Abstract
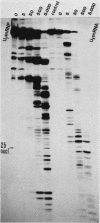
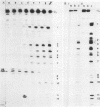
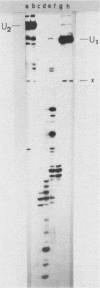
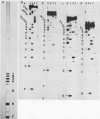
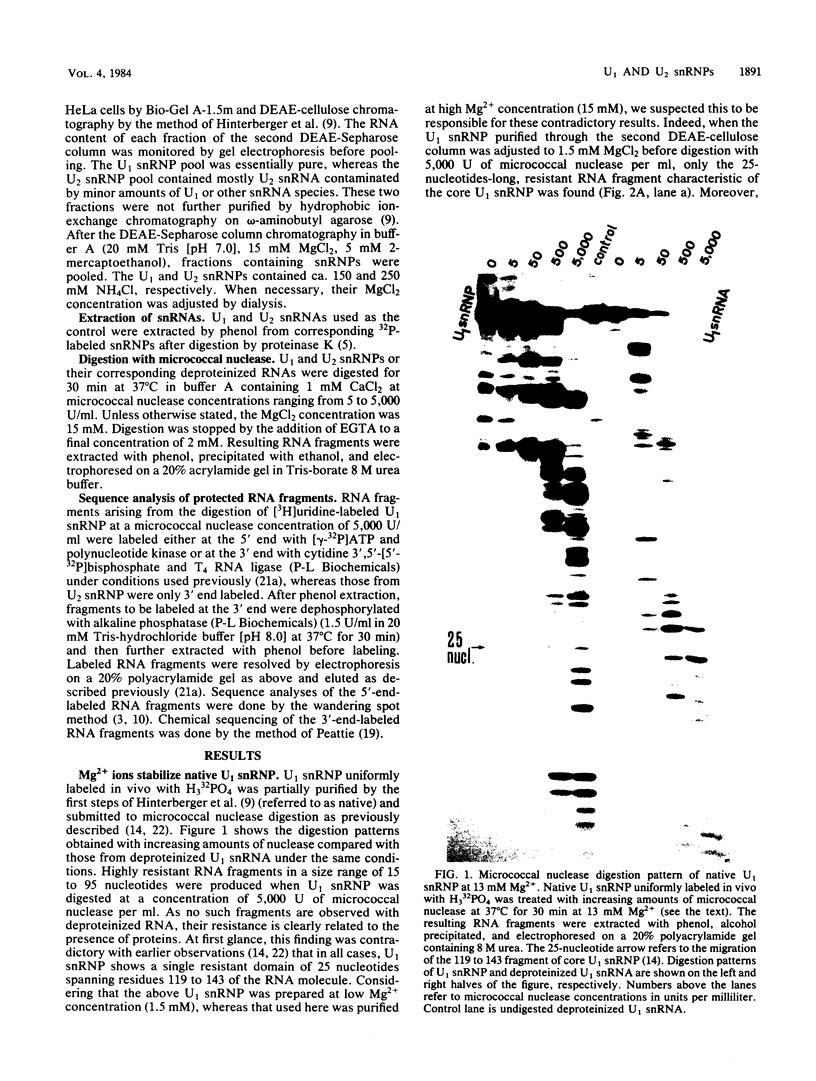
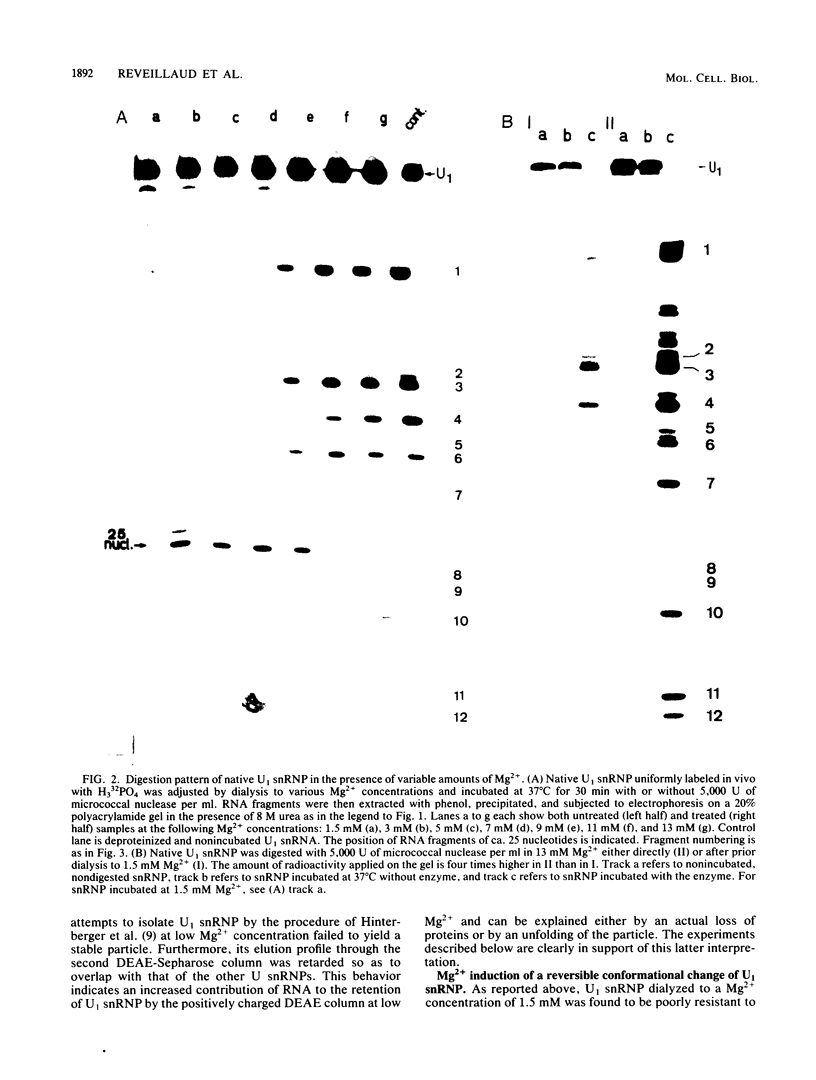
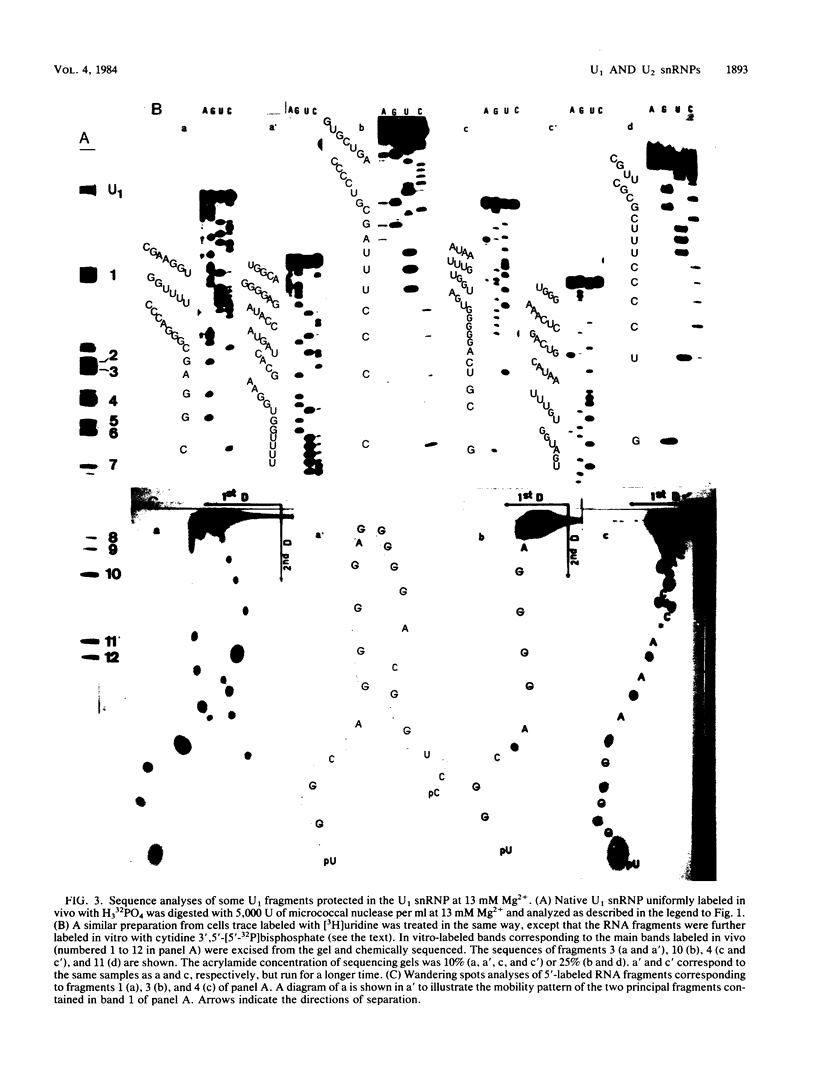
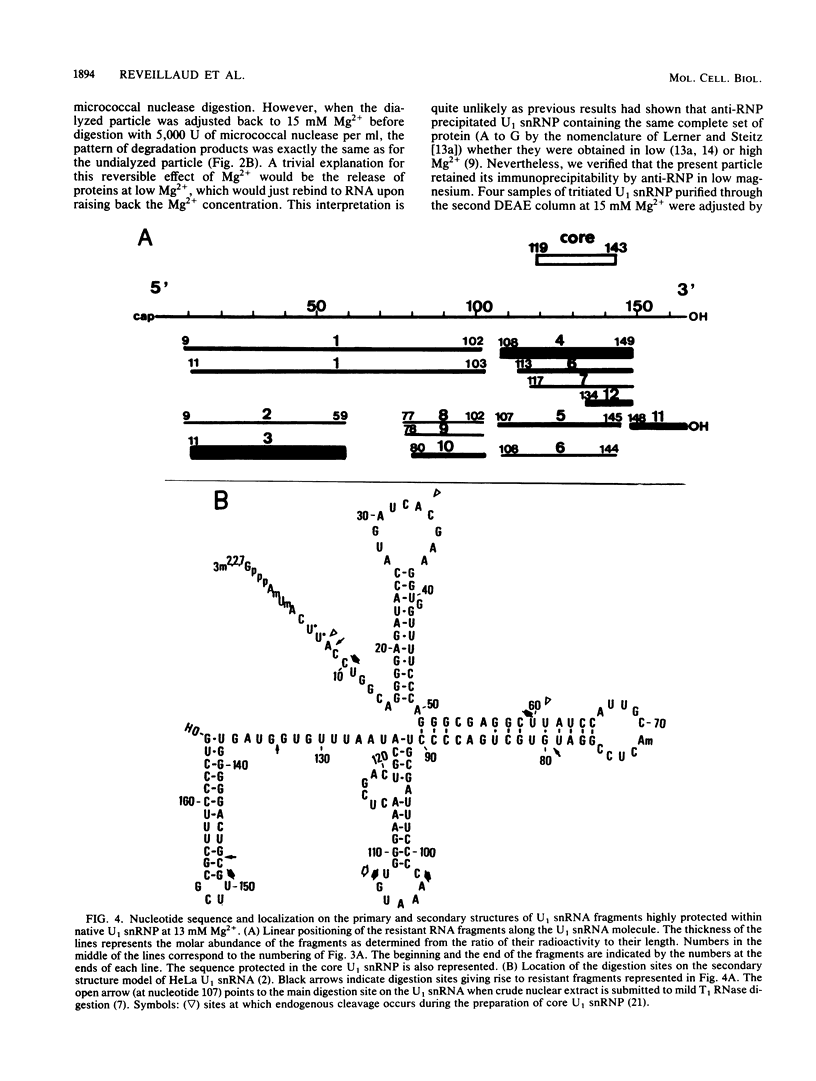
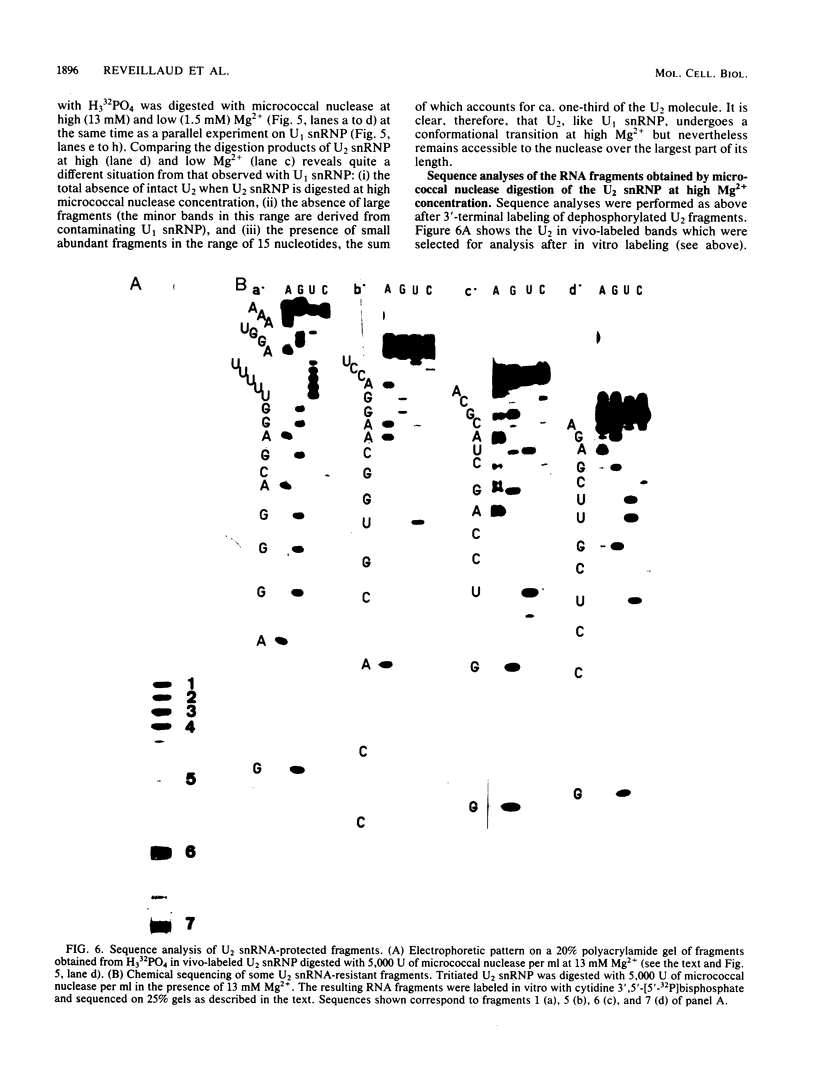
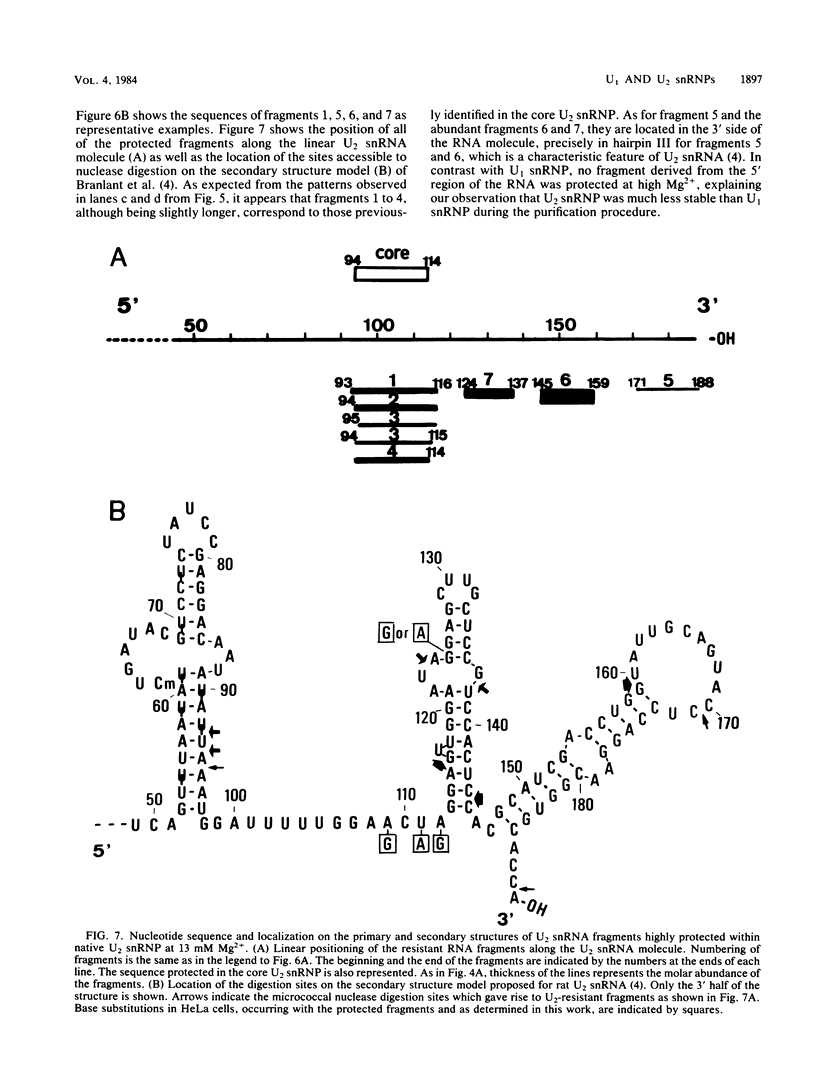
When U1 and U2 small nuclear ribonucleoproteins (snRNPs) purified by a procedure which preserves their immunoprecipitability by autoimmune antibodies (Hinterberger et al., J. Biol. Chem. 258:2604-2613, 1983), were submitted to extensive digestion with micrococcal nuclease, we found that their degradation pattern was sharply dependent upon magnesium concentration, indicating that they undergo a profound structural modification. At low Mg2+ (less than or equal to 5 