Background: Sphingosine-1-phosphate phosphatase 1, encoded by Sgpp1, dephosphorylates the signaling lysophospholipid, S1P; however, its in vivo functions are not well understood.
Results: Deletion of Sgpp1 causes epidermal defects with intrinsic keratinocyte abnormalities.
Conclusion: Sgpp1 deficiency in keratinocytes causes S1P elevation and accelerates their differentiation.
Significance: Intracellular S1P metabolism regulates keratinocyte differentiation and epidermal homeostasis.
Keywords: Epidermis, Keratinocytes, Metabolism, Sphingolipid, Sphingosine-1-phosphate, Sgpp1
Abstract
Sphingosine 1-phosphate (S1P) is a bioactive lipid whose levels are tightly regulated by its synthesis and degradation. Intracellularly, S1P is dephosphorylated by the actions of two S1P-specific phosphatases, sphingosine-1-phosphate phosphatases 1 and 2. To identify the physiological functions of S1P phosphatase 1, we have studied mice with its gene, Sgpp1, deleted. Sgpp1−/− mice appeared normal at birth, but during the 1st week of life they exhibited stunted growth and suffered desquamation, with most dying before weaning. Both Sgpp1−/− pups and surviving adults exhibited multiple epidermal abnormalities. Interestingly, the epidermal permeability barrier developed normally during embryogenesis in Sgpp1−/− mice. Keratinocytes isolated from the skin of Sgpp1−/− pups had increased intracellular S1P levels and displayed a gene expression profile that indicated overexpression of genes associated with keratinocyte differentiation. The results reveal S1P metabolism as a regulator of keratinocyte differentiation and epidermal homeostasis.
Introduction
Sphingosine 1-phosphate (S1P)2 is a bioactive lipid that directly participates in both intracellular and extracellular signaling (1–4). Among the first signaling activities identified for S1P was its intracellular action to mobilize Ca2+ from internal stores (5, 6). More recently, several other intracellular targets have been identified (7, 8). Extracellular S1P exerts its effects through interactions with the family of S1P G protein-coupled receptors (S1PR1–5) that are carried by most cells, either singly or in different combinations. These receptors trigger distinctive cellular signaling pathways and exert control within the immune, vascular, and nervous systems (1–3). In addition to its important role in cellular signaling, S1P also functions as a key intermediate in lipid metabolic pathways (9).
S1P levels are tightly controlled by the regulation of its synthesis and degradation (see scheme in Fig. 1A) (10). After the formation of ceramide by de novo synthesis or degradation of complex sphingolipids, sphingosine is liberated by the action of ceramidase. S1P is then synthesized via the ATP-dependent phosphorylation of sphingosine by the sphingosine kinases, Sphk1 or Sphk2. Intracellularly, S1P is degraded in either of two ways, through dephosphorylation by the S1P phosphatases (SPP) SPP1 and SPP2 to yield sphingosine or through irreversible cleavage by S1P lyase. Extracellularly, S1P is dephosphorylated by the lipid phosphate phosphatases (LPP) LPP1, LPP2, and LPP3.
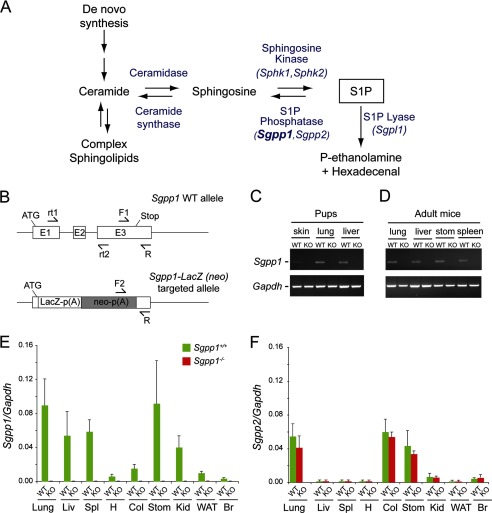
FIGURE 1.
Generation of Sgpp1 knock-out mouse line by Sgpp1 gene disruption. A, intracellular metabolic pathway of S1P. B, schematic representation of the Sgpp1 gene targeting strategy. The structure of the mouse Sgpp1 locus is shown at the top, and the structure of the Sgpp1 targeted allele is shown at the bottom. Arrows F1, F2, and R represent the primers used for genotyping; arrows rt1 and rt2 represent the primers used in semiquantitative RT-PCR. C and D, mRNA expression for Sgpp1 (top gels) and Gapdh (bottom gels) determined by semiquantitative RT-PCR of various tissues from Sgpp1+/+ (WT) and Sgpp1−/− (KO) 2-day-old (C) and adult (D) mice. E and F, mRNA expression for Sgpp1 (E) and Sgpp2 (F) determined by RT-qPCR of various tissues from Sgpp1+/+ and Sgpp1−/− adult mice. LIv, liver; Spl, spleen; H, heart; Col, colon; Stom, stomach; Kid, kidney; WAT, white adipose tissue; Br, brain. The relative level of Gapdh mRNA expression in each sample was set to 1. Bars represent mean values ± S.D. Student's t test, n = 4.
Both the SPPs and LPPs are members of the superfamily of phosphohydrolases (11–14). SPPs are highly specific for sphingoid-base 1-phosphate substrates, including S1P, dihydro-S1P, and phyto-S1P (15), whereas LPPs have a broader specificity that includes S1P but also ceramide 1-phosphate, lysophosphatidic acid, and phosphatidic acid. SPPs are integral enzymes of the endoplasmic reticulum (ER) (16–19) with a lumenal active site. In contrast, the LPPs are found on the plasma membrane with their active site oriented to the cell exterior (11, 20). Their localization suggests that SPPs and LPPs have distinct pools of S1P as substrate, with the SPPs dephosphorylating intracellular S1P, and the LPPs dephosphorylating S1P in the extracellular space. Indeed, deletion of LPP3 blocked the egress of lymphocytes from the thymus, an important extracellular signaling function of S1P, supporting the notion that LPPs are ecto-enzymes that regulate extracellular levels of S1P for signaling (20).
Within the sphingolipid metabolic pathway, SPP activity has been shown to influence the levels of both S1P and ceramide, presumably by diverting the sphingoid-base substrate from S1P formation back to ceramide synthesis. Functionally, the SPPs have been shown to have diverse cellular activities. SPP1 has been shown to regulate apoptosis by elevating ceramide levels (15), ER-to-Golgi ceramide trafficking (21), and autophagy induced by ER stress (22, 23). In addition, its effects on S1P levels influence resistance artery tone (24) and cell migration (25). SPP2 has been found to be elevated during immune responses (26). However, despite being implicated in diverse activities, the roles of the SPPs in physiology are not well understood.
Sphingolipids are recognized for their importance in skin biology as both structural and signaling molecules (27–30). Recently, S1P has been shown to have signaling properties that regulate keratinocyte cell function (31–36). Here, we report that the deletion of Sgpp1, the gene encoding SPP1, greatly disturbs epidermal homeostasis in mice. Keratinocytes lacking Sgpp1 have elevated S1P levels and overexpress genes associated with differentiation. These results link Sgpp1 expression with skin biology through the regulation of keratinocyte differentiation by S1P metabolism.
EXPERIMENTAL PROCEDURES
Mouse Generation and Procedures
Sgpp1−/− mice were generated by the Knock-out Mouse Project Repository (University of California, Davis) from ES cells created by Velocigene Regeneron Pharmaceuticals, Inc. (Tarrytown, NY). The mice had a C57Bl6 background and were maintained by heterozygous mating. Mice were genotyped by multiplex PCR of tail-snip DNA using three primers as follows: 5′-CGTGTCATGACATTCGCCAAGC-3′ (F1); 5′-GGAAATGGCTCCCAGAGGAAA-3′ (F2); and 5′-GCACCTCTGTTCCACATACAC-3′ (R) (Fig. 1B). The following conditions were used: denaturation, 94 °C for 5 min; amplification, 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1.5 min; and extension, 72 °C for 3 min (35 cycles). The WT Sgpp1 gene fragment was 588 bp in length, and the mutant Sgpp1 gene fragment was 402 bp in length.
Pups were analyzed at days 1 or 2 after birth, unless otherwise specified. Adult Sgpp1−/− mice were analyzed when they were between 7 and 10 months old. Littermate WT (Sgpp1+/+) mice were used as controls for each experiment. All animal procedures were approved by the NIDDK Animal Care and Use Committee and were performed in accordance with National Institutes of Health guidelines.
Histology and Immunohistochemistry
Skin tissue from Sgpp1+/+ and Sgpp1−/− mice was fixed with 10% formalin and embedded in paraffin. Sections were stained with H&E and examined on a Leica DMLB microscope (Leica Microsystems Inc., Buffalo Grove, IL). The epidermis was divided into two regions as follows: the stratum corneum, which is the top flaky layers stained by eosin and characterized by the lack of nuclei; and the subcorneal layers, right below the stratum corneum, which included the rest of the epidermis (from the top: stratum granulosum, stratum spinosum, and stratum basale) and were distinguished by hematoxylin staining. Quantification of epidermal thickness was done on photographs acquired from H&E-stained sections with a ×10 objective, using the image analysis program ImageJ. A scale was made within ImageJ from an ocular ruler photographed using the same microscopic field as the skin sections. Ten perpendicular lines were drawn from the bottom edge of the subcorneal layers to the top edge of the stratum corneum. The mean length of the lines was determined for two photographs/mouse.
