Background: Signaling events affected by disease-associated mutations in TRPC6 are poorly defined.
Results: Expression of mutant TRPC6 induces ERK1/2 activation via both cell-autonomous and non-cell-autonomous mechanisms.
Conclusion: Mutant TRPC6 activates complex signaling pathways that lead to the release of paracrine factors activating ERK.
Significance: Understanding the signaling pathways downstream of gain-of-function TRPC6 is crucial for understanding TRPC6-mediated biology and pathology.
Keywords: Calcium, Epidermal Growth Factor Receptor (EGFR), Kidney, Protein Kinase A (PKA), TRP Channels, Focal Segmental Glomerulosclerosis
Abstract
Gain-of-function mutations in the canonical transient receptor potential 6 (TRPC6) gene are a cause of autosomal dominant focal segmental glomerulosclerosis (FSGS). The mechanisms whereby abnormal TRPC6 activity results in proteinuria remain unknown. The ERK1/2 MAPKs are activated in glomeruli and podocytes in several proteinuric disease models. We therefore examined whether FSGS-associated mutations in TRPC6 result in activation of these kinases. In 293T cells and cultured podocytes, overexpression of gain-of-function TRPC6 mutants resulted in increased ERK1/2 phosphorylation, an effect dependent upon channel function. Pharmacologic inhibitor studies implicated several signaling mediators, including calmodulin and calcineurin, supporting the importance of TRPC6-mediated calcium influx in this process. Through medium transfer experiments, we uncovered two distinct mechanisms for ERK activation by mutant TRPC6, a cell-autonomous, EGF receptor-independent mechanism and a non-cell-autonomous mechanism involving metalloprotease-mediated release of a presumed EGF receptor ligand. The inhibitors KN-92 and H89 were able to block both pathways in mutant TRPC6 expressing cells as well as the prolonged elevation of intracellular calcium levels upon carbachol stimulation seen in these cells. However, these effects appear to be independent of their effects on calcium/calmodulin-dependent protein kinase II and PKA, respectively. Phosphorylation of Thr-70, Ser-282, and Tyr-31/285 were not necessary for ERK activation by mutant TRPC6, although a phosphomimetic TRPC6 S282E mutant was capable of ERK activation. Taken together, these results identify two pathways downstream of mutant TRPC6 leading to ERK activation that may play a role in the development of FSGS.
Introduction
The canonical transient receptor potential 6 (TRPC6) channel is a member of the transient receptor potential (TRP) superfamily of six transmembrane cation channels (1, 2). TRPC subunits assemble to form functional homo- and/or heterotetramers, with TRPC6 capable of oligomerizing with TRPC1, -3, -7, and itself (3). Several stimuli have been shown to activate the channel, including Gαq-coupled receptors (4, 5) and receptor tyrosine kinases (6) as well as direct binding of diacylglycerol (7). In the case of receptor-mediated activation, regulated exocytosis of the channel is at least partially responsible for increasing channel activity (5, 6, 8). Membrane deformation has also been reported to activate the channel directly (9), although this mechanism remains controversial (10).
Mutations in the TRPC6 gene are a cause of autosomal dominant focal segmental glomerulosclerosis (FSGS),2 a progressive kidney disease frequently leading to end-stage kidney disease (11, 12). The majority of mutations identified to date have been shown to be gain-of-function, increasing whole cell current amplitudes and/or decreasing channel inactivation (11–14). The mutations map to various segments of the protein, predominantly the ankyrin repeats in the amino-terminal cytoplasmic domain and a putative coiled-coil sequence in the carboxyl-terminal cytoplasmic domain. The mechanism(s) whereby these mutations enhance channel activity remains unclear, although there are suggestions that some mutations may alter cell surface localization (12) and binding to nephrin (8). Additional evidence suggests that TRPC6 plays an important role in podocyte function. TRPC6 is up-regulated in several acquired proteinuric kidney diseases (15), transgenic overexpression of wild-type or mutant TRPC6 in podocytes induces a mild glomerular phenotype (16), and TRPC6-deficient mice show some resistance to angiotensin II-induced proteinuria (17). TRPC6 has also been implicated in regulating blood pressure (18), pulmonary vascular tone and permeability (19–24), cardiac hypertrophy (25–27), myofibroblast differentiation and associated wound healing (28, 29), neuronal growth cone guidance (30), and platelet function (31). Why mutations in TRPC6 lead specifically to kidney disease is unknown.
Several signaling pathways have been shown to be regulated downstream of TRPC6. For example, in cardiac myoblasts and fibroblasts, TRPC6 activates the calcineurin-NFAT (nuclear factor of activated T-cells) pathway (25–27), whereas in podocytes, FSGS-associated mutations in TRPC6 increase basal activation of this pathway (32). Calcineurin activation in podocytes has, in turn, been implicated in mediating proteinuria in several murine models (33), whereas forced expression of constitutively active NFATc1 in podocytes rapidly leads to podocyte effacement and proteinuria (34). In addition to activating calcineurin, TRPC6 activates the small GTPase Rho, while antagonizing the activation of Rac, in podocytes and other cell types (35–37). Excess Rho activity in podocytes induces proteinuria and glomerular dysfunction (38, 39). The importance of calcineurin and Rho activation in mediating TRPC6-induced glomerular pathology has not been established in vivo and awaits investigation in an animal model. Finally, TRPC6 has been shown to be involved in activation of ERK downstream of PDGF signaling in dopaminergic neurons (40).
The Raf-MEK-ERK pathway is one of three canonical mitogen-activated protein kinase signaling pathways and plays a central role in multiple essential cellular functions, including proliferation, survival, and differentiation (41–44). The pathway represents one of the first kinase cascades identified, with Raf phosphorylating MEK, which in turn phosphorylates, and thereby activates, ERK1 and -2. Numerous proteins and signaling pathways can influence this cascade through input at its various levels, with activation of Raf by the small GTPase Ras representing the classical pathway. Similarly, the output can be wildly disparate, with over 160 substrates for ERK1 and -2 identified (45–47) as capable of affecting diverse responses (46). The mechanisms whereby the pathway is able to execute specific responses to different stimuli remain incompletely understood, although the use of scaffolding proteins and distinct pools of kinases in different subcellular locales appears critical (48).
Several lines of evidence suggest that ERK activation occurs in glomeruli, and in podocytes and mesangial cells specifically, in response to pathological stresses. ERK phosphorylation, and thereby activation, is increased in glomeruli and podocytes in response to angiotensin II infusion (49–52), salt-sensitive hypertension (53), puromycin aminoglycoside nephropathy (54–58), passive Heymann nephritis (59, 60), rapidly progressive glomerulonephritis (61–64), and diabetic nephropathy (65–67). Phospho-ERK levels in mesangial cells have been correlated with glomerular damage in IgA nephropathy (68). In podocytes, ERK has been implicated in mediating actin reorganization, cell migration, and proliferation (61) and in acting as a both proapoptotic (56, 69–71) and antiapoptotic/prosurvival (58, 60, 72–74) signal.
In the present study, we demonstrate that disease-associated, gain-of-function mutations in TRPC6 induce ERK activation in several cell types, including cultured podocytes, with at least two distinct pathways involved, one cell-autonomous and one non-cell-autonomous. Both pathways and calcium influx downstream of mutant TRPC6 activation are inhibited by the small molecules KN-92 and H89, although the targets of their actions remain unclear. Finally, several reported phosphorylation sites on TRPC6 are not required for the action of mutant TRPC6, although a phosphomimetic S282E mutation is sufficient to induce ERK activation. These results identify ERK as a potential mediator of focal segmental glomerulosclerosis induced by TRPC6 mutations.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids
Pharmacologic reagents were purchased from the following vendors: carbachol, cyclosporine A, forskolin, H89, KN-92, KN-93, tetracycline, and Y-27632 from Sigma-Aldrich; U0126 and tyrphostin AG 1478 from Cell Signaling Biotechnology; bisindolylmaleimide I/Gö6850, Compound C, Gö6976, Gö6983, GW 5074, STO-609, and W-7 from EMD Biosciences; batimastat, marimastat, and GW6001 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); conjugated, cell-permeable C3 transferase (Rho inhibitor I) from Cytoskeleton, Inc.
Antibodies used in this study were from the following commercial sources: Cell Signaling Technology (rabbit phospho-ERK1/2, catalog no. 4370; rabbit ERK1/2, catalog no. 4695; mouse ERK1/2, catalog no. 9107; rabbit and mouse anti-HA antibodies, catalog nos. 3724 and 2367; phospho-Ser/Thr PKA substrate antibody, catalog no. 9621; HRP-conjugated anti-rabbit and anti-mouse IgG secondary antibodies, catalog nos. 7074 and 7076), Santa Cruz Biotechnology, Inc. (rabbit PKA Cα, sc-903), Sigma-Aldrich (FLAG M2, unconjugated (F3165), FITC-labeled (F4049), and conjugated to agarose (A2220); HA mouse monoclonal conjugated to agarose (A2095)). Fluorescence-conjugated secondary antibodies and phalloidin were from Jackson ImmunoResearch and Invitrogen, respectively.
The plasmids pcDNA3.1 and pcDNA4/TO/myc-His B (Invitrogen), containing the full-length human TRPC6 ORF with an amino-terminal FLAG tag sequence and various FSGS-associated mutations as well as the dominant-negative pore mutation changing amino acids LFW to AAA, have been described previously (32). HA tagged wild-type and R895C mutant TRPC6 were generated by standard PCR-based strategies and cloned into pcDNA3/myc-His A (Invitrogen). Phosphorylation site mutations were introduced using the Invitrogen QuikChange XL kit, following the manufacturer's protocols, and confirmed by sequencing. Plasmids for expressing GFP fused to the calcium/calmodulin-dependent protein kinase II (CaMKII)-inhibitory peptide (GFP-AC3-I) or scrambled control peptide (GFP-AC3-C) were provided by Dr. M. Anderson (75). FLAG-tagged wild-type and kinase mutant (K73M) PKA Cα expression constructs were kindly provided by Drs. Murshid and Calderwood (76).
Cell Culture
M1R cells, generated by expressing the M1 acetylcholine receptor in T-REx-293 cells (Invitrogen), as well as stable clones of M1R cells expressing tetracycline-inducible wild-type FLAG-TRPC6 or mutant FLAG-TRPC6 R895C were maintained as previously described (32). TRPC6 expression was induced by growing cells in medium supplemented with 2 μg/ml tetracycline or 0.1 μg/ml doxycycline for 18–48 h prior to use. HEK 293T cells were obtained from GeneHunter and maintained in DMEM with 4.5 g/liter glucose and supplemented with 10% fetal bovine serum (Atlanta Biologicals) and antibiotics. A conditionally immortalized murine podocyte cell line was generated from a temperature-sensitive SV40 large T antigen transgenic mouse (Charles River, St. Louis) as reported previously (77) and was maintained on collagen I-coated tissue culture plates in RPMI medium with 10% FBS and interferon γ (20 units/ml; Sigma-Aldrich) at 33 °C; cells were shifted to medium without interferon and cultured at 37 °C to induce differentiation. Human conditionally immortalized podocytes were provided by Dr. M. Saleem and cultured as recommended (78).
Transient transfections of M1R cell derivatives and 293T cells were performed using FuGENE6 or XtremeGENE 9 (Roche Applied Science) following the manufacturer's protocol. Cells were processed 18–48 h after transfection. Murine podocytes were transfected 48–72 h after differentiation using an Amaxa Nucleofector apparatus with program T-13 and the Basic Nucleofector Kit for Primary Mammalian Epithelial Cells (Lonza) and replated onto collagen I-coated plates for 24 h prior to analysis.
The siRNA experiments were performed by reverse transfection using Lipofectamine RNAiMax and pools of either control siRNA or siRNA targeting PKA Cα (Santa Cruz Biotechnology, Inc.). 36–48 h after transfection, cells were treated with or without doxycycline overnight to induce TRPC6 expression.
For inhibitor studies, cells were first treated with or without tetracycline (2 μg/ml) for 16–24 h. The medium was then replaced with complete medium containing the indicated inhibitors at the following concentrations: 25 μm W-7, 5 μm KN-92 and -93, 5 μm STO-609, 10 μm cyclosporine A, 40 μm Compound C, 1 μm Gö6976, 1 μm Gö6983, 5 μm U0126, 10 μm GW5074, 10 μm H89, 100 nm tyrphostin AG 1478, 20 μm batimastat, 20 μm marimastat, 20 μm GW6001, 1 μg/ml C3 transferase, 10 μm Y-27632. Equal final concentrations of DMSO carrier were present under all conditions. After 3 h of treatment with inhibitors, cells were lysed and processed for analysis.
