Background: Hepatitis B virus (HBV) infection has been linked to the development of hepatocellular carcinoma (HCC) in humans.
Results: Specific microRNAs inhibit HBV replication, and HBV infection also reprograms microRNA expression.
Conclusion: The viral HBx RNA directly mediates the down-regulation of the tumor suppressor microRNA miR-15a/miR-16-1.
Significance: The functional interplay between HBV infection and microRNA may contribute to HCC development.
Keywords: Gene Regulation, Hepatitis Virus, MicroRNA, Tumor Suppressor Gene, Viral DNA
Abstract
Hepatitis B virus (HBV) is a key risk factor for the development of hepatocellular carcinoma (HCC). Recent work suggests a functional link between HCC and microRNA expression, but the mechanism underlying the functional interaction between microRNA and HBV infection has remained largely elusive. Here we present evidence that the microRNA machinery serves as a defense system against HBV infection, which, in turn, reprograms the expression of specific microRNAs. We demonstrate a critical role of miR-15a/miR-16-1 in this functional interplay between microRNA and HBV infection, but in contrast to various indirect mechanisms mediated by viral proteins, we unexpectedly found that the HBx transcript directly triggers the down-regulation of miR-15a/miR-16-1 via the microRNA targeting sequences in the viral RNA. Because miR-15a and miR-16-1 are well known tumor suppressor microRNAs in multiple human cancers, our findings raise the intriguing possibility that viral RNA-mediated down-regulation of specific tumor suppressor microRNAs may contribute to HCC development in HBV-infected cells.
Introduction
Liver cancer is one of the leading causes of mortality in humans, especially in developing countries. A key risk factor is the infection of hepatitis B virus (HBV),4 which has been tightly linked to the development of hepatocellular carcinoma (HCC) (1, 2). HBV is a partially double-stranded DNA virus that contains a 3.2-kb circular genome in which four overlapping open reading frames encode for viral enzymes, core proteins, and surface antigens (3). Particularly important is the evidence for the causal role of the HBV-encoded regulatory protein HBx in hepatocarcinogenesis, including its ability to induce cell transformation, enhance cell cycle progression, and accelerate tumor development (4, 5). At the molecular level, the HBx protein has been shown to modulate cell signaling, alter transcription programs, interfere with DNA repair, and inhibit apoptosis (6, 7). HBx appears to interact with a variety of transcription factors, including the multiple basal transcription factors TFIIB, TFIIH, the TATA binding protein TBP, and various regulatory transcription factors such as p53, cAMP response element-binding protein, and CBP/p300 (8, 9). Although previous works suggest multiple molecular pathways that may orchestrate the development of HCC in HBV-infected cells, it has been challenging to determine which of these events trigger hepatocarcinogenesis in humans, given the fact that chronically HBV-infected patients take a long time to develop liver cancer in only a subset of virus carriers.
MicroRNAs have emerged as key components of regulatory networks by targeting up to 60% of transcribed genes in mammalian cells (10). Increasing evidence suggests intensive functional interplay between viruses and microRNAs in virally infected cells (11). Specific microRNAs may inhibit viral replication by targeting viral transcripts or cellular factors critical for viral replication or enhance viral replication by compromising the host defense system or negative regulators of viral replication (12, 13). In the case of HBV, multiple microRNAs have been shown to target specific HBV transcripts to inhibit HBV replication (14–17). Importantly, chronic viral infection can, in turn, alter the expression of specific microRNAs, which may directly contribute to the development of hepatocarcinoma because some of these microRNAs are known to function as oncogenes (i.e. miR-602) (18), whereas others function as tumor suppressors (i.e. miR-15a/miR-16, Let-7a, and 199a-3p) (16, 18–20).
It has been poorly understood how HBV reprograms the miRNome in infected cells. Such reprogramming may be elicited directly by specific viral gene products or triggered indirectly by the host response to viral infection. In HBV-infected hepatocytes, the HBx protein has been implicated in playing a direct role in cell transformation, at least in part by antagonizing p53 expression and function (21). More recently, HBx has been found to cause down-regulation of miR-15a/miR-16-1, which appears to account for HBx-induced anchorage-independent cell growth and suppression of cellular apoptosis in hepatocytes (20). The mechanism was suggested to be due to the induction of c-Myc, shown previously to mediate the transcriptional repression of the miR-15a/miR-16-1 gene cluster in B cell lymphomas (22). However, it has been unclear how HBx activates c-Myc, and there is also evidence to suggest that c-Myc induction may not be the only mechanism for the down-regulation of miR-16-1 in HBx-overproducing HepG2 cells (20).
In this study, we aimed to address functional interplay between HBV and the microRNA machinery hoping to identify potential initiating events for HCC development. We first established that the microRNA machinery is inhibitory to HBV infection on the basis of multiple lines of functional evidence, which is contrary to the conclusion reached in a recent report (23). We found that miR-15a/miR-16-1 is able to directly target HBV transcripts, particularly that encoding for HBx, which is also responsible for reprogramming the expression of multiple microRNAs, including miR-15a/miR-16-1. We found, unexpectedly, that a frame-shifted HBx expression unit that produced no protein but expressed an equivalent amount of RNA could also mediate the down-regulation of miR-15a/miR-16-1 via the microRNA-targeting sequence in the viral RNA. These findings provide a biological paradigm for mRNA to mediate microRNA trimming initially observed in fly extracts (24) and, together with other reported cases of RNA-induced microRNA down-regulation (25, 26), suggest an emerging molecular strategy for viral RNA-mediated pathogen-host interactions.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Luciferase Assays
Human hepatocellular carcinoma HepG2 cells were cultured in DMEM (Invitrogen) plus 10% FBS in a humidified incubator with 5% CO2 at 37 °C. HepG2 cells were seeded in 6-well plates, and 72 h after transfection with Lipofectamine 2000 (Life Technology), cells were harvested to monitor viral replication. For luciferase assays, cells seeded in 24-well plates were collected 48 h after transfection. Luciferase activities were measured using the dual-luciferase reporter assay system (Promega). Luciferase activity was normalized against the firefly luciferase activity in cotransfected cells.
Plasmid Construction and Oligonucleotide Synthesis
To construct plasmids expressing the wild-type or mutant versions of HBx protein or RNA, the HBx cDNA was PCR-amplified from the pCH9-3091 plasmid that contains the full-length HBV genome. Fragments containing the predicted miR-15a target site in the HBx transcript were inserted into pRL-TK in the protein-coding region right before the stop codon. A series of DNA oligos used for cloning are listed in supplemental Table S1. Various miRs and anti-miRs, as described previously, were synthesized at Genepharma (Shanghai, China) and are listed in supplemental Table S2.