mM), both particles only exhibit a core-resistant structure previously identified as being common to all but U6 snRNAs (Liautard et al., J. Mol. Biol. 162: 623-643, 1982). At high Mg2+ (greater than or equal to 7 mM), U1 and U2 snRNPs behave differently from one another. In U1 snRNP, most U1 snRNA sequence is protected, except for the 10 5'-terminal nucleotides presumably involved in splicing and a short sequence between nucleotides 102 and 108. Another region spanning nucleotides 60 to 79 is only weakly protected. This structural modification was demonstrated to be reversible. In U2 snRNP, the U2 snRNA sequence remains exposed in its 5' part up to nucleotide 92, and the 3'-terminal hairpin located outside the core structure becomes protected.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assens C., Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Highly purified snRNPs retain antigenic determinants towards anti Sm antibodies. Biochem Biophys Res Commun. 1982 Jun 15;106(3):953–960. doi: 10.1016/0006-291x(82)91803-4. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel C., Widada J. S., Lelay M. N., Jeanteur P., Liautard J. P. Purification and characterization of a simple ribonucleoprotein particle containing small nucleoplasmic RNAs (snRNP) as a subset of RNP containing heterogenous nuclear RNA (hnRNP) from HeLa cells. Nucleic Acids Res. 1981 Feb 25;9(4):815–830. doi: 10.1093/nar/9.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducamp C., Jeanteur P. Characterization of nuclear RNP particles from Hela cells. Analysis of protein and RNA constituents. Presence of poly (A). Biochimie. 1973;55(10):1235–1243. doi: 10.1016/s0300-9084(74)80328-7. [DOI] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Busch H. Site-specific cleavage by T1 RNase of U-1 RNA in u-1 ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1562–1566. doi: 10.1073/pnas.78.