Abstract
Due to the lack of success in small-diameter (<6 mm) prosthetic vascular grafts, a variety of strategies have evolved utilizing a tissue-engineering approach. Much of this work has focused on enhancing the endothelialization of these grafts. A healthy, confluent endothelial layer provides dynamic control over homeo-stasis, influencing and preventing thrombosis and smooth muscle cell proliferation that can lead to intimal hyperplasia. Strategies to improve endothelialization of biodegradable polymeric grafts have encompassed both chemical and physical modifications to graft surfaces, many focusing on the recruitment of endothelial and endothelial progenitor cells. This review aims to provide a compilation of current and developing strategies that utilize in situ endothelialization to improve vascular graft outcomes, providing a context for the future directions of vascular tissue-engineering strategies that do not require preprocedural cell seeding.
Introduction
Coronary artery disease is a leading cause of death and morbidity worldwide.1 Angioplasty and stenting procedures are used in cases of limited occlusion. For more occluded vessels, bypass surgery is required in ∼250,000 patients per year.2 Autologous transplantation of conduits such as saphenous veins and mammary arteries is often used in cases necessitating arterial bypass. Patients requiring bypass surgery, however, may not have vessels available due to disease or previous surgery. Synthetic, nondegradable vessels have been used in such cases. Success rates in large-diameter grafts (>6 mm) have been satisfactory with patency rates of 95% after 5 years.3 Conversely, the patency rate of small-diameter grafts (<6 mm) was reported to be only 30% in the same study.3 Other studies demonstrated patency rates such as 0% to 25% after only weeks or months of implantation in various animal models.4–6 Complications resulting from noncompliance, thrombogenicity, intimal hyperplasia, aneurysms, and calcium deposition contribute to these low patency rates.7–9 While much graft research has focused on coronary artery bypass procedures, the need for small-diameter vascular grafts extends far beyond coronary artery disease. Each year, over 500,000 patients are found to have end-stage renal disease and 8 million with peripheral artery disease,2 prompting research into small-diameter grafts for hemodialysis access and peripheral artery bypass.10,11
To improve the long-term patency and functionality of small-diameter grafts, biodegradable grafts have emerged over the years as a chief solution to the complications associated with commonly used biologically stable materials such as poly-tetrafluoroethylene (PTFE) or Dacron. The focus of biodegradable grafts is to promote native tissue ingrowth and replacement of the graft while the scaffold material degrades over a sustained period of time necessary for adequate vessel repair and growth. Tissue-engineering approaches to the problem of developing small-diameter, biodegradable vascular grafts have been numerous. Approaches aim to achieve acceptable patency rates through the development of grafts that best mimic or promote the extracellular environment and mechanical properties of native blood vessels. A tissue-engineered, small-diameter vascular graft is based on three basic principles: (1) base scaffold matrix; (2) biofunctional molecules; (3) cells (seeded or recruited in vivo).
To promote a fully functional native tissue replacement, grafts must promote the establishment of cellular and tissue organization similar to a native vessel. A blood vessel contains three identifiable layers called the tunica intima, the tunica media, and the tunica adventitia, from the lumen outward. Endothelial cells (ECs) make up the monolayer intimal lining, often called the endothelial layer or endothelium. Smooth muscle cells (SMCs) are predominately located in the media, while the adventitia primarily consists of fibroblasts.7 Of these vessel layers, the primary focus is to establish the endothelium on a graft due to the endothelial layer's crucial role in vascular biology. The endothelial layer provides dynamic control of interactions with blood flowing through the vessel, maintaining hemostasis by regulating inflammation, permeability, thrombosis, and fibrinolysis.13,14 Establishment of a healthy endothelium on an implanted graft is thought to be crucial in the prevention of complications such as reduced patency due to intimal hyperplasia and thrombogenicity. The endothelium plays a direct role in the regulation of the coagulation cascade and thus thrombosis.15 While intimal hyperplasia is caused by the ingrowth of SMCs, the endothelium plays a crucial role in regulating SMC growth from the media layer. Inflammation and thrombosis, both regulated by the endothelium, can trigger intimal hyperplasia. A healthy endothelium also has the capability to inhibit excess SMC proliferation and migration.16 Although prevention of intimal hyperplasia is crucial to endothelialization, the media layer does play an integral role in endothelialization. Mechanical stability provided by the medial layer can prevent anastomosis, while extracellular matrix (ECM) production and remodeling can further promote the development of neovessel tissue and support endothelial growth.17,18
Many strategies for seeding cells on vascular grafts have been developed to establish a complete endothelium before implantation. Even when these strategies are successful, the hurdles of cell seeding may limit clinical applicability, drastically increase graft costs, and/or require a lengthy amount of time.19,20 Obtaining an adequate number of mature ECs for proper cell seeding may be difficult without causing donor-site morbidity.21 Instead of relying on autologous or allogous cell seeding for the production and implantation of vascular grafts, many current small-diameter, vascular graft strategies incorporate biofunctional molecules or components to mobilize autologous cells within the vasculature to the graft postimplantation.22,23
This review will focus on current and developing strategies to promote the adhesion, differentiation, and proliferation of ECs and endothelial progenitor cells (EPCs) to form a complete endothelium on biodegradable, small-diameter, vascular grafts in situ. We have chosen to focus on polymeric grafts due to their relative ease of production, ability to be tuned for mechanical properties, and availability of surface modification techniques. Techniques of surface modification will be reviewed, examining chemical and topographical factors that contribute to successful endothelialization and how such strategies have or can be applied to biodegradable, small-diameter grafts. In addition, alternative strategies, treatments, and concepts will be covered that can be applied to promote endothelialization of such grafts. This review will attempt to provide a framework useful for combining these approaches in the ultimate development of a biodegradable, small-diameter vascular graft that can effectively enhance and expedite the endothelialization process postimplantation.
Targeted Cells for In Situ Endothelialization
Endothelial cells
EC adhesion and proliferation are vital to the establishment of a thromboresistant cellular layer and the prevention of intimal hyperplasia by inhibiting SMC growth into the inner lumen of a vascular graft.24,25 If ECs are not seeded on the graft, migration and adhesion of ECs occur in one, or a combination, of several manners. ECs may migrate over the anastomosis site of the graft from the neighboring vessel structure. ECs may also migrate through pores in the graft via ingrowth of capillaries.26 However, EC ingrowth beyond the anastomosis site into the graft is often restricted to 1–2 cm.7 Thus, relying on the passive migration of neighboring ECs may be insufficient for expedited endothelialization of an implanted graft.
Endothelial progenitor cells
Circulating EPCs may also contribute to the endothelialization of the graft via adhesion, proliferation, and differentiation into ECs. EPCs were first identified in 1997 as a population of cells capable of neovascularization derived from the bone marrow.27 EPCs appear to play a significant role in vascular homeostasis and blood vessel formation.28–30 EPCs are capable of expressing various EC characteristics and markers such as CD31, vascular endothelial (VE) cadherin, vascular endothelial growth factor receptor-2 (VEGFR-2), and von Willebrand factor (vWF).27,31,32 These markers contribute to vascular permeability, cell–cell adhesion, and controlling other cellular responses during neovascularization. It has been suggested that EPCs migrate to ischemic tissues and sites of vascular injury to promote neovascularization and vessel healing, responding to hemodynamics and chemical stimuli to differentiate into mature endothelial phenotype cells.33–35 Endothelial injury coagulation activation and platelet response provide mobilization and homing of EPCs. Among EPCs, distinct populations have been identified: early-outgrowth and late-outgrowth cells. The early-outgrowth EPCs, though exhibiting several endothelial markers, are unable to form vascular structures, though they have been found to impact homeostasis and neoangiogenesis.36,37 Late-outgrowth EPCs both display endothelial markers and have been found to form vessel structures.36 Despite their differences when cultured alone, these two populations of EPCs have been found to interact synergistically to promote neovascularization.38 Despite the unclear nature of neighboring ECs versus EPCs in endothelial repair, research still suggests that administration of EPCs can lead to enhanced vascular function.39 Besides contributing to the endothelium through proliferation and differentiation, EPCs also release soluble factors that enhance the migration of ECs.40 A basic schematic demonstrating these pathways of EPC and EC migration can be observed in Figure 1. Optimal endothelialization of a small-diameter vascular graft may be achieved through utilization of EPC mobilization, homing, adhesion, and differentiation into ECs.
FIG. 1.