Immunoprecipitation and Immunoblotting
Cells were rinsed once in phosphate-buffered saline with calcium and phosphate and lysed in TBS with 1% Triton X-100 (50 mm Tris, pH 7.4, 150 mm NaCl, 1% (v/v) Triton X-100), modified radioimmune precipitation assay lysis buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate), or low salt lysis buffer (50 mm Tris, pH 8.0, 100 mm NaCl, 5 mm EDTA, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate), all supplemented with Complete protease inhibitor mixture and PhosStop (Roche Applied Science). Lysates were cleared by centrifugation at 17,000 × g for 15 min at 4 °C. An aliquot was mixed with 4× SDS-sample loading buffer with β-mercaptoethanol, incubated at 95 °C for 5 min, and used to assay protein expression by Western blot.
For immunoprecipitation, cleared lysates were incubated with 15 μl of FLAG M2 or anti-HA-agarose slurry (Sigma-Aldrich) and incubated with constant agitation at 4 °C for 2–3 h. Immunoprecipitated complexes were washed three times with lysis buffer and eluted off of the beads by boiling in SDS-sample loading buffer.
Cell lysates and immunoprecipitated materials were separated by SDS-PAGE using TGX gels (Bio-Rad) and transferred to PVDF membrane (Bio-Rad). The membrane was blocked with 5% bovine serum albumin (BSA) in TBST (TBS with 0.05% Tween-20) for 1 h at room temperature, followed by incubation with primary antibody in 5% BSA TBST using dilutions recommended by the supplier. After three washes in TBST, blots were incubated with the appropriate secondary antibody conjugated to HRP (Cell Signaling Technology) in TBST at room temperature, followed by detection with SuperSignal West Dura chemiluminescent substrate (Pierce). Images were obtained using autoradiography film or with a FluorChem Q imager (Cell Biosciences).
In Vitro Kinase Assay
HA-TRPC6 was immunoprecipitated from cell lysates with anti-HA antibody-conjugated agarose and washed extensively with lysis buffer followed by 1× PKA phosphorylation buffer (50 mm Tris-HCl, 10 mm MgCl2, pH 7.5). The sample was divided into two sets and incubated in PKA buffer with 200 μm ATP with or without 2,500 units of PKA catalytic subunit (New England Biolabs) at 30 °C for 1 h. The samples were then washed twice with lysis buffer, incubated in 2× sample loading buffer at 95 °C, and processed for Western blot analysis.
Immunofluorescence
Cells were cultured on 12-mm poly-d-lysine-covered coverslips (BD 354086) in 24-well plates until 60–70% confluent, followed by the addition of tetracycline were indicated. Twenty-four hours later, coverslips were passed to clean 24-well plates, and cells were washed twice with cold PBS, fixed in 2% formaldehyde, and permeabilized with 0.3% Triton X-100. Cells were blocked for 1 h in PBS with 1% BSA and 0.5% Tween 20 and then incubated sequentially with primary and secondary antibodies in the same blocking solution for 1 h each. Nuclei were counterstained with 2 μg/ml Hoechst solution, and coverslips were mounted on glass slides with Prolong Gold antifade reagent (Invitrogen). Digital images were captured using an RT Slider Diagnostic Instruments digital camera connected to an Olympus BX60 microscope.
Calcium Imaging
Intracellular calcium concentration analysis of cell populations was performed using Fura-2/AM (Invitrogen) following the manufacturer's general recommendations. Briefly, cells were cultured in 96-well black, clear bottom plates (Costar) until 70% densities and then treated with or without tetracycline (2 μg/ml) for 24 h to induce FLAG-TRPC6 WT or R895C expression. Cells were dye-loaded with 2 μm Fura-2 in 50 μl of calcium uptake buffer (1× Hanks' balanced salt solution, 20 mm HEPES, 2.5 mm probenecid) for 30 min at 37 °C in a cell incubator. Medium was then exchanged for 75 μl of calcium uptake buffer alone for another 30 min. For inhibitor studies, inhibitors or DMSO carrier was present during this incubation.
Fura-2 fluorescence signal capture was performed in a FlexStation 3 multimode microplate reader (Molecular Devices) calibrated to 37 °C using SoftMax Pro version 5.4 software. Fluorescence signals were sequentially measured with excitation at 340 and 380 nm and emission at 510 nm to obtain a 340/380 ratio as a surrogate for intracellular calcium concentrations. Data were collected every 5 s. The base-line ratio was obtained for 1 min, followed by the addition of 25 μl of calcium uptake buffer with or without carbachol, and signal was captured every 5 s for another 5 min.
Transactivation Assay
Tetracycline-inducible FLAG-TRPC6 cells were grown with or without tetracycline or doxycycline for 18–24 h. Conditioned medium from the cells was harvested and cleared of cell debris by centrifugation. Various inhibitors were either present during the entire medium conditioning step or added after medium harvest, as indicated. HEK 293T cells were exposed to either unconditioned or conditioned medium as indicated, followed by lysis and Western blot analysis.
Statistical Analysis
Western blot band intensities were quantified using AlphaView Q SA version 3.2.2 (Cell Biosciences) and normalized to control conditions. Statistical analysis was performed using GraphPad Prism 5.
RESULTS
Gain-of-function TRPC6 Mutations Activate ERK
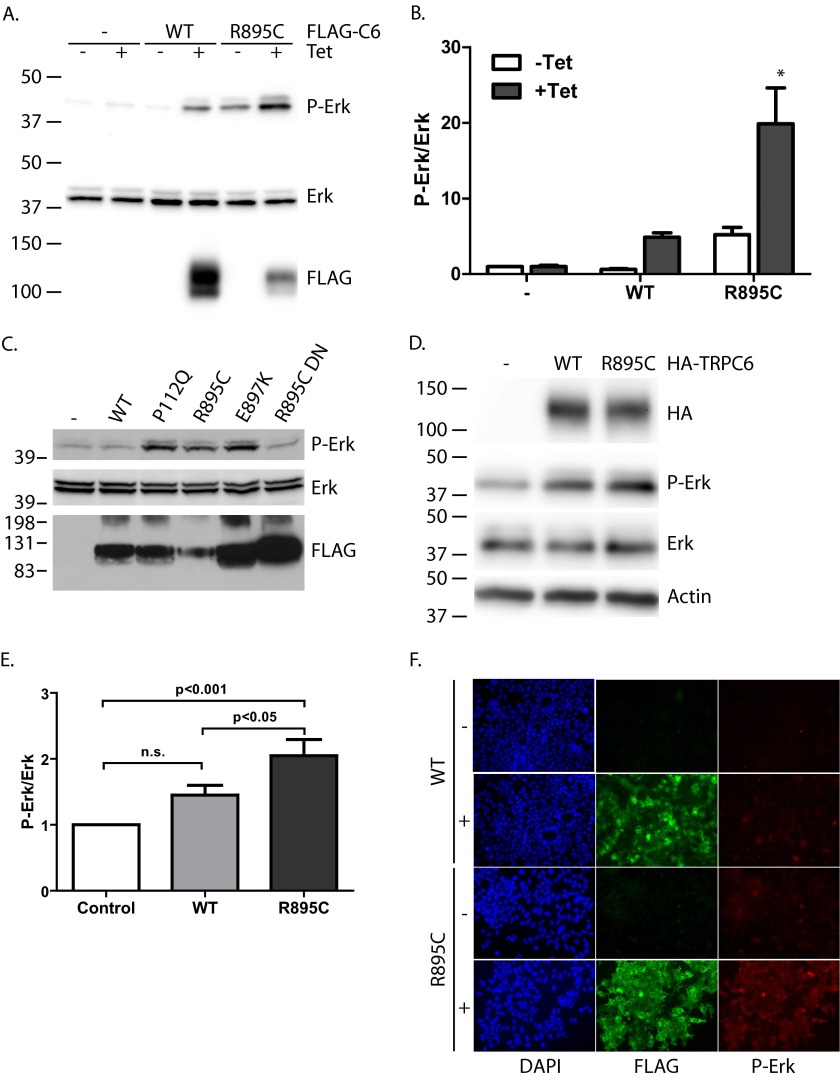
We examined the levels of phosphorylated, activated ERK1/2 in stable cell lines expressing either no TRPC6 (M1R), FLAG-tagged wild-type TRPC6 (WT), or FLAG-tagged TRPC6 harboring the FSGS-associated R895C mutation (R895C) under a tetracycline-inducible promoter (Fig. 1, A and B). Inducing expression of wild-type TRPC6 led to a small increase in basal ERK activation, although this did not reach statistical significance. Similarly, transient transfection of wild-type TRPC6 had only a minimal effect on phospho-ERK levels (Figs. 1C and 9). In contrast, expression of the R895C mutant TRPC6 induced a pronounced increase in phospho-ERK levels (Fig. 1, A–C). The reason for the higher basal level of phospho-ERK seen in the R895C-inducible cell line even in the absence of tetracycline is unclear, but it may be due to low level expression of the channel under uninduced conditions (as ascertained by RT-PCR and immunoprecipitation/Western blotting; data not shown).
FIGURE 1.
Mutant TRPC6 induces ERK activation. A, parental M1R cells or subclones expressing the indicated FLAG-TRPC6 construct under a tetracycline-inducible promoter were treated with or without tetracycline for 16–24 h, as indicated, before lysis. Whole lysate blots were probed for FLAG, phosphorylated ERK1/2 (P-Erk), and total ERK1/2 as indicated. B, average normalized ratio of phosphorylated ERK1/2 to total ERK signal in different stable cell lines without or with tetracycline induction. One-way ANOVA with Tukey's multiple comparison test was used; *, p < 0.001 versus all other groups; n = 5. C, phosphorylated ERK, total ERK, and FLAG-TRPC6 levels in M1R cells transiently transfected with the indicated FLAG-TRPC6 constructs. D, whole cell lysates from differentiated murine podocytes transfected with the indicated HA-TRPC6 expression constructs or control vector were blotted for the expression of HA-TRPC6, phosphorylated ERK1/2, total ERK, and β-actin, as indicated. E, average normalized ratio of phosphorylated ERK1/2 to total ERK signal in podocytes expressing the indicated HA-TRPC6 protein. One-way ANOVA with Tukey's multiple comparison test; n.s., not statistically significant; n = 7. F, immunofluorescence of inducible M1R subclones expressing wild-type or R895C mutant FLAG-TRPC6 treated without (−) or with (+) tetracycline as indicated. Images were taken under identical exposure conditions. Error bars, S.E.
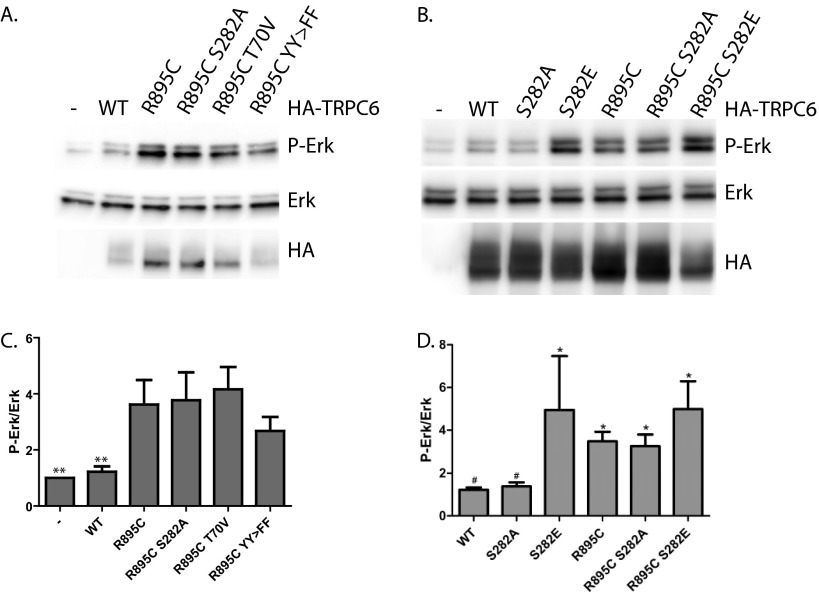
FIGURE 9.
Effect of TRPC6 phosphorylation site mutations on activation of ERK by R895C TRPC6. 293T cells were transfected with the indicated HA-tagged TRPC6 constructs. YY>FF, Y31F/Y285F double mutation. A and B, Western blots for phospho-ERK (P-Erk), total ERK, and HA-TRPC6. C and D, average normalized phospho-ERK/total ERK ratios for cells expressing the indicated HA-TRPC6 constructs. C, one-way ANOVA with Dunnett's multiple comparison test; **, p < 0.01 versus R895C; n = 7. D, Bonferroni's multiple-comparison test against wild-type and R895C; *, p < 0.05 versus WT; #, p < 0.05 versus R895C; n = 9–17. Error bars, S.E.