Analysis of HBV Replication
For ELISA detection of HBsAg and HBeAg, the supernatants of HepG2 cells transfected with pCH9-3091 were harvested for quantifying the levels of HBsAg and HBeAg using ELISA kits (InTec Products, Shanghai, China). Absorbance was recorded with dual-wavelength measurements (450/630 nm).
For quantification of extracellular HBV DNA, the supernatant of pCH9-3091-transfected HepG2 cells was collected, and DNA was precipitated with an equal volume of isopropanol at −20 °C for 1 h. Precipitated DNA was collected by centrifugation at 10,000 × g for 20 min at 4 °C, washed with cold 70% ethanol, dried, and resuspended in 50 μl of H2O. The DNA copy number was determined by real-time PCR using the primer pair in supplemental Table S3.
To quantify intracellular replicative intermediate HBV DNA, HBV-infected cells were harvested and resuspended in 0.5 ml of 0.5% Nonidet P-40, 20 mm Tris-HCl (pH7.5), 150 mm NaCl, and 1 mg/ml BSA. After centrifugation to remove insoluble materials, the supernatant was precipitated in a final concentration of 20% PEG-8000 for 2 h at 4 °C. The pellet, which contained the intermediate DNA of HBV, was resuspended in 0.4 ml of 0.4 m NaCl, 10 mm Tris-HCl (pH8.0), and 2 mm EDTA, followed by the addition of 40 μl of 20% SDS and 16 μl of 10 mg/ml proteinase K. After incubation at 60 °C for 3 h, 0.3 ml of 6 m NaCl was added, insoluble materials were removed by centrifugation, and the intermediate DNA in the supernatant was precipitated with an equal of volume of isopropanol at −20 °C for 1 h. The viral DNA was collected by centrifugation at 10,000 × g at 4 °C for 20 min, washed with 70% ethanol, dried, and dissolved in 50 μl of double-distilled H2O. The nature of the intermediate DNA was confirmed on agarose gel and quantified by real-time PCR using the primer pair listed in supplemental Table S3.
To detect and quantify covalently closed circular HBV DNA, HBV-transfected cells were lysed in 0.5 ml of 6% SDS and 100 mm NaOH and incubated at 37 °C for 30min. After neutralization with an equal volume of 3 m potassium acetate (pH4.8), cell debris was removed by centrifugation for 20 min at 10,000 × g at 4 °C, and the HBV DNA in the supernatant was extracted with phenol/chloroform and precipitated with ethanol in the presence of 10 μg of tRNA. Pelleted DNA was resuspended in 50 μl of TE buffer (10 mm Tris-HCl (pH 7.5), 1 mm EDTA) and digested with 10 units of plasmid-safe DNase (Epicenter) for 2 h at 37 °C. The HBV covalently closed circular DNA, confirmed on agarose gel, was quantified by real-time PCR using the primer pair listed in supplemental Table S3.
RT-PCR and Quantitative RT-PCR Analysis of Cellular mRNA and MicroRNA
Total RNA was extracted with TRIzol reagent (Life Technology) and treated with RQ1 DNase I (Promega). For RT reactions, 2 μg of total RNA was used in each 20-μl reaction containing 1 μl of the RT primer. After the RT reaction, 1 μl of cDNA was used for real-time PCR analysis in the presence of the SYBR® premix ExTaqTM II (TaKaRa, Otst, Shiga, Japan) at 94 °C for 3 min followed by 40 cycles at 94 °C for 10 s, 60 °C for 15 s, and 72 °C for 20 s in a Rotor Gene 6000 real-time PCR system. Real-time PCR results were analyzed and expressed as relative microRNA expression after converting the CT values to fold changes. Specific mRNAs and microRNAs were normalized against actin mRNA and U6 snRNA, respectively. The primers used to analyze specific mRNAs and microRNAs are listed in supplemental Table S4.
Western Blotting Analysis
Proteins in cell lysate were resolved in 12% SDS-PAGE, transferred to a PVDF membrane, and probed with specific antibodies. Anti Bcl-2, Ccnd-1, c-Myc, and GFP antibodies were purchased from Proteintech. Anti-FLAG antibody was purchased from Sigma.
Bio-miR Pull-down assays
HepG2 cells seeded in 6-well plates were transfected with 100 pmol Bio-miR and 1 μg of pcDNA3.0-HBx-w-R or pcDNA3.0-HBx-m-R. Harvested cells were lysed in 0.2 ml of PBS plus 0.1% SDS and 0.5% Nonidet P-40, and cell debris was removed by centrifugation. The supernatant was mixed with 10 μl of streptavidin beads (New England Biolabs) and incubated on a tube roller at room temperature for 1 h. After washing the beads with the lysis buffer (0.1% SDS and 0.5% Nonidet P-40), RNA was extracted with TRIzol, and recovered RNA was analyzed by RT-PCR. Specific Bio-miRs used in this study are listed in supplemental Table S5.
RESULTS
The MicroRNA Machinery Is Inhibitory to HBV Replication
A large amount of evidence has documented that specific microRNAs can target viral transcripts, which was initially thought to serve as an innate immune system against viral infection. However, some microRNAs have also been found to stimulate the expression of viral RNA, as exemplified by miR-122 in hepatocytes, where it enhances the translation of hepatitis C virus (HCV) by targeting the 5′ UTR of the viral RNA (27). Furthermore, many microRNAs can enhance viral infection by suppressing the interferon system or modulating cellular gene products critical for viral replication in host cells (11). Therefore, the net effect of the microRNA system may be either positive or negative, depending on specific viruses. In hepatocytes, for example, HCV replication was attenuated after knocking down Dicer or other key components of the microRNA machinery, suggesting a positive net effect of the microRNA machinery on HCV replication (13, 28).