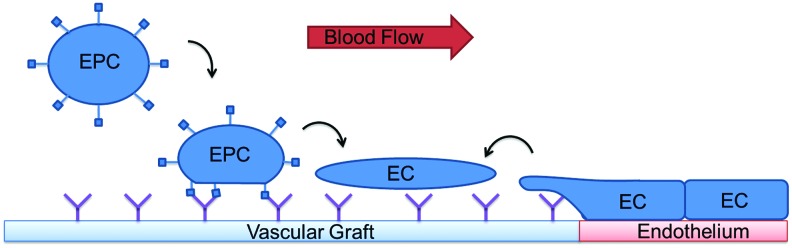
Paradigms of endothelialization of vascular grafts. Endothelial cells (ECs) migrate from neighboring tissues over the anastomosis, while endothelial progenitor cells (EPCs) respond to functionalized vascular graft surfaces. EPCs adopt an EC phenotype, under influence of shear stress, growth factors, and immobilization to the graft surface. Color images available online at www.liebertpub.com/teb
EPC Homing, Mobilization, and Migration
EPCs circulate the bloodstream in relatively low abundance in normal, physiological conditions, so graft designs utilizing the accelerated endothelialization potential of these cells must incorporate methods of increased mobilization of EPCs.41
Growth factor incorporation
Growth factors such as VEGF,42 stromal cell-derived factor-1 (SDF-1),43 and granulocyte colony-stimulating factor (G-CSF)44 have been found to increase mobilization of EPCs from the bone marrow and used in vascular graft applications. Nerve growth factor (NGF) has also been found to promote EPC migration in vitro and mobilization and homing in vivo to a collagen-modified decellularized blood vessel matrix.45 NGF was bound to the blood vessels, resulting in significantly increased endothelialization and patency rates in a mouse model.45 Recently, brain-derived neurotrophic factor (BDNF) has been found to enhance graft patency rates in rats when bound to small-diameter tissue-engineered blood vessels.46 BDNF was found to increase EPC mobilization and capture in vitro and in vivo. VEGF production was found to have been increased twofold in BDNF-treated cultures with EPCs, which demonstrated the paracrine effects that contribute to the enhanced mobilization and migration of EPCs due to BDNF. In vivo, EPC migration to BDNF-modified grafts experienced a fivefold increase over control grafts.
While not studying biodegradable grafts, improved endothelialization and reduced thrombosis were demonstrated for two commercially available polyester grafts (Gelsoft™ from Vacutek® and POLYMAILLE® C from Perouse Medical).43 Grafts were coated with fibronectin and, subsequently, homing factor SDF-1α to promote homing and adhesion of hematopoietic stem cells and EPCs. Significantly, less thrombotic material accumulated on both grafts after coating, and EC coverage of the grafts was almost doubled compared to the uncoated controls. In addition, tests demonstrated increased proliferation, differentiation, and homing of EPCs due to BDNF. Star poly(ethylene glycol) (PEG)–heparin hydrogels were modified by immobilizing a novel, biased-like SDF-1 derivative to mobilize EPCs.47 The SDF-1 derivative (AAV-[S4V]-SDF-1α) was found to be more effective at increasing EPC migration compared to SDF-1α. Research into the use of growth factors for EPC homing, mobilization, and migration has been increasingly robust. Future work may include tuning the presentation of these growth factors by combining several factors immobilized on a graft, controlling their release in the case of soluble factors, and varying local concentrations of the factors on the graft's surface.
External treatments
Treatments exogenous to vascular grafts can augment endothelialization through paracrine effects, resulting in increased mobilization of EPCs. Increased EPC circulation and vessel healing have been found by administering treatments such as atorvastatins48 and erythropoietins.49,50 Providing an exogenous erythropoietin delivery to mice to improve transplanted EPC survival to treat myocardial infarctions was found to be effective.50 Not only was the survival of transplanted EPCs supported, but there was an increase in autologous EPC mobilization as well. Such methods of mobilizing EPCs may be used in conjunction with adhesion and homing mechanisms on grafts to expedite endothelialization.
In some vascular graft applications, treatments involving these EPC mobilization stimulants have led to increased EPC presence in the bloodstream and endothelialization of luminal graft surfaces. The benefits of combining atorvastatin treatment (30 mg/d) in a canine model were observed in the improved patency of implanted expanded poly-tetrafluoroethylene (ePTFE) grafts.51 Atorvastatin therapy increased the amount of circulating EPCs, while improving EPC adherence and migration on the grafts. With the therapy, a discontinuous EC monolayer was established on the grafts after 4 weeks in treated canines, and a completely confluent EC monolayer was achieved after 8 weeks of implantation. Control animals without atorvastatin treatment displayed only a thin acellular tissue on luminal graft surfaces.
While these treatments have been administered externally, one study demonstrated the safety and efficacy of antibiotic delivery in polyester vascular grafts coated with hydroxypropyl-β-cyclodextrin (BPβCD).52 The BPβCD is a cage molecule with a hydrophilic exterior and a hydrophobic interior suitable for encapsulating drugs or other bioactive molecules. The group showed that the BPβCD-based polymer coating sustained degradation and antibiotic activity, showed no significantly detrimental effects on native tissues, and showed an overall efficacy in a canine model. This drug delivery system could be adapted to release an EPC-mobilizing drug such as atorvastatin or a mobilizing and homing growth factor such as VEGF. Such a system could eliminate the need for external treatments, while increasing the effective endothelialization of the graft of the graft.
Adhesion and Proliferation
Biofunctional molecules
Adhesion and supported proliferation of ECs and EPCs are essential to graft endothelialization. As previously mentioned, ECs may migrate from neighboring tissues to the graft. EPC adhesion may be a more complex process. A current model of EPC recruitment involves EPC rolling and adhesion, similar to leukocytes, followed by migration and differentiation. Adhesion of EPCs to ECs involves adhesive bonds formed by a variety of adhesive molecule interactions, dependent on the substrate stiffness and shear flow in the vascular environment.53 Incorporating molecules into a graft to simulate these effects has proven to be an effective method of increasing EC and EPC adhesion and subsequent proliferation.
Antibodies
Immobilization of antibodies has been pursued in a variety of cardiovascular implant applications. Antibodies targeting markers for ECs and EPCs have been explored for increasing the adhesion of cells to the inner lumen of vascular grafts. For EPCs, two identified surface markers have been utilized in graft applications: CD34 and kinase insert domain receptor (KDR).22,54 KDR, also known as VEGFR-2, and CD34 are present in circulating EPCs. CD31 antibodies and VEGFR-2 antibodies have been used to target ECs.55,56
Coating poly(caprolactone) (PCL) grafts with anti-CD31 antibodies has been shown to induce EC-specific binding, promoting attachment and long-term adhesion of ECs.55 human umbilical vein endothelial cells (HUVEC) adhesion to the modified graft was found to be 14.9-fold higher than adhesion to bare PCL films. Cell viability was also higher over a 5-day experiment. CD31 is also expressed on platelets, granulocytes, lymphocytes, and monocytes,57–59 which could cause deleterious immune and inflammation responses when implanted in vivo.
Anti-CD34 antibodies have been used in a variety of applications to aid in the endothelialization of vascular stents.60–63 Anti-CD34 end-grafted to covalently immobilized PEG on titanium stents to enhance EPC migration and proliferation to improve endothelialization.62 Similarly, anti-CD34 antibodies have been immobilized on a heparin/collagen coating for a stent, accelerating the attachment of cells and expediting endothelialization.61
While CD34 antibodies bind to EPCs, some debate remains asserting that solely recruiting CD34 cells can be detrimental to long-term graft patency. CD34+ cells can differentiate into other cell types, including cardiomyocytes, ECs, and vascular SMCs.64 Nonspecific adhesion of all CD34+ cells can lead to problems such as restenosis due to SMC proliferation from the captured CD34+ population. Because it is thought a small percent of circulating CD34+ cells are EPCs,65 recruitment of non-EPC cells to a graft relying on CD34+ capture raises the potential for undesired cell attachment and graft patency rates.
VEGFRs are located on both the EC and EPC membranes. A VEGFR-2 (or anti-Flk-1) antibody for the VEGFR has been utilized through surface immobilization.56 The group found a 2.5-fold increase in HUVEC capture with the molecule by orienting it with a G-protein rather than through passive coating. The anti-FLk-1 antibody was utilized in an attempt to capture circulating EPCs and ECs.66 The group used a radial-flow chamber with three regions coated with fibronectin, VEGF, or anti-Flk-1 antibody. Cell spreading was greatest with fibronectin, followed by VEGF and anti-Flk-1 antibody. Cell adhesion was independent of the bound protein. However, the group did not orient the anti-Flk-1 antibody binding like the strategy utilized in the previously mentioned study.56 Passive absorption could lead to a lower quantity of available antigen-specific binding sites. It is possible that orienting the anti-Flk-1 antibody could lead to more promising results for cell adhesion via VEGFR binding in ECs and EPCs. For example, protection of the antigen-binding site of an antibody led to an almost 10-fold increase in number of active sites available for binding compared to nonprotected, randomly immobilized antibodies in one study.67 Another study utilized hydrophilic spacer arms for antibody attachment to increase the activity of antibodies.68 Employing techniques such as these in the immobilization of antibodies could increase the binding activity and efficiency on graft surfaces, resulting in better endothelialization rates over randomly immobilized antibody applications.