To ascertain whether the effect on ERK phosphorylation was specific to the R895C mutant or a common effect of disease-associated, gain-of-function mutations, we transiently transfected the parental cell line (M1R cells) with either wild type or one of several mutant forms of TRPC6 (Fig. 1C). Expression of three well established gain-of-function disease mutants located on opposite ends of the protein (P112Q, R895C, and E897K) led to an increase in ERK phosphorylation. Introduction of a dominant negative pore mutation (LFW to AAA (3)) into the R895C mutant protein (R895C DN) abolished ERK1/2 activation, suggesting that the channel activity of TRPC6 is necessary for ERK activation.
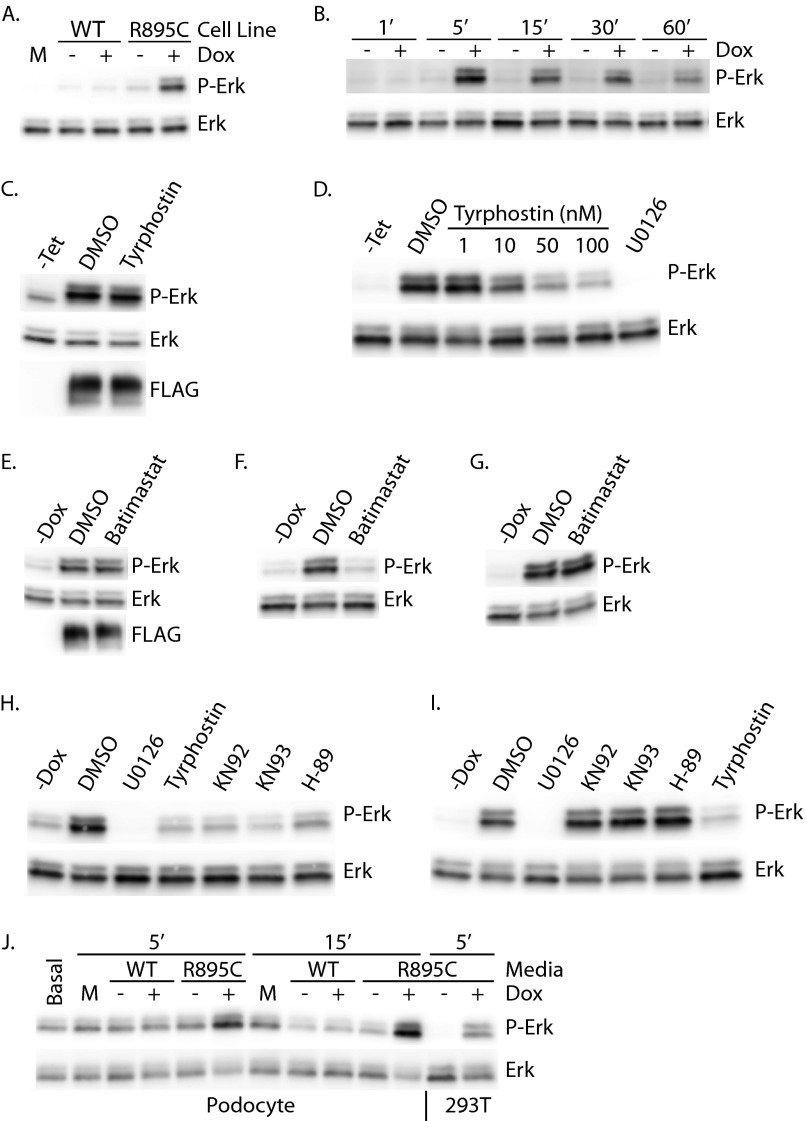
To ascertain whether mutant TRPC6 had a similar effect in a more disease-relevant cell line, we ascertained phospho-ERK levels in murine podocytes transiently transfected with either control vector or HA-tagged wild-type or R895C mutant TRPC6 (Fig. 1, D and E). Again, expression of mutant TRPC6 led to a significant increase in phospho-ERK levels compared with both control and wild-type TRPC6-expressing cells. The more modest relative increase in phospho-ERK levels in podocytes relative to 293T cells may in part be due to the higher basal levels of phospho-ERK in these cells (Fig. 5J) (data not shown).
FIGURE 5.
Soluble factor induces TRPC6 R895C-mediated ERK activation. A, 293T cells were treated for 5 min with unconditioned medium (M) or conditioned medium from cells uninduced (−) or induced (+) to express the indicated FLAG-TRPC6. Phospho-ERK (P-Erk) and total ERK levels were assessed by Western blot. B, 293T cells were incubated for the indicated times with medium conditioned by FLAG-TRPC6 R895C cells cultured in the presence or absence of tetracycline, as indicated. C, FLAG-TRPC6 R895C-inducible M1R cells were treated with 100 nm tyrphostin or DMSO, as indicated, for 3 h prior to lysis and Western blotting. D, medium conditioned by FLAG-TRPC6 R895C-expressing cells was supplemented with the indicated inhibitors immediately prior to the addition to 293T cells. Phosphorylated and total ERK levels in 293T cells were assessed after a 5-min incubation. E, Western blot analysis of FLAG-TRPC6 R895C cells grown overnight in the absence (−Dox) or presence of doxycycline and either carrier (DMSO) or 20 μm batimastat. Medium from the cells was collected and used to treat 293T cells in F for 5 min. G, conditioned medium from TRPC6 R895C expressing cells was supplemented with carrier (DMSO) or 20 μm batimastat immediately prior to treating 293T cells for 5 min. H and I, 293T cells were treated for 5 min with FLAG-TRPC6 R895C conditioned medium, which was supplemented with 5 μm KN-92, 5 μm KN-93, or 10 μm H89 either during the overnight medium conditioning period (H) or immediately prior to transfer onto 293T cells (I). J, podocytes or 293T cells (as indicated at the bottom) were exposed to unconditioned medium (M), medium conditioned by cells inducibly expressing FLAG-TRPC6 wild type (WT) or R895C, or left in standard podocyte medium (Basal) for the times indicated prior to lysis and immunoblotting.
We further examined ERK activation in TRPC6 R895C-expressing M1R cells by immunocytochemistry (Fig. 1F). In the absence of tetracycline, minimal phospho-ERK signal was seen in cells. Expressing wild-type TRPC6 did not appreciably increase phospho-ERK levels. In contrast, when mutant TRPC6 expression was induced, the majority of cells showed an increase in phospho-ERK signal, although there was significant heterogeneity in phospho-ERK levels.
Pharmacologic Inhibition of Mutant TRPC6-mediated ERK Activation
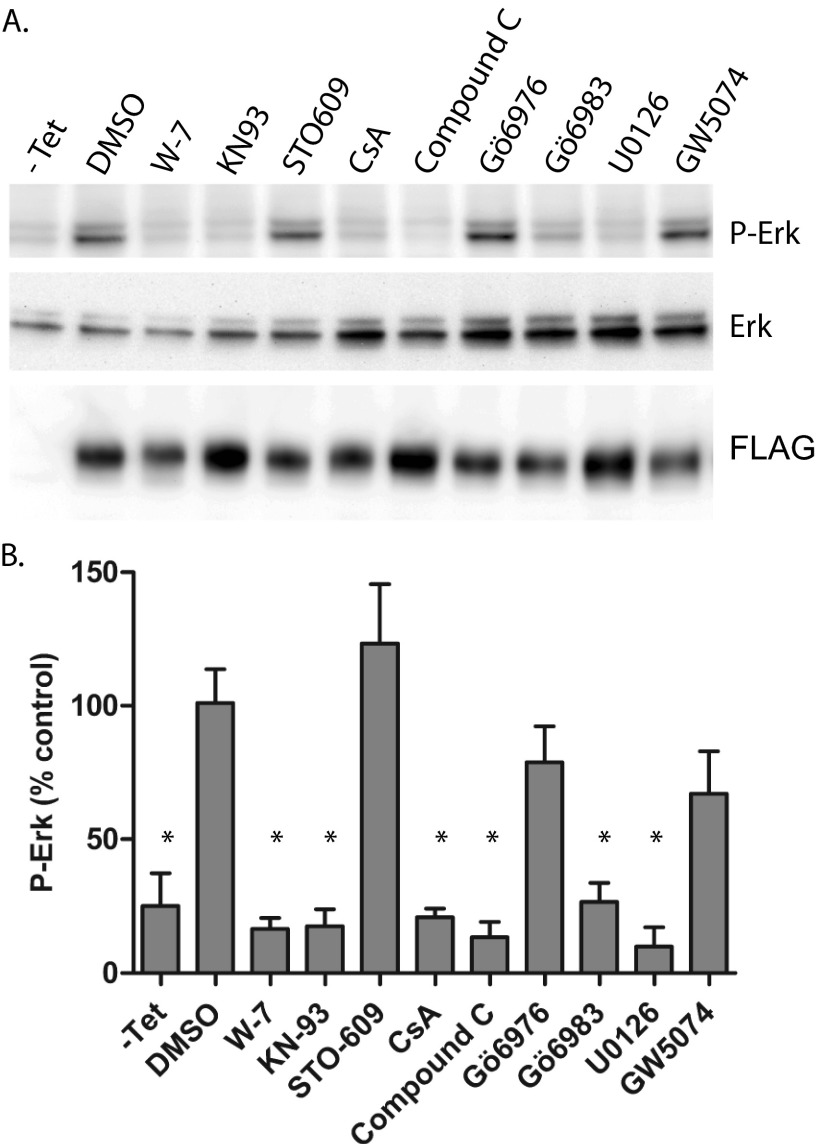
To begin to address what pathways might be involved in ERK1/2 activation downstream of mutant TRPC6, we examined the effect of various pharmacologic inhibitors (Table 1) on this process (Fig. 2, A and B). As expected, U0126, an inhibitor of MEK, the kinase directly responsible for ERK phosphorylation, effectively abrogated the increase in phospho-ERK due to TRPC6 R895C. The calmodulin inhibitor W-7, the CaMKII inhibitor KN-93, and the calcineurin inhibitor cyclosporine A, but not the calcium/calmodulin-dependent protein kinase kinase inhibitor STO-609, all abolished the effect of R895C TRPC6 on ERK activation. Compound C, an inhibitor of AMP-activated protein kinase (although with additional off-target effects (79)), also blocked ERK activation. Interestingly, the broad PKC inhibitor Gö6983 (and bisindolylmaleimide I/Gö6850; data not shown), but not Gö6976, which targets the calcium-dependent, conventional PKCs, also inhibited ERK activation, suggesting that one of the atypical PKCs may be required for this effect of TRPC6. Finally, GW5074, which inhibits c-Raf in vitro (80), was not an effective inhibitor of mutant TRPC6-mediated ERK activation.
TABLE 1.
Pharmacological inhibitors used in this study and their major reported targets
| Inhibitor | Target |
|---|---|
| Batimastat | Zinc-dependent metalloproteases |
| C3 transferase | Rho |
| Compound C | AMP-activated protein kinase, mitochondrial ROS production |
| Cyclosporine A | Calcineurin |
| Gö6976 | Calcium-dependent PKC (PKCα, PKCβ1) |
| Gö6983 | PKC (broad spectrum) |
| GW5074 | c-Raf |
| H89 | PKA, ROCK-II |
| KN-93 | CaMKII, L-type calcium channel |
| KN-92 | “Negative control” for KN-93 effect on CaMKII, L-type calcium channel |
| STO-609 | Calcium/calmodulin-dependent protein kinase kinase |
| Tyrphostin AG 1478 | EGFR, ErbB4 |
| U0126 | MEK |
| W-7 | Calmodulin |
| Y-27632 | ROCK |
FIGURE 2.
Pharmacological inhibition of ERK activation. M1R cells stably expressing tetracycline-inducible FLAG-TRPC6 R895C were treated without (−Tet) or with tetracycline (all other samples) overnight before being treated with the indicated inhibitors for 3 h prior to cell lysis. A, Western blot analysis of phosphorylated ERK (P-Erk), total ERK1/2, and FLAG-TRPC6. B, average normalized phospho-ERK levels in cells exposed to various inhibitors. One-way ANOVA with Dunnett's multiple comparison test was used; *, p < 0.05 versus DMSO-treated; n = 3. Table 1 summarizes the major targets of inhibitors used. Error bars, S.E.
The effect of KN-93 on phospho-ERK levels suggested that CaMKII might be involved in this signaling cascade. To further address this possibility, we first tested the effect of KN-92, a derivative of KN-93 without inhibitory activity against CaMKII (81), on phospho-ERK levels (Fig. 3, A and B). Surprisingly, we found that KN-92 was almost as effective at decreasing phospho-ERK levels in cells expressing TRPC6 R895C as KN-93. To further examine the potential role of CaMKII, we made use of a CaMKII-inhibitory peptide fused to GFP (GFP-AC3-I) (75). Expressing either this inhibitory peptide or a scrambled control peptide (GFP-AC3-C), together with TRPC6 R895C, did not appreciably alter phospho-ERK levels (Fig. 3C), further arguing against a role for CaMKII in mediating ERK activation by mutant TRPC6 and suggesting that the effect of KN-93 on this process is mediated, at least in part, independently of its effect on CaMKII activity.