For immunostaining, deparaffinized sections of back skin from pups and adult mice were incubated separately with the following antibodies: rabbit anti-mouse keratin 6 (PRB-169P-100); keratin 10 (PRB-159P-100); keratin 14 (PRB-155P-100); loricrin (PRB-145P-100); filaggrin (PRB-417P-100) (all from Covance, Philadelphia, PA); and Ki67 antigen (ab66155; from Abcam, Cambridge, MA), followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody (A11034; from Invitrogen) and DAPI. Sections were examined using a confocal laser scanning microscope with a Duo-Scan system (LSM 5 LIVE; Carl Zeiss, Inc., Thornwood, NY) using a ×20 objective. Excitation and filters were as follows: Alexa Fluor 488, laser 489 nm for excitation, emission collected with BP 520–555-nm filter; and DAPI, laser 405 nm for excitation, emission collected with BP 415–480-nm filter. Images were acquired using the ZEN 2007 software (Carl Zeiss, Inc.).
β-Galactosidase expression from the lacZ reporter was determined on the back skin of 2-day-old Sgpp1+/− mice using 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) as described previously (37). Sgpp1+/+ skin sections were used as negative control.
Determination of Ca2+ Distribution in Situ in Mouse Skin
Ca2+ distribution was detected in intact frozen skin sections obtained from 4-day-old pups as described previously (33, 38). Briefly, a 10-μm frozen skin section was placed on a glass slide covered by a thin layer of 2% agarose containing 10 μg/ml Calcium Green-1 (Invitrogen). Sections were observed using a Leica DMLB microscope within the next 2 h.
Primary Keratinocyte Cultures
Primary keratinocyte cultures were prepared as described previously (39, 40). Briefly, skin was removed from 1-day-old pups and placed in 0.25% trypsin without EDTA overnight at 4 °C. The epidermis was peeled away from the dermis with forceps, then minced, and filtered. Cells were plated on collagen-coated plates. Using this protocol, only keratinocytes from the stratum basale attach to the plates. Keratinocytes were grown in 0.05 mm Ca2+-containing Eagle's minimum essential medium (Lonza, Walkersville, MD) plus 8% fetal calf serum (low Ca2+ medium) for 3–4 day and then harvested. Alternatively, WT mouse keratinocytes were obtained from (CELLnTEC Advance Cell Systems, Bern, Switzerland) and were grown in CnT7 medium containing 0.07 mm Ca2+ (CELLnTEC Advance Cell Systems).
When indicated, WT keratinocytes were treated with 10−7 m S1P (Avanti Polar Lipids, Inc., Alabaster AL) for 2 days in CnT2 medium (CELLnTEC Advance Cell Systems) containing 1.2 mm Ca2+. Conditioned medium from 5-day-old Sgpp1+/+ and Sgpp1−/− keratinocyte cultures grown in CnT7 medium were collected and centrifuged at 200 × g for 10 min.
Lipid Analysis
Skin dissected from the back of 2-day-old Sgpp1+/+ and Sgpp1−/− mice was placed on top of calcium/magnesium-free PBS containing 10 mm EDTA (pH 7.4) with the dermal side facing downward. After incubating for 40 min at 37 °C, the epidermis was peeled away from the dermis with forceps (41). Epidermal sheets were quickly rinsed with normal saline and frozen at −80 °C. Keratinocytes growing in culture were washed, harvested, and cell pellets frozen at −80 °C. Plasma was prepared from blood from adult Sgpp1+/+ and Sgpp1−/− mice. Sphingolipids were extracted from the epidermal sheets, keratinocyte pellets, and plasma, and S1P, dihydro-S1P, sphingosine, and ceramides containing different nonhydroxy amide-linked fatty acid species were measured by HPLC-tandem MS by the Lipidomics Core at the Medical University of South Carolina on a Thermo Finnigan (Waltham, MA) TSQ 7000 triple quadrupole mass spectrometer, operating in a multiple reaction-monitoring positive ionization mode as described previously (42). Sphingolipid concentration was normalized using inorganic phosphate measurements of the lipid extracts.
For sphingomyelin determinations, lipid extracts were prepared from epidermis and keratinocyte pellets as described previously (43), applied to high performance thin layer chromatography (HPTLC) plates (Merck), and developed in chloroform/methanol/water (65:35:8). After running, HPTLC plates were sprayed with 1.5% cupric sulfate in acetic acid/sulfuric acid/orthophosphoric acid/water (50:10:10:30) and baked at 110 °C for 10 min. Bands were identified using known standards run simultaneously with the samples and quantified using the Carestream Molecular Imaging Software (Carestream Health, Inc., Rochester, NY). Sphingomyelin concentrations were normalized using inorganic phosphate measurements of the lipid extracts.
S1P-induced Down-modulation of S1PR1-GFP Receptor from the Plasma Membrane
HEK293 cells stably expressing the S1PR1-GFP chimeric receptor (44) were grown in 10% charcoal-stripped FBS-containing DMEM (C-DMEM) for 24 h and treated for 60 min with 10−7 m S1P, 5-day Sgpp1+/+ and Sgpp1−/− keratinocyte-conditioned medium, or C-DMEM alone. Cells were then fixed with 1% paraformaldehyde in PBS for 15 min and examined for GFP and DAPI using a Zeiss confocal laser scanning microscope under a ×63 oil objective.
Skin Permeability Assays
Embryos obtained by Caesarean-section at days 16.5 and 17.5 postcoitum (which was determined by the presence of a vaginal plug at day 0.5) and 4-day-old pups were euthanized. Mice were fixed in methanol for 5 min at room temperature, washed five times with PBS, and incubated with 0.1% toluidine blue in normal saline for 10 min (41). After washing with PBS, mice were examined for the penetration of the blue dye into the skin and photographed.
Gene Expression
Total RNA from mouse tissues was purified using TRIzol (Invitrogen) and from keratinocyte cultures cells using the RNeasy mini kit (Qiagen, Valencia, CA). Total RNA (1 μg) was first digested with DNase I and subsequently reverse-transcribed with the SuperScript First-Strand Synthesis System (Invitrogen) by following the manufacturer's instructions.
For semiquantitative RT-PCR, the forward primer (rt1, 5′-tacatgggaatgcattctat-3′) was located on exon 1 of the Sgpp1 transcript, and the reverse primer (rt2, 5′-GCTTTTCCAAACAGAGTTACA-3′) was located on exon 3 (Fig. 1B). The following conditions were used: denaturation, 95 °C for 5 min; amplification, 95 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min; and extension, 72 °C for 3 min (35 cycles). For amplification of Gapdh mRNA, the forward primer was at the exon 4–5 junction (5′-ACCACAGTCCATGCCATCAC-3′) and the reverse primer was in exon 7 (5′-CACCACCCTGTTGCTGTAGCC-3′). The following conditions were used: denaturation, 95 °C for 5 min; amplification, 95 °C for 1 min, 65 °C for 1 min, 72 °C for 1 min; and extension, 72 °C for 3 min (35 cycles). PCR products were visualized on a 1.2% agarose gel.
Alternatively, mRNA expression levels were determined by real time-quantitative PCR (RT-qPCR) using predesigned Assay-on-Demand probes and primers (Applied Biosystems, Foster City, CA) on an ABI Prism 7700 Sequence Detection System (Applied Biosystems) as follows: for mouse Sgpp1 (Mm00473016_m1); Sgpp2 (Mm01158866_m1); Sgpl1 (Mm00486079_m1); Flg (Mm01716522_m1); Lor (Mm01219285_m1); Krt10 (Mm03009921_m1); Krt14 (Mm00516876_m1); Lce1c (Mm00783426_s1); Rptn (Mm00485857_m1); Tgm3 (Mm00436999_m1); Cdsn (Mm01275230_m1); Lor (Mm01962650_s1); Hrnr (Mm00808280_s1); Spink5 (Mm00511522_m1); Ppap2a (Mm00477016_m1); Ppap2b (Mm005404516_m1); Ppap2c (Mm00479290_m1), and Gapdh (Mm99999915_g1).
For microarray analysis, total RNA purified from primary keratinocyte cultures of Sgpp1+/+ and Sgpp1−/− cells was analyzed on Affymetrix GeneChip Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) as described previously (9). The National Center for Biotechnology Information Gene Expression Omnibus (GEO) accession number for the microarray data is GSE40838.
Statistical Analysis
Statistical significance was determined using the Student's t test. In all cases, p < 0.05 was considered statistically significant.
RESULTS
Sgpp1 Deficiency Results in Neonatal Lethality and Abnormal Skin in Newborn Pups
The structure of the targeted Sgpp1 allele mouse is shown in Fig. 1B. The targeting vector replaced the entire Sgpp1 coding region, beginning at the start codon in exon 1 and extending into exon 3, with lacZ and neo cassettes (Fig. 1B). This targeting procedure is expected to result in a null Sgpp1 allele and a lacZ reporter under the control of the native Sgpp1 promoter elements.
Expression of Sgpp1 was readily detectable in tissues of Sgpp1+/+ mice by semiquantitative RT-PCR (Fig. 1, C and D) and RT-qPCR (Fig. 1, E and F), but it was below the level of detection in Sgpp1−/− mice, confirming that the gene targeting procedure had successfully generated a null Sgpp1 allele. Expression levels of Sgpp2, the gene encoding the second SPP isozyme, were not significantly altered in Sgpp1−/− mouse tissues (Fig. 1F).
Sgpp1+/− mice were bred, and offspring were genotyped at a few days after birth or at 3 weeks of age. Newborn Sgpp1−/− pups were found in a Mendelian ratio (from a total of 182 pups, 26% were Sgpp1+/+, 51% Sgpp1+/−, and 23% Sgpp1−/−). However, only 3.5% of the offspring were Sgpp1−/− when they were weaned at 3 weeks of age. These results indicated that a substantial fraction of the Sgpp1−/− mice died between birth and 3 weeks of age.