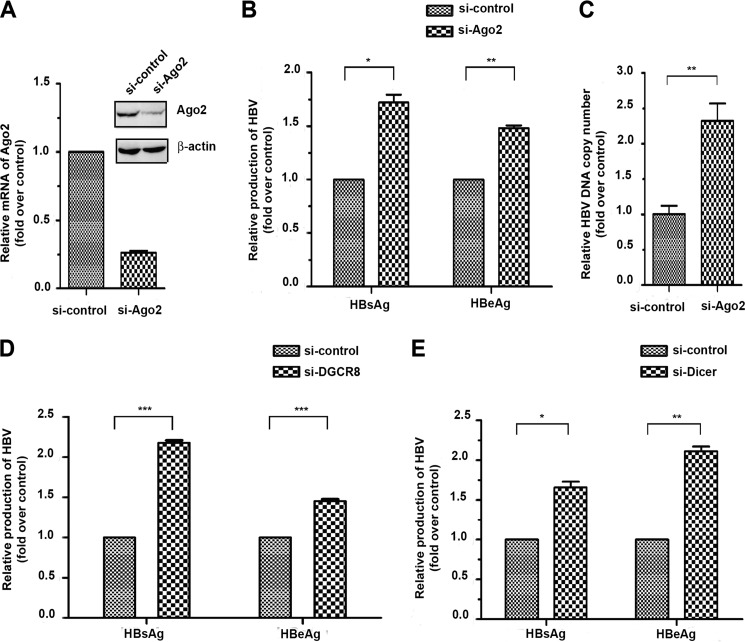
Similar to the HCV system, different microRNAs in the same cells appear to have positive or negative effects on HBV replication. For instance, while most microRNAs characterized to date inhibit viral replication by targeting specific HBV transcripts, miR-1 has been shown to enhance HBV replication by targeting the host gene HDAC4 (14). To determine the net effect of the microRNA machinery on HBV replication, we knocked down the key component of the RISC complex Ago2 in hepatocytes (Fig. 1A) and observed that the inactivation of the microRNA system caused a marked enhancement in the production of both the s and e antigens (HBsAg and HBeAg) expressed from the HBV genome (B). The enhancement likely resulted from more efficient viral replication, as indicated by increased viral DNA released from transfected cells (Fig. 1C).
FIGURE 1.
Negative net impact of the microRNA machinery on HBV replication in HepG2 cells. A, siRNA-mediated knockdown of Ago2 was verified by real-time RT-PCR and Western blotting. B and C, Ago2 knockdown enhanced the production of both s (HBs) and e (HBe) antigens (B) and increased HBV replication (C). D and E, enhanced HBV replication was also observed after knocking down DGCR8 (D) or Dicer (E). *, p < 0.05; **, p < 0.01; ***, p < 0.001 on the basis of experiments performed in triplicate.
Our data suggest a net negative impact of the microRNA machinery on HBV replication, which is contradictory to a recent report indicating a positive role of Ago2 in HBV replication (23). To address this discrepancy, we further tested knocking down the essential component of the microprocessor DGCR8 and the microRNA maturation factor Dicer, both of which were efficiently down-regulated in siRNA-treated cells (data not shown). Under these conditions, we also detected increased production of HBsAg and HBeAg (Fig. 1, D and E). Therefore, these data are fully consistent with the effect of Ago2 knockdown and, coupled with further functional analyses (see below), strongly suggest that the microRNA system is generally defensive against HBV infection, which is opposite to the well documented impact of the microRNA machinery on HCV replication in hepatocytes.
Multiple MicroRNAs, Including miR-15a/miR-16-1, Inhibit HBV Replication
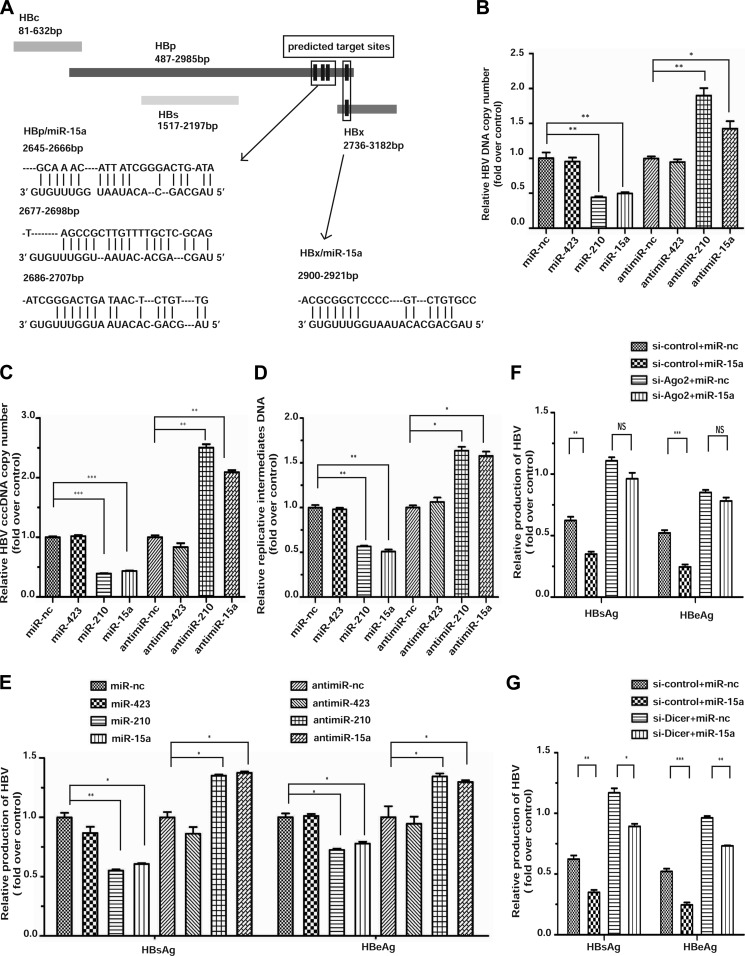
Because of the overall negative effect of the microRNA system on HBV replication, we suspected that many specific microRNAs might exert a negative impact on HBV transcripts in a collective fashion, thus offsetting other, potentially positive effects through modulating host factors. Using TargetScan (29), we confirmed multiple microRNAs that have been characterized previously to target specific sites in HBV transcripts (14). In these analyses, we additionally identified three sites in the coding region for HBp and one in the overlapping region between HBp and HBx that could be targeted by miR-15a and miR-16-1 (Fig. 2A) (note that these two microRNAs differ by only one base outside of the seed region and, thus, are likely to have similar targeting specificity), despite the fact that these microRNAs appear to target HBp and HBx transcripts via non-canonical base-pairing in the seed region, which is now known to be widespread in mammalian cells (30). We were particularly interested in these microRNAs because they have been reported recently to be down-regulated in HBV-infected cells (20). Furthermore, these microRNAs are well characterized for their tumor suppressor functions in B lymphocytes (31), and the loss of the microRNA locus has been linked to HCC (32). Thus, these observations raise an intriguing possibility that the functional interplay between these microRNAs and HBV replication may contribute to the development of HCC in HBV-infected cells.
FIGURE 2.
