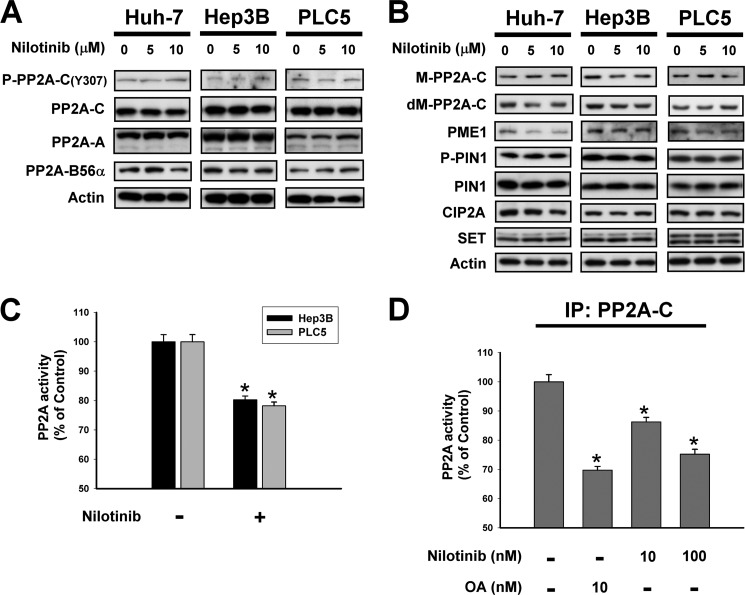
Nilotinib Reduces the Activity of PP2A
Next, we investigated the effect of nilotinib on PP2A. As shown in Fig. 5A, the expression of the PP2A subunits, including PP2A-C, phosphorylated PP2A-C at tyrosine 307, PP2A-A and PP2A-B56α, were not changed significantly after the treatment of nilotinib in three HCC cell lines. In addition to phosphorylation, the activity of PP2A may also be regulated by the methylation of PP2A-C (27). As shown in Fig. 5B nilotinib did not affect the methylated PP2A-C (M-PP2A-C), demethylated PP2A-C (dM-PP2A-C), and PP2A methyltransferase (PME) in HCC cells, indicating that nilotinib did affect the activity of PP2A by targeting the methylation of PP2A-C. As prolyl isomerase PIN1 is known a regulator of PP2A (28), we tested the role of PIN1 in nilotinib-treated cells and found that the phosphorylated PIN1 and total PIN1 were not affect by nilotinib (Fig. 5B). We next examined other cellular regulators of PP2A including cancerous inhibitor of PP2A (CIP2A) and SET (17). Nilotinib did not alter the expression of CIP2A and SET in our cells (Fig. 5B). Next, we examined whether nilotinib affect the activity of PP2A. Interestingly, we found that nilotinib reduced the activity of PP2A significantly in both Hep3B and PLC5 cells (Fig. 5C). To investigate whether nilotinib down-regulated the activity by direct interactions, we first immunoprecipated HCC cells by PP2A-C then treated the cells with nilotinib at 10 nm or 100 nm or okadaic acid at 10 nm for 24 h before measure the activity of PP2A. As shown in Fig. 5D, nilotinib reduced the activity of PP2A-C in a dose-dependent manner in PP2A-C containing cell lysates, suggesting that nilotinib, like okadaic acid, might inhibit the activity of PP2A through direct interactions. At present, it is still unclear that the interaction between nilotinib and PP2A is direct or indirect. Further biochemical experiments are needed to elucidate the interactions between nilotinib and PP2A.
FIGURE 5.
Nilotinib reduced the activity of PP2A. A, dose-dependent effects of nilotinib on P-PP2A-C, PP2A-C, PP2A-A, and PP2A-B56α. B, dose-dependent effects of nilotinib on PP2A-related proteins. C, treatment of nilotinib reduced the activity of PP2A. D, effects of nilotinib on PP2A activity in PP2A-C-containing lysates. Hep3B cells were immunoprecipitated with anti-PP2A-C then incubated with drugs for 24 h. Columns, mean; bars, S.D. (n = 3). *, p < 0.05.