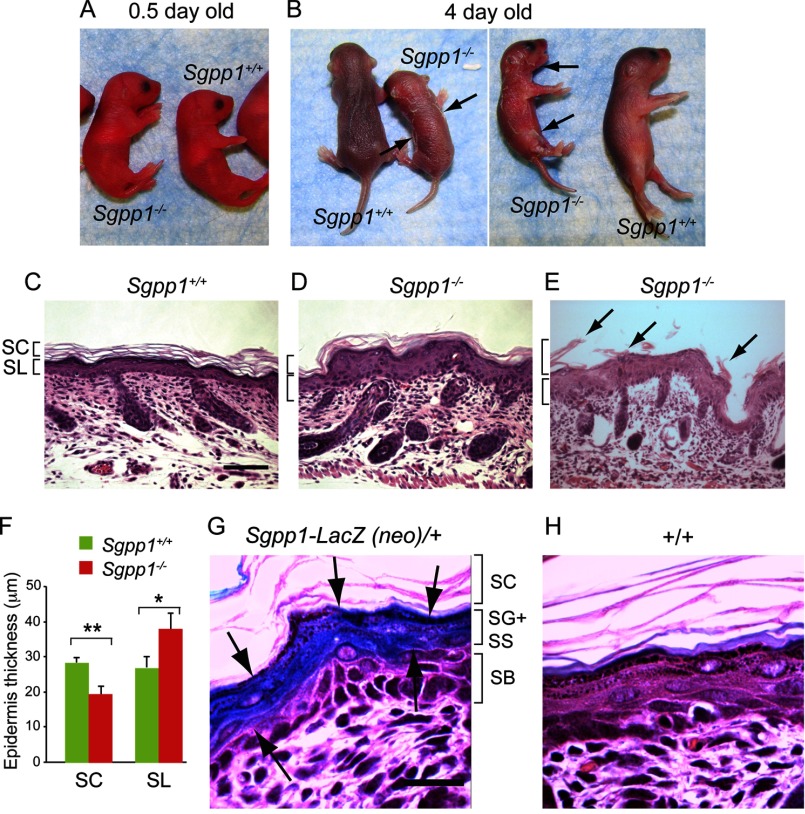
Immediately after birth, Sgpp1−/− pups could not be distinguished from Sgpp1+/+ littermates (Fig. 2A). However, starting at 3 days of age, Sgpp1−/− mice appeared smaller than their littermates. In addition, they showed skin desquamation on the trunk and around the joints of the upper and lower extremities that progressively worsened with time (Fig. 2B). Skin sections from 2-day-old pups showed that Sgpp1−/− epidermis was thickened compared with WT epidermis (Fig. 2, C and D). Histological analysis of Sgpp1−/− back skin undergoing peeling showed the detachment of the stratum corneum (Fig. 2E). Measurements demonstrated that, in Sgpp1−/− mice, the subcorneal layers (which included the stratum granulosum, stratum spinosum, and stratum basale) were significantly thicker, whereas the stratum corneum was thinner than in Sgpp1+/+ mice (Fig. 2F). These results indicate that the deletion of the Sgpp1 gene induces epidermal abnormalities in newborn mice.
FIGURE 2.
Sgpp1−/− pups exhibit focal desquamation and abnormal epidermal histology. A and B, Sgpp1+/+ and Sgpp1−/− mice at 0.5 days (A) and 4 days after birth (B). The arrows in B indicate sites of desquamation. C–E, histology of Sgpp1-deficient mouse skin. Paraffin sections of back skin from 2-day-old Sgpp1+/+ (C) and Sgpp1−/− (D and E) mice were stained with H&E. E represents a region of back skin undergoing peeling of the stratum corneum (marked by arrows). Bar, 200 μm. SC, stratum corneum; SL, subcorneal layer. F, stratum corneum and subcorneal layer thicknesses in 2-day-old Sgpp1+/+ and Sgpp1−/− mice. Bars represent mean values ± S.D. n = 4 for each genotype. Student's t test; *, p < 0.05; **, p < 0.01. G and H, activity of β-galactosidase, a surrogate marker for Sgpp1 expression, in 2-day-old mouse skin section. Sgpp1+/− (Sgpp1-LacZ (neo)/+) (G) and Sgpp1+/+ (+/+) (H) skin sections are shown. Arrows in G mark the keratinocyte layers that express β-galactosidase as a marker of Sgpp1 expression. This staining is absent in the +/+ skin section (H). Bar, 50 μm. SC, stratum corneum; SG, stratum granulosum; SS, stratum spinosum; SB, stratum basale.
Expression of Sgpp1 in Epidermis
The expression of Sgpp1 in the epidermis was determined using a surrogate marker, β-galactosidase, the product of the lacZ gene, which had been placed under the control of the native Sgpp1 regulatory elements in the Sgpp1 gene-targeted mice (see targeting scheme in Fig. 1B). In skin sections from 2-day-old Sgpp1+/− mice stained for β-galactosidase activity, intense blue reaction product was observed in the stratum granulosum and the stratum spinosum (Fig. 2G). Sgpp1+/+ skin sections without the lacZ reporter were largely negative for β-galactosidase activity (Fig. 2H). This result suggests that the expression of the Sgpp1 gene is up-regulated as keratinocytes differentiate within the epidermis.
We also determined the relative mRNA expression of the three specific S1P degradation enzymes, the two SPPs (Sgpp1 and Sgpp2) and S1P lyase (Sgpl1), in the skin of 2-day-old Sgpp1+/+ and Sgpp1−/− mice (supplemental Fig. S2A). Sgpp1 mRNA was readily detected in Sgpp1+/+ skin, but it was below the detection limit in Sgpp1−/− samples. Sgpp2 and Sgpl1 mRNA signals in Sgpp1+/+ skin were substantially lower than that of Sgpp1, suggesting that Sgpp1 was the most highly expressed of the three S1P degradative enzymes (supplemental Fig. S2A). Compensatory increases of Sgpp2 or Sgpl1 mRNA expression were not detected in Sgpp1−/− skin (supplemental Fig. S2A). Neither were there significant changes detected in the mRNA levels of the broad specificity LPPs (Ppap1a, Ppap2b, and Ppap2c) (supplemental Fig. S2B).
Skin Barrier Formation in the Absence of Sgpp1
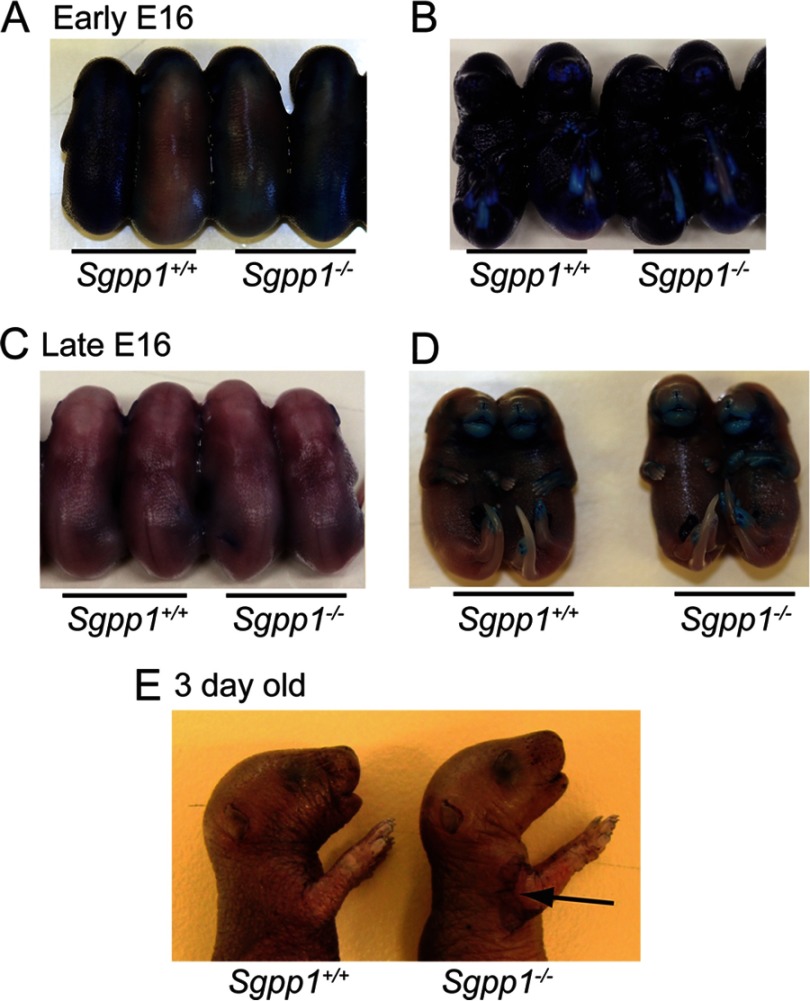
We next assessed the development of the epidermal permeability barrier in Sgpp1−/− mice by the ability of their skin to exclude toluidine blue. Normally, the skin permeability barrier begins to form at around embryonic day (E) 16.5, initiating from the dorsal surface and developing ventrally until it is complete by E17.5 (45). Overall, Sgpp1−/− embryos displayed a developmental pattern of skin permeability barrier formation indistinguishable from the Sgpp1+/+ embryos (Fig. 3, A–D). Sgpp1+/+ and Sgpp1−/− early stage E16 embryos stained equally blue, indicating a lack of a permeability barrier at this time (Fig. 3, A and B). However, by late stage E16 both Sgpp1+/+ and Sgpp1−/− embryos largely excluded the blue dye except for the anterior portions of their limbs and snout (Fig. 3, C and D). These results indicate that the skin permeability barrier develops normally in the Sgpp1−/− embryos. Three days after birth, Sgpp1−/− pups showed normal exclusion of toluidine blue in areas where the skin remained intact (Fig. 3E). However, there was visible dye uptake in desquamated areas, indicating a focally disrupted permeability barrier (Fig. 3E).