Besides the orientation of antibodies, it is also important to consider an appropriate combination of antibodies or other factors that will better uniquely encourage EPC attachment. As described, the incorporation of solely antibodies for CD34 may encourage the attachment of cells inappropriate for expedited endothelialization. It may be necessary to include other antibodies or biofunctional molecules to better isolate and encourage the attachment of EPCs and ECs.
Peptides
ECM proteins have been a popular addition to vascular grafts, permanent and biodegradable, to mimic native vessel architecture and achieve enhanced endothelialization.23 Significant evidence exists, supporting the notion that the ECM regulates EC and EPC function through integrin interactions.69–72 Laminin type 1 has been utilized in ePTFE grafts, demonstrating enhanced endothelialization over unmodified grafts.73 While laminin-derived RGD continues to be one of the more popular ECM-derived peptides to enhance endothelialization,74 a variety of other peptide sequences derived from fibronectin, laminin, and collagen type I have been incorporated into vascular grafts.
EC adhesion to polyurethane (PU) surfaces was improved via the cross-linking of elastin-like polypeptide 4 macromolecules.75 In addition to the favorable cell morphology and increased expression of endothelial nitric oxide synthase, platelet adhesion and activation were reduced compared to control PU surfaces. ECs and EPCs were shown to have adhered well to L-selectin and VE-cadherin chimera proteins that were coated on titanium surfaces.76 Compared to single-protein-coated and uncoated surfaces, a 50:50 ratio of the proteins on a coated surface showed the highest HUVEC and EPC adherence, viability, and proliferation. One group described 2.2-mm-diameter electrospun PCL grafts modified with Nap-FFGRGD via dip coating.77 The surface of the PCL films was modified by self-assembly of Nap-FFGRGD utilizing a hydrogelator (Nap-FF).78 After 2 and 4 weeks of implantation in rabbits, a threefold increase in EC coverage of the luminal side of the grafts was observed compared to unmodified grafts.77
Besides modifying the surface, an alternative approach is to incorporate isolated adhesion peptide sequences into the scaffold material. In a rat model, the accelerated endothelialization of a graft that incorporated the peptide cysteine-alanine-glycine (CAG) into PCL fibers was demonstrated.79 CAG peptide was shown to selectively enhance attachment of ECs while rejecting SMC adhesion.80 CAG was mixed with PCL and electrospun into fibers used to form a graft with an inner diameter of 0.7 mm. The area of endothelialization of the CAG-PCL grafts compared with PCL grafts was higher at 1, 2, and 6 weeks after implantation.79
An inherent weakness of peptide modification is the unselective nature of peptide-promoted cell adhesion. Sequences, such as RGD, allow for the adhesion of a variety of cell types.74 In one study, CAG was chosen for incorporation into the graft design based on the results from an array-based peptide–cell interaction assay methodology.79,81 Approaches, like this one, to identify EC- and EPC-selective adhesion peptides or peptide combinations should be employed to improve small-diameter vascular graft success. Since most adhesive peptide sequences are nonspecific, it may be appropriate to include other, more specific biofunctional molecules, such as antibodies or aptamers, to better encourage EPC and EC adhesion.
In addition to being used for its direct cellular adhesion interactions, recent research has examined the effectiveness of biomimetic proteins and peptides to immobilize other functional molecules to graft surfaces. For example, dopamine is a key functional group in mussels, which allows the animals to attach to virtually any material. Researchers have developed a method of mimicking this behavior through co-polypeptides containing 3,4-dihydroxyphenylalinine and l-lysine.82 PCL grafts were also coated with poly(dopamine) (PDA).83 The PDA-coated PCL nanofibers demonstrated highly enhanced HUVEC adhesion and viability compared to unmodified PCL nanofibers. In another study, 3,4-dihydroxyphenylalinine and l-lysine co-polypeptide was used to immobilize anti-CD34 antibodies on a PCL substrate, enhancing EC and EPC attachment, growth, and adhesion.63 VEGF was also immobilized through a simple dipping methodology after PDA was deposited on the surface of a poly(l-lactide-co-ɛ-caprolactone) (PLCL) film.84 VEGF–dopamine coatings demonstrated accelerated HUVEC migration and proliferation compared to dopamine-coated or uncoated PLCL films. The group also was able to immobilize basic fibroblast growth factor, demonstrating the versatility of the PDA immobilization techniques. The technology appears promising for in situ endothelialization. In vivo studies will be informative to determine the feasibility of this technology in the future of small-diameter vascular grafts.
Oligonucleotides and aptamers
Incorporation of nucleotides to control cell adhesion has been more recently investigated. DNA molecules can be synthesized rapidly and can allow for further graft functionalization via accessible functional groups.85 In a recent study, DNA–oligonucleotide coatings were immobilized by adsorption on parylene-coated ePTFE and polystyrene using vapor deposition.86 Murine EPCs, HUVECs, and adult human ECs were seeded. Dynamic culture conditions were maintained to apply constant shear stress. All cell groups experienced significantly enhanced adhesion on the coated surfaces compared to uncoated graft materials, while also demonstrating low thrombogenicity in human blood.
Aptamers are ligands consisting of nucleic acids with a high affinity and specificity to a target. Nucleotide sequences are isolated through systemic evolution of ligands by exponential enrichment (SELEX) that fold into a three-dimensional (3D) structure with the greatest binding affinity to the target and were first shown to promote osteoblast cell capture and adhesion.87 Aptamers with a high affinity for EPCs have been identified and used to capture porcine EPCs onto a star-PEG coating. Differentiation of the EPCs into endothelial-like cells was observed within 10 days in vitro.88 Aptamers capturing ECs have been used to coat permanent coronary stents. Coated stents were implanted in swine with uncoated cobalt–chromium controls, and polymer-coated controls. Aptamer-coated stents displayed no apparent advantages over either of the groups, showing restenosis rates and neointimal proliferation similar between all groups.89
In vivo studies of these type of modifications would elucidate some of the challenges that oligonucleotide and aptamer functionalization face. Nucleolytic degradation and DNA's solubility in aqueous environments may be detrimental to the successful EC and EPC adhesion to graft surfaces modified with these materials.90,91 The potential to synthesize highly selective nucleic acid ligands is still an enormous advantage with oligonucleotide and aptamer surface modification techniques.
Oligosaccharides and phospholipids
The popularity of identifying native biomolecules to enhance endothelialization has included the investigation of grafted oligosaccharides. Sialyl Lexisx has a high affinity for L-selectin present on circulating EPCs and has been shown to enhance mobilization and recruitment of EPCs.92 Enhanced adhesion of EPCs utilizing was demonstrated utilizing a Sialyl Lexisx–collagen matrix in vitro and in a rat model.92 More recently, hyaluronic acid (HA) oligosaccharide, a glycosaminoglycan with angiogenic and antithrombogenic properties, chains of varying lengths were grafted to PU-based films.93 While all three different lengths of HA proved more effective at limiting platelet adhesion and protein adsorption than PEG- or heparin-modified PU films, it was found that endothelial growth on the films was dependent on the molecular weight of the HA chains. Smaller chains of HA appeared to support better EC growth.
A 2-methacryloyloxyethyl phosphorylcholine copolymer was immobilized on the surface of an electrospun poly(ester urethane)urea graft and subsequently implanted in a rat. A 10-fold decrease in platelet adhesion compared to an uncoated graft was observed. A confluent layer of cells formed within the cells, though this was only qualitatively documented.94 Phosphorylcholine-modified chitosan has also demonstrated survival and differentiation of EPCs in increased numbers compared to a fibronectin control.95 While EPC adherence was increased, mesenchymal stem cell adherence was decreased, demonstrating that the modified chitosan matrix provides a possibly more selective surface for EPC attachment and proliferation.
The investigation of oligosaccharides and phospholipids has been less broadly researched than more extensively applied molecules such as antibodies and peptides. However, they do offer viable alternatives. While the phospholipid applications covered here are not EPC-specific, the Sialyl Lexisx oligosaccharides do offer binding to specific EC and EPC markers, though these markers are shared with leukocytes that may competitively inhibit EPC binding.
Magnetic molecules
The incorporation of magnetic molecules into target cell populations has been pursued to enable the use of a magnet to attract cells to increase cell-homing efficiency to grafts. Commercially available magnetic particles, Dynabeads (Life Technologies), have the capability of targeting specific molecules, proteins, or cells. CD31-coated Dynabeads to target HUVECs have been used, demonstrating a 90% seeding efficiency on a tubular collagen scaffold in vitro.96 However, using high concentrations of the beads to increase efficiency results in decreased EC proliferation and metabolic activities.97 Another group demonstrated the use of magnetic nanoparticles produced by a bacterium to direct EPCs in a microfluidic environment.98 After cellular uptake of the particles, EPCs were injected into a microchannel under a controlled flow rate. The study demonstrated that EPCs could be successfully directed to a specific location within the channel, successfully targeting ∼40% of the labeled EPCs. More recently, enhanced homing of injected EPCs labeled with bacterium-derived magnetic nanoparticles was demonstrated in a mouse model.99 EPCs were magnetically directed toward an ischemic hind limb. Magnetically homing EPCs demonstrated significantly improved perfusion through the ischemic area compared to injection of nonmagnetic EPCs. While such techniques offer an alternative method of homing EPCs to graft sites, clinical implementation of magnetic homing would require injected magnetic particle labeling of an EPC or EC population, potentially increasing the complexity of preparation for the grafting procedure.