Effect of Nilotinib on PLC5 Xenograft Tumor Growth in Vivo
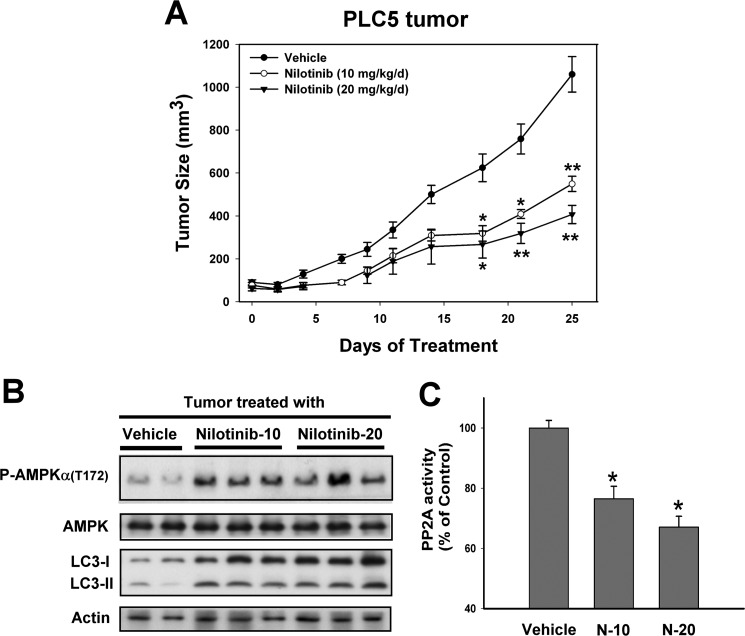
To investigate whether nilotinib-induced autophagy in HCC cell lines has potentially clinically relevant implications, we assessed the in vivo effect of nilotinib on the growth of HCC xenograft tumors. Tumor-bearing mice were treated with vehicle or nilotinib p.o. at doses of 10 or 20 mg/kg/day daily. All animals tolerated the treatments well without observable signs of toxicity and had stable body weights throughout the course of study. No gross pathologic abnormalities were noted at necropsy. Tumor growth was significantly suppressed by treatment with 10 or 20 mg/kg/day nilotinib (versus control, p < 0.01 at day 25) (Fig. 6A). As shown in Fig. 6B, treating PLC5 tumors with nilotinib up-regulated both P-AMPK and LC3 processing. In addition, nilotinib also decreased PP2A activity significantly (Fig. 6C), indicating that PP2A plays a role in mediating the antitumor effects of nilotinib in the xenograft tumor.
FIGURE 6.
In vivo effect of nilotinib on PLC5 xeonograft nude mice. A, tumor growth curves of PLC5. Points, mean (n = 6); bars, S.E. *, p < 0.05; **, p < 0.01. B, Western blot analysis of P-AMPK, AMPK, and LC-3 in PLC5 tumors. C, analysis of PP2A activity. Columns, mean; bars, S.D. (n = 6). *, p < 0.05 versus vehicle group.