FIGURE 3.
Epidermal permeability barrier formation was normal in Sgpp1−/− mice. Epidermal barrier acquisition was tested by resistance to toluidine blue staining at early embryonic (E) day E16 (A and B), late day E16 (C and D), and at 3 days after birth (E). Embryos are viewed dorsally (A and C) and ventrally (B and D). In the 3-day-old pups (E), the arrow indicates an area of desquamation that stained with toluidine blue.
Sphingolipid Levels in Epidermis
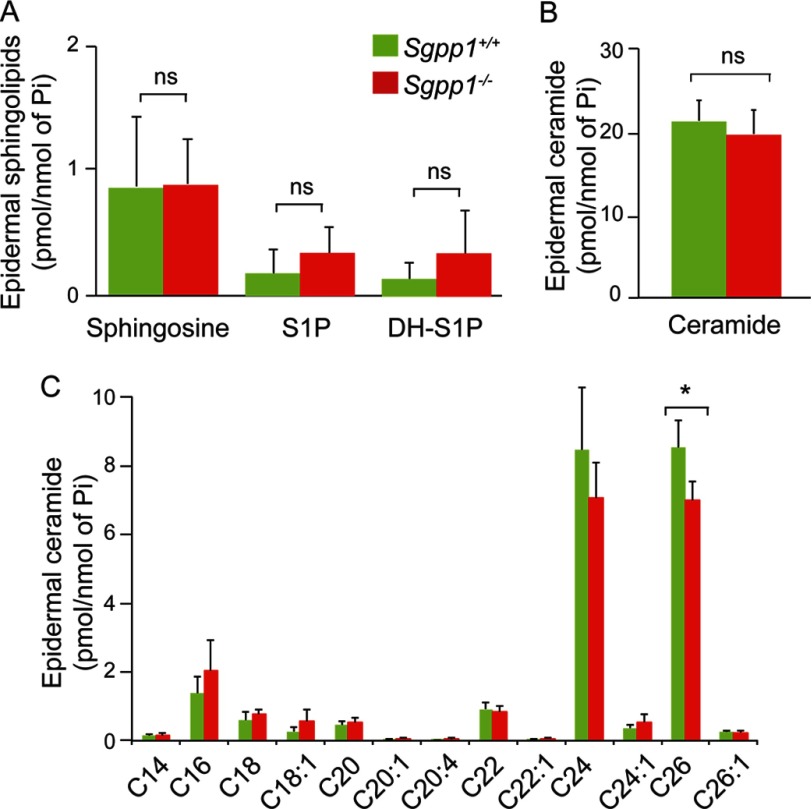
Sphingolipids were extracted from epidermal sheets obtained from 1- to 2-day-old Sgpp1+/+ and Sgpp1−/− mice, and levels of ceramide species, sphingosine, S1P, and dihydro-S1P were determined by mass spectrometry. Total levels of these sphingolipids were not significantly different in Sgpp1−/− compared with Sgpp1+/+ epidermis (Fig. 4, A and B). In addition, total epidermal sphingomyelin levels were not significantly different in Sgpp1−/− compared with Sgpp1+/+ mice (supplemental Fig. S1A). When levels of individual ceramide species were examined, the only significant difference was a decrease in the content of C26-fatty acid-containing ceramide in Sgpp1−/− compared with Sgpp1+/+ epidermis (Fig. 4C).
FIGURE 4.
Sphingolipid profile in Sgpp1−/− mouse epidermis. The sphingolipid profile was determined by HPLC-tandem MS on epidermis isolated from Sgpp1+/+ and Sgpp1−/− 2-day-old pups. Levels of sphingosine, S1P, and dihydro-S1P (A), total ceramide (B), and individual ceramide species with different fatty-acid chain lengths (C) were determined. Values are expressed as picomoles of sphingolipid per nmol of inorganic phosphorous (Pi). Bars represent mean values ± S.D. n = 5 for each genotype. Student's t test; *, p < 0.05. ns, not significant.
Abnormal Keratinocyte Proliferation and Differentiation in Sgpp1−/− Mice
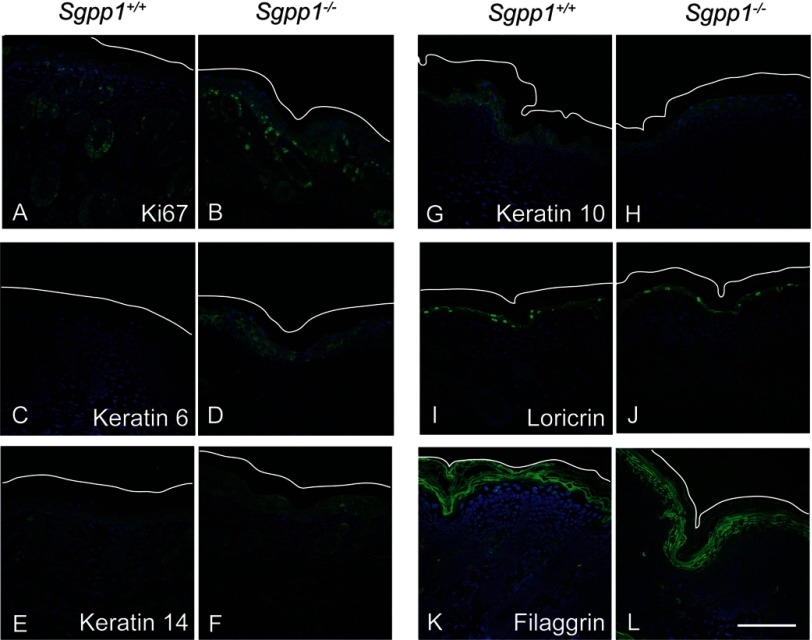
The thicker epidermal layers observed for Sgpp1-deficient skin suggested that increased keratinocyte proliferation was occurring in these mice. To address this possibility, we determined the expression of proliferative markers in skin sections from 2-day-old mice. In Sgpp1−/− skin, the expression of the proliferative antigen Ki67 was markedly increased in the stratum basale compared with Sgpp1+/+ skin, consistent with increased keratinocyte cell division (Fig. 5, A and B). We found highly elevated expression of hyperproliferation-associated keratin 6 (46–48) in Sgpp1−/− skin sections throughout the subcorneal epidermal layers (Fig. 5, C and D). Keratin 14, which is expressed in undifferentiated, mitotically active keratinocytes (49), was slightly increased throughout several epidermal layers in Sgpp1−/− skin compared with Sgpp1+/+ skin (Fig. 5, E and F). Significantly increased expression of keratin 14 mRNA in the skin of 2-day-old Sgpp1 null mice compared with WT mice was confirmed by RT-qPCR (supplemental Fig. S3A). These results indicate that the deletion of Sgpp1 leads to epidermal hyperplasia.
FIGURE 5.
Expression of proliferation and keratinocyte differentiation markers are increased in Sgpp1−/− skin. Paraffin sections of 2-day-old skin from Sgpp1+/+ and Sgpp1−/− mice were stained with antibodies against Ki67 (A and B), keratin 6 (C and D), keratin 14 (E and F), keratin 10 (G and H), loricrin (I and J), filaggrin (K and L) (green staining), and DAPI (blue). The white line represents the outer edge of the stratum corneum to mark the skin limit on the top of the image. Bar, 100 μm.
We also assessed markers of keratinocyte differentiation in skin sections of Sgpp1+/+ and Sgpp1−/− mice. Expression of keratin 10, a marker of differentiating keratinocytes after growth arrest (50), was expressed similarly in Sgpp1+/+ and Sgpp1−/− epidermis (Fig. 5, G and H). Expression of loricrin and filaggrin, two major proteins of the cornified cell envelope in the stratum corneum (51), was also determined. Loricrin expression appeared normal in Sgpp1−/− mice (Fig. 5, I and J), although filaggrin expression was expanded into the subcorneal epidermal layers and was relatively weakly expressed in the stratum corneum of the Sgpp1−/− mice (Fig. 5, K and L). We also quantified the expression of these differentiation-related genes by RT-qPCR. We found that mRNA expression of keratin 10 and loricrin was not significantly different between Sgpp1+/+ and Sgpp1−/− skin, although filaggrin mRNA levels were significantly increased in Sgpp1−/− skin compared with skin from WT mice (supplemental Fig. S3, B–D). Together, these results show that the epidermis of the Sgpp1−/− mice exhibits abnormalities both in keratinocyte differentiation and proliferation.