Targeting of HBp and HBx by miR-15a/miR-16-1. A, the open reading frames for the four HBV-encoded genes are illustrated along with their gene coordinates. Three predicted target sites in HBp and one shared target site in HBp and HBx by miR-15a are highlighted below. B–F, reciprocal effects of the miR-15a mimic and anti-miR-15a on HBV replication measured by extracellular HBV DNA (B), intracellular covalently closed circular HBV DNA (cccDNA) (C), and intermediate HBV DNA (D) and production of the s and e antigens (E). The effects of the miR-423 mimic and anti-miR-423 were analyzed as negative controls, whereas the miR-210 mimic and anti-miR-210 were tested as positive controls in these assays. F and G, the effect of miR-15a in conjunction with knockdown of Ago2 (G) or Dicer (H) on HBV replication was measured on the basis of the production of the s and e antigens. *, p < 0.05; **, p < 0.01; ***, p < 0.001 on the basis of experiments performed in triplicate.
We therefore committed to determine whether miR-15a could indeed modulate HBV replication by overexpressing a miR-15a mimic or blocking miR-15a with an anti-miR-15a. As expected, we found that the miR-15a mimic inhibited the production of both HBsAg and HBeAg and, conversely, that the anti-miR-15a enhanced HBsAg and HBeAg production, both in a dose-dependent fashion (data not shown). To thoroughly evaluate the impact of miR-15a and its anti-miR on HBV infection, we measured the extracellular DNA in HBV-infected cells (Fig. 2B), the levels of intracellular DNA, including the covalently closed circular DNA (C) and replication intermediate DNA (D), and the production of the s and e antigens (E). In these analyses, the miR-210 mimic and anti-miR-210 served as positive controls, as documented previously (33), and the miR-423 mimic and anti-miR-423 were similarly examined as negative controls that lacked a detectable effect on HBV replication at all concentrations tested. Through these analyses, we observed the expected opposite effects of the miR-15a mimic and its anti-miR on viral replication. We similarly tested the effect of the miR-16-1 mimic, obtaining the same inhibitory effect as the miR-15a mimic to HBV replication (data not shown).
To further demonstrate that these microRNAs function as part of the RISC complex, we tested the effects of miR-15a in Ago2 siRNA-treated cells. We found that Ago2 is required for miR-15a to suppress the production of the s and e antigens (Fig. 2F). In contrast, although siDicer impaired the function of endogenous microRNAs, thus causing enhanced HBV replication, miR-15a could still function in Dicer knockdown cells (Fig. 2G). Together, these data demonstrate that miR-15a functions as part of the RISC complex to suppress HBV replication in hepatocytes.
miR-15a Targets the Protein Coding Region for HBp and HBx
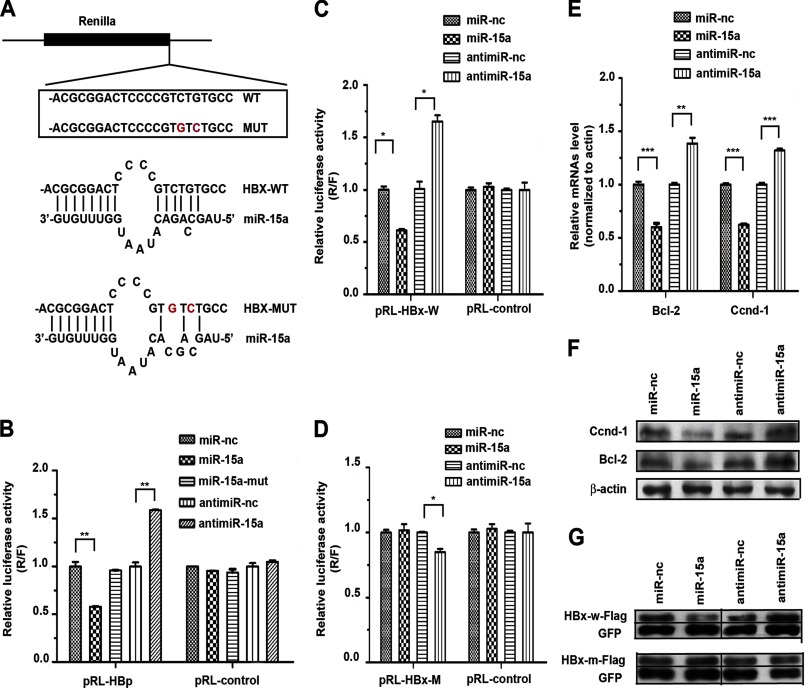
To demonstrate that miR-15a targets specific HBV transcripts through the classic targeting mechanism via base-pairing, we prepared a set of luciferase reporters that contain the wild-type or mutant miR-15a targeting sequences (Fig. 3A). Because the predicted targeting sites reside in the coding region of the HBp and HBx transcripts, we wanted to closely mimic the sequence configuration by inserting the miR-15a targeting site(s) right before the stop codon in the Renilla reporter. We found that the miR-15a mimic was able to suppress the activity of the luciferase reporter containing all three targeting sites from HBp but that the mutant miR-15a mimic lacked such an effect. Conversely, anti-miR-15a enhanced the activity of the luciferase reporter (Fig. 3B). We made similar observations on the luciferase reporter containing the single predicated miR-15a-targeting site in the HBx transcript (Fig. 3C). Because HBp contains three predicated targeting sites for miR-15a/miR-16-1 and HBx contains a single site, we focused on determining the requirement for base-pairing in the seed region on the HBx-based reporter (Fig. 3A). We found that the mutant luciferase reporter was no longer sensitive to modulation by the miR-15a mimic and anti-miR-15a (note some nonspecific effect of anti-miR-15a) (Fig. 3D). These data demonstrate that the predicted target in HBx is indeed susceptible to targeting by miR-15a in the luciferase reporter-based assay.
FIGURE 3.
Inhibition of HBV gene expression by targeting miR-15a to the coding regions of HBp and HBx. A, the luciferase reporter containing the miR-15a targeting site was inserted near the end of the Renilla coding region. Three targets in HBp were similarly tested as a group. B, response of the HBp reporter to the miR-15a mimic or anti-miR-15a. C, response of the HBx reporter to the miR-15a mimic or anti-miR-15a. D, lack of response of the mutant HBx reporter to the miR-15a mimic. Anti-miR-15a showed a minor inhibitory effect on the reporter. E and F, response of the endogenous miR-15a target genes Bcl-2 and Ccnd-1 to the miR-15a mimic or anti-miR-15a measured by real-time RT-PCR (E) or Western blotting (F). G, response of the transfected HBx expression unit to the miR-15a mimic or anti-miR-15a. The FLAG-tagged HBx expression from the wild-type construct (HBx-w-FLAG) was inhibited by miR-15a but enhanced by anti-miR-15a. No response was recorded with the mutant HBx expression unit lacking the miR-15a targeting site (HBx-m-FLAG). The levels of HBx were normalized against GFP expressed from a cotransfected expression plasmid. *, p < 0.05; **, p < 0.01 on the basis of experiments performed in triplicate.