Inhibition of Autophagy Reduced the Anti-tumor Effect of Nilotinib in Vitro and in Vivo
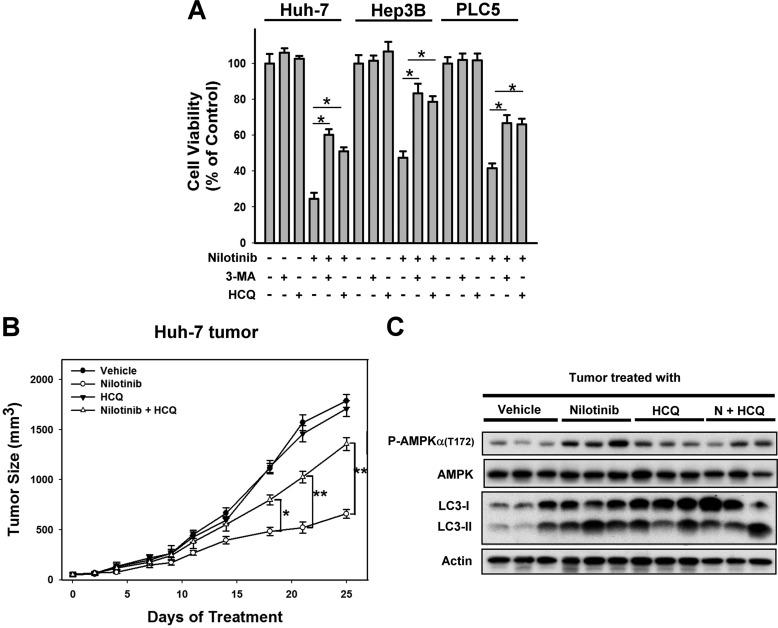
To prove that nilotinib-induced autophagy was responsible for nilotinib-induced cell death in HCC, we examined the effect of nilotinib in combination with autophagy inhibitors. As shown in Fig. 7A, co-treatment of autophagy inhibitors (3-MA or HCQ) reduced the effect of nilotinib on cell viability significantly in three tested HCC cells. Next, our in vivo data showed that adding HCQ, an autophagy inhibitor, reduced the effect of nilotinib on tumor growth and autophagy in Huh-7 tumors (Fig. 7, B and C). These data suggest that inhibition of autophagy reduced the effect of nilotinib on HCC in vitro and in vivo. Together, our data suggest that nilotinib exhibits good anti-cancer activity in vivo via AMPK activation regulated by PP2A. Further clinical investigation is warranted.
FIGURE 7.
Inhibition of autophagy reduced the anti-tumor effect of nilotinib in vitro and in vivo. A, co-treatment with autophagy inhibitors, 3-methyladenine (3-MA, 1 mm) or hydroxychloroquine (HCQ, 10 μm), reduced the effect of nilotinib on cell death. Cells were treated with nilotinib at10 μm and/or 3-MA/HCQ for 24 h. Cell viability was analyzed by MTT assay. B, combination of nilotinib and HCQ reduced the effect of nilotinib on Huh-7 tumors. Points, mean (n = 6); bars, S.E. *, p < 0.05; **, p < 0.01. (nilotinib group versus nilotinib plus HCQ group). C, Western blot analysis of P-AMPK, AMPK, and LC-3 in Huh-7 tumors.
DISCUSSION
This study showed that treatment with nilotinib, a multiple kinase inhibitor formally approved as a therapy for CML, induces autophagy, but not apoptosis, in human HCC tumors. As previous studies only demonstrated that nilotinib induces cytotoxicity via apoptosis (29–30), we first showed that nilotinib triggers autophagic cell death in Huh-7, Hep3B, and PLC5 cells. Our data also revealed that PP2A-mediated AMPK phosphorylation contributed to nilotinib-induced autophagy. Nilotonib suppressed PP2A activity and subsequently increased P-AMPK expression by direct interaction with PP2A. The xenograft tumor model further showed that nilotinib inhibited PLC5 tumor growth. These findings suggest that the cytotoxic effect of nilotinib in vivo occurs, at least in part, through autophagy.
Our results indicate that nilotinib can induce autophagic cell death and suppress xenograft HCC tumor growth through AMPK activation. Nilotinib is a novel TKI that is 30-times more potent than its previous-generation compound, imatinib, against, wild-type Bcr-Abl kinase, while maintaining activity against KIT and PDGFR (30). In Bcr-Abl-positive CML cells, nilotinib increased cytosolic calcium concentrations resulting from both membrane influx and ER release, which resulted in a change in mitochondrial membrane permeability leading to apoptosis (31). KIT/PDGFR overexpression is often observed in GIST, and treatment with nilotinib provided better progression-free survival (PFS) and overall survival (OS) in patients with advanced or metastatic GIST (11). In human breast cancer, deprivation of estrogen resulted in overexpression of PDGFR/Abl pathway-related proteins which became the target of nilotinib in turn inhibiting the growth of estrogen-deprived breast cancer cells and increasing the phosphorylation of AKT and ERK (15). Numerous pathways have been found to be deregulated in human HCC, including growth factors (e.g. epidermal growth factor (EGF), insulin-like growth factor (IGF), and hepatocyte growth factor (HGF)), cytoplasmic intermediates (e.g. AKT/MTOR and RAS/MAPK), angiogenesis [e.g. vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and PDGF], and differentiation cascades (WNT/s-catenin, Hedgehog, Notch) (32). As a multiple kinase inhibitor, we found nilotinib also exerts an antiproliferative effect on HCC cells. Although the exact targets of nilotinib in HCC cells remain elusive, our data revealed that the PP2A-AMPK signaling axis may play important role in nilotinib-mediated autophagic cell death.