Adult Sgpp1−/− Mice Have an Ichthyosis-like Phenotype
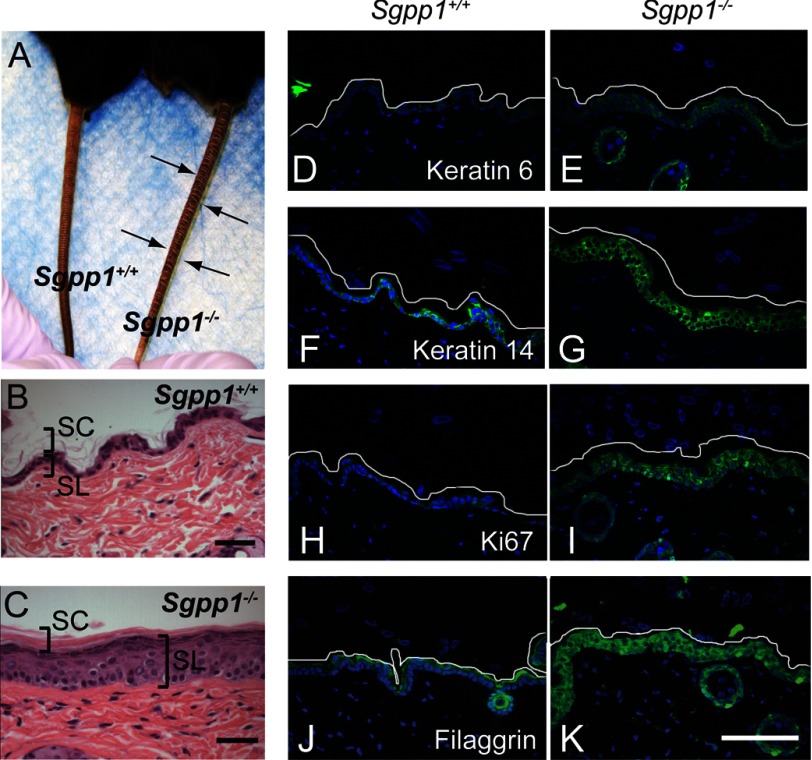
The rare surviving adult Sgpp1−/− mice were similar to Sgpp1+/+ mice in size (data not shown). However, the skin on their tails had an ichthyotic appearance (Fig. 6A). Histological analysis of dorsal skin (Fig. 6, B and C) from adult Sgpp1+/+ and Sgpp1−/− mice revealed that, as in the newborns, the Sgpp1−/− epidermis was significantly thicker than that from Sgpp1+/+ mice. The stratum corneum appeared dense and compact (Fig. 6C).
FIGURE 6.
Surviving Sgpp1−/− adult mice displayed abnormal skin phenotype. A, surviving adult Sgpp1−/− mouse and its Sgpp1+/+ littermate. Arrows indicate the deep constriction rings found on the tails of Sgpp1−/− mice. B and C, paraffin sections from Sgpp1+/+ (B) and Sgpp1−/− (C) adult mouse back skin stained with H&E. Bar, 200 μm. SC, stratum corneum; SL, subcorneal layer. D–K, immunohistochemistry of Sgpp1+/+ and Sgpp1−/− adult mouse skin. Paraffin sections were stained with antibodies against keratin 6 (D and E), keratin 14 (F and G), Ki67 antigen (H and I), filaggrin (J and K) (green staining), and DAPI (blue). The white line represents the edge of the stratum corneum to mark the skin limit on the top of the image. Bar, 100 μm.
Expression of keratin 6 (Fig. 6, D and E), keratin 14 (Fig. 6, F and G), and Ki67 antigen (Fig. 6, H and I) was elevated in subcortical layers of the Sgpp1−/− epidermis from adult mice, consistent with epidermal hyperplasia. In Sgpp1−/− epidermis, filaggrin expression was highly elevated and extended into deeper epidermal layers (Fig. 6, J and K) compared with Sgpp1+/+ epidermis.
We quantified S1P levels in Sgpp1+/+ and Sgpp1−/− plasma, and we found no significant difference (supplemental Fig. S4).
Sgpp1 Deletion Increases S1P Levels and Alters the Gene Expression Profile of Keratinocytes in Vitro
Because it was possible that the keratinocyte abnormalities observed in the Sgpp1−/− mice might be the result of extrinsic factors, such as an altered stratum corneum or an effect of the Sgpp1 deletion on other skin cell types, we wanted to determine whether the effect of the Sgpp1 deletion was intrinsic to keratinocytes. To investigate this, we isolated and grew keratinocytes from Sgpp1+/+ and Sgpp1−/− mice under low Ca2+ conditions, which impede differentiation, for 3–4 days (39).
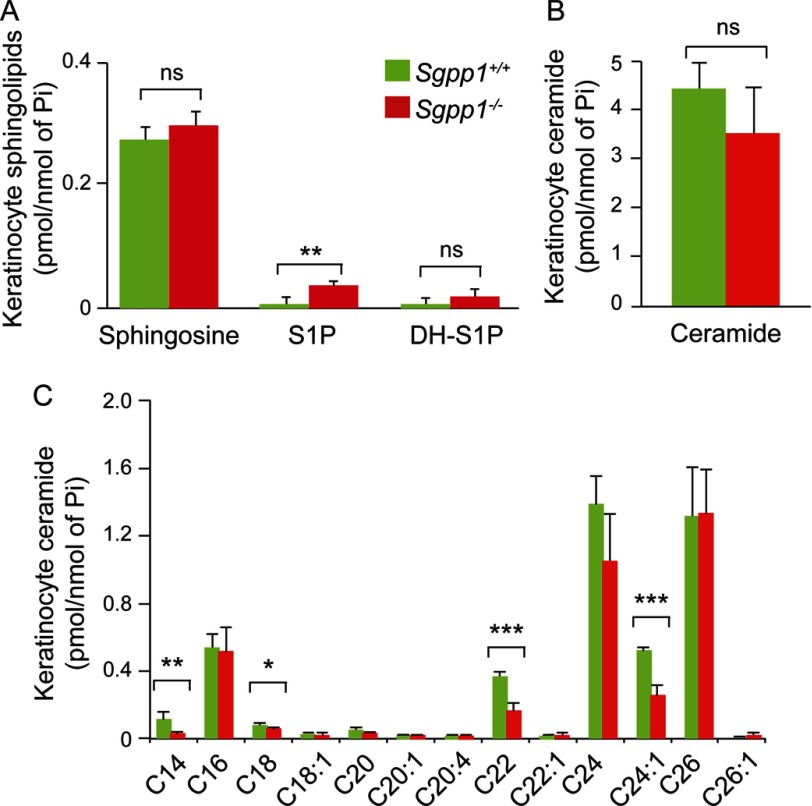
We first determined the sphingolipid profile of cultured keratinocytes by HPLC-tandem MS. We found that the S1P levels in the Sgpp1−/− keratinocytes were significantly increased compared with Sgpp1+/+ mice, although dihydro-S1P and sphingosine levels (Fig. 7A) as well as sphingomyelin levels (supplemental Fig. S1B) were unchanged. Although total ceramide levels were similar in keratinocytes from Sgpp1+/+ and Sgpp1−/− mice (Fig. 7B), the ceramide species containing fatty acid lengths of C14, C18, C22, and C24:1 in Sgpp1−/− keratinocytes were all significantly decreased compared with Sgpp1+/+ keratinocytes (Fig. 7C).
FIGURE 7.
Sphingolipid profile of Sgpp1−/− keratinocytes. Primary keratinocytes from skin of 1-day-old Sgpp1+/+ and Sgpp1−/− mice were grown in low Ca2+ media for 3–4 days. The sphingolipid profile of each culture was determined by HPLC-tandem MS. Levels of sphingosine, S1P, and dihydro-S1P (A), total ceramide (B), and individual ceramide fatty acid chain species (C) were determined. Values are expressed as picomoles of sphingolipid per nmol of inorganic phosphorous (Pi). Bars represent mean values ± S.D. n = 5 for each genotype. Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant.
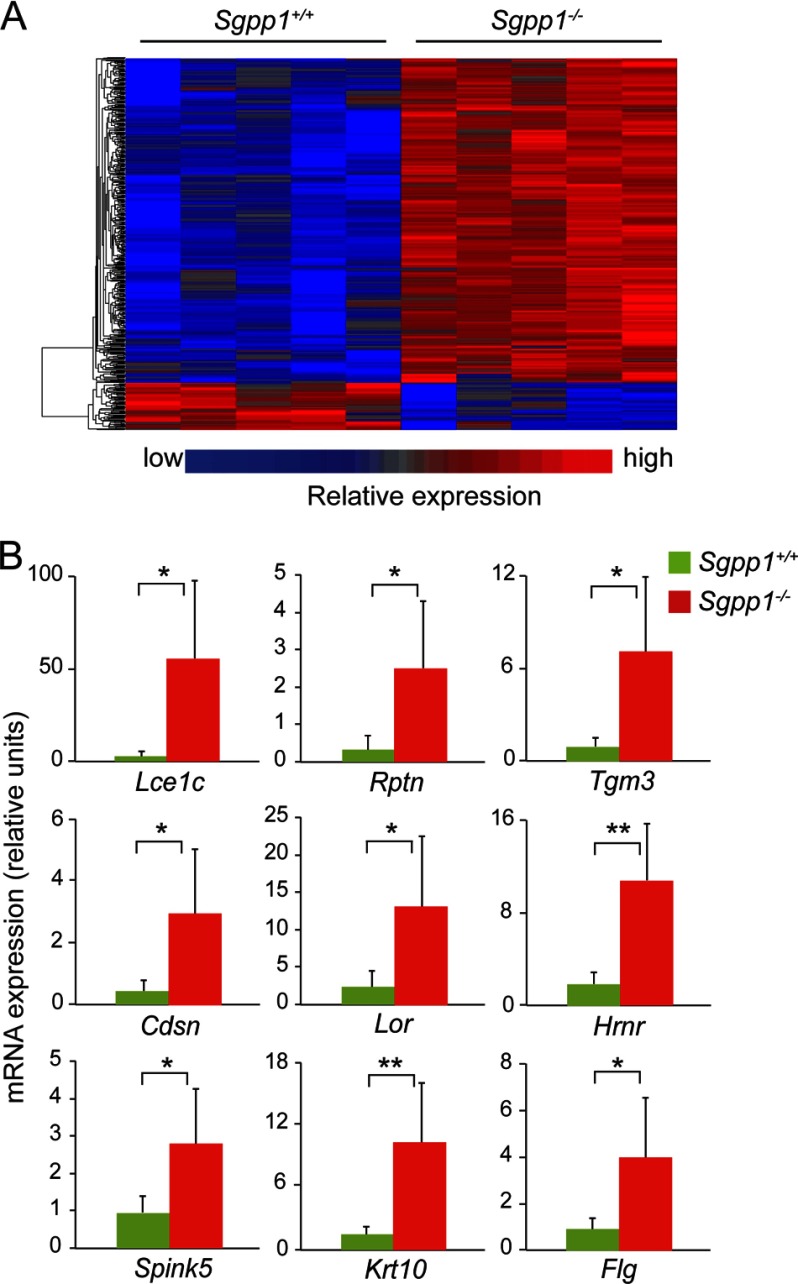
We next performed gene expression profiling on mRNA from cultured keratinocytes from Sgpp1+/+ and Sgpp1−/− mice using Affymetrix GeneChip Mouse Genome 430 2.0 arrays. From the microarray data, we identified more than 700 gene annotated probe sets that showed significant changes between keratinocytes from Sgpp1+/+ and Sgpp1−/− mice, using a cutoff of p < 0.05 and a fold change of greater than 2 (Fig. 8A). Examination of the top genes that were significantly overexpressed in the Sgpp1−/− keratinocytes revealed that many of them were associated with keratinocyte differentiation (Fig. 8B and supplemental Table 1). These included several genes of the late cornified envelope family (51) (Fig. 8B and supplemental Table 1). Indeed, the Gene Ontology categories “keratinization” (1.8-fold; p < 3.2E-09) and “cornified envelope” (2.7-fold; p < 6.7E-10) were significantly elevated in Sgpp1−/− keratinocytes when compared with Sgpp1+/+ keratinocytes (supplemental Fig. S5, A and B). These results indicate that the deletion of Sgpp1 has an intrinsic effect on keratinocytes that causes an elevated expression of genes associated with their differentiation.