FIGURE 3.
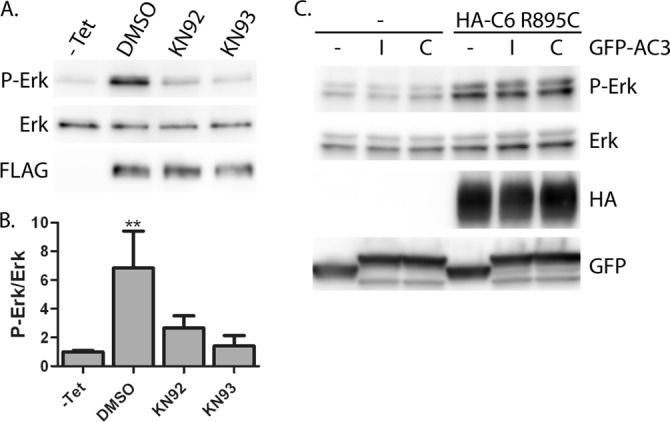
Effect of KN-92 and KN-93 on ERK activation by TRPC6 R895C. M1R cells expressing FLAG-TRCP6 R895C, or uninduced cells (−Tet) were treated with the indicated inhibitors or DMSO as a carrier control for 3 h prior to cell lysis. A, Western blot analysis of phospho-ERK (P-Erk), ERK, and FLAG-TRPC6 levels. B, average normalized phospho-ERK levels in cells exposed to the indicated inhibitors. One-way ANOVA with Tukey's multiple comparison test was used; **, p < 0.001 versus all other conditions; all other comparisons, p > 0.05; n = 4. C, Western blot analysis of 293T cells transfected with HA-TRPC6 R895C and either GFP (−), GFP-AC3 inhibitor (I), or control (C) constructs, as indicated. Error bars, S.E.
Previous studies have suggested that PKA and cGMP-dependent protein kinase (PKG) might play a role in limiting the activity of the TRPC6 channel (82–87). We therefore examined whether inhibition of PKA through H89 might further enhance ERK phosphorylation in cells expressing TRPC6 R895C. Contrary to our expectations, treating cells with H89 abolished the increase in phospho-ERK in cells expressing mutant TRPC6 (Fig. 4A). To investigate the potential role of PKA in mediating ERK activation by mutant TRPC6, we co-transfected TRPC6 R895C together with either wild-type or dominant negative, kinase-dead PKA Cα (Fig. 4B). Unlike the effect of H89, dominant negative PKA Cα did not diminish phospho-ERK levels, although overexpression of wild-type PKA Cα did increase phospho-ERK levels both in the presence and absence of TRPC6. To further address the discrepancy between the effect of H89 and dominant negative PKA, we knocked down the expression of endogenous PKA Cα. PKA levels, normalized to actin, were significantly decreased after transfecting PKA siRNA compared with scrambled control siRNA (PKA/actin ratio, 46.8 ± 2.3% versus 100 ± 6.1%, respectively; p < 0.0001 by paired t test, n = 10). Upon PKA knockdown, phospho-ERK levels were modestly increased in R895C TRPC6-expressing cells compared with control siRNA-treated cells (Fig. 4, C and D). The increase in phospho-ERK levels seen upon PKA knockdown in the absence of TRPC6 induction did not reach statistical significance. Taken together, these results suggest that H89 is able to inhibit mutant TRPC6-mediated increases in phospho-ERK levels independently of its effect on PKA. We cannot exclude the possibility that PKA may, in fact, have a small inhibitory effect on mutant TRPC6.
FIGURE 4.
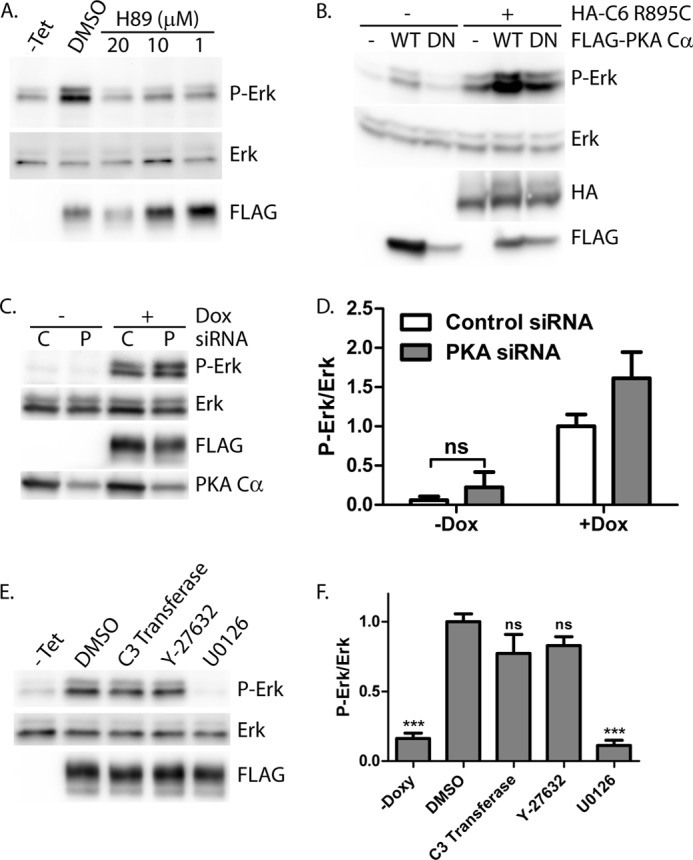
PKA inhibitor H89, but not dominant negative PKA Cα, PKA knockdown, or RhoA pathway inhibitors, blocks ERK activation by R895C TRPC6. A, M1R cells expressing R895C TRPC6 in a tetracycline-inducible manner were treated without (−Tet) or with tetracycline, followed by incubation with H89 at increasing concentrations or control carrier (DMSO). Phospho-ERK (P-Erk), ERK, and FLAG-TRPC6 expression were examined by Western blot of whole cell lysates. B, Western blot for phosphorylated ERK, ERK, HA, and FLAG of 293T cells transfected with HA-TRPC6 R895C and FLAG-PKA Cα wild type (WT) or dominant negative (DN) constructs, as indicated. C, R895C TRPC6-expressing cells were transfected with control (C) siRNA or siRNA targeting PKA Cα (P), followed by treatment with or without doxycycline to induce TRPC6 expression. Levels of phosphorylated and total ERK, FLAG-TRPC6, and endogenous PKA Cα were analyzed by Western blot. D, average normalized phospho-ERK/ERK levels in M1R cells expressing R895C TRPC6 under doxycycline treatment and transfected with the indicated siRNA. One-way ANOVA with Tukey's multiple comparison test was used; all pairwise comparisons with p < 0.001 except where indicated by ns (not significant); n = 3–7. E, tetracycline-inducible FLAG-TRPC6 R895C-expressing cells were treated without (−Tet) or with tetracycline, followed by incubation with carrier only (DMSO), Rho inhibitor (C3 transferase), ROCK inhibitor (Y-27632), or MEK inhibitor (U0126). Phospho-ERK and total ERK levels were analyzed by Western blot. F, average normalized phospho-ERK/ERK levels in cells treated as in E. One-way ANOVA with Dunnett's multiple comparison test was used versus DMSO; ***, p < 0.0001; ns, p > 0.05; n = 3–5. Error bars, S.E.
H89 has been reported to inhibit kinases in addition to PKA, including Rho-dependent protein kinase II (ROCK-II) (88). TRPC6 is known to activate RhoA (35, 37). We therefore examined whether the Rho-ROCK pathway was necessary for mutant TRPC6-mediated ERK activation. Neither membrane-permeable C3 transferase (a Rho inhibitor) nor the ROCK inhibitor Y-27632 significantly altered phospho-ERK levels (Fig. 4, E and F). These results suggest that mutant TRPC6-mediated ERK activation occurs independently of PKA or the Rho-ROCK pathway.
Mutant TRPC6 Induces Paracrine ERK Activation via Release of EGFR Ligand
Calcium-activated signals play a role in G-protein-coupled receptor autocrine and paracrine signaling pathways, including those activating ERK through tyrosine kinase receptor transactivation (reviewed in Refs. 89 and 90). Furthermore, TRPC1 has been shown to be involved in the amplification of the EGFR signaling cascade, including the activation of ERK (91). We therefore investigated whether a paracrine signaling pathway might be involved in ERK activation due to TRPC6 R895C expression (Fig. 5). HEK293T cells were exposed to unconditioned medium or medium conditioned by uninduced or induced TRPC6 wild-type or R895C cells. Exposure to conditioned medium from cells expressing TRPC6 R895C induced ERK activation within 5 min, whereas medium from uninduced cells, medium from cells expressing wild-type TRPC6, or control medium had no effect (Fig. 5A). A time course analysis revealed peak activation of ERK after 5 min of exposure to conditioned medium from TRPC6 R895C cells, with a lower level of ERK activation persisting through 60 min (Fig. 5B). We next examined whether TRPC6-mediated ERK activation was dependent upon EGFR kinase activity. Treating TRPC6 R895C-expressing cells with tyrphostin AG 1478, an EGFR (92) and ErbB4 (93) kinase inhibitor, failed to decrease phospho-ERK levels (Fig. 5C). In contrast, adding tyrphostin AG 1478 to TRPC6 R895C conditioned medium inhibited ERK activation in 293T cells exposed to the medium (Fig. 5D), suggesting that distinct signaling pathways are involved in cell-autonomous and non-cell-autonomous ERK activation by mutant TRPC6.
Because metalloprotease mediated shedding of EGF family growth factors is a frequent mechanism involved in transactivation (89, 94), we next examined the ability of metalloprotease inhibitors to affect ERK activation. Incubating FLAG-TRPC6 R895C-expressing cells with the metalloprotease inhibitor batimastat did not alter ERK activation in these cells (Fig. 5E), but the conditioned medium generated from these cells failed to activate ERK in 293T cells (Fig. 5F). Adding batimastat after conditioning did not alter ERK activation in the 293T cells (Fig. 5G). Similar results were obtained with two additional metalloprotease inhibitors, marimastat and GM6001 (data not shown). Together these results strongly suggest that a metalloprotease-dependent signaling step is required in TRPC6 R895C-expressing cells to allow efficient medium conditioning.
We also examined the ability of KN-92, KN-93, and H89 to affect paracrine ERK activation. Consistent with a model in which these inhibitors block early signaling downstream of the mutant channel, medium conditioned in the presence of these inhibitors did not activate ERK in 293T cells (Fig. 5H), whereas adding inhibitors to preconditioned medium did not prevent ERK activation (Fig. 5I).
Cultured human podocytes were also exposed to cultured media from various TRPC6-expressing 293T cell lines (Fig. 5J). Similar to results with 293T cells, phospho-ERK levels were increased only by medium conditioned by TRPC6 R895C-expressing cells. The time course of activation was slightly different, with higher phospho-ERK levels seen at 15 min compared with 5 min. In addition, the relative increase in ERK phosphorylation was lower than in 293T cells, due to higher basal phospho-ERK levels in podocytes compared with 293T cells (Fig. 5J, compare second and fourth lanes from the right).
In aggregate, these results suggest that mutant TRPC6 can lead to ERK activation through at least two distinct mechanisms: 1) a cell-autonomous mechanism independent of metalloproteases and EGFR and 2) a non-cell-autonomous process that involves the metalloprotease-dependent release of a soluble factor that signals through a tyrphostin AG 1478-sensitive receptor kinase.
TRPC6 R895C-mediated Calcium Elevations Are Inhibited by H89 and KN-92
The ability of KN-92 and H89 to inhibit both cell-autonomous ERK activation in TRPC6 R895C-expressing cells and release of paracrine signaling factors suggests that they are acting relatively proximal to channel activation and the resulting calcium influx. We therefore addressed whether these inhibitors might interfere with TRPC6-mediated calcium influx.