To directly demonstrate that the predicted miR-15a target site in the coding region of HBx is functional in its native sequence context, we further examined the expression of FLAG-tagged HBx protein in response to transfected miR-15a mimics. We first showed that transfected miR-15a and anti-miR-15a exhibited the intended effects on the expression of the endogenous target genes Bcl-2 and Ccnd-1, as reported previously (34), showing that the miR-15a mimic suppressed, whereas the anti-miR-15a enhanced, the expression of these genes at both the RNA and protein levels (Fig. 3, E and F). Under these conditions, we found that the miR-15a mimic reduced the expression of the FLAG-tagged HBx protein, whereas anti-miR-15a showed the opposite effect in transfected cells (Fig. 3G, top panel). Both the miR-15a mimic and anti-miR-15a lost their effect on the expression of HBx from the construct that carries mutations in the miR-15a targeting site (Fig. 3G, bottom panel). Together, these data demonstrated that miR-15a could efficiently repress HBx expression through its targeting site within the HBx coding region.
HBV Infection Modulates MicroRNA Expression
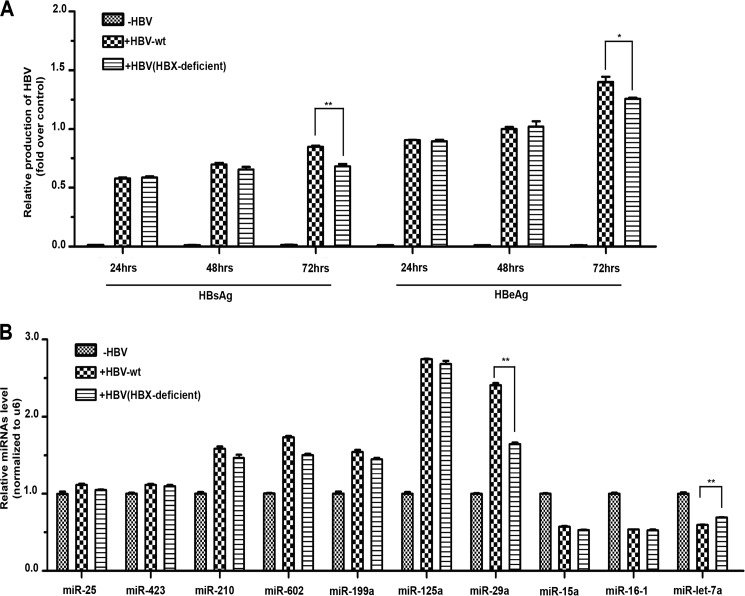
To further investigate the functional interplay between HBV and microRNA, we asked how HBV infection might alter microRNA expression in HepG2 cells, aiming to obtain clues about how the virus might cope with the cellular defense system to establish the chronic state of infection in host cells. We selected a panel of microRNAs demonstrated previously to target HBV transcripts (14) and quantified their expression in response to HBV replication by real-time RT-PCR. In this analysis, we also created a mutant HBV strain in which the coding capacity for HBx protein was abolished by an engineered stop codon. This HBx-deficient HBV replicated equally as well as wild-type HBV except for a mild replication deficiency 72 h after infection (Fig. 4A), which is consistent with the largely dispensable role of the HBx protein in HBV replication in infected cell lines (35). As expected, wild-type HBV induced five microRNAs (miR-210, miR-602, miR-199a, miR-125a, and miR-29a) and suppressed three (miR-15a, miR-16-1, and miR-Let-7a) (Fig. 4B).
FIGURE 4.
Reprogramming of microRNA expression by wild-type and HBx-deficient HBV. A, viral replication was monitored in HepG2 cells transfected with wild-type or HBx-deficient HBV. Time course HBV replication was determined on the basis of the production of the s and e antigens. HBx-deficient HBV showed only a minor defect after 72 h. B, a panel of microRNAs implicated previously in the regulation of HBV replication were analyzed in HepG2 cells infected with wild-type and HBx-deficient HBV. HBx-deficient HBV only showed a reduced induction of miR-29a and reduced suppression of miR-Let-7a compared with wild-type HBV. *, p < 0.05; **, p < 0.01 on the basis of experiments performed in triplicate.
Multiple studies have implicated HBx as the major viral protein that interacts extensively with cellular factors to alter the transcriptome, including microRNA, in HBV-infected cells (8, 9). Particularly relevant is the recent finding that the miR-15a/miR-16-1 cluster is down-regulated in HepG2 cells ectopically expressing the HBx protein (20). Unexpectedly, however, we found that the HBx-deficient HBV still modulated microRNA expression like its wild-type HBV, with the exception of the reduced induction of miR-29a and slightly compromised repression of miR-Let-7a (Fig. 4B). This result implies that the impact of HBV infection on microRNA expression may result largely from viral infection but not directly by the HBx protein.