FIGURE 8.
Gene expression profile of keratinocytes in vitro. A, microarray gene expression analysis was performed with RNA from five Sgpp1+/+ and five Sgpp1−/− keratinocyte cultures, isolated from individual mice, grown in low Ca2+ media for 3–4 days. The heat map shows the raw signal values of genes that are significantly different between Sgpp1+/+ and Sgpp1−/− groups, using a cutoff of p < 0.05 and a fold change of greater than 2. B, expression levels of nine genes among the top 50 determined by microarray analysis to be overexpressed in Sgpp1−/− keratinocytes were validated by RT-qPCR. Results are expressed as mean value ± S.D. n = 6 for both genotypes. Student's t test; *, p < 0.05; **, p < 0.01.
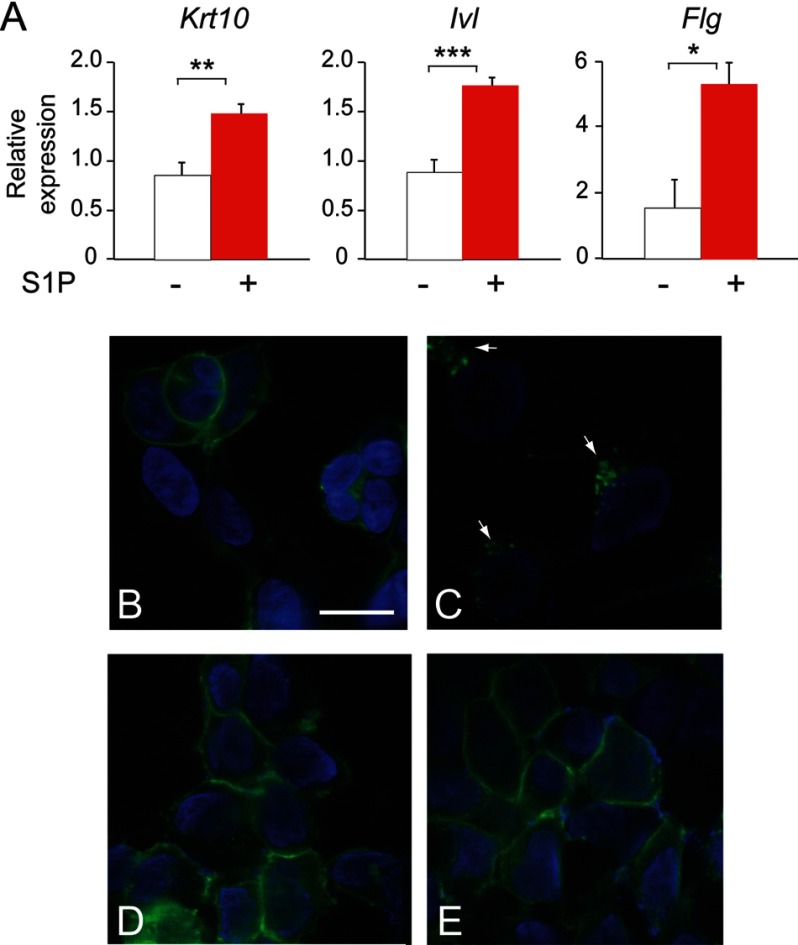
Treatment of WT keratinocytes with 10−7 m S1P also led to an increase in the expression of the differentiation-related genes keratin 10, involucrin, and filaggrin (Fig. 9A). This result indicates that elevating S1P levels is able to recapitulate the enhanced differentiation observed in Sgpp1 null keratinocytes and points to elevated S1P levels generated by the Sgpp1 deletion as responsible for the accelerated differentiation phenotype.
FIGURE 9.
Role of S1P in keratinocyte differentiation. A, mouse keratinocytes were incubated with 10−7 m S1P for 2 days, and gene expression was determined by RT-qPCR. Data are expressed as mean value ± S.D., n = 3 for each phenotype, and is representative of three independent experiments. Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001. B–E, S1PR1-GPF receptor-expressing HEK293 cells were incubated for 60 min with C-DMEM (B), 10−7 m S1P (C), Sgpp1+/+ conditioned medium (D), and Sgpp1−/− conditioned medium (E). Cells were then fixed and examined using a Zeiss confocal laser scanning microscope under a ×63 oil objective. Bar, 20 μm.
To further characterize the mechanism through which S1P regulates keratinocyte differentiation, we studied whether S1P was acting “outside-in” (52) by ligation of S1P receptors. We determined if there was sufficient S1P released by keratinocytes to induce internalization of a S1PR1-GFP chimeric receptor expressed on the plasma membrane of HEK293 cells (44). Addition of 10−7 m S1P completely induced internalization of the receptor from the plasma membrane to perinuclear vesicles as reported (44) (Fig. 9, B and C). However, after the addition of 5-day conditioned medium from Sgpp1+/+ or Sgpp1−/− keratinocytes, the S1PR1-GFP receptor remained at the plasma membrane (Fig. 9, D and E). The results show that there is insufficient S1P in keratinocyte cultures to induce S1PR1-GFP internalization, a surrogate for receptor activation.
Sgpp1 Deletion Increases Ca2+ Levels within the Epidermis
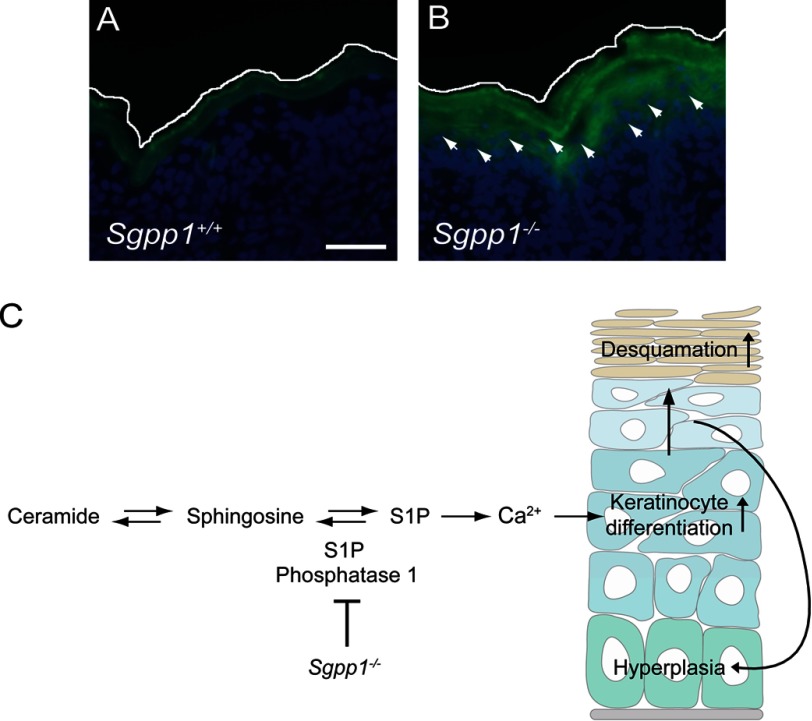
S1P has been shown to induce differentiation in keratinocytes by controlling intracellular Ca2+ levels (6, 32, 33, 34, 53). To determine whether epidermal Ca2+ was increased in vivo by the Sgpp1 deletion, Calcium Green-1, which exhibits a fluorescence intensity increase upon binding Ca2+, was used to detect Ca2+ in skin sections. Sgpp1+/+ skin showed fluorescence largely confined to the outer epidermal layers as described (Fig. 10A) (33, 54, 55). In contrast, Sgpp1−/− skin displayed higher fluorescence that extended well into the nucleated layers of the epidermis (Fig. 10B). This result indicates that the Sgpp1 deletion increases Ca2+ levels within epidermis.