Different studies have reported contrasting roles for AMPK activation in autophagy. Activation of AMPK by addition of the cell-permeable nucleotide analog AICA riboside (AICAR) to hepatocytes was reported to strongly inhibit autophagy (33). Another report also showed that increased AMPK phosphorylation suppressed autophagy and caused intracellular accumulation of ubiquitinated proteins within neurons (34). By contrast, in yeast and various mammalian cells, AMPK activation has been shown to stimulate autophagy (35–36). The results of this study suggest that nilotinib-induced cytotoxicity in HCC cells via AMPK-activation triggered autophagy, thus agreeing with the latter set of reports. HCC cells treated with nilotinib showed increased LC3 cleavage in the presence of AMPK activator (AICAR) and decreased LC3 cleavage when AMPK expression was silenced, clearly validating AMPK as a major trigger of autophagy in HCC. This finding was further demonstrated in the in vivo xenograft PLC5 tumors, which were significantly reduced in size when treated with nilotinib and also showed a concomitant increase in P-AMPK and LC3 processing. Together, these results show that nilotinib induces autophagy and autophagic cell death by activation of AMPK.
Next, we showed that nilotinib-mediated AMPK activation is through PP2A inhibition, rather than being regulated by the canonical LKB1/STAND/Mo-25-complex or CAMKK pathways. It has been reported that PP2A regulates the interaction between the AMPKα and γ subunits (37), and dephosphorylates AMPKα in a cell-free system (38). Recently, AMPK inhibition induced by glucose, palmitate, or ethanol was also found to be mediated by PP2A activation (39–41). One previous finding also revealed that PP2A mediates AMPK inhibition to up-regulate heat shock protein (HSP) 70 expression during stress (26). Exploration of the AMPK-downstream signaling cascades revealed that nilotinib-mediated AMPK activation to induce autophagy is via an mTOR-independent pathway. The serine/theroine kinase mammalian target of rapamycin (mTOR) is a major negative regulator of autophagy (42), and AMPK serves as one of the main mTOR regulators. Activation of AMPK inhibits mTOR phosphorylation and elicits autophagy. However, recent studies have documented the existence of mTOR-indepenent autophagy (43). For example, a decrease in intracellular inositol or inositol 1,4,5-triphosphate (IP3) levels by lithium is known to induce autophagy in neurobalstoma cells (44).
The correlation between autophagy and tumorigenesis has been explored extensively, but whether autophagy acts as a pro-tumorigenic or antitumor player in tumor development and cancer therapy is still unclear. Recently, it has been suggested that cancer cells have evolved to require autophagy under basal conditions, implying cell-autonomous roles for autophagy in tumor maintenance. For example, autophagy has been demonstrated to be required for continued cell growth in pancreatic cancers (45). Inhibition of autophagy also results in metabolic turbulence, reduced oxidative phosphorylation and decreased ATP production (46). In contrast, accumulating evidence shows that suppression of the proteins involved in autophagy such as Beclin-1 and Atg-5 may cause acceleration of tumorigenesis. Deletion of one copy of an autophagy-related gene (47), or reduced expression level of such genes has been found in certain types of cancer cells (48). Our present data show that nilotinib induces autophagy which contributes to cytotoxicity, rather than drug-resistance in HCC cells. This finding indicates that autophagy plays a tumor-suppressive role in liver cancer cells treated with nilotinib. Furthermore, as we observed no significant changes in autophagy-related proteins such as Atg-5,-7, or Beclin-1, we speculate that nilotinib induces an unconventional type autophagy. Atg-5/Atg-7-independent alternative autophagy has been discovered in several embryonic tissues revealing that autophagy can occur through at least two different pathways (49). Further investigation into the specific pathway(s) involved in nilotinib-mediated autophagy in HCC is warranted.