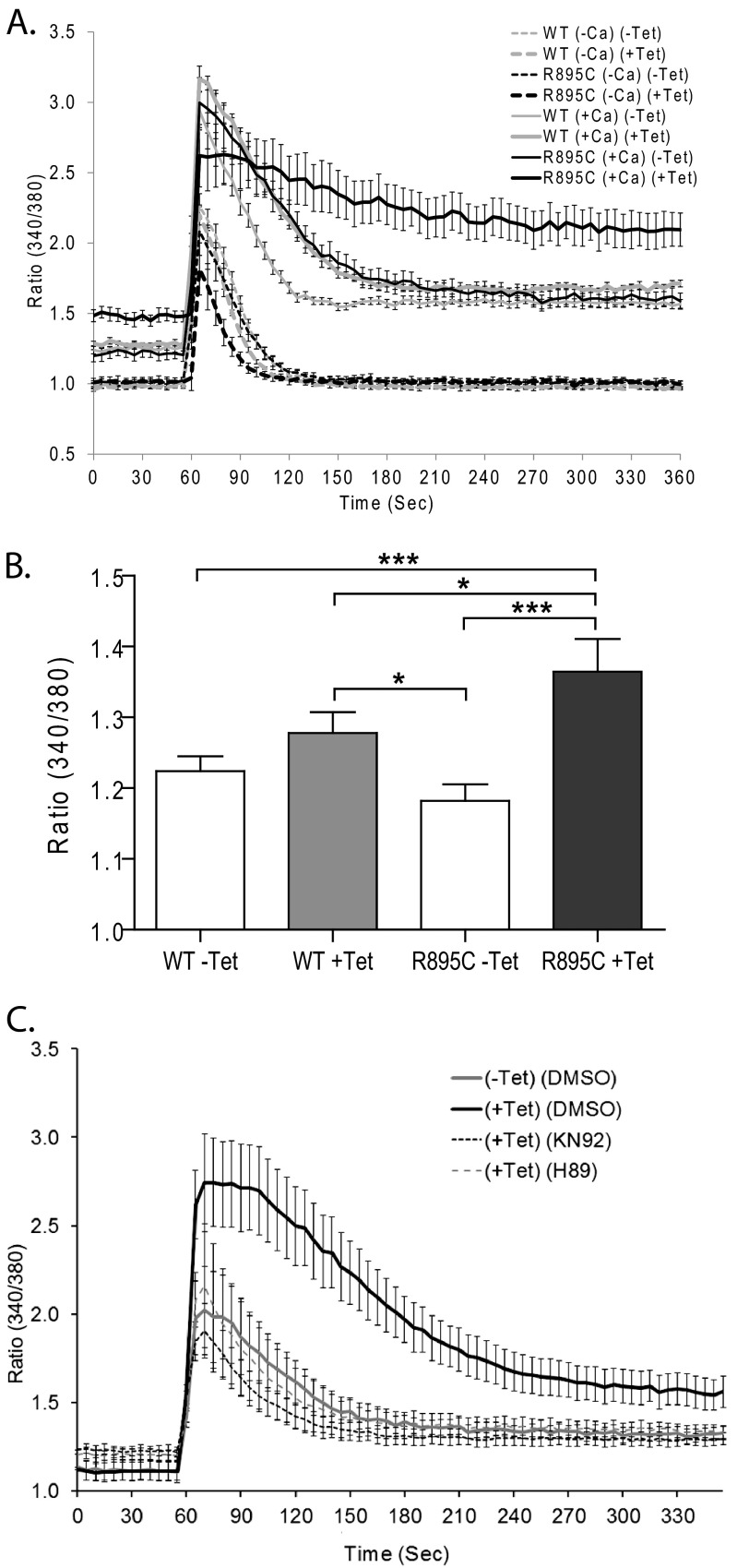
As measured by the ratiometric fluorescent dye Fura-2 (Fig. 6A), we found that in the presence of extracellular calcium, uninduced cells had similar basal fluorescence ratios measured at 30 s (Fig. 6, A and B). Cells expressing TRPC6 R895C had, on average, a higher basal calcium level, although this difference was variable and not seen consistently in all experiments (e.g. compare basal levels in Fig. 6, A and C) or in our previously published findings (32). In the absence of extracellular calcium (Fig. 6A, −Ca), all cells had a similar basal fluorescence ratio that was significantly lower than in the presence of calcium (p < 0.0001, one-way ANOVA with Tukey's multiple comparison test, n = 8).
FIGURE 6.
Enhanced calcium influx by TRPC6 R895C is inhibited by H89 and KN-92. Shown is the ratio of Fura-2 fluorescence upon excitation at 340 and 380 nm in the indicated M1R stable cell lines. Carbachol was added to a final concentration of 100 μm after 60 s. A, comparison of tetracycline-inducible TRPC6 wild-type and R895C cell lines in the presence or absence of extracellular calcium. Basal Fura-2 ratios (measured at 30 s) were not different between cell types in the absence of extracellular calcium but were significantly higher in the presence of extracellular calcium. In addition, with extracellular calcium, TRPC6 R895C-expressing cells had slightly higher basal calcium levels than other cells. After carbachol stimulation, the peak ratio (70 s) was significantly higher in the presence rather than the absence of extracellular calcium but was not significantly different among cells within these groups. At 210 s, R895C (+Tet) cells demonstrated higher Fura-2 ratios than the other three cell populations in the presence of calcium but not in the absence of exogenous calcium. See “Results” for details of statistical analysis. B, average basal Fura-2 fluorescence ratios (measured at 30 s) in the indicated M1R cell lines in the presence of extracellular calcium. Paired one-way ANOVA with Tukey's multiple comparison test was used; ***, p < 0.0001; *, p < 0.05; all other pairwise comparisons with p > 0.05; n = 21. C, Fura-2 fluorescence ratios of TRPC6 R895C-expressing cells pretreated with H89 or KN-92 as indicated. Cells were stimulated with carbachol at 60 s. At 210 s, R895C (+Tet) cells had higher Fura-2 ratios relative to the other three cell populations; one-way ANOVA with Dunnett's multiple comparison test; p < 0.01; n = 9–12. Peak ratio differences between the groups did not reach statistical significance. Error bars, S.E.
Stimulating cells with carbachol (at time 60 s, Fig. 6A) led to a rapid rise in intracellular calcium in all cells in the presence or absence of extracellular calcium. Peak 340/380 ratios (measured at 70 s) in the presence of extracellular calcium were significantly higher than in the absence of extracellular calcium (p < 0.0001, one-way ANOVA with Bonferroni's multiple-comparison test; n = 8). Inducing wild-type or mutant TRPC6 expression did not significantly alter peak ratios among the groups of cells treated with extracellular calcium or among the groups treated without extracellular calcium. In the absence of extracellular calcium, intracellular calcium levels rapidly returned back to base line. In contrast, in the presence of extracellular calcium, the TRPC6 R895C-expressing cells showed a slower and smaller drop in the fluorescence ratio over the next several min (p < 0.0001, one-way ANOVA with Tukey's multiple comparison test, compared with all other groups at 210 s; n = 8). These results are consistent with previously published electrophysiological data suggesting that this mutation leads to increased current amplitudes (11) and suggest a possible effect on time-dependent channel inactivation similar to that seen with other FSGS-associated mutations (13).
We next addressed whether KN-92 and H89 have any effect on TRPC6 R895C-mediated calcium influx (Fig. 6C). Both inhibitors prevented the prolonged elevation in the Fura-2 fluorescence ratio seen with expression of TRPC6 R895C (p < 0.01 by one-way ANOVA comparing (+Tet) (DMSO) versus all other groups at 210 s). The apparent blunting of the initial peak rise in the fluorescence ratio upon carbachol stimulation in cells treated with H89 or KN-92 did not reach statistical significance. Taken together, these data suggest that the ability of both inhibitors to block ERK activation by mutant TRPC6 may be due to their ability to blunt calcium influx mediated by mutant TRPC6 activation. Our data do not allow us to differentiate between a direct effect of these compounds on channel activation/activity and an indirect effect on calcium influx (such as through reversal of the sodium-calcium exchanger (95, 96)).
PKA Cα Binds and Phosphorylates TRPC6
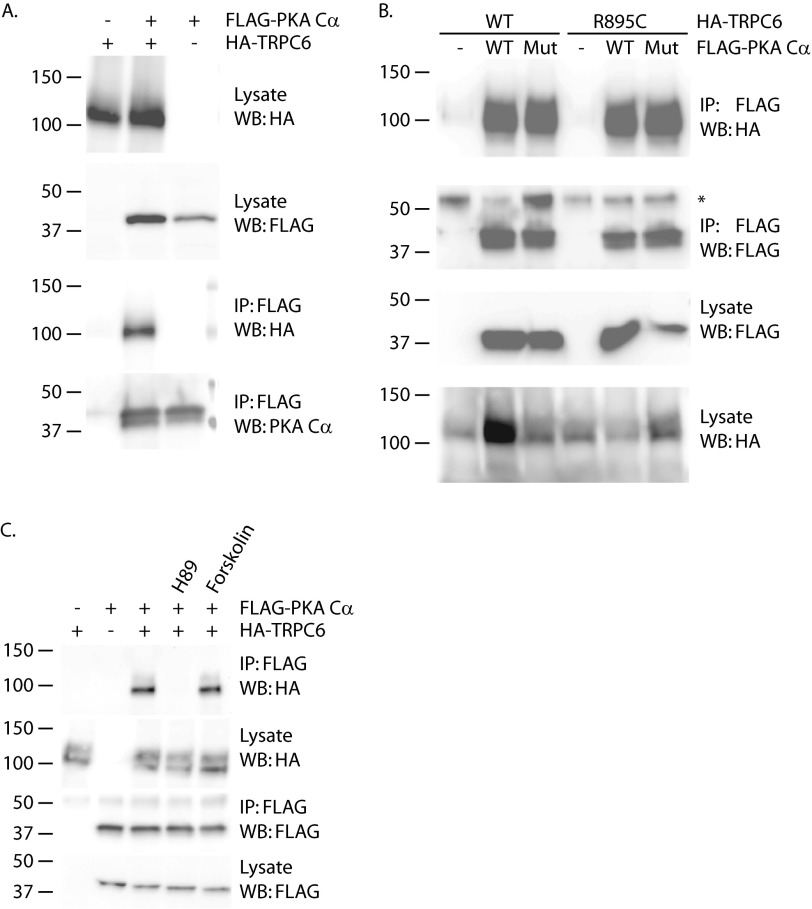
To further investigate the disparate effects of H89 and dominant negative PKA Cα or PKA siRNA on phospho-ERK levels, we examined whether TRPC6 and PKA can interact. TRPC6 was readily co-immunoprecipitated with PKA Cα when co-expressed in 293T cells (Fig. 7A). The ability of PKA Cα to co-precipitate TRPC6 was not affected by introducing the R895C mutation into TRPC6 or by abolishing the kinase activity of PKA Cα (Fig. 7B). In contrast to mutating the kinase domain of PKA Cα, treating cells with H89 did abolish the TRPC6-PKA interaction (Fig. 7C). This result was surprising in light of reports that H89 acts by competing for ATP but not substrate, binding to PKA (97). Forskolin-treating cells to activate adenylate cyclase did not appreciably enhance the interaction between the two overexpressed proteins (Fig. 7C).
FIGURE 7.
TRPC6 binds PKA Cα. A, 293T cells were transfected with expression plasmids for HA-TRPC6 and FLAG-PKA Cα, as indicated. Expression was confirmed by Western blotting (WB) of lysates with anti-HA and anti-FLAG antibodies. HA-TRPC6 was detected in FLAG immunoprecipitated (IP) complexes only in the presence of FLAG-PKA Cα. B, co-immunoprecipitation of wild-type and R895C mutant HA-TRPC6 with both wild-type and kinase-inactive mutant FLAG-tagged PKA Cα. Lysates were probed to confirm expression of the various proteins. The asterisk in the second panel indicates IgG heavy chain. C, effect of PKA inhibitor H89 and adenylate cyclase activator forskolin on the interaction of overexpressed TRPC6 and PKA Cα.