FIGURE 10.
Mechanism for the skin phenotype found in Sgpp1−/− mice. A and B, Ca2+ distribution was visualized in situ by Calcium Green-1 staining on frozen skin sections from Sgpp1+/+ (A) and Sgpp1−/− (B) pups. Arrows indicate staining of nucleated cells in the Sgpp1−/− sections. The white line represents the outer edge of the stratum corneum to mark the skin limit on the top of the image. Bar, 100 μm. B and C, proposed mechanism. The S1P phosphatase encoded by Sgpp1 is relatively highly expressed in differentiating keratinocytes. Its deletion in keratinocytes raises S1P levels, triggering Ca2+-induced keratinocyte differentiation, resulting in a compensatory increase in keratinocyte proliferation leading to increased desquamation.
DISCUSSION
Here, we have demonstrated a novel role for Sgpp1 in the regulation of epidermal homeostasis. Mice with an inactive Sgpp1 gene developed an ichthyosis-like phenotype, characterized by desquamation, epidermal hyperplasia, and abnormal expression of keratinocyte differentiation markers. Focally severe desquamation began a few days after birth and is a possible cause of the demise of most of the Sgpp1−/− mice. Those Sgpp1−/− mice that survived developed ichthyotic skin that was evident on their tails. Other than the skin-related phenotype, adult Sgpp1−/− mice appeared grossly normal.
The lack of any other apparent major phenotypic consequences may be due to alternative pathways that serve to maintain low S1P levels in most tissue compartments. A major S1P degradative pathway is via S1P lyase-mediated cleavage, a process that occurs in many cells and tissues (56). S1P lyase-deficient mice exhibit highly elevated S1P levels in circulation and in tissues, along with diverse phenotypic aberrations, notably in the immune system (9, 57), but, interestingly, not the skin phenotype exhibited by the Sgpp1 null mice. The phenotypic expression of a defect within the epidermis in the Sgpp1−/− mice suggests that Sgpp1 might serve a unique function within differentiating keratinocytes.
As keratinocytes differentiate within the epidermis, they synthesize and package complex sphingolipids, such as sphingomyelin and β-glucosylceramide, along with other lipids, into lamellar bodies that are secreted by cells of the outer layers in the stratum granulosum (28). These complex sphingolipids in the lamellar bodies are then processed in the extracellular space into ceramides. Epidermal ceramides are critical for barrier function, accounting for 30–40% of the lipids in the stratum corneum (27, 58–60). The acquisition of a normal skin permeability barrier during embryonic development in the Sgpp1 null mice indicated that the sphingolipid metabolic pathway for the production of extracellular ceramides of the stratum corneum was not adversely impacted by the deletion of Sgpp1. Consistent with the acquisition of a normal permeability barrier were the normal ceramide and sphingomyelin levels in Sgpp1−/− skin.
Our results point to an intrinsic defect in the Sgpp1−/− keratinocytes as a mechanism to account for the abnormal epidermal phenotype in these mice (Fig. 10C). This conclusion is supported by the microarray analysis showing that Sgpp1−/− keratinocytes exhibit a gene expression profile distinct from WT keratinocytes, in which genes associated with keratinocyte differentiation and cornified envelope formation were significantly elevated. An intrinsic cellular defect resulting in accelerated keratinocyte differentiation would be consistent with the epidermal skin phenotype of the Sgpp1−/− mice, characterized by elevated keratinocyte differentiation markers, such as filaggrin and keratin 10. The keratinocyte hyperplasia in the Sgpp1 null epidermis may be explained as a secondary compensatory effect due to increased differentiation (Fig. 10C). The heightened keratinocyte growth and differentiation could then lead to the abnormal desquamation seen in the pups and the ichthyotic condition observed in the adult mice. Although accumulation of S1P was not found in the whole Sgpp1−/− epidermis, a severalfold increase in cellular S1P was detected in cultured Sgpp1−/− keratinocytes, which is consistent with increased intracellular levels of S1P as the cause of the keratinocyte defects (Fig. 10C). Moreover, S1P addition to WT keratinocytes enhanced their differentiation, as has been described (6, 32, 33, 53), further implicating S1P elevation in keratinocytes as the cause of the Sgpp1−/− epidermal phenotype.
S1P has been reported to induce Ca2+ signaling, a key process for epidermal and keratinocyte differentiation (5, 6, 31). The regulated release of Ca2+ from ER and Golgi stores, as well as the Ca2+ influx through Ca2+-permeable ion channels, induces the transcription of genes associated with keratinocyte differentiation, such as keratin 1 and 10, filaggrin, and loricrin (61). S1P has long been known to induce elevations of intracellular Ca2+ levels through the release from internal stores (5, 6), and its metabolism is believed to serve as a complementary Ca2+ signaling pathway to promote keratinocyte differentiation (31). Elevating S1P levels through sphingosine kinase activation or lowering S1P lyase levels has been shown to increase Ca2+ levels and promote differentiation (33, 34). Furthermore, the up-regulation of alkaline and acid ceramidases (see scheme in Fig. 1A) to increase sphingosine and S1P production mediates Ca2+-induced keratinocyte differentiation (62). The Sgpp1 deletion caused a striking elevation of Ca2+ indicator staining within the epidermis consistent with an abnormally enhanced Ca2+ response inducing the differentiation of keratinocytes. S1P exerts functional effects on keratinocytes through both direct intracellular and extracellular receptor-mediated signaling pathways (6). Our results are most consistent with an intracellular mechanism of S1P-induced differentiation, rather than an extracellular receptor-mediated effect, because we could not detect extracellular, bioactive S1P secreted by Sgpp1−/− keratinocytes.
The expression of Sgpp1 on the differentiating layers of the epithelium supports the notion that SPP1 activity may be important in controlling S1P levels as keratinocytes differentiate within the skin. Overall, the results are consistent with the possibility that the activation of sphingolipid metabolism signals keratinocytes to differentiate via the attendant production of bioactive S1P. De novo sphingolipid synthesis in epidermis is up-regulated after epidermal damage (63). One function of this increased de novo synthesis after injury is believed to be for the replenishment of barrier lipids. Another may be to provide a lipid signal, via S1P, to promote a keratinocyte-differentiation program.
The functions of the SPPs, in a physiological context, have been enigmatic. Here, we have defined a novel functional role for SPP1 in keratinocyte differentiation and in skin biology. The importance of SPP1 in keratinocytes may identify a key role for S1P metabolism and signaling in their function and may suggest therapeutic avenues for skin diseases with defects in keratinocyte differentiation.
Supplementary Material
Acknowledgment
We thank Maria Irene Morasso for useful discussions and for providing reagents.
This work was supported, in whole or in part, by National Institutes of Health grants from NIDDK, Grant P30 CA138313 (to Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina), and Grant P20 RR017677 (to Lipidomics Core in the South Carolina Lipidomics and Pathobiology COBRE).
This article was selected as a Paper of the Week.

This article contains supplemental Figs. S1–S5 and Table S1.
- S1P
- sphingosine 1-phosphate
- SPP
- sphingosine-1-phosphate phosphatase
- LPP
- lipid phosphate phosphatase
- ER
- endoplasmic reticulum
- RT-qPCR
- real time-quantitative PCR
- E
- embryonic day.