In conclusion, our data show that nilotinib effectively inhibits HCC growth in vitro and in vivo through autophagy induction that is mediated by the PP2A-AMPK signaling pathway. This study suggests nilotinib-induced cytotoxicity occurs through a novel mechanism, and supports its clinical potential as a component of therapeutic strategies for HCC.
This work was supported by Grants NTUH 101P01 (to K.-F. C.) from the National Taiwan University Hospital and NSC99-2314-B-002-017-MY2 (to K.-F. C.), and NSC101-2325-B-002-032 (to K.-F. C.) from the National Science Council, Taiwan.
- HCC
- hepatocellular carcinoma
- AMPK
- 5′ adenosine monophosphate-activated protein kinase
- PP2A
- protein phosphatase 2A
- CIP2A
- cancerous inhibitor of PP2A
- PI3K
- phosphatidylinositol-3-kinase
- PDK1
- phosphatidylinositol-3-kinase dependent 1
- PARP
- poly (ADP-ribose) polymerase
- s.c.
- subcutaneous
- PME
- PP2A methyltransferase
- HCQ
- hydroxychloroquine
- 3-MA
- 3-methyladenine.
REFERENCES
- 1. Adnane L., Trail P. A., Taylor I., Wilhelm S. M. (2006) Sorafenib (BAY 43–9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407, 597–612 [DOI] [PubMed] [Google Scholar]
- 2. Al-Kandari F. A., Owunwanne A., Syed G. M., Ar Marouf R., Elgazzar A. H., Shiekh M., Rizui A. M., Al-Ajmi J. A., Mohammed A. M. (2007) Regional cerebral blood flow in patients with sickle cell disease: study with single photon emission computed tomography. Ann. Nucl. Med. 21, 439–445 [DOI] [PubMed] [Google Scholar]
- 3. Auclair D., Miller D., Yatsula V., Pickett W., Carter C., Chang Y., Zhang X., Wilkie D., Burd A., Shi H., Rocks S., Gedrich R., Abriola L., Vasavada H., Lynch M., Dumas J., Trail P. A., Wilhelm S. M. (2007) Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia 21, 439–445 [DOI] [PubMed] [Google Scholar]
- 4. Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R. A., Schwartz B., Simantov R., Kelley S. (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5, 835–844 [DOI] [PubMed] [Google Scholar]
- 5. Cheng A. L., Kang Y. K., Chen Z., Tsao C. J., Qin S., Kim J. S., Luo R., Feng J., Ye S., Yang T. S., Xu J., Sun Y., Liang H., Liu J., Wang J., Tak W. Y., Pan H., Burock K., Zou J., Voliotis D., Guan Z. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 [DOI] [PubMed] [Google Scholar]
- 6. Manley P. W., Drueckes P., Fendrich G., Furet P., Liebetanz J., Martiny-Baron G., Mestan J., Trappe J., Wartmann M., Fabbro D. (2010) Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim. Biophys. Acta 1804, 445–453 [DOI] [PubMed] [Google Scholar]
- 7. Govender V. M., Ghaffar A., Nishtar S. (2007) Measuring the economic and social consequences of CVDs and diabetes in India and Pakistan. Biosci. Trends 1, 121–127 [PubMed] [Google Scholar]
- 8. Martinelli G., Iacobucci I., Soverini S., Palandri F., Castagnetti F., Rosti G., Baccarani M. (2007) Nilotinib: a novel encouraging therapeutic option for chronic myeloid leukemia patients with imatinib resistance or intolerance. Biologics 1, 121–127 [PMC free article] [PubMed] [Google Scholar]
- 9. Barnard G., Hopkins L., Moorthie S., Seilly D., Tonks P., Dabaghian R., Clewley J., Coward J., McConnell I. (2007) Direct detection of disease associated prions in brain and lymphoid tissue using antibodies recognizing the extreme N terminus of PrPC. Prion 1, 121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cauchi C., Somaiah N., Engstrom P. F., Litwin S., Lopez M., Lee J., Davey M., Bove B., von Mehren M. (2012) Evaluation of nilotinib in advanced GIST previously treated with imatinib and sunitinib. Cancer Chemother. Pharmacol. 69, 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Italiano A., Cioffi A., Coco P., Maki R. G., Schöffski P., Rutkowski P., Le Cesne A., Duffaud F., Adenis A., Isambert N., Bompas E., Blay J. Y., Casali P., Keohan M. L., Toulmonde M., Antonescu C. R., Debiec-Rychter M., Coindre J. M., Bui B. (2012) Patterns of care, prognosis, and survival in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. Ann. Surg. Oncol. 19, 1551–1559 [DOI] [PubMed] [Google Scholar]