Topography and physical properties
Cells are influenced by mechanical cues in their environment. These cues can affect cellular adhesion, proliferation, differentiation, and morphology.100 As such, surface topography of materials has been an important topic in various cardiovascular prosthetics. To improve stent designs, the topography of stent surfaces has been examined to improve endothelialization.101 Such insights and strategies have been adapted to vascular grafts. One study examined the effects of nanostructured poly(lactic-co-glycolic acid) (PLGA) surfaces.102 Nanostructured surface features were found to significantly enhance EC densities compared to microstructured and untreated PLGA surfaces. Substrate rigidity has been found to affect cellular differentiation, as well. For example, one group enhanced the differentiation and proliferation of ECs from cardiosphere-derived cells (CDCs) by testing polyacrylamide gels coated with fibronectin with Young's modulus values ranging from 8 to 21 kPa.103 When matching the rigidity of native tissue, greater numbers of CD31+ cells were present on the substrates after initial seeding of CDCs. Substrate rigidity appeared to influence the expression of p190RhoGAP, which promotes VEGFR expression. This caused a cascade of events known to control endothelial differentiation. Table 1 provides examples of several strategies implemented by research groups utilizing techniques to control surface topography and the experimental outcomes.
Table 1.
Recent Examples of Topographical Features and Methods of Topography Modification Used to Achieve Enhanced Endothelialization on Vascular Prosthetics
| Author | Feature(s) | Method | Result |
|---|---|---|---|
| Dickinson et al.112 | Micropillars | Lithography | Micropillars >3 μm significantly decrease EC adhesion and spreading; 1-μm high fibronectin micropillars promoted EC adhesion and alignment |
| Le Saux et al.119 | Nano- to microscaled pyramids | Chemical etching | Only microscaled pyramids inhibited EC migration when present with low RGD density on surface; size of pyramids appeared to control EC adhesion, while RGD density controlled cell spreading |
| Wu et al.132 | Nanofiber alignment | Electrospinning | Directed EC alignment on scaffold |
| Pareta et al.140 | Nanoroughness and surface energy | Plasma deposition | Increased EC adhesion until optimal surface energy and nanoroughness reached followed by decrease in EC adhesion |
| Ranjan et al.111 | Micropatterned nanoroughness | Vapor deposition and mold casting | EC function enhanced on a patterned surface with widest spacing and greatest surface area of nanoroughness, compared to narrower spacing and nonpatterned surface |
EC, endothelial cells.
Fiber alignment
With the popularity of electrospun fiber designs in vascular grafts, it is crucial to examine the architecture of such grafts and the resulting effects on endothelialization. It has been thought that the architecture of the nanofibers can influence cell proliferation, migration, and differentiation.104–106 In native vessels, fiber architecture varies by layer. The medial layer consists of a circumferential orientation, while the intimal layer of ECs is aligned longitudinally with the direction of the blood vessel.104 Creating a vascular graft with distinctly oriented fiber layers could be crucial to better endothelialization and long-term graft outcomes. Recently, electrospun tubular scaffolds with an inner layer of aligned poly (lactic acid) PLA fibers and an outer layer of random PCL and PLA fibers were fabricated, in an attempt to replicate native architecture.107 HUVECs and SMCs were cocultured on the grafts. HUVECs were oriented in the direction of the fibers, and SMCs proliferated and spread throughout the random fibers. Another group fabricated oriented nanofibrous PCL scaffolds that were aminolyzed to promote the immobilization of HA for EC attachment.108 On aligned nanofiber scaffolds, investigations revealed that nanofiber alignment influenced the pattern of f-actin organization in HUVECs. Spindle-shaped morphology and bipolar extension of HUVECs were more significantly facilitated with aligned nanofibers. Combining aligned nanofibers with the HA surface modification promoted a confluent HUVEC monolayer and greater expression levels of vWF compared to random-oriented unmodified scaffolds and scaffolds with only HA modification or aligned fibers. Simply put, it is certainly advantageous to mimic the cellular orientation present in native tissue via electrospun fibers to help ensure EPC and EC orientation, proliferation, migration, and differentiation.
Surface roughness and features
It was found that nanometer-scale roughness, even at 10–100 nm, could enhance HUVEC adhesion and growth.109 Endothelial and SMC densities were found to be increased on nanostructured PLGA surfaces.102 Spherical features of 200 nm proved more beneficial for fibronectin spreading and both SMC and EC adhesion and growth on a PLGA surface.110 On polydimethylsiloxane (PDMS) films, grooves and ridges (500 nm in depth) were created in alternating nano- and micron roughness regions in linear patterns for one study.111 The space between these grooves ranged from 22 to 80 μm created via electron beam physical vapor deposition method on a flat titanium surface preceding polymer casting. Rat aortic EC adhesion was most adherent on patterned films with the greatest spacing in the sample group. Elongation of these cells was about twice as great as those on nonpatterned films. According to the results, the optimal adhesion and elongation can be tuned on a 45-μm spacing micron-rough and 80-μm spacing nanorough-patterned PDMS films.111
In a recent study, one group modified PDMS with an array of micropillars.112 They found that using 1-μm-high micropillars of fibronectin allowed HUVEC adhesion and promoted cell alignment when testing a variety of heights and diameters of pillars. Endothelial colony-forming cells (ECFCs), a subset of EPCs, and HUVECs were seeded on the substrates, showing much greater viability with micropillars of heights 1 and 3 μm than those 6 or 8 μm in height. Cell spreading was best achieved on micropillars of height 1 μm. Next, diameters and spacing between pillars were tested ranging from 1 to 0.56 μm and 0.6 to 15 μm, respectively. It was reported that fewer HUVEC extensions were observed in larger pillars with small spacing (diameters of 2.8 μm with spacing of 0.8 μm, for example), whereas smaller diameter micropillars with wider spacing yielded more-pronounced adhesive protrusions (diameters of 2 μm with spacing of 4 μm). Otherwise, HUVEC adhesion and alignment occurred on most other substrates. ECFC adhesion and elongation were optimum on 1–2-μm-diameter pillars. Finally, EC elongation and alignment were also found to be greater on PDMS with micropillar arrangements than a stiff SiO2 substrate with a similar topography.
In summary, it is crucial to consider the surface roughness and features of the vascular graft. It would appear that patterns with spaces on the scale of ∼45–80-μm spacing with features of heights no more than 1–2 μm may be optimal for EC and EPC adhesion, proliferation, and spreading.
Porosity
Porosity has long been an important factor in vascular graft design. Inflammatory reactions can be instigated by grafts with pore sizes below the so-called critical porosity size. Example sizes include 1.0 μm for PTFE, 0.8 μm for cellulose acetate and acrylic copolymer, and 1.2 μm for mixed esters cellulose.113 Additionally, it has been shown that the ideal pore size is between 10 and 45 μm to support EC coverage and reduce fibrous tissue infiltration. ECs could not bridge pores greater than cell-sized diameters.114 In another study, PU grafts were prepared with an average pore size between 5 and 30 μm. They found the highest rate of endothelialization with grafts of pore size 30 μm in the abdominal aortas of rats.115 It was thought that ingrowth of perigraft collagenous tissues aided in the establishment of the endothelium by presenting an ECM suitable for establishment of an endothelial layer. The most significant impact of porosity on the rate of endothelialization may be the establishment of these subendothelium tissues that are crucial to native tissue replacement of a biodegradable small-diameter graft. While most strategies for endothelialization techniques are focused on surface features, it is vital to consider the effect porosity has on cell migration through the graft.
Micropatterning of molecules
Combining the advantages of physical and chemical modifications of graft surfaces is an important step to induce expedited endothelialization. For example, it is well established that the spatial arrangement and organization of RGD are crucial to promoting cell adhesion and impacting the strength of cell adhesion, including in ECs.116–118 In one study, the effects of nanotopography modification were compared to RGD binding to a silicon surface. Varying the size of nano- and microscale pyramids on the material surface appeared to better control the initial adherence of ECs to the silicon film. However, RGD density better controlled EC spreading and length of focal adhesions.119 As the authors indicate, these data point to the possibility that endothelialization may follow a two-step process. It can be inferred that surface features guide the initial adhesion to cells, and the immobilized molecules predominately influence cell spreading. Thus, controlling spatial presentation of molecules and surface features may be crucial to optimizing endothelialization of a small-diameter vascular graft. One group used micropatterned lanes with selective collagen type I deposition to control EC and EPC adhesion, encouraging cells to form either elongated or cobblestone morphologies.120 Elongated EPCs and ECs experienced good ECM deposition and maintained aligned actin skeletal formation. EC deposition of ECM was largely dependent on morphology; cobblestone patterns produced more collagen type IV and fibronectin. Elongated EPCs were found to deposit and remodel significantly more than the elongated ECs. Another study demonstrated that the patterning of fibronectin supported EPC elongation and subsequent tube formation.121 Patterning of HUVECs has also been achieved with micropatterned-immobilized VEGF.122 Through micropatterning, EC and EPC adhesion, elongation, and growth can be more precisely controlled. Optimization of micropatterns can lead to a geometrically, chemically, and mechanically functional graft that better replicates native blood vessel architecture and biological cues for endothelialization.