HBx Viral RNA Directly Mediates miR-15a/miR-16-1 Down-regulation
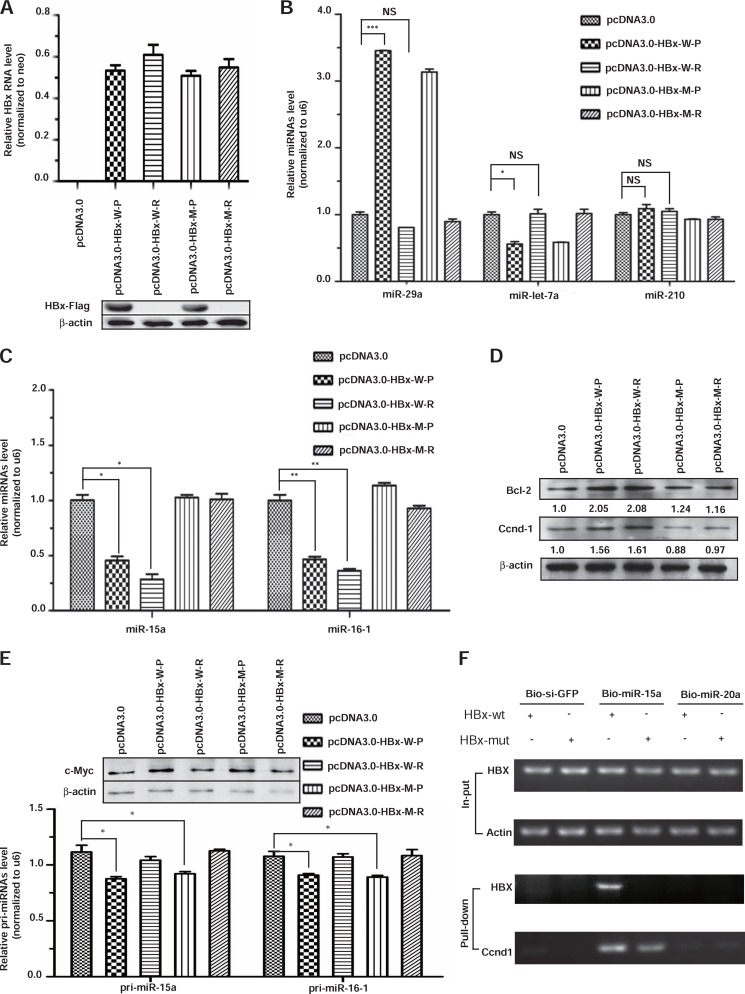
Because overexpressed HBx clearly induced the repression of miR-15a/miR-16-1 (20), we addressed this puzzle by first repeating the observation by using the HBx expression unit in transfected HepG2 cells, and, for comparison, we also wished to test a frame-shifted HBX gene that expresses HBx RNA but not protein. Thus, we created the protein (donated as HBx-w-P) and RNA (donated as HBx-w-R) versions from the HBx expression vector. In addition, we also generated the corresponding expression units that carry synonymous mutations in the target site for miR-15a/miR-16-1 (donated as HBx-m-P and HBx-m-R). All four constructs expressed an equivalent amount of HBx RNA, but only the protein version of the HBx expression units produced the anticipated HBx protein in transfected cells (Fig. 5A).
FIGURE 5.
Induction of miR-15a/miR-16-1 down-regulation by HBx RNA. A, all four constructs expressed an equivalent amount of RNA (upper panel), but only the two protein-expressing constructs generated the expected FLAG-tagged HBx protein detected by Western blotting (lower panel). B, representative microRNAs responded to the expression of wild-type HBx protein (HBx-w-P) or RNA (HBx-wt-R) but not to the mutant HBx protein or RNA (HBx-m-P or HBx-m-R) in the miR-15a targeting site. C, the effects of HBx protein and RNA on the expression of miR-15a and miR-16-1 were quantified by real-time RT-PCR. D, the miR-15a/miR-16-1 cellular targets Bcl-2 and Ccnd-1 were only induced by wild-type HBx protein and RNA. The enhancing effects (quantified by scanning the protein gel) were quantified on the basis of the average of two independent experiments and are shown at the bottom of each gel. E, the effects of HBx protein and RNA on the expression of pri-miR-15a and pri-miR-16-1 were determined by real-time RT-PCR, and the degrees of c-Myc induction were quantified by Western blotting (top panel, top gel). F, capture of exogenous HBx and endogenous Ccnd1 transcripts with transfected biotinylated miR-15a but not bio-siGFP or bio-miR-20a. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS > 0.05 on the basis of experiments performed in triplicate.
Focusing on several representative microRNAs that responded to HBV infection, we found that the overexpressed HBx protein, but not its RNA version, induced miR-29a, repressed Let-7a, and had no effect on miR-210 (Fig. 5B). These results indicate that the HBx protein can alter the expression of specific microRNAs under overexpression conditions. Significantly, we observed that both the protein and RNA versions of HBx were able to down-regulate miR-15a and miR-16-1 and that the effects were lost with the mutations in their microRNA targeting site (Fig. 5C). Therefore, the HBx RNA appears to be sufficient to down-regulate its targeting microRNAs. We next tested the prediction that down-regulation of miR-15a/miR-16-1 would relieve the inhibition of their cellular target genes, such as Bcl-2 and Ccnd-1. Indeed, we found consistently that both of these cellular genes were up-regulated in HepG2 cells transfected with either the protein or the RNA version of HBx but not with the mutation versions in the microRNA targeting site (Fig. 5D).
Because a previous study has implicated c-Myc induction in HBx-overexpressed HepG2 cells and suggested that induced c-Myc contributed to transcription repression of pri-miR-15a and pri-miR-16-1 (20), we additionally examined c-Myc induction and the expression of the primary transcripts for miR-15a and miR-16-1. Indeed, HBx protein, but not RNA, caused a mild induction of c-Myc and detectable repression of the primary miR-15a and miR-16-1 transcripts, suggesting that the HBx protein did contribute to the reduction of miR-15a/miR-16-1 transcription to some extent under overexpression conditions (Fig. 5E).
Finally, we wished to provide direct evidence for the interaction between the HBX RNA and miR-15a. For this purpose, we prepared biotinylated miR-15a (bio-miR-15a) and two negative controls (bio-siGFP and bio-miR-20a) and cotransfected these synthetic RNAs with wild-type and mutant (in the miR-15a targeting site) HBx expression units into HepG2 cells. By analyzing the captured RNA on the biotinylated small RNA, we found that bio-miR-15a was associated specifically with both exogenous wild-type HBX RNA and endogenous Ccnd-1 RNA but not the exogenous mutant HBX RNA (Fig. 5F). No RNA was captured with bio-siGFP or bio-miR-20a. These results strongly suggest that the HBX RNA, with or without the coding potential, is able to bind and trigger the decay of the corresponding microRNAs, which is reminiscent of induced down-regulation of miR-122 by HBV RNA (26) and enhanced microRNA instability by the herpesvirus non-coding RNA (25). Together, these findings suggest an emerging biological paradigm where target RNA-directed microRNA decay is employed to regulate the microRNA network in mammalian cells.
DISCUSSION
Previous studies have identified multiple microRNAs capable of targeting HBV transcripts to inhibit viral replication (14). However, because microRNAs are also known to target many cellular cofactors critical for viral replication, as demonstrated in the case of HCV (13), the microRNA machinery in host cells may have a net positive or negative impact on viral replication. Through attenuating the microRNA processing machineries (i.e. by knocking down DGCR8 or Dicer) or inactivating the RISC complex (i.e. by knocking down Ago2), we demonstrated that inactivation of the microRNA machinery benefits HBV replication in HepG2 cells. Our results disagree with a recent report indicating that a siRNA against Ago2 impaired HBV replication in T23 cells (23). Although the source for this discrepancy remains unclear, we note that T23 cells carry an integrated HBV genome whereas our experiments were performed on freshly transfected HepG2 cells. Therefore, T23 cells might reflect a latent state of HBV-infected cells where some reprogramming might have taken place. Importantly, compared with the experiment on T23 cells treated with a single siAgo2, our conclusion was on the basis of interference of the microRNA machinery at multiple points, from microRNA biogenesis to execution. Thus, the consistent results argue strongly against any potential off-targets in our analysis.