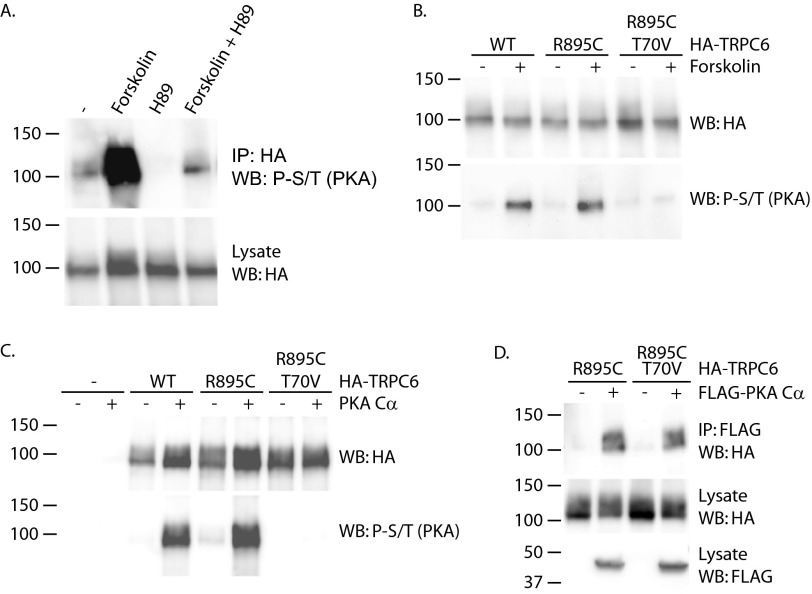
We next examined whether PKA could phosphorylate TRPC6, making use of a phospho-PKA substrate antibody developed to specifically recognize phosphoserine and -threonine residues within the context of the consensus PKA site RRX(S/T) (Cell Signaling Technology). Under basal conditions, some amount of TRPC6 phosphorylation could be detected using this antibody (Fig. 8A). This signal was greatly increased by treating cells with forskolin to activate adenylated cyclase. To confirm that these phosphorylation events were mediated by PKA, cells were treated with H89. The presence of this compound abolished both the basal and forskolin-induced phospho-Ser/Thr signals (Fig. 8A, compare lane 1 with lane 3 and lane 2 with lane 4). TRPC6 contains several potential consensus PKA phosphorylation sites, including Ser-13, Thr-70, and Ser-322 (84). Phosphorylation of threonine 70 in TRPC6 and of the corresponding threonine in TRPC3 by both PKA and PKG has been reported (85, 86, 98). The role of threonine 70 as a site for PKA phosphorylation was therefore investigated (Fig. 8B). Both wild-type and R895C mutant TRPC6 showed increased phospho-S/T signal upon treating cells with forskolin. In contrast, introducing the T70V mutation into the R895C TRPC6 protein completely abolished this effect of forskolin, indicating that threonine 70 is a major TRPC6 phosphorylation site after forskolin treatment. To ascertain whether threonine 70 is in fact a direct target of PKA phosphorylation, we performed in vitro kinase assays (Fig. 8C). Incubating wild-type and R895C mutant TRPC6 with recombinant PKA increased signal using the phospho-PKA substrate antibody. In contrast, no phosphoserine or phosphothreonine was detected in TRPC6 containing the T70V mutation. Although threonine 70 appears to be a site of PKA-mediated phosphorylation in vitro and in vivo, the site was not required for binding of PKA Cα (Fig. 8D).
FIGURE 8.
TRPC6 is phosphorylated by PKA at threonine 70. A, TRPC6 phosphorylation at PKA consensus sites (phospho-Ser/Thr; P-S/T). 293T cells expressing HA-TRPC6 were treated with 20 μm forskolin and/or 20 μm H89, as indicated, for 30 min prior to cell lysis and immunoprecipitation (IP). Immunoprecipitated HA-TRPC6 was probed with phospho-PKA substrate antibody. WB, Western blot. B, threonine 70 is a major site of TRPC6 phosphorylation in response to forskolin treatment. The indicated HA-TRPC6 constructs were expressed in 293T cells exposed to forskolin or carrier for 30 min prior to lysis and HA immunoprecipitation. TRPC6 phosphorylation at PKA consensus sites was monitored with phospho-PKA substrate antibody. C, in vitro phosphorylation of TRPC6 by PKA catalytic subunit. The indicated HA-TRPC6 proteins were purified from cell lysates and incubated with or without recombinant PKA catalytic subunit, followed by immunoblotting with phospho-PKA substrate antibody. D, threonine 70 is not required for TRPC6 binding to PKA Cα. The indicated HA-TRPC6 constructs were co-immunoprecipitated with FLAG-PKA Cα.
Evaluation of TRPC6 Phosphorylation Site Mutations on ERK Activation
Several phosphorylation sites on TRPC6 have been reported to play potential roles in regulating channel function. The potential ERK phosphorylation site on Ser-282 has been reported to be required for activating TRPC6 currents in response to the cAMP analog, 8-bromo-cyclic AMP (99). Introduction of the S282A mutation into the TRPC6 R895C construct did not significantly alter phospho-ERK levels (Fig. 9, A and C). In addition, abolishing the PKA/PKG phosphorylation site on Thr-70 (86, 100) or the dual tyrosine phosphorylation sites at Tyr-31 and Tyr-285 (8) failed to significantly alter ERK activation by TRPC6 R895C (Fig. 9, A and C). However, when we introduced a phosphomimetic S282E mutation into wild-type TRPC6, phospho-ERK levels were elevated to a level comparable with that induced by the R895C mutation (Fig. 9, B and D). Neither the S282E nor the S282A mutation significantly altered phospho-ERK levels in the context of the R895C mutation. This suggests that although the S282E mutation induces ERK activation similarly to R895C and other disease-associated mutations, phosphorylation of the Ser-282 residue is not required for ERK activation in the context of the R895C mutant.
DISCUSSION
Here we report several novel observations: 1) gain-of-function TRPC6 mutants activate ERK in the absence of exogenous stimuli; 2) ERK activation by mutant TRPC6 can be mediated by two distinct pathways, a cell-autonomous pathway resistant to metalloprotease and EGFR inhibitors and a non-cell-autonomous pathway sensitive to these inhibitors; 3) two pharmacological inhibitors, KN-92 and H89, are able to block the prolonged elevations in intracellular calcium concentrations mediated by mutant TRPC6 as well as block both pathways leading to ERK activation; 4) dominant negative PKA Cα or knockdown of endogenous PKA Cα does not replicate the effect of H89, whereas H89 disrupts the interaction of TRPC6 and PKA Cα; and 5) introducing a phosphomimetic S282E mutation into TRPC6 induces ERK activation, but abolishing potential phosphorylation sites at Thr-70, Ser-282, and Tyr-31/285 does not prevent ERK activation by TRPC6 R895C.
Although our results clearly demonstrate ERK activation downstream of gain-of-function TRPC6 mutants, it remains unclear whether activation of wild-type TRPC6 is also able to activate the two signaling pathways leading to ERK phosphorylation. Addressing this point has been hampered by the lack of specific TRPC6 agonists; treatment with stimuli known to activate TRPC6, including Hyp9 (101), leads to ERK activation even in cells not expressing TRPC6.3 However, several prior studies have reported links between TRPC channels and ERK. A role for TRPC channels, including TRPC6, in activating ERK was first suggested in the context of PDGF-mediated neuroprotection (40). More recently, TRPC1 has been shown to augment EGFR-mediated signaling, including ERK activation (91), whereas TRPC3 appears to play a role in ERK activation in cardiac fibroblasts (102). Our data suggest that multiple distinct pathways may connect TRPC6 activity to ERK activation, including a paracrine signaling pathway probably involving an EGFR ligand. It is interesting to speculate whether a similar paracrine signaling mechanism is involved in the reported augmentation of EGFR-mediated ERK activation by TRPC1 (91).
Several lines of evidence implicate calcium influx in mutant TRPC6-mediated ERK activation. Specifically, pore mutations abolishing channel activity prevent ERK activation, inhibitors of the calcium-dependent proteins calmodulin and calcineurin block ERK activation, and KN-92 and H89 block both ERK activation and calcium influx downstream of mutant TRPC6. However, although average basal calcium levels are slightly higher in cells expressing TRPC6 R895C, the differences are small and not consistently seen either in this or previous (32) studies. How might these discrepant results be reconciled? We postulate that in contrast to the global and simultaneous activation of TRPC6 channels upon stimulation with carbachol, under basal conditions, there may be stochastic activation of TRPC6 within a subset of cells and/or localized to a small area of a cell. These localized increases in calcium concentration may be sufficient to induce ERK activation but not to always significantly alter the average calcium concentration as measured across the entire cell population. Consistent with this possibility, in our prior study (32), a small minority of TRPC6 R895C-expressing cells demonstrate elevated basal calcium levels without significantly altering the average calcium level across the population. Transient calcium spikes could induce prolonged periods of ERK activation and therefore lead to an increase in global phospho-ERK levels. Confirming this hypothesis will require the development of assays to simultaneously monitor intracellular calcium concentrations and ERK activity within individual cells across time.
Besides its potential role downstream of TRPC signaling, ERK has been reported to be required for TRPC6 activation by cAMP analogues (99). In addition, disrupting a putative ERK phosphorylation site at Ser-281 in mouse TRPC6 (corresponding to Ser-282 in humans) prevented TRPC6 activation by cAMP, although phosphorylation of this site was not demonstrated (99). Although we found that Ser-282 is not necessary for ERK activation by TRPC6 R895C, a phosphomimetic S282E mutation in wild-type TRPC6 did induce ERK activation. These results suggest that phosphorylation at Ser-282, although not necessary for TRPC6 R895C actions, might activate signaling pathways downstream of TRPC6. The effect of the S282E mutation on TRPC6 channel activity and evidence that the Ser-282 residue is in fact phosphorylated in vivo await further investigation.
In aggregate, the studies focusing on the interplay between TRPC6 and ERK suggest the possibility of a potential feed-forward loop involving these two proteins. Such a loop could potentially parallel or augment the well established positive feedback loop involving the TRPC6-calcineurin-NFAT pathway (25, 26, 103), especially because ERK activation in our system was inhibited by cyclosporine A.
Our data add to the conflicting reports regarding the potential effect of PKA, its inhibitors, and phosphorylation of Thr-70 on TRPC6 activity. TRPC6 was first reported to be phosphorylated by PKA in human platelets, but this does not appear to alter 1-oleoyl-2-acetyl-glycerol-stimulated calcium influx (104). Similarly, PKA has been reported to inhibit TRPC5 but not TRPC6 currents when the proteins are expressed in 293T cells (105), and activation of TRPC6 by cAMP analogues is insensitive to H89 (99). In contrast, in pituitary cells, nonspecific cation channels, probably including TRPC6, are involved in PKA-stimulated calcium influx (106). PKA has been reported to phosphorylate mouse TRPC6 at two sites, Ser-28 and Thr-69 (corresponding to Thr-70 in humans), leading to channel inhibition (86, 100). However, in one study, Ser-28 was felt to be the critical inhibitory site (100), whereas the other study noted that phosphorylation of Thr-69 was key (98). In support of the role of Thr-69, the same site has been reported to be the target of inhibitory phosphorylation by PKG (86, 87). We confirmed that PKA is capable of phosphorylating TRPC6 at Thr-70 in vivo and in vitro and that in vivo phosphorylation at this site is inhibited by H89. In our system, H89 blocked mutant TRPC6-mediated ERK activation, medium conditioning, and prolonged intracellular calcium elevations after carbachol stimulation and also interfered with the interaction of TRPC6 and the catalytic subunit of PKA. Overexpression of dominant negative, kinase-dead PKA Cα, in contrast, did not block ERK activation, whereas knockdown of endogenous PKA modestly enhanced ERK activation by mutant TRPC6. These results strongly suggest that the ability of H89 to inhibit mutant TRPC6-mediated ERK activation is independent of its inhibition of PKA. Our results are consistent with an inhibitory role for PKA as well, although if there is such a role, it does not depend upon phosphorylation of Thr-70 on TRPC6. Although our data do not fully elucidate the role of PKA or H89 in regulating TRPC6, they strongly caution against the use of H89 in studying the interplay of TRPC6 and PKA.
The ability of KN-92, initially used as a negative control for KN-93, to inhibit mutant TRPC6-mediated ERK activation and calcium influx was unexpected. The effect on calcium influx, in particular, suggests that it may be acting either on TRPC6 channel activation/activity directly or indirectly on calcium influx subsequent to TRPC6 activation. Consistent with the latter possibility, KN-92 and KN-93 have both been shown to inhibit L-type calcium channels (107), and nimodipine-sensitive L-type calcium channels have been proposed to be activated in response to TRPC6-mediated depolarization in smooth muscle cells (108). Dedicated electrophysiological studies will be needed to address these possibilities.
Ultimately, these studies beget the question of whether ERK activation plays a role in the pathogenesis of TRPC6-mediated FSGS. As noted earlier, ERK activation is clearly a common manifestation in multiple human glomerular diseases and mouse models, although whether its role is as a mediator or mitigator of disease is uncertain. No studies addressing the effect of ERK1/2 deletion in the kidney or podocytes on glomerular function have been published to date. Addressing the importance of ERK activation in TRPC6-mediated FSGS will need to be addressed in mouse models.
In summary, we have identified activation of the ERK pathway via at least two distinct pathways as a consequence of FSGS-associated mutations in TRPC6. These results open up novel lines of investigation, including those focusing on a role for TRPC6 in EGFR transactivation and paracrine signaling and on a role for ERK activation in TRPC6-mediated kidney disease.
Acknowledgments
We thank M. Anderson, A. Murshid, and S. Calderwood for sharing expression plasmids and M. Saleem for providing the human podocyte cell line. We are grateful to the Institute of Chemistry and Cell Biology-Longwood screening facility and the Beth Israel Deaconess Microscopy Core for providing excellent core facilities and technical support.
This work was supported, in whole or in part, by National Institutes of Health, NIDDK, Grant K08DK80947. This work was also supported by a Carl W. Gottschalk Research Grant from the American Society of Nephrology.