Surface Modification and Graft Fabrication Techniques
Surface modification and control of small-diameter vascular grafts are vital to influencing cellular response, hemocompatibility, and overall success of the graft once implanted. The modifications influencing cellular responses to the graft can be broken down into two categories: chemical and physical surface modifications. Such modifications can be controlled via biofunctionalization or through graft fabrication to control surface architecture.
Biofunctional surface modification
Biofunctionalization surface modification involves immobilizing molecules via methods such as surface coatings and covalent linking. These techniques involve a chemical change in the interface between the cells and the material surfaces, to induce adhesion, proliferation, and differentiation. Table 2 provides examples of several biofunctionalization strategies implemented by research groups utilizing these techniques.
Table 2.
Recent Examples of Molecules and Methods of Immobilization Used to Achieve Enhanced Endothelialization
| Author | Biofunctionalization molecule | Immobilization method | Model | Effects |
|---|---|---|---|---|
| Shin et al.84 | VEGF | PDA/Dipping | In vitro | VEGF did not produce significantly more HUVEC adhesion than PDA alone; VEGF did support increased CD31 expression |
| Du et al.108 | Hyaluronic acid | Covalently bound | In vitro | Increased HUVEC attachment |
| Yin et al.63 | Anti-CD34 | 3,4-dihydroxyphenyalinine and l-lysine co-polypeptide linking | In vitro | Attachment and growth of ECs and EPCs increased |
| Kuwabara et al.79 | CAG peptide | Mixed into PCL solution for electrospinning fibers | Rat | Higher rate of confluent endothelialization of grafts |
| De Visscher et al.43 | SDF-1α | Immersed in fibronectin solution, followed by SDF-1α immersion | Sheep | Four times higher fraction of CD34+ cells adhered; all grafts patent after 3 months |
| Williams et al.73 | Laminin type 1 | Covalently bound | Rat | Accelerated neovascularization and endothelialization |
| Zheng et al.77 | Nap-FFGRGD | Molecular self-assembly of a hydrogelator75 | Rabbit | Threefold increase in endothelial coverage; 100% patency at 2 and 4 weeks compared to 60% patency in uncoated grafts |
ECs, endothelial cells; EPCs, endothelial progenitor cells; PDA, poly(dopamine), VEGF, vascular endothelial growth factor; HUVEC, human umbilical vein endothelial cells; CAG, cysteine-alanine-glycine; SDF, stromal cell-derived factor; PCL, poly(caprolactone).
Passive coating
In one attempt to modify material surfaces passively, hydrophobins were utilized to modify PDMS.123 Hydrophobins are a family of fungal proteins with the ability to self-assemble into amphiphilic membranes.124,125 The molecules self-assembled on the surface of the PDMS, significantly increasing the hydrophilicity of the surface.123 Hydrophobins alone have been found to improve cell adhesion to materials used for vascular grafts. In one study, improved cell adhesion was demonstrated on PLGA scaffolds modified with hydrophobins.126 In addition, the group demonstrated that collagen immobilization could be improved on PLGA surfaces modified with hydrophobins. A technique was recently developed for the immobilization of anti-CD31 antibodies on electrospun PCL scaffolds by utilizing hydrophobins. Hydrophobin coating improved the hydrophilicity of the hydrophobic PCL surface, along with providing immobilization of the anti-CD31 antibody in a simple and efficient immersion technique. HUVEC adhesion to the hydrophobin-antibody-modified PCL films was 14.9-fold higher than uncoated PCL films.55 Hydrophobins provide a backbone for surface modification that not only allows the mobilization of cell-specific bound molecules but also improves cell adhesion to the surface of the material on its own. This technology could be promising in the development of in situ endothelializing grafts. However, studies have not yet encompassed in vivo studies that would be highly informational regarding the clinical potential of this surface modification technique.
Covalently linked
Covalent binding of molecules for surface modification offers the ability to more uniformly distribute bioactive molecules and functional groups on graft surfaces. Plasma surface modification has been utilized significantly in vascular graft engineering to aid in the development of hemocompatible, bioactive, and biomimetic graft surfaces, especially on permanent polymeric grafts.127 However, it is vital to consider a covalent immobilization technique's effect on biodegradable polymeric grafts. Modification can affect the surface chemistry and aging of polymers, as well as the effective function of the attached biomolecule and the stability of their attachment.128 Vapor-phase grafting has been demonstrated to initiate covalent VEGF functionalization of grafts on PLLA and PCL in a nondestructive manner.42 One group covalently immobilized sulfated silk fibroin to PLGA scaffolds using γ-irradiation, supporting in vitro hemocompatibility and endothelialization.129 Another study examined the effectiveness of various strategies for biofunctionalizing PCL using ammonia plasma, oxygen plasma/aminopropyl triethoxysilane (APTESI), and 4,4′-methylene-bis(phenyl isocyanate)/water to add terminal amino groups to the PCL.130 An anti-inflammatory and antithrombogenic drug, acetylsalicylic acid (ASA), and VEGF were immobilized via an N,N-disucciniidyl carbonate (DSC) crosslinker. Highest functionality was observed in the APTESI, group and immobilization of ASA and VEGF was greatly improved with the DSC crosslinker compared to passive adsorption. NH3 plasma-activated PCL had the highest ASA loading, while APTES modification provided the highest attachment of VEGF. This study makes it clear that choosing the proper methodology of immobilizing molecules for biofunctionalization of a graft can be just as important as the choice of biomolecule. Many covalent techniques have been proven to be effective for modifying the surface of graft materials and immobilizing functional molecules to encourage cell homing and binding. Covalent binding offers good control over the orientation of the ligands, but it is important to consider the impacts of covalent modification on the biodegradable graft material, so as to avoid deleterious side effects.
Methods of graft fabrication to control graft architecture
As described, physical surface modification allows the manipulation of surface characteristics such as roughness, patterns, features, and overall topography of the material surface that still consists of the bulk graft material. Methods of fabricating grafts to utilize the effects outlined in the Topography and physical properties section on topography, and physical properties are outlined below.
Solvent casting
To solvent cast a graft, a polymer is dissolved in a solvent. A porogen may be added to the solution, and then the solution is added to a 3D mold. After the solvent is evaporated, the porogen may be leached, resulting in somewhat controlled porosity. The configuration of the mold enables some control over surface topography. Porous PU scaffolds were developed by one group using solvent casting and subsequent salt leaching. Porosity uniformity was controlled via centrifugation.131 While solvent casting is not the most prevalent method of graft fabrication and physical surface control, it is a simple and relatively easy method of fabrication. Still, other methods, such as electrospinning and even stereolitheography, may enable more precise control over fabricating grafts to mimic the native architecture of the vascular ECM.
Electrospinning
Fabrication of electrospun grafts to mimic the native ECM is an important consideration in small-diameter vascular grafts. An increasingly investigated area of graft fabrication is the control of nanofiber orientation. Nanofibrous PCL scaffolds were fabricated in aligned and random orientations, followed by surface modification with HA.108 Importantly, to align the PCL nanofibers, the rotating drum for collecting the fibers was operated at a high speed of rotation (2000 rpm) to generate well-aligned fibers. A low speed of rotation (20 rpm) produced random PCL nanofibers. Another study demonstrated the feasibility of controlling nanofiber layers in a PCL vascular graft by altering the rotation of the nanofiber collector and the electric field during the electrospinning process.132 This enabled the fabrication of interspaced layers aligned circumferentially, axially, and as a controlled mixture of orientations. Such studies demonstrate the expanding capabilities of electrospun vascular grafts. Electrospinning offers robust material selection, low cost, simplicity, high surface-to-volume ratios, and favorable, controllable porosity.133–136 With these advantages, electrospinning is a proven framework allowing for the improved endothelialization of vascular grafts through precise fiber alignment and organization.