Besides multiple microRNAs characterized to date, we found that HBV is also subjected to attack by miR-15a/miR-16-1. We showed that the target site in the HBx transcript is susceptible to microRNA attack either in its native sequence context or in the coding region of a luciferase-based reporter. This is important because most previous studies only relied on the 3′UTR-based reporter system, but nearly all microRNA targeting sites characterized to date reside in the coding region of different HBV transcripts. We believe that such an analysis of microRNA targeting sites ought to be extended to all microRNAs that have been implicated in the regulation of HBV replication.
Perhaps the most unexpected finding of this work is the role of the HBx RNA in mediating the down-regulation of its targeting microRNAs. Target RNA has been found to induce microRNA tailing and trimming in fly extracts (24). However, it has been unclear how extensively such a mutual regulation is employed in different biological systems. The biological relevance of such a target RNA-induced down-regulation of microRNA was first recognized in herpesvirus-infected cells where two virus-encoded non-coding RNAs mediate the down-regulation of a specific microRNA (25). Given the extensive sponge effect of endogenous transcripts on microRNA in mammalian cells (36), such a mutual regulation between microRNA and target RNA might be more widespread than previously thought. Indeed, a recent study showed such an RNA-dependent down-regulation of miR122 in HBV-infected hepatocytes (26). We now provide further evidence for this mechanism.
Importantly, our finding suggests that such an HBx RNA-mediated down-regulation of miR-15a/miR-16-1 may correspond to a mechanism suspected previously to exist in a stable HBx-expressing HepG2 cell line because HBx-induced down-regulation of miR-15a could be rescued efficiently by knocking down c-Myc, but c-Myc down-regulation appears to have a much lesser effect on miR-16-1 (20). Our findings suggest that such a missing mechanism might be due to the HBx RNA. Therefore, both HBx protein and RNA may act in a synergistic fashion to modulate the cellular microRNA program in HBV-infected cells. It will be interesting to determine in future studies how this and other HBV transcripts might cause down-regulation of multiple other microRNAs implicated previously in inhibiting HBV replication.
HBV infection has been tightly linked to HCC development, but the causal role of viral infection to the etiology of hepatocarcinoma has remained unclear. Given the chronic nature of HBV infection, it is likely that liver tumor development may result from progressive cellular transformation as a result of altered regulatory programs in HBV-infected cells. Increasing evidence points to a key role of the oncogenic HBx protein in this process because its overexpression has been shown to enhance cell cycle progression and impair cellular apoptosis (1), which might be due to the repression of p53 pathways (21). Our current work further strengthens the proposed role of HBx in oncogenic transformation of hepatocytes by showing that the HBX viral RNA is able to regulate the expression of specific microRNAs, thus enlarging the biological and pathological properties of the oncogenic HBX gene in the HBV genome.
Particularly important is the ability of both HBx protein and RNA to down-regulate yet another tumor suppressor pathway that involves miR-15a/miR-16-1. Indeed, it has been demonstrated that down-regulation of these tumor suppressor microRNAs is sufficient to enhance cell proliferation and transformation in hepatocytes (20). We confirmed these biological effects with the miR-15a/miR-16-1 mimic-transfected cells (data not shown). Unfortunately, despite continuous efforts, we have not yet obtained stable cell lines that can express the HBX RNA at the level comparable with that in HBV-infected cells, which would permit further determination of the biological effects of the HBX RNA. Nonetheless, it is conceivable that, during the early phase of HBV infection, miR-15a/miR-16-1 may first serve to antagonize HBV infection, which may cause the virus to enter the chronic state in infected cells, and some cells may even clear the virus as a result of microRNA-mediated inhibition of viral replication. However, if the virus somehow escapes such a line of cellular defense, efficient HBV replication, which can produce up to 105 copies of viral RNA in the cell (37, 38), may, in turn, induce the down-regulation of inhibitory microRNAs, thereby contributing to cellular reprogramming that eventually leads to oncogenic transformation. This study demonstrates the HBx RNA as part of such a regulatory circuitry and underscores the potential regulatory function of viral RNA in HCC development.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM049369 and GM052872. This work was also supported by China 973 Program Grants 2011CB811300 and 2012CB910800) and by Chinese 111 Program Grant B06018.

This article contains supplemental Tables S1–S5.
- HBV
- hepatitis B virus
- HBx
- hepatitis B virus X protein
- HCC
- hepatocellular carcinoma
- miR
- microRNA
- HCV
- hepatitis C virus.