D. Chiluiza, unpublished observation.
- FSGS
- focal segmental glomerulosclerosis
- CaMKII
- calmodulin-dependent protein kinase II
- EGFR
- epidermal growth factor receptor
- PKA Cα
- cAMP-dependent protein kinase catalytic subunit α
- PKG
- cGMP-dependent protein kinase
- ROCK
- Rho-dependent protein kinase
- ANOVA
- analysis of variance.
REFERENCES
- 1. Clapham D. E., Runnels L. W., Strübing C. (2001) The TRP ion channel family. Nat. Rev. Neurosci. 2, 387–396 [DOI] [PubMed] [Google Scholar]
- 2. Ramsey I. S., Delling M., Clapham D. E. (2006) An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647 [DOI] [PubMed] [Google Scholar]
- 3. Hofmann T., Schaefer M., Schultz G., Gudermann T. (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulay G., Zhu X., Peyton M., Jiang M., Hurst R., Stefani E., Birnbaumer L. (1997) Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 272, 29672–29680 [DOI] [PubMed] [Google Scholar]
- 5. Cayouette S., Lussier M. P., Mathieu E. L., Bousquet S. M., Boulay G. (2004) Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J. Biol. Chem. 279, 7241–7246 [DOI] [PubMed] [Google Scholar]
- 6. Hisatsune C., Kuroda Y., Nakamura K., Inoue T., Nakamura T., Michikawa T., Mizutani A., Mikoshiba K. (2004) Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 279, 18887–18894 [DOI] [PubMed] [Google Scholar]
- 7. Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 [DOI] [PubMed] [Google Scholar]
- 8. Kanda S., Harita Y., Shibagaki Y., Sekine T., Igarashi T., Inoue T., Hattori S. (2011) Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-γ1 and nephrin. Effect of mutations associated with focal segmental glomerulosclerosis. Mol. Biol. Cell 22, 1824–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spassova M. A., Hewavitharana T., Xu W., Soboloff J., Gill D. L. (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. U.S.A. 103, 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottlieb P., Folgering J., Maroto R., Raso A., Wood T. G., Kurosky A., Bowman C., Bichet D., Patel A., Sachs F., Martinac B., Hamill O. P., Honoré E. (2008) Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. Eur. J. Physiol. 455, 1097–1103 [DOI] [PubMed] [Google Scholar]
- 11. Reiser J., Polu K. R., Möller C. C., Kenlan P., Altintas M. M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C., McGee M., Sugimoto H., Brown D., Kalluri R., Mundel P., Smith P. L., Clapham D. E., Pollak M. R. (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winn M. P., Conlon P. J., Lynn K. L., Farrington M. K., Creazzo T., Hawkins A. F., Daskalakis N., Kwan S. Y., Ebersviller S., Burchette J. L., Pericak-Vance M. A., Howell D. N., Vance J. M., Rosenberg P. B. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 [DOI] [PubMed] [Google Scholar]
- 13. Heeringa S. F., Möller C. C., Du J., Yue L., Hinkes B., Chernin G., Vlangos C. N., Hoyer P. F., Reiser J., Hildebrandt F. (2009) A novel TRPC6 mutation that causes childhood FSGS. PloS One 4, e7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu B., Chen N., Wang Z. H., Pan X. X., Ren H., Zhang W., Wang W. M. (2009) Identification and functional analysis of a novel TRPC6 mutation associated with late onset familial focal segmental glomerulosclerosis in Chinese patients. Mutat. Res. 664, 84–90 [DOI] [PubMed] [Google Scholar]
- 15. Möller C. C., Wei C., Altintas M. M., Li J., Greka A., Ohse T., Pippin J. W., Rastaldi M. P., Wawersik S., Schiavi S., Henger A., Kretzler M., Shankland S. J., Reiser J. (2007) Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 [DOI] [PubMed] [Google Scholar]
- 16. Krall P., Canales C. P., Kairath P., Carmona-Mora P., Molina J., Carpio J. D., Ruiz P., Mezzano S. A., Li J., Wei C., Reiser J., Young J. I., Walz K. (2010) Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PloS One 5, e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eckel J., Lavin P. J., Finch E. A., Mukerji N., Burch J., Gbadegesin R., Wu G., Bowling B., Byrd A., Hall G., Sparks M., Zhang Z. S., Homstad A., Barisoni L., Birbaumer L., Rosenberg P., Winn M. P. (2011) TRPC6 enhances angiotensin II-induced albuminuria. J. Am. Soc. Nephrol. 22, 526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dietrich A., Mederos Y., Gollasch M., Gross V., Storch U., Dubrovska G., Obst M., Yildirim E., Salanova B., Kalwa H., Essin K., Pinkenburg O., Luft F. C., Gudermann T., Birnbaumer L. (2005) Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol. Cell. Biol. 25, 6980–6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tauseef M., Knezevic N., Chava K. R., Smith M., Sukriti S., Gianaris N., Obukhov A. G., Vogel S. M., Schraufnagel D. E., Dietrich A., Birnbaumer L., Malik A. B., Mehta D. (2012) TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 209, 1953–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weissmann N., Dietrich A., Fuchs B., Kalwa H., Ay M., Dumitrascu R., Olschewski A., Storch U., Mederos y Schnitzler M., Ghofrani H. A., Schermuly R. T., Pinkenburg O., Seeger W., Grimminger F., Gudermann T. (2006) Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc. Natl. Acad. Sci. U.S.A. 103, 19093–19098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weissmann N., Sydykov A., Kalwa H., Storch U., Fuchs B., Mederos y Schnitzler M., Brandes R. P., Grimminger F., Meissner M., Freichel M., Offermanns S., Veit F., Pak O., Krause K. H., Schermuly R. T., Brewer A. C., Schmidt H. H., Seeger W., Shah A. M., Gudermann T., Ghofrani H. A., Dietrich A. (2012) Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 3, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samapati R., Yang Y., Yin J., Stoerger C., Arenz C., Dietrich A., Gudermann T., Adam D., Wu S., Freichel M., Flockerzi V., Uhlig S., Kuebler W. M. (2012) Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am. J. Respir. Crit. Care Med. 185, 160–170 [DOI] [PubMed] [Google Scholar]
- 23. Loot A. E., Fleming I. (2011) Cytochrome P450-derived epoxyeicosatrienoic acids and pulmonary hypertension. Central role of transient receptor potential C6 channels. J. Cardiovasc. Pharmacol. 57, 140–147 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs B., Rupp M., Ghofrani H. A., Schermuly R. T., Seeger W., Grimminger F., Gudermann T., Dietrich A., Weissmann N. (2011) Diacylglycerol regulates acute hypoxic pulmonary vasoconstriction via TRPC6. Respir. Res. 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., Olson E. N. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116, 3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. (2006) TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 25, 5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X., Eder P., Chang B., Molkentin J. D. (2010) TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 107, 7000–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis J., Burr A. R., Davis G. F., Birnbaumer L., Molkentin J. D. (2012) A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 23, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishida M., Onohara N., Sato Y., Suda R., Ogushi M., Tanabe S., Inoue R., Mori Y., Kurose H. (2007) Gα12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J. Biol. Chem. 282, 23117–23128 [DOI] [PubMed] [Google Scholar]
- 30. Li Y., Jia Y. C., Cui K., Li N., Zheng Z. Y., Wang Y. Z., Yuan X. B. (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 [DOI] [PubMed] [Google Scholar]
- 31. Paez Espinosa E. V., Murad J. P., Ting H. J., Khasawneh F. T. (2012) Mouse transient receptor potential channel 6. Role in hemostasis and thrombogenesis. Biochem. Biophys. Res. Commun. 417, 853–856 [DOI] [PubMed] [Google Scholar]
- 32. Schlöndorff J., Del Camino D., Carrasquillo R., Lacey V., Pollak M. R. (2009) TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am. J. Physiol. 296, C558–C569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Faul C., Donnelly M., Merscher-Gomez S., Chang Y. H., Franz S., Delfgaauw J., Chang J. M., Choi H. Y., Campbell K. N., Kim K., Reiser J., Mundel P. (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y., Jarad G., Tripathi P., Pan M., Cunningham J., Martin D. R., Liapis H., Miner J. H., Chen F. (2010) Activation of NFAT signaling in podocytes causes glomerulosclerosis. J. Am. Soc. Nephrol. 21, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tian D., Jacobo S. M., Billing D., Rozkalne A., Gage S. D., Anagnostou T., Pavenstädt H., Hsu H. H., Schlondorff J., Ramos A., Greka A. (2010) Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci. Signal. 3, ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh I., Knezevic N., Ahmmed G. U., Kini V., Malik A. B., Mehta D. (2007) Gαq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 282, 7833–7843 [DOI] [PubMed] [Google Scholar]
- 37. Jiang L., Ding J., Tsai H., Li L., Feng Q., Miao J., Fan Q. (2011) Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp. Biol. Med. 236, 184–193 [DOI] [PubMed] [Google Scholar]
- 38. Wang L., Ellis M. J., Gomez J. A., Eisner W., Fennell W., Howell D. N., Ruiz P., Fields T. A., Spurney R. F. (2012) Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 81, 1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu L., Jiang R., Aoudjit L., Jones N., Takano T. (2011) Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao H., Peng F., Fan Y., Zhu X., Hu G., Buch S. J. (2009) TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 16, 1681–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubinfeld H., Seger R. (2004) The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol. Biol. 250, 1–28 [DOI] [PubMed] [Google Scholar]
- 42. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Mitogen-activated protein kinase. Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 43. Aouadi M., Binetruy B., Caron L., Le Marchand-Brustel Y., Bost F. (2006) Role of MAPKs in development and differentiation. Lessons from knockout mice. Biochimie 88, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 44. Roskoski R., Jr. (2012) ERK1/2 MAP kinases. Structure, function, and regulation. Pharmacol. Res. 66, 105–143 [DOI] [PubMed] [Google Scholar]
- 45. Carlson S. M., Chouinard C. R., Labadorf A., Lam C. J., Schmelzle K., Fraenkel E., White F. M. (2011) Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci. Signal. 4, rs11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoon S., Seger R. (2006) The extracellular signal-regulated kinase. Multiple substrates regulate diverse cellular functions. Growth Factors 24, 21–44 [DOI] [PubMed] [Google Scholar]
- 47. Kosako H., Yamaguchi N., Aranami C., Ushiyama M., Kose S., Imamoto N., Taniguchi H., Nishida E., Hattori S. (2009) Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat. Struct. Mol. Biol. 16, 1026–1035 [DOI] [PubMed] [Google Scholar]
- 48. Wortzel I., Seger R. (2011) The ERK cascade. Distinct functions within various subcellular organelles. Genes Cancer 2, 195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X. C., Carretero O. A., Zhuo J. L. (2006) Cross-talk between angiotensin II and glucagon receptor signaling mediates phosphorylation of mitogen-activated protein kinases ERK 1/2 in rat glomerular mesangial cells. Biochem. Pharmacol. 71, 1711–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flannery P. J., Spurney R. F. (2006) Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron. Exp. Nephrol. 103, e109–e118 [DOI] [PubMed] [Google Scholar]
- 51. Gorin Y., Ricono J. M., Wagner B., Kim N. H., Bhandari B., Choudhury G. G., Abboud H. E. (2004) Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem. J. 381, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hamaguchi A., Kim S., Yano M., Yamanaka S., Iwao H. (1998) Activation of glomerular mitogen-activated protein kinases in angiotensin II-mediated hypertension. J. Am. Soc. Nephrol. 9, 372–380 [DOI] [PubMed] [Google Scholar]
- 53. Hamaguchi A., Kim S., Izumi Y., Iwao H. (2000) Chronic activation of glomerular mitogen-activated protein kinases in Dahl salt-sensitive rats. J. Am. Soc. Nephrol. 11, 39–46 [DOI] [PubMed] [Google Scholar]
- 54. Bijian K., Takano T., Papillon J., Khadir A., Cybulsky A. V. (2004) Extracellular matrix regulates glomerular epithelial cell survival and proliferation. Am. J. Physiol. Renal Physiol. 286, F255–F266 [DOI] [PubMed] [Google Scholar]
- 55. Koshikawa M., Mukoyama M., Mori K., Suganami T., Sawai K., Yoshioka T., Nagae T., Yokoi H., Kawachi H., Shimizu F., Sugawara A., Nakao K. (2005) Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 16, 2690–2701 [DOI] [PubMed] [Google Scholar]