- 12. Allen K. D., Mata B. A., Gabr M. A., Huebner J. L., Adams S. B., Jr., Kraus V. B., Schmitt D. O., Setton L. A. (2012) Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res. Ther. 14, R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganguly S. S., Fiore L. S., Sims J. T., Friend J. W., Srinivasan D., Thacker M. A., Cibull M. L., Wang C., Novak M., Kaetzel D. M., Plattner R. (2012) c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene 31, 1804–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao M., Yeh P. Y., Lu Y. S., Hsu C. H., Chen K. F., Lee W. C., Feng W. C., Chen C. S., Kuo M. L., Cheng A. L. (2008) OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 68, 9348–9357 [DOI] [PubMed] [Google Scholar]
- 15. Weigel M. T., Ghazoui Z., Dunbier A., Pancholi S., Dowsett M., Martin L. A. (2012) Preclinical and clinical studies of estrogen deprivation support the PDGF/Abl pathway as a novel therapeutic target for overcoming endocrine resistance in breast cancer. Breast Cancer Res. 14, R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen K. F., Yu H. C., Liu C. Y., Chen H. J., Chen Y. C., Hou D. R., Chen P. J., Cheng A. L. (2011) Bortezomib sensitizes HCC cells to CS-1008, an antihuman death receptor 5 antibody, through the inhibition of CIP2A. Mol. Cancer Ther. 10, 892–901 [DOI] [PubMed] [Google Scholar]
- 17. Chen K. F., Liu C. Y., Lin Y. C., Yu H. C., Liu T. H., Hou D. R., Chen P. J., Cheng A. L. (2010) CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene 29, 6257–6266 [DOI] [PubMed] [Google Scholar]
- 18. Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., Suzuki K., Tokuhisa T., Ohsumi Y., Yoshimori T. (2001) Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanzawa T., Germano I. M., Komata T., Ito H., Kondo Y., Kondo S. (2004) Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 11, 448–457 [DOI] [PubMed] [Google Scholar]
- 21. Hardie D. G., Carling D. (1997) The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 22. Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Mäkelä T. P., Alessi D. R., Hardie D. G. (2003) Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeqiraj E., Filippi B. M., Deak M., Alessi D. R., van Aalten D. M. (2009) Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 326, 1707–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Höyer-Hansen M., Jäättelä M. (2007) AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3, 381–383 [DOI] [PubMed] [Google Scholar]
- 25. Carling D. (2004) The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem. Sci. 29, 18–24 [DOI] [PubMed] [Google Scholar]
- 26. Wang T., Yu Q., Chen J., Deng B., Qian L., Le Y. (2010) PP2A-mediated AMPK inhibition promotes HSP70 expression in heat shock response. PLoS One 5, e13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stanevich V., Jiang L., Satyshur K. A., Li Y., Jeffrey P. D., Li Z., Menden P., Semmelhack M. F., Xing Y. (2011) The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell 41, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michniewicz M., Zago M. K., Abas L., Weijers D., Schweighofer A., Meskiene I., Heisler M. G., Ohno C., Zhang J., Huang F., Schwab R., Weigel D., Meyerowitz E. M., Luschnig C., Offringa R., Friml J. (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056 [DOI] [PubMed] [Google Scholar]
- 29. Hiwase D. K., White D. L., Saunders V. A., Kumar S., Melo J. V., Hughes T. P. (2009) Short-term intense Bcr-Abl kinase inhibition with nilotinib is adequate to trigger cell death in BCR-ABL(+) cells. Leukemia 23, 1205–1206 [DOI] [PubMed] [Google Scholar]