REFERENCES
- 1. Neuveut C., Wei Y., Buendia M. A. (2010) Mechanisms of HBV-related hepatocarcinogenesis. J. Hepatol. 52, 594–604 [DOI] [PubMed] [Google Scholar]
- 2. Tiollais P., Pourcel C., Dejean A. (1985) The hepatitis B virus. Nature 317, 489–495 [DOI] [PubMed] [Google Scholar]
- 3. Seeger C., Mason W. S. (2000) Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64, 51–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kremsdorf D., Soussan P., Paterlini-Brechot P., Brechot C. (2006) Hepatitis B virus-related hepatocellular carcinoma. Paradigms for viral-related human carcinogenesis. Oncogene 25, 3823–3833 [DOI] [PubMed] [Google Scholar]
- 5. Tang H., Oishi N., Kaneko S., Murakami S. (2006) Molecular functions and biological roles of hepatitis B virus X protein. Cancer Sci. 97, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arbuthnot P., Capovilla A., Kew M. (2000) Putative role of hepatitis B virus X protein in hepatocarcinogenesis: effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J. Gastroenterol. Hepatol. 15, 357–368 [DOI] [PubMed] [Google Scholar]
- 7. Bouchard M. J., Schneider R. J. (2004) The enigmatic X gene of hepatitis B virus. J. Virol. 78, 12725–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cougot D., Wu Y., Cairo S., Caramel J., Renard C. A., Lévy L., Buendia M. A., Neuveut C. (2007) The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J. Biol. Chem. 282, 4277–4287 [DOI] [PubMed] [Google Scholar]
- 9. Levrero M., Pollicino T., Petersen J., Belloni L., Raimondo G., Dandri M. (2009) Control of cccDNA function in hepatitis B virus infection. J. Hepatol. 51, 581–592 [DOI] [PubMed] [Google Scholar]
- 10. Bartel D. P. (2009) MicroRNAs. Target recognition and regulatory functions. Cell 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottwein E., Cullen B. R. (2008) Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshikawa T., Takata A., Otsuka M., Kishikawa T., Kojima K., Yoshida H., Koike K. (2012) Silencing of microRNA-122 enhances interferon-α signaling in the liver through regulating SOCS3 promoter methylation. Sci. Rep. 2, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Randall G., Panis M., Cooper J. D., Tellinghuisen T. L., Sukhodolets K. E., Pfeffer S., Landthaler M., Landgraf P., Kan S., Lindenbach B. D., Chien M., Weir D. B., Russo J. J., Ju J., Brownstein M. J., Sheridan R., Sander C., Zavolan M., Tuschl T., Rice C. M. (2007) Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. U.S.A. 104, 12884–12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu W. H., Yeh S. H., Chen P. J. (2011) Role of microRNAs in hepatitis B virus replication and pathogenesis. Biochim. Biophys. Acta 1809, 678–685 [DOI] [PubMed] [Google Scholar]
- 15. Gramantieri L., Fornari F., Callegari E., Sabbioni S., Lanza G., Croce C. M., Bolondi L., Negrini M. (2008) MicroRNA involvement in hepatocellular carcinoma. J. Cell Mol. Med. 12, 2189–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou J., Lin L., Zhou W., Wang Z., Ding G., Dong Q., Qin L., Wu X., Zheng Y., Yang Y., Tian W., Zhang Q., Wang C., Zhang Q., Zhuang S. M., Zheng L., Liang A., Tao W., Cao X. (2011) Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 19, 232–243 [DOI] [PubMed] [Google Scholar]
- 17. Chen Y., Shen A., Rider P. J., Yu Y., Wu K., Mu Y., Hao Q., Liu Y., Gong H., Zhu Y., Liu F., Wu J. (2011) A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication. FASEB J. 25, 4511–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang L., Ma Z., Wang D., Zhao W., Chen L., Wang G. (2010) MicroRNA-602 regulating tumor suppressive gene RASSF1A is overexpressed in hepatitis B virus-infected liver and hepatocellular carcinoma. Cancer Biol. Ther. 9, 803–808 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y., Lu Y., Toh S. T., Sung W. K., Tan P., Chow P., Chung A. Y., Jooi L. L., Lee C. G. (2010) Lethal-7 is down-regulated by the hepatitis B virus X protein and targets signal transducer and activator of transcription 3. J. Hepatol. 53, 57–66 [DOI] [PubMed] [Google Scholar]
- 20. Wu G., Yu F., Xiao Z., Xu K., Xu J., Tang W., Wang J., Song E. (2011) Hepatitis B virus X protein downregulates expression of the miR-16 family in malignant hepatocytes in vitro. Br. J. Cancer 105, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S. G., Rho H. M. (2000) Transcriptional repression of the human p53 gene by hepatitis B viral X protein. Oncogene 19, 468–471 [DOI] [PubMed] [Google Scholar]
- 22. Chang T. C., Yu D., Lee Y. S., Wentzel E. A., Arking D. E., West K. M., Dang C. V., Thomas-Tikhonenko A., Mendell J. T. (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 40, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayes C. N., Akamatsu S., Tsuge M., Miki D., Akiyama R., Abe H., Ochi H., Hiraga N., Imamura M., Takahashi S., Aikata H., Kawaoka T., Kawakami Y., Ohishi W., Chayama K. (2012) Hepatitis B virus-specific miRNAs and Argonaute2 play a role in the viral life cycle. PLoS ONE 7, e47490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ameres S. L., Horwich M. D., Hung J. H., Xu J., Ghildiyal M., Weng Z., Zamore P. D. (2010) Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cazalla D., Yario T., Steitz J. A. (2010) Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li C., Wang Y., Wang S., Wu B., Hao J., Fan H., Ju Y., Ding Y., Chen L., Chu X., Liu W., Ye X., Meng S. (2012) HBV mRNAs-mediated miR-122 inhibition up-regulates PTTG1-binding protein which promotes HCC tumor growth and cell invasion. J. Virol. 87, 2193–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henke J. I., Goergen D., Zheng J., Song Y., Schüttler C. G., Fehr C., Jünemann C., Niepmann M. (2008) microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 27, 3300–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jopling C. L., Yi M., Lancaster A. M., Lemon S. M., Sarnow P. (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309, 1577–1581 [DOI] [PubMed] [Google Scholar]
- 29. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 30. Loeb G. B., Khan A. A., Canner D., Hiatt J. B., Shendure J., Darnell R. B., Leslie C. S., Rudensky A. Y. (2012) Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 48, 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calin G. A., Croce C. M. (2006) MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866 [DOI] [PubMed] [Google Scholar]
- 32. Wong C. M., Lee J. M., Lau T. C., Fan S. T., Ng I. O. (2002) Clinicopathological significance of loss of heterozygosity on chromosome 13q in hepatocellular carcinoma. Clin Cancer Res. 8, 2266–2272 [PubMed] [Google Scholar]
- 33. Zhang G. L., Li Y. X., Zheng S. Q., Liu M., Li X., Tang H. (2010) Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210. Antiviral Res. 88, 169–175 [DOI] [PubMed] [Google Scholar]
- 34. Bonci D., Coppola V., Musumeci M., Addario A., Giuffrida R., Memeo L., D'Urso L., Pagliuca A., Biffoni M., Labbaye C., Bartucci M., Muto G., Peschle C., De Maria R. (2008) The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 14, 1271–1277 [DOI] [PubMed] [Google Scholar]
- 35. Melegari M., Scaglioni P. P., Wands J. R. (1998) Cloning and characterization of a novel hepatitis B virus X binding protein that inhibits viral replication. J. Virol. 72, 1737–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebert M. S., Sharp P. A. (2010) Emerging roles for natural microRNA sponges. Curr. Biol. 20, R858–R861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X., Zhang E., Ma Z., Pei R., Jiang M., Schlaak J. F., Roggendorf M., Lu M. (2011) Modulation of hepatitis B virus replication and hepatocyte differentiation by MicroRNA-1. Hepatology 53, 1476–1485 [DOI] [PubMed] [Google Scholar]
- 38. Wang S., Qiu L., Yan X., Jin W., Wang Y., Chen L., Wu E., Ye X., Gao G. F., Wang F., Chen Y., Duan Z., Meng S. (2012) Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1)- modulated P53 activity. Hepatology 55, 730–741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.