- 56. Liu S., Ding J., Fan Q., Zhang H. (2010) The activation of extracellular signal-regulated kinase is responsible for podocyte injury. Mol. Biol. Rep. 37, 2477–2484 [DOI] [PubMed] [Google Scholar]
- 57. Park S. J., Jeong K. S. (2004) Cell-type-specific activation of mitogen-activated protein kinases in PAN-induced progressive renal disease in rats. Biochem. Biophys. Res. Commun. 323, 1–8 [DOI] [PubMed] [Google Scholar]
- 58. Wada T., Pippin J. W., Nangaku M., Shankland S. J. (2008) Dexamethasone's prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron. Exp. Nephrol. 109, e8–e19 [DOI] [PubMed] [Google Scholar]
- 59. Cybulsky A. V., Takano T., Papillon J., Bijian K., Guillemette J. (2005) Activation of the extracellular signal-regulated kinase by complement C5b-9. Am. J. Physiol. Renal Physiol. 289, F593–F603 [DOI] [PubMed] [Google Scholar]
- 60. Pippin J. W., Durvasula R., Petermann A., Hiromura K., Couser W. G., Shankland S. J. (2003) DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J. Clin. Invest. 111, 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bollée G., Flamant M., Schordan S., Fligny C., Rumpel E., Milon M., Schordan E., Sabaa N., Vandermeersch S., Galaup A., Rodenas A., Casal I., Sunnarborg S. W., Salant D. J., Kopp J. B., Threadgill D. W., Quaggin S. E., Dussaule J. C., Germain S., Mesnard L., Endlich K., Boucheix C., Belenfant X., Callard P., Endlich N., Tharaux P. L. (2011) Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat. Med. 17, 1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bokemeyer D., Guglielmi K. E., McGinty A., Sorokin A., Lianos E. A., Dunn M. J. (1997) Activation of extracellular signal-regulated kinase in proliferative glomerulonephritis in rats. J. Clin. Invest. 100, 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bokemeyer D., Ostendorf T., Kunter U., Lindemann M., Kramer H. J., Floege J. (2000) Differential activation of mitogen-activated protein kinases in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 11, 232–240 [DOI] [PubMed] [Google Scholar]
- 64. Bokemeyer D., Panek D., Kramer H. J., Lindemann M., Kitahara M., Boor P., Kerjaschki D., Trzaskos J. M., Floege J., Ostendorf T. (2002) In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 13, 1473–1480 [DOI] [PubMed] [Google Scholar]
- 65. Sakai N., Wada T., Furuichi K., Iwata Y., Yoshimoto K., Kitagawa K., Kokubo S., Kobayashi M., Hara A., Yamahana J., Okumura T., Takasawa K., Takeda S., Yoshimura M., Kida H., Yokoyama H. (2005) Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am. J. Kidney Dis. 45, 54–65 [DOI] [PubMed] [Google Scholar]
- 66. Toyoda M., Suzuki D., Honma M., Uehara G., Sakai T., Umezono T., Sakai H. (2004) High expression of PKC-MAPK pathway mRNAs correlates with glomerular lesions in human diabetic nephropathy. Kidney Int. 66, 1107–1114 [DOI] [PubMed] [Google Scholar]
- 67. Awazu M., Ishikura K., Hida M., Hoshiya M. (1999) Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J. Am. Soc. Nephrol. 10, 738–745 [DOI] [PubMed] [Google Scholar]
- 68. Tamouza H., Chemouny J. M., Raskova Kafkova L., Berthelot L., Flamant M., Demion M., Mesnard L., Paubelle E., Walker F., Julian B. A., Tissandié E., Tiwari M. K., Camara N. O., Vrtovsnik F., Benhamou M., Novak J., Monteiro R. C., Moura I. C. (2012) The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 82, 1284–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang H., Ding J., Fan Q., Liu S. (2009) TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-κB translocation. Exp. Biol. Med. 234, 1029–1036 [DOI] [PubMed] [Google Scholar]
- 70. Chen C. A., Chen T. S., Chen H. C. (2012) Extracellular signal-regulated kinase plays a proapoptotic role in podocytes after reactive oxygen species treatment and inhibition of integrin-extracellular matrix interaction. Exp. Biol. Med. 237, 777–783 [DOI] [PubMed] [Google Scholar]
- 71. Wang Y., Deb D. K., Zhang Z., Sun T., Liu W., Yoon D., Kong J., Chen Y., Chang A., Li Y. C. (2012) Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J. Am. Soc. Nephrol. 23, 1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brinkkoetter P. T., Olivier P., Wu J. S., Henderson S., Krofft R. D., Pippin J. W., Hockenbery D., Roberts J. M., Shankland S. J. (2009) Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Invest. 119, 3089–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bijian K., Takano T., Papillon J., Le Berre L., Michaud J. L., Kennedy C. R., Cybulsky A. V. (2005) Actin cytoskeleton regulates extracellular matrix-dependent survival signals in glomerular epithelial cells. Am. J. Physiol. Renal Physiol. 289, F1313–F1323 [DOI] [PubMed] [Google Scholar]
- 74. Doublier S., Lupia E., Catanuto P., Periera-Simon S., Xia X., Korach K., Berho M., Elliot S. J., Karl M. (2011) Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 79, 404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang R., Khoo M. S., Wu Y., Yang Y., Grueter C. E., Ni G., Price E. E., Jr., Thiel W., Guatimosim S., Song L. S., Madu E. C., Shah A. N., Vishnivetskaya T. A., Atkinson J. B., Gurevich V. V., Salama G., Lederer W. J., Colbran R. J., Anderson M. E. (2005) Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 11, 409–417 [DOI] [PubMed] [Google Scholar]
- 76. Murshid A., Chou S. D., Prince T., Zhang Y., Bharti A., Calderwood S. K. (2010) Protein kinase A binds and activates heat shock factor 1. PloS One 5, e13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schumacher V. A., Schlötzer-Schrehardt U., Karumanchi S. A., Shi X., Zaia J., Jeruschke S., Zhang D., Pavenstädt H., Drenckhan A., Amann K., Ng C., Hartwig S., Ng K. H., Ho J., Kreidberg J. A., Taglienti M., Royer-Pokora B., Ai X. (2011) WT1-dependent sulfatase expression maintains the normal glomerular filtration barrier. J. Am. Soc. Nephrol. 22, 1286–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saleem M. A., O'Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 79. Emerling B. M., Viollet B., Tormos K. V., Chandel N. S. (2007) Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 581, 5727–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lackey K., Cory M., Davis R., Frye S. V., Harris P. A., Hunter R. N., Jung D. K., McDonald O. B., McNutt R. W., Peel M. R., Rutkowske R. D., Veal J. M., Wood E. R. (2000) The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 10, 223–226 [DOI] [PubMed] [Google Scholar]
- 81. Tombes R. M., Grant S., Westin E. H., Krystal G. (1995) G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase). Cell Growth Differ. 6, 1063–1070 [PubMed] [Google Scholar]
- 82. Chen J., Crossland R. F., Noorani M. M., Marrelli S. P. (2009) Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am. J. Physiol. Heart Circ. Physiol. 297, H417–H424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kinoshita H., Kuwahara K., Nishida M., Jian Z., Rong X., Kiyonaka S., Kuwabara Y., Kurose H., Inoue R., Mori Y., Li Y., Nakagawa Y., Usami S., Fujiwara M., Yamada Y., Minami T., Ueshima K., Nakao K. (2010) Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 106, 1849–1860 [DOI] [PubMed] [Google Scholar]
- 84. Koitabashi N., Aiba T., Hesketh G. G., Rowell J., Zhang M., Takimoto E., Tomaselli G. F., Kass D. A. (2010) Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J. Mol. Cell. Cardiol. 48, 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kwan H. Y., Huang Y., Yao X. (2004) Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc. Natl. Acad. Sci. U.S.A. 101, 2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nishida M., Watanabe K., Sato Y., Nakaya M., Kitajima N., Ide T., Inoue R., Kurose H. (2010) Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J. Biol. Chem. 285, 13244–13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takahashi S., Lin H., Geshi N., Mori Y., Kawarabayashi Y., Takami N., Mori M. X., Honda A., Inoue R. (2008) Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J. Physiol. 586, 4209–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liebmann C. (2011) EGF receptor activation by GPCRs. An universal pathway reveals different versions. Mol. Cell. Endocrinol. 331, 222–231 [DOI] [PubMed] [Google Scholar]
- 90. Werry T. D., Sexton P. M., Christopoulos A. (2005) “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol. Metab. 16, 26–33 [DOI] [PubMed] [Google Scholar]
- 91. Tajeddine N., Gailly P. (2012) TRPC1 protein channel is major regulator of epidermal growth factor receptor signaling. J. Biol. Chem. 287, 16146–16157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Levitzki A., Gazit A. (1995) Tyrosine kinase inhibition. An approach to drug development. Science 267, 1782–1788 [DOI] [PubMed] [Google Scholar]
- 93. Fukazawa R., Miller T. A., Kuramochi Y., Frantz S., Kim Y. D., Marchionni M. A., Kelly R. A., Sawyer D. B. (2003) Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell. Cardiol. 35, 1473–1479 [DOI] [PubMed] [Google Scholar]
- 94. Ohtsu H., Dempsey P. J., Eguchi S. (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. 291, C1–C10 [DOI] [PubMed] [Google Scholar]
- 95. Lemos V. S., Poburko D., Liao C. H., Cole W. C., van Breemen C. (2007) Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem. Biophys. Res. Commun. 352, 130–134 [DOI] [PubMed] [Google Scholar]
- 96. Rosker C., Graziani A., Lukas M., Eder P., Zhu M. X., Romanin C., Groschner K. (2004) Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J. Biol. Chem. 279, 13696–13704 [DOI] [PubMed] [Google Scholar]
- 97. Engh R. A., Girod A., Kinzel V., Huber R., Bossemeyer D. (1996) Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J. Biol. Chem. 271, 26157–26164 [DOI] [PubMed] [Google Scholar]
- 98. Nishioka K., Nishida M., Ariyoshi M., Jian Z., Saiki S., Hirano M., Nakaya M., Sato Y., Kita S., Iwamoto T., Hirano K., Inoue R., Kurose H. (2011) Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel. Arterioscler. Thromb. Vasc. Biol. 31, 2278–2286 [DOI] [PubMed] [Google Scholar]
- 99. Shen B., Kwan H. Y., Ma X., Wong C. O., Du J., Huang Y., Yao X. (2011) cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J. Biol. Chem. 286, 19439–19445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Horinouchi T., Higa T., Aoyagi H., Nishiya T., Terada K., Miwa S. (2012) Adenylate cyclase/cAMP/protein kinase A signaling pathway inhibits endothelin type A receptor-operated Ca2+ entry mediated via transient receptor potential canonical 6 channels. J. Pharmacol. Exp. Ther. 340, 143–151 [DOI] [PubMed] [Google Scholar]
- 101. Leuner K., Heiser J. H., Derksen S., Mladenov M. I., Fehske C. J., Schubert R., Gollasch M., Schneider G., Harteneck C., Chatterjee S. S., Müller W. E. (2010) Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators. Identification of a novel pharmacophore. Mol. Pharmacol. 77, 368–377 [DOI] [PubMed] [Google Scholar]
- 102. Harada M., Luo X., Qi X. Y., Tadevosyan A., Maguy A., Ordog B., Ledoux J., Kato T., Naud P., Voigt N., Shi Y., Kamiya K., Murohara T., Kodama I., Tardif J. C., Schotten U., Van Wagoner D. R., Dobrev D., Nattel S. (2012) Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nijenhuis T., Sloan A. J., Hoenderop J. G., Flesche J., van Goor H., Kistler A. D., Bakker M., Bindels R. J., de Boer R. A., Möller C. C., Hamming I., Navis G., Wetzels J. F., Berden J. H., Reiser J., Faul C., van der Vlag J. (2011) Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179, 1719–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hassock S. R., Zhu M. X., Trost C., Flockerzi V., Authi K. S. (2002) Expression and role of TRPC proteins in human platelets. Evidence that TRPC6 forms the store-independent calcium entry channel. Blood 100, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 105. Sung T. S., Jeon J. P., Kim B. J., Hong C., Kim S. Y., Kim J., Jeon J. H., Kim H. J., Suh C. K., Kim S. J., So I. (2011) Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am. J. Physiol. 301, C823–832 [DOI] [PubMed] [Google Scholar]
- 106. Tomić M., Kucka M., Kretschmannova K., Li S., Nesterova M., Stratakis C. A., Stojilkovic S. S. (2011) Role of nonselective cation channels in spontaneous and protein kinase A-stimulated calcium signaling in pituitary cells. Am. J. Physiol. Endocrinol. Metab. 301, E370–E379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gao L., Blair L. A., Marshall J. (2006) CaMKII-independent effects of KN93 and its inactive analog KN92. Reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 345, 1606–1610 [DOI] [PubMed] [Google Scholar]
- 108. Soboloff J., Spassova M., Xu W., He L. P., Cuesta N., Gill D. L. (2005) Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J. Biol. Chem. 280, 39786–39794 [DOI] [PubMed] [Google Scholar]