Stereolithography and 3D printing
While elecrospinning has dominated the present direction of vascular graft production, there has been some investigation into controlling graft fabrication and surface characteristics via techniques such as stereolithography and 3D printing. As previously mentioned, architecture of a graft should resemble native vessel conditions. The nature of electrospun fiber fabrication necessitates porosity in grafts, which increases the surface area for cell attachment and allows cell invasion. However, 3D scaffold architecture and porosity can also be controlled via freeform fabrication techniques as demonstrated by one group.137 Using microstereolithography, they studied the internal pore and architecture relationship of a poly(propylene fumarate) graft intended for bone scaffolding. More recently, the feasibility of controlling graft architecture through a 3D computer-aided design was translated for vascular grafts. Three-dimensional microarchitectural features were created using a new stereobiofabrication method on PEG diacrylate and gelatin methacrylate.138 Another group printed positive molds for the grafts and poured the graft material into the molds.139 All materials used were photocrosslinkable polymers based on urethane diacrylate monomers. UV light was applied to the filled molds to activate crosslinking of the materials. Currently, these techniques are not being widely pursued in the field of vascular grafts, presumably due to the difficult of adapting such technologies to graft applications and the relative ease and low cost of electrospinning. Advances in the technology may also enable more precise control of graft architecture over electrospinning, allowing further investigating into better control of graft surfaces to encourage EC and EPC adhesion, differentiation, and proliferation.
Chemical vapor deposition
Vapor deposition can be used to deposit small particles onto graft material surfaces to create micro- and nanoscale patterns and structures. One group studied plasma-modified nanostructures on a variety of polymeric and metallic surfaces.140 The process altered the nanoroughness and surface energy of the materials, affecting EC adhesion. E-beam evaporation has been used to generate surface features on a titanium substrate.141 In addition, vapor deposition was used to modify surfaces used in a mold for polymer casting.111
Chemical vapor deposition can also be used to allow for functionalization of graft surfaces. Surface chemistry can be altered, or reactive groups can be added, to allow for covalent immobilization of biomolecules to material surfaces.142 One study demonstrated plasma modification to modify a biodegradable graft material for enhanced endothelialization.143 The group introduced polyvinyl acetic acid groups to the PLLA substrate to immobilize fibronectin. Cell adhesion and proliferation were improved, along with increased cell retention under shear stress. Chemical vapor deposition has been more extensively researched in permanent materials, and this study offers a glimpse of the potential application in biodegradable, small-diameter grafts.
Controlling EPC Differentiation into EC-Like Cells
Shear stress
In mature blood vessels, ECs are exposed to shear stress resulting from blood flow. This shear stress affects a variety of mechanotransduction pathways, including cell alignment and biochemical functions.144 Shear stress has been found to induce EPC differentiation into adhesive ECs.145 Recent research indicates that shear stress affects EPCs through the VEGF-R2 and PI3K/Akt/mTOR signal transduction pathways, increasing adhesion, differentiation, migration, and proliferation of circulating EPCs.146 While shear stress is innately applied due to blood flow, it is important to ensure that graft designs preserve hemodynamics similar to native vessels. Fluid dynamic analyses of novel graft architectural structures or surface patterns should not be neglected.
Chemokines, cytokines, and growth factors
Besides assisting in homing and mobilizing of EPCs, growth factors are also integral to the differentiation of EPCs into EC-like cells. SDF-1,147,148 matrix metallopeptidase-9,147 VEGF,149,150 erythropoietin,151 and interleukin-8152 have been identified for their roles in vascular remodeling, neovasculogenesis, and EPC differentiation. Research is ongoing to determine other factors that influence EPC differentiation into EC-like phenotypes and inclusion of these factors in graft applications. For example, BDNF has been found to support EC viability and neoangiogensis, thought to have a significant regulatory role in EC development.153 BDNF was found to improve the patency rate of a graft fabricated from decellularized rat carotid arteries when incorporated on the inner lumen.46 Improved isolation and immobilization of factors could greatly increase the effectiveness of EPC homing, recruitment, and, most importantly, differentiation to EC-like phenotypes. Additionally, continued research into the biology of EPCs is necessary to better understand these pathways and how these chemokines, cytokines, and growth factors interact with EPCs to better control EPC response in vivo.
It is important to consider the effects of chemokines, cytokines, and growth factors in environments more similar to in vivo environments, as well. Static culture studies may not be representative of the actual effects on EPCs. Exposure to shear stress has been found to augment EPC differentiation into EC-like cells on fibronectin and VEGF-bound surfaces, compared to modified surfaces in static cultures or shear stress alone. An increase in VEGFR-2 and VE-cadherin expression indicative of EC differentiation of EPCs augmented was found on fibronectin-coated surfaces when shear stress was applied.154 Furthermore, another study demonstrated that EPC expression of vWF, CD31, and ephribB2 signals, markers for arterial ECs, were significantly increased on VEGF-bound surfaces when shear stress was applied.155 Comparing marker expression in these two studies, VEGF-bound surfaces appeared to better support EPC differentiation under shear stress conditions than fibronectin-bound surfaces.155 Evaluation of prototype grafts should include shear stress conditions to better understand EPC differentiation under the influence of various immobilized biofunctional molecules.
Material and surface properties
Material and topographical properties alone have been found to influence the differentiation of EPCs into EC-like cells. Recently, it was demonstrated that growth of EPCs on fibrin displays significantly increased levels of cytokine release associated with angiogenesis compared to EPCs grown on fibronectin, though EC marker expression was similar between both groups.156 It was recently demonstrated that EPCs produced varying levels of markers influencing EC function depending on the surfaces on which the cells were seeded.157 The group-modified titanium surfaces for the following experimental groups are acid-etched, sand-blasted and acid-etched, hydrophilic acid-etched, and hydrophilic acid-etched and sand-blasted. These groups were compared to cell-culture-compatible plastic and fibronectin-coated plastic controls. Lower levels of VEGF expression were measured on acid-etched titanium, while the highest VEGF expression was found in hydrophilic acid-etched and sand-blasted. The highest EC endothelial nitric oxide synthetase expression was also found on hydrophilic acid-etched and sand-blasted titanium.158 Combining the material properties encountered by the EPCs along with controlling surface textures could work to selectively promote EPC growth. A recent study demonstrated the differentiation of adipocyte-derived stem cells (ADSC) into ECs, and subsequent EC marker expression has been found to be significantly upregulated by nanopographical modification on quartz substrates with 250-nm ridges and 500-nm grooves.159 While ADSCs are not a cell type suitable for in situ endothelialization, this study demonstrates the importance of nanotopography of graft surfaces in differentiation of endothelial lineages. Further work into the effects of nanotopography on EPC differentiation could further elucidate this phenomenon to promote expedited EPC differentiation. Combining these material and surface property stimuli along with the presentation of appropriate growth factors can work to achieve optimal EPC growth and differentiation.
Future Directions and Conclusions
Many strategies have been studied to support in situ endothelialization of small-diameter vascular grafts. Likely, the optimal vascular graft will incorporate several strategies. The graft must effectively support EPC mobilizing and homing. This may be accomplished by presentation and release of a growth factor like VEGF or G-CSF or an external treatment such as injection of atorvastatin. The graft surface will need to have molecules suitable for selective adhesion of EPCs and ECs while maintaining thromboresistance and hemocompatibility. Nanotopography can also aid in adhesion of ECs and EPCs, while affecting cell geometry, growth, and differentiation. Porosity and hierarchical design of grafts incorporating various materials will also be necessarily optimized to allow for adequate cell infiltration to support subendothelial tissues to support EC and EPC growth and differentiation. After the graft degrades, establishment of these tissues will be crucial to long-term success of the graft and the new vessel.
Despite the increasing number of small-diameter graft strategies utilizing EPCs, there is still controversy surrounding the biological nature of these cells. There is no specific marker that uniquely identifies EPCs, and thus a variety of EPC subpopulations have been identified under markers generally presented by EPCs.160 This can make EPC-specific cell attachment difficult. One group has taken the stance of abandoning EPCs almost entirely for their endothelialization potential, instead urging research to focus on the paracrine effects of these cells.161 They maintain that the ECs are primarily responsible for arterial repair and regenerations, and EPC research has not yielded data sufficient for supporting the idea that EPCs contribute directly to endothelial regeneration. Still, other perspectives offer that a particular subset of cells that have been labeled EPCs may have a significant role in vascular repair: ECFCs.160,162 More research into the unique markers that identify these cells will be necessary to better take advantage of their endothelialization properties in a small-diameter graft.