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Keller W. Splicing of in vitro synthesized messenger RNA precursors in HeLa cell extracts. Cell. 1983 Nov;35(1):89–99. doi: 10.1016/0092-8674(83)90211-8. [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlaw C. S., Robberson B. L., Berget S. M. Fractionation and characterization of human small nuclear ribonucleoproteins containing U1 and U2 RNAs. J Biol Chem. 1983 Jun 10;258(11):7181–7189. [PubMed] [Google Scholar]
- Lelay M. N., Brunel C., Jeanteur P. Peptide mapping of in vivo and in vitro phosphorylated sites of proteins from HeLa hnRNP. FEBS Lett. 1978 Jun 1;90(1):54–56. doi: 10.1016/0014-5793(78)80296-8. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Hardy S. F., Sharp P. A. Splicing of adenovirus RNA in a cell-free transcription system. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5230–5234. doi: 10.1073/pnas.80.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri-Widada J., Assens C., Liautard J. P., Jeanteur P., Brunel C. Isolation of a pure U1 snRNP from HeLa cells. Biochem Biophys Res Commun. 1982 Jan 29;104(2):457–462. doi: 10.1016/0006-291x(82)90659-3. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Assens C., Brunel C. Primary structure identification of snRNAs present in highly purified snRNPs from HeLa cells. Mol Biol Rep. 1981 Nov 30;8(1):29–36. doi: 10.1007/BF00798382. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Brunel C., Jeanteur P. Interaction of snRNAs with rapidly sedimenting nuclear sub-structures (hnRNPs) from HeLa cells. Nucleic Acids Res. 1983 Oct 11;11(19):6631–6646. doi: 10.1093/nar/11.19.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]