- 30. Quintás-Cardama A., Cortes J. (2008) Nilotinib: a phenylamino-pyrimidine derivative with activity against BCR-ABL, KIT and PDGFR kinases. Future Oncol. 4, 611–621 [DOI] [PubMed] [Google Scholar]
- 31. Forchap S. L., Pirmohamed M., Clark R. E. (2012) Release of intracellular calcium primes chronic myeloid leukaemia cells for tyrosine kinase inhibitor-induced apoptosis. Leukemia 26, 490–498 [DOI] [PubMed] [Google Scholar]
- 32. Villanueva A., Newell P., Chiang D. Y., Friedman S. L., Llovet J. M. (2007) Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 27, 55–76 [DOI] [PubMed] [Google Scholar]
- 33. Samari H. R., Seglen P. O. (1998) Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J. Biol. Chem. 273, 23758–23763 [DOI] [PubMed] [Google Scholar]
- 34. Magnaudeix A., Wilson C. M., Page G., Bauvy C., Codogno P., Leveque P., Labrousse F., Corre-Delage M., Yardin C., Terro F. (2013) PP2A blockade inhibits autophagy and causes intraneuronal accumulation of ubiquitinated proteins. Neurobiol. Aging 34, 770–790 [DOI] [PubMed] [Google Scholar]
- 35. Meley D., Bauvy C., Houben-Weerts J. H., Dubbelhuis P. F., Helmond M. T., Codogno P., Meijer A. J. (2006) AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 281, 34870–34879 [DOI] [PubMed] [Google Scholar]
- 36. Wang Z., Wilson W. A., Fujino M. A., Roach P. J. (2001) Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21, 5742–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gimeno-Alcañiz J. V., Sanz P. (2003) Glucose and type 2A protein phosphatase regulate the interaction between catalytic and regulatory subunits of AMP-activated protein kinase. J. Mol. Biol. 333, 201–209 [DOI] [PubMed] [Google Scholar]
- 38. Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett. 377, 421–425 [DOI] [PubMed] [Google Scholar]
- 39. Ravnskjaer K., Boergesen M., Dalgaard L. T., Mandrup S. (2006) Glucose-induced repression of PPARα gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J. Mol. Endocrinol. 36, 289–299 [DOI] [PubMed] [Google Scholar]
- 40. Wu Y., Song P., Xu J., Zhang M., Zou M. H. (2007) Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 282, 9777–9788 [DOI] [PubMed] [Google Scholar]
- 41. Liangpunsakul S., Sozio M. S., Shin E., Zhao Z., Xu Y., Ross R. A., Zeng Y., Crabb D. W. (2010) Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G1004–G1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarkar S., Ravikumar B., Floto R. A., Rubinsztein D. C. (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 16, 46–56 [DOI] [PubMed] [Google Scholar]
- 44. Sarkar S., Floto R. A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L. J., Rubinsztein D. C. (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang S., Wang X., Contino G., Liesa M., Sahin E., Ying H., Bause A., Li Y., Stommel J. M., Dell'antonio G., Mautner J., Tonon G., Haigis M., Shirihai O. S., Doglioni C., Bardeesy N., Kimmelman A. C. (2011) Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou S., Zhao L., Kuang M., Zhang B., Liang Z., Yi T., Wei Y., Zhao X. (2012) Autophagy in tumorigenesis and cancer therapy: Dr. Jekyll or Mr. Hyde? Cancer Lett. 323, 115–127 [DOI] [PubMed] [Google Scholar]
- 47. Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 [DOI] [PubMed] [Google Scholar]
- 48. Kang M. R., Kim M. S., Oh J. E., Kim Y. R., Song S. Y., Kim S. S., Ahn C. H., Yoo N. J., Lee S. H. (2009) Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. J. Pathol. 217, 702–706 [DOI] [PubMed] [Google Scholar]
- 49. Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., Komatsu M., Otsu K., Tsujimoto Y., Shimizu S. (2009) Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 [DOI] [PubMed] [Google Scholar]