REFERENCES
- 1. Rivera J., Proia R. L., Olivera A. (2008) The alliance of sphingosine 1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8, 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fyrst H., Saba J. D. (2010) An update on sphingosine 1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 6, 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine 1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 5. Spiegel S., Milstien S. (2003) Sphingosine 1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 6. Meyer Zu Heringdorf D. (2004) Lysophospholipid receptor-dependent and -independent calcium signaling. J. Cell Biochem. 92, 937–948 [DOI] [PubMed] [Google Scholar]
- 7. Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine 1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine 1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bektas M., Allende M. L., Lee B. G., Chen W., Amar M. J., Remaley A. T., Saba J. D., Proia R. L. (2010) Sphingosine-1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 285, 10880–10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merrill A. H., Jr. (2011) Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111, 6387–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pyne S., Long J. S., Ktistakis N. T., Pyne N. J. (2005) Lipid phosphate phosphatases and lipid phosphate signalling. Biochem. Soc. Trans. 33, 1370–1374 [DOI] [PubMed] [Google Scholar]
- 12. Le Stunff H., Peterson C., Liu H., Milstien S., Spiegel S. (2002) Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta 1582, 8–17 [DOI] [PubMed] [Google Scholar]
- 13. Sciorra V. A., Morris A. J. (2002) Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim. Biophys. Acta 1582, 45–51 [DOI] [PubMed] [Google Scholar]
- 14. Brindley D. N., Pilquil C. (2009) Lipid phosphate phosphatases and signaling. J. Lipid Res. 50, S225–S230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mandala S. M., Thornton R., Galve-Roperh I., Poulton S., Peterson C., Olivera A., Bergstrom J., Kurtz M. B., Spiegel S. (2000) Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine 1-phosphate and induces cell death. Proc. Natl. Acad. Sci. U.S.A. 97, 7859–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mao C., Wadleigh M., Jenkins G. M., Hannun Y. A., Obeid L. M. (1997) Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem. 272, 28690–28694 [DOI] [PubMed] [Google Scholar]
- 17. Mandala S. M., Thornton R., Tu Z., Kurtz M. B., Nickels J., Broach J., Menzeleev R., Spiegel S. (1998) Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. U.S.A. 95, 150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Stunff H., Peterson C., Thornton R., Milstien S., Mandala S. M., Spiegel S. (2002) Characterization of murine sphingosine-1-phosphate phosphohydrolase. J. Biol. Chem. 277, 8920–8927 [DOI] [PubMed] [Google Scholar]
- 19. Ogawa C., Kihara A., Gokoh M., Igarashi Y. (2003) Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hsp26. J. Biol. Chem. 278, 1268–1272 [DOI] [PubMed] [Google Scholar]
- 20. Breard B., Ramos-Perez W. D., Mendoza A., Salous A. K., Gobert M., Huang Y., Adams R. H., Lafaille J. J., Escalante-Alcalde D., Morris A. J., Schwab S. R. (2011) Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giussani P., Maceyka M., Le Stunff H., Mikami A., Lépine S., Wang E., Kelly S., Merrill A. H., Jr., Milstien S., Spiegel S. (2006) Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-Golgi trafficking of ceramide. Mol. Cell. Biol. 26, 5055–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lépine S., Allegood J. C., Edmonds Y., Milstien S., Spiegel S. (2011) Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. J. Biol. Chem. 286, 44380–44390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lépine S., Allegood J. C., Park M., Dent P., Milstien S., Spiegel S. (2011) Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. 18, 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peter B. F., Lidington D., Harada A., Bolz H. J., Vogel L., Heximer S., Spiegel S., Pohl U., Bolz S. S. (2008) Role of sphingosine-1-phosphate phosphohydrolase 1 in the regulation of resistance artery tone. Circ. Res. 103, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Stunff H., Mikami A., Giussani P., Hobson J. P., Jolly P. S., Milstien S., Spiegel S. (2004) Role of sphingosine-1-phosphate phosphatase 1 in epidermal growth factor-induced chemotaxis. J. Biol. Chem. 279, 34290–34297 [DOI] [PubMed] [Google Scholar]
- 26. Mechtcheriakova D., Wlachos A., Sobanov J., Kopp T., Reuschel R., Bornancin F., Cai R., Zemann B., Urtz N., Stingl G., Zlabinger G., Woisetschläger M., Baumruker T., Billich A. (2007) Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell. Signal. 19, 748–760 [DOI] [PubMed] [Google Scholar]
- 27. Feingold K. R. (2009) The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50, S417–S422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holleran W. M., Takagi Y., Uchida Y. (2006) Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 580, 5456–5466 [DOI] [PubMed] [Google Scholar]
- 29. Elias P. M., Feingold K. R. (1992) Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin. Dermatol. 11, 176–182 [PubMed] [Google Scholar]
- 30. Schuette C. G., Pierstorff B., Huettler S., Sandhoff K. (2001) Sphingolipid activator proteins: proteins with complex functions in lipid degradation and skin biogenesis. Glycobiology 11, 81R–90R [DOI] [PubMed] [Google Scholar]
- 31. Herzinger T., Kleuser B., Schäfer-Korting M., Korting H. C. (2007) Sphingosine 1-phosphate signaling and the skin. Am. J. Clin. Dermatol. 8, 329–336 [DOI] [PubMed] [Google Scholar]
- 32. Vogler R., Sauer B., Kim D. S., Schäfer-Korting M., Kleuser B. (2003) Sphingosine 1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J. Invest. Dermatol. 120, 693–700 [DOI] [PubMed] [Google Scholar]
- 33. Hong J. H., Youm J. K., Kwon M. J., Park B. D., Lee Y. M., Lee S. I., Shin D. M., Lee S. H. (2008) K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J. Invest. Dermatol. 128, 2166–2178 [DOI] [PubMed] [Google Scholar]
- 34. Celli A., Mackenzie D. S., Zhai Y., Tu C. L., Bikle D. D., Holleran W. M., Uchida Y., Mauro T. M. (2012) SERCA2-controlled Ca2+-dependent keratinocyte adhesion and differentiation is mediated via the sphingolipid pathway: a therapeutic target for Darier's disease. J. Invest. Dermatol. 132, 1188–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim D. S., Kim S. Y., Kleuser B., Schäfer-Korting M., Kim K. H., Park K. C. (2004) Sphingosine 1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell. Signal. 16, 89–95 [DOI] [PubMed] [Google Scholar]
- 36. Schmitz E. I., Potteck H., Schuppel M., Manggau M., Wahydin E., Kleuser B. (2012) Sphingosine 1-phosphate protects primary human keratinocytes from apoptosis via nitric oxide formation through the receptor subtype S1P(3). Mol. Cell. Biochem. 371, 165–176 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., Liu C. H., Hla T., Spiegel S., Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denda M., Hosoi J., Asida Y. (2000) Visual imaging of ion distribution in human epidermis. Biochem. Biophys. Res. Commun. 272, 134–137 [DOI] [PubMed] [Google Scholar]
- 39. Lichti U., Anders J., Yuspa S. H. (2008) Isolation and short-term culture of primary keratinocytes, hair follicle populations, and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 3, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hwang J., Kita R., Kwon H. S., Choi E. H., Lee S. H., Udey M. C., Morasso M. I. (2011) Epidermal ablation of Dlx3 is linked to IL-17-associated skin inflammation. Proc. Natl. Acad. Sci. U.S.A. 108, 11566–11571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vasireddy V., Uchida Y., Salem N., Jr., Kim S. Y., Mandal M. N., Reddy G. B., Bodepudi R., Alderson N. L., Brown J. C., Hama H., Dlugosz A., Elias P. M., Holleran W. M., Ayyagari R. (2007) Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 16, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 43. Saitoh M., Sakakihara Y., Mizuguchi M., Itoh M., Takashima S., Iwamori M., Kamoshita S., Igarashi T. (2007) Increase of ceramide monohexoside and dipalmitoylglycerophospholipids in the brain of Zellweger syndrome. Neurosci. Lett. 417, 165–170 [DOI] [PubMed] [Google Scholar]
- 44. Liu C. H., Thangada S., Lee M. J., Van Brocklyn J. R., Spiegel S., Hla T. (1999) Ligand-induced trafficking of the sphingosine 1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hardman M. J., Sisi P., Banbury D. N., Byrne C. (1998) Patterned acquisition of skin barrier function during development. Development 125, 1541–1552 [DOI] [PubMed] [Google Scholar]
- 46. Weiss R. A., Eichner R., Sun T. T. (1984) Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kilodalton keratin as molecular markers for hyperproliferative keratinocytes. J. Cell Biol. 98, 1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mansbridge J. N., Knapp A. M. (1987) Changes in keratinocyte maturation during wound healing. J. Invest. Dermatol. 89, 253–263 [DOI] [PubMed] [Google Scholar]
- 48. Stoler A., Kopan R., Duvic M., Fuchs E. (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J. Cell Biol. 107, 427–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fuchs E., Cleveland D. W. (1998) A structural scaffolding of intermediate filaments in health and disease. Science 279, 514–519 [DOI] [PubMed] [Google Scholar]
- 50. Fuchs E., Green H. (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042 [DOI] [PubMed] [Google Scholar]
- 51. Candi E., Schmidt R., Melino G. (2005) The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 [DOI] [PubMed] [Google Scholar]
- 52. Spiegel S., Milstien S. (2000) Sphingosine 1-phosphate: signaling inside and out. FEBS Lett. 476, 55–57 [DOI] [PubMed] [Google Scholar]
- 53. Kwon Y. B., Kim C. D., Youm J. K., Gwak H. S., Park B. D., Lee S. H., Jeon S., Kim B. J., Seo Y. J., Park J. K., Lee J. H. (2007) Novel synthetic ceramide derivatives increase intracellular calcium levels and promote epidermal keratinocyte differentiation. J. Lipid Res. 48, 1936–1943 [DOI] [PubMed] [Google Scholar]
- 54. Menon G. K., Grayson S., Elias P. M. (1985) Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J. Invest. Dermatol. 84, 508–512 [DOI] [PubMed] [Google Scholar]
- 55. Elias P. M., Nau P., Hanley K., Cullander C., Crumrine D., Bench G., Sideras-Haddad E., Mauro T., Williams M. L., Feingold K. R. (1998) Formation of the epidermal calcium gradient coincides with key milestones of barrier ontogenesis in the rodent. J. Invest. Dermatol. 110, 399–404 [DOI] [PubMed] [Google Scholar]
- 56. Olivera A., Allende M. L., Proia R. L. (2013) Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta 183, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Allende M. L., Bektas M., Lee B. G., Bonifacino E., Kang J., Tuymetova G., Chen W., Saba J. D., Proia R. L. (2011) Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Holleran W. M., Man M. Q., Gao W. N., Menon G. K., Elias P. M., Feingold K. R. (1991) Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J. Clin. Invest. 88, 1338–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elias P. M., Menon G. K. (1991) Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 24, 1–26 [DOI] [PubMed] [Google Scholar]
- 60. Uchida Y., Holleran W. M. (2008) ω-O-Acylceramide, a lipid essential for mammalian survival. J. Dermatol. Sci. 51, 77–87 [DOI] [PubMed] [Google Scholar]
- 61. Li L., Tucker R. W., Hennings H., Yuspa S. H. (1995) Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J. Cell Physiol. 163, 105–114 [DOI] [PubMed] [Google Scholar]
- 62. Sun W., Xu R., Hu W., Jin J., Crellin H. A., Bielawski J., Szulc Z. M., Thiers B. H., Obeid L. M., Mao C. (2008) Up-regulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J. Invest. Dermatol. 128, 389–397 [DOI] [PubMed] [Google Scholar]
- 63. Harris I. R., Farrell A. M., Grunfeld C., Holleran W. M., Elias P. M., Feingold K. R. (1997) Permeability barrier disruption coordinately regulates mRNA levels for key enzymes of cholesterol, fatty acid, and ceramide synthesis in the epidermis. J. Invest. Dermatol. 109, 783–787 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.