Strategies to promote in situ endothelialization of a small-diameter, biodegradable vascular graft have been robust, but much work remains to make these techniques clinically viable. The biology behind in situ endothelialization techniques, especially those utilizing EPCs and, specifically, ECFCs, should be further investigated. In addition, combinatorial methods of graft coating, topography, porosity, and external treatments should be investigated to optimize graft environments that enable efficient and thorough endothelialization of graft surfaces.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under the Award Number R01 AR061460. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. World Health Statistics 2012. 2012. www.who.int/gho/publications/world_health_statistics/2012/en www.who.int/gho/publications/world_health_statistics/2012/en
- 2.Lloyd-Jones D. Adams R.J. Brown T.M. Carnethon M. Dai S. De Simone G., et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Abbott W.M. Callow A. Moore W. Rutherford R. Veith F. Weinberg S. Evaluation and performance standards for arterial prostheses. J Vasc Surg. 1993;17:746. doi: 10.1067/mva.1993.45222. [DOI] [PubMed] [Google Scholar]
- 4.Budd J.S. Allen K.E. Hartley G. Bell P.R.F. The effect of preformed confluent endothelial cell monolayers on the patency and thrombogenicity of small calibre vascular grafts. Eur J Vasc Surg. 1991;5:397. doi: 10.1016/s0950-821x(05)80171-9. [DOI] [PubMed] [Google Scholar]
- 5.Pasic M. Müller-Glauser W. Von Segesser L.K. Lachat M. Mihaljevic T. Turina M.I. Superior late patency of small-diameter Dacron grafts seeded with omental microvascular cells: an experimental study. Ann Thorac Surg. 1994;58:677. doi: 10.1016/0003-4975(94)90726-9. [DOI] [PubMed] [Google Scholar]
- 6.Demiri E.C. Iordanidis S.L. Mantinaos C.F. Experimental use of prosthetic grafts in microvascular surgery. Handchirurgie, Mikrochirurgie, Plastische Chirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse. Organ. 1999;31:102. doi: 10.1055/s-1999-13504. [DOI] [PubMed] [Google Scholar]
- 7.Zilla P. Bezuidenhout D. Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Wang X. Lin P. Yao Q. Chen C. Development of small-diameter vascular grafts. World J Surg. 2007;31:682. doi: 10.1007/s00268-006-0731-z. [DOI] [PubMed] [Google Scholar]
- 9.Nieponice A. Soletti L. Guan J. Hong Y. Gharaibeh B. Maul T.M., et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A. 2010;16:1215. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAllister T.N. Maruszewski M. Garrido S.A. Wystrychowski W. Dusserre N. Marini A., et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 11.Kakisis J.D. Liapis C.D. Breuer C. Sumpio B.E. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W.J. Liu W. Cui L. Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubanyi G.M. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22(Suppl. 4):S1. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 14.Van Hinsbergh V.W.M. The endothelium: vascular control of haemostasis. Eur J Obstet Gynecol Reprod Biol. 2001;95:198. doi: 10.1016/s0301-2115(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 15.Pearson J.D. Endothelial cell function and thrombosis. Best Pract Res Clin Haematol. 1999;12:329. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 16.Patel S.D. Waltham M. Wadoodi A. Burnand K.G. Smith A. The role of endothelial cells and their progenitors in intimal hyperplasia. Ther Adv Cardiovasc Dis. 2010;4:129. doi: 10.1177/1753944710362903. [DOI] [PubMed] [Google Scholar]
- 17.Naito Y. Williams-Fritze M. Duncan D.R. Church S.N. Hibino N. Madri J.A., et al. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs. 2012;195:60. doi: 10.1159/000331405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarbiv G. Sc M. Sarig U. Karram T. Hoffman A. Machluf M., et al. Porcine small diameter arterial extracellular matrix supports endothelium formation and media remodeling forming a promising vascular engineered biograft. Tissue Eng Part A. 2012;18:411. doi: 10.1089/ten.TEA.2011.0173. [DOI] [PubMed] [Google Scholar]
- 19.Schmedlen R.H. Elbjeirami W.M. Gobin A.S. West J.L. Tissue engineered small-diameter vascular grafts. Clin Plast Surg. 2003;30:507. doi: 10.1016/s0094-1298(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 20.Berglund J.D. Galis Z.S. Designer blood vessels and therapeutic revascularization. Br J Pharmacol. 2003;140:627. doi: 10.1038/sj.bjp.0705457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain R.K. Au P. Tam J. Duda D.G. Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 22.Avci-Adali M. Ziemer G. Wendel H.P. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—a review of current strategies. Biotechnol Adv. 2010;28:119. doi: 10.1016/j.biotechadv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 23.De Mel A. Jell G. Stevens M.M. Seifalian A.M. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9:2969. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- 24.Berger K. Sauvage L.R. Rao A.M. Wood S.J. Healing of arterial prostheses in man: its incompleteness. Ann Surg. 1972;175:118. doi: 10.1097/00000658-197201000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquinelli G. Freyrie A. Preda P. Curti T. D'Addato M. Laschi R. Healing of prosthetic arterial grafts. Scan Microsc. 1990;4:351. [PubMed] [Google Scholar]
- 26.Clowes A.W. Kirkman T.R. Reidy M.A. Mechanisms of arterial graft healing. Rapid transmural capillary ingrowth provides a source of intimal endothelium and smooth muscle in porous PTFE prostheses. Am J Pathol. 1986;123:220. [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara T. Murohara T. Sullivan A. Silver M. Van der Zee R. Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T. Kalka C. Masuda H. Chen D. Silver M. Kearney M., et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 29.Rafii S. Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 30.Crosby J.R. Kaminski W.E. Schatteman G. Martin P.J. Raines E.W. Seifert R.A., et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 31.Ladhoff J. Fleischer B. Hara Y. Volk H.-D. Seifert M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovasc Res. 2010;88:121. doi: 10.1093/cvr/cvq109. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q. Rafii S. Wu M.H. Wijelath E.S. Yu C. Ishida A., et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362. [PubMed] [Google Scholar]
- 33.Lee S.P. Youn S.W. Cho H.J. Li L. Kim T.Y. Yook H.S., et al. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 34.Oh I.Y. Yoon C.H. Hur J. Kim J.H. Kim T.Y. Lee C.S., et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt D. Breymann C. Weber A. Guenter C.I. Neuenschwander S. Zund G., et al. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann Thorac Surg. 2004;78:2094. doi: 10.1016/j.athoracsur.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Investig. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirschi K.K. Ingram D.A. Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon C.-H. Hur J. Park K.-W. Kim J.-H. Lee C.-S. Oh I.-Y., et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 39.Reed D.M. Foldes G. Hardling S.E. Mitchell J.A. Stem cell derived endothelial cells for cardiovascular disease; a therapeutic perspective. Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04361.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urbich C. Aicher A. Heeschen C. Dernbach E. Hofmann W.K. Zeiher A.M., et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Aicher A. Zeiher A.M. Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 42.Edlund U. Sauter T. Albertsson A.-C. Covalent VEGF protein immobilization on resorbable polymeric surfaces. Polym Adv Technol. 2011;22:166. [Google Scholar]
- 43.De Visscher G. Mesure L. Meuris B. Ivanova A. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater. 2011;8:1330. doi: 10.1016/j.actbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M. Liu Z. Li K. Qiao W. Jiang X. Ran F., et al. Beneficial effects of granulocyte-colony stimulating factor on small-diameter heparin immobilized decellularized vascular graft. J Biomed Mater Res Part A. 2010;95:600. doi: 10.1002/jbm.a.32864. [DOI] [PubMed] [Google Scholar]
- 45.Zeng W. Yuan W. Li L. Mi J. Xu S. Wen C., et al. The promotion of endothelial progenitor cells recruitment by nerve growth factors in tissue-engineered blood vessels. Biomaterials. 2010;31:1636. doi: 10.1016/j.biomaterials.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 46.Zeng W. Wen C. Wu Y. Li L. Zhou Z. Mi J., et al. The use of BDNF to enhance the patency rate of small-diameter tissue-engineered blood vessels through stem cell homing mechanisms. Biomaterials. 2012;33:473. doi: 10.1016/j.biomaterials.2011.09.066. [DOI] [PubMed] [Google Scholar]
- 47.Baumann L. Prokoph S. Gabriel C. Freudenberg U. Werner C. Beck-Sickinger A.G. A novel, biased-like SDF-1 derivative acts synergistically with starPEG-based heparin hydrogels and improves eEPC migration in vitro. J Control Release. 2012;162:68. doi: 10.1016/j.jconrel.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 48.Wang B. Sun L. Tian Y. Li Z. Wei H. Wang D., et al. Effects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J Neurol Sci. 2012;319:117. doi: 10.1016/j.jns.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Heeschen C. Aicher A. Lehmann R. Fichtlscherer S. Vasa M. Urbich C., et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y. Hu R. Lv L. Ling L. Jiang S. Erythropoietin improves the efficiency of endothelial progenitor cell therapy after myocardial infarction in mice: effects on transplanted cell survival and autologous endothelial progenitor cell mobilization. J Surg Res. 2012;176:e47. doi: 10.1016/j.jss.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 51.Li J. Lu W.M. Li X.X. Wang S.M. Yu J.X. Zhu Y.F., et al. Intensive statin therapy: a favorable adjunct to the improvement of small-diameter vascular grafts. Angiology. 2010;61:427. doi: 10.1177/0003319709356422. [DOI] [PubMed] [Google Scholar]
- 52.Jean-Baptiste E. Blanchemain N. Martel B. Neut C. Hildebrand H.F. Haulon S. Safety, healing, and efficacy of vascular prostheses coated with hydroxypropyl-β-cyclodextrin polymer: experimental in vitro and animal studies. Eur J Vasc Endovasc Surg. 2012;43:188. doi: 10.1016/j.ejvs.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Gong X.B. Li Y.Q. Gao Q.C. Cheng B.B. Shen B.R. Yan Z.Q., et al. Adhesion behavior of endothelial progenitor cells to endothelial cells in simple shear flow. Acta Mech Sin. 2011;27:1071. [Google Scholar]
- 54.Ciraci E. Della Bella S. Salvucci O. Rofani C. Segarra M. Bason C., et al. Adult human circulating CD34-Lin-CD45-CD133- cells can differentiate into hematopoietic and endothelial cells. Blood. 2011;119:2105. doi: 10.1182/blood-2010-10-316596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M. Wang Z. Wang Z. Feng S. Xu H. Zhao Q., et al. Immobilization of anti-CD31 antibody on electrospun poly(ɛ-caprolactone) scaffolds through hydrophobins for specific adhesion of endothelial cells. Colloids Surfaces B Biointerfaces. 2011;85:32. doi: 10.1016/j.colsurfb.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 56.Markway B.D. McCarty O.J.T. Marzec U.M. Courtman D.W. Hanson S.R. Hinds M.T. Capture of flowing endothelial cells using surface-immobilized anti-kinase insert domain receptor antibody. Tissue Eng Part C Methods. 2008;14:97. doi: 10.1089/ten.tec.2007.0300. [DOI] [PubMed] [Google Scholar]
- 57.Muller W.A. Ratti C.M. McDonnell S.L. Cohn Z.A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockinger H. Gadd S.J. Eher R. Majdic O. Schreiber W. Kasinrerk W., et al. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990;145:3889. [PubMed] [Google Scholar]
- 59.Tanaka Y. Albelda S.M. Horgan K.J. Van Seventer G.A. Shimizu Y. Newman W., et al. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992;176:245. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakazawa G. Granada J.F. Alviar C.L. Tellez A. Kaluza G.L. Guilhermier M.Y., et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC. 2010;3:68. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Lin Q. Ding X. Qiu F. Song X. Fu G. Ji J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials. 2010;31:4017. doi: 10.1016/j.biomaterials.2010.01.092. [DOI] [PubMed] [Google Scholar]
- 62.Chen J. Cao J. Wang J. Maitz M.F. Guo L. Zhao Y., et al. Biofunctionalization of titanium with PEG and anti-CD34 for hemocompatibility and stimulated endothelialization. J Colloid Interface Sci. 2012;368:636. doi: 10.1016/j.jcis.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Yin M. Yuan Y. Liu C. Wang J. Combinatorial coating of adhesive polypeptide and anti-CD34 antibody for improved endothelial cell adhesion and proliferation. J Mater Sci Mater Med. 2009;20:1513. doi: 10.1007/s10856-009-3715-3. [DOI] [PubMed] [Google Scholar]
- 64.Yeh E.T.H. Zhang S. Wu H.D. Körbling M. Willerson J.T. Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 65.Pearson J.D. Endothelial progenitor cells—hype or hope? J Thromb Haemost. 2009;7:255. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 66.Kawahara D. Matsuda T. Hydrodynamic shear-stress-dependent retention of endothelial and endothelial progenitor cells adhered to vascular endothelial growth factor-fixed surfaces. J Biomed Mater Res Part B Appl Biomater. 2012;100:1218. doi: 10.1002/jbm.b.32686. [DOI] [PubMed] [Google Scholar]
- 67.Yoon M. Hwang H.J. Kim J.H. Immobilization of antibodies on the self-assembled monolayer by antigen-binding site protection and immobilization kinetic control. J Biomed Sci Eng. 2011;04:242. [Google Scholar]
- 68.Shmanai V.V. Nikolayeva T.A. Vinokurova L.G. Litoshka A.A. Oriented antibody immobilization to polystyrene macrocarriers for immunoassay modified with hydrazide derivatives of poly(meth)acrylic acid. BMC Biotechnol. 2001;1:4. doi: 10.1186/1472-6750-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caiado F. Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogen Tissue Repair. 2012;5:4. doi: 10.1186/1755-1536-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iivanainen E. Kähäri V.-M. Heino J. Elenius K. Endothelial cell-matrix interactions. Microsc Res Tech. 2003;60:13. doi: 10.1002/jemt.10238. [DOI] [PubMed] [Google Scholar]
- 71.Short S.M. Talbott G.A. Juliano R.L. Integrin-mediated signaling events in human endothelial cells. Mol Biol Cell. 1998;9:1969. doi: 10.1091/mbc.9.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X. Groopman J.E. Wang J.F. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- 73.Williams S.K. Kleinert L.B. Patula-Steinbrenner V. Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J Biomed Mater Res Part A. 2011;99:67. doi: 10.1002/jbm.a.33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hersel U. Dahmen C. Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 75.Blit P.H. McClung W.G. Brash J.L. Woodhouse K.A. Santerre J.P. Platelet inhibition and endothelial cell adhesion on elastin-like polypeptide surface modified materials. Biomaterials. 2011;32:5790. doi: 10.1016/j.biomaterials.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 76.Behjati M. Kazemi M. Hashemi M. Zarkesh-Esfahanai S.H. Bahrami E. Hashemi-Beni B., et al. Double-chimera proteins to enhance recruitment of endothelial cells, their progenitor cells. Int J Cardiol. 2012 doi: 10.106/j.ijcard.2012.04.094. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Zheng W. Wang Z. Song L. Zhao Q. Zhang J. Li D., et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials. 2012;33:2880. doi: 10.1016/j.biomaterials.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z. Wang H. Zheng W. Zhang J. Zhao Q. Wang S., et al. Highly stable surface modifications of poly(3-caprolactone) (PCL) films by molecular self-assembly to promote cells adhesion and proliferation. Chem Commun. 2011;47:8901. doi: 10.1039/c1cc11564b. [DOI] [PubMed] [Google Scholar]
- 79.Kuwabara F. Narita Y. Yamawaki-Ogata A. Kanie K. Kato R. Satake M., et al. Novel small-caliber vascular grafts with trimeric peptide for acceleration of endothelialization. Ann Thorac Surg. 2011;93:156. doi: 10.1016/j.athoracsur.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 80.Kanie K. Narita Y. Zhao Y. Kuwabara F. Satake M. Honda S., et al. Collagen type IV-specific tripeptides for selective adhesion of endothelial and smooth muscle cells. Biotechnol Bioeng. 2012;109:1808. doi: 10.1002/bit.24459. [DOI] [PubMed] [Google Scholar]
- 81.Kato R. Kaga C. Kunimatsu M. Kobayashi T. Honda H. Peptide array-based interaction assay of solid-bound peptides and anchorage-dependant cells and its effectiveness in cell-adhesive peptide design. J Biosci Bioeng. 2006;101:485. doi: 10.1263/jbb.101.485. [DOI] [PubMed] [Google Scholar]
- 82.Wang J. Liu C. Lu X. Yin M. Co-polypeptides of 3,4-dihydroxyphenylalanine and L-lysine to mimic marine adhesive protein. Biomaterials. 2007;28:3456. doi: 10.1016/j.biomaterials.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Ku S.H. Park C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31:9431. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 84.Shin Y.M. Lee Y.B. Kim S.J. Kang J.K. Park J.-C. Jang W., et al. Mussel-inspired immobilization of vascular endothelial cell growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules. 2012;13:2020. doi: 10.1021/bm300194b. [DOI] [PubMed] [Google Scholar]
- 85.Saccà B. Niemeyer C.M. Functionalization of DNA nanostructures with proteins. Chem Soc Rev. 2011;40:5910. doi: 10.1039/c1cs15212b. [DOI] [PubMed] [Google Scholar]
- 86.Schleicher M. Hansmann J. Elkin B. Kluger P.J. Liebscher S. Huber A.J.T., et al. Oligonucleotide, parylene surface coating of polystyrene, ePTFE for improved endothelial cell attachment, hemocompatibility. Int J Biomater. 2012;2012 doi: 10.1155/2012/397813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo K. Wendel H.P. Scheideler L. Ziemer G. Scheule A.M. Aptamer-based capture molecules as a novel coating strategy to promote cell adhesion. J Cell Mol Med. 2005;9:731. doi: 10.1111/j.1582-4934.2005.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoffman J. Paul A. Harwardt M. Groll J. Reeswinkel T. Klee D., et al. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J Biomed Mater Res Part A. 2008;84:614. doi: 10.1002/jbm.a.31309. [DOI] [PubMed] [Google Scholar]
- 89.Strahm Y. Flueckiger A. Billinger M. Meier P. Mettler D. Weisser S., et al. Endothelial-cell-binding aptamer for coating of intracoronary stents. J Invasive Cardiol. 2010;22:481. [PubMed] [Google Scholar]
- 90.Fukushima T. Inoue Y. Hayakawa T. Taniguchi K. Miyazaki K. Okahata Y. Preparation of and tissue response to DNA-lipid films. J Dent Res. 2001;80:1772. doi: 10.1177/00220345010800081801. [DOI] [PubMed] [Google Scholar]
- 91.Uhlmann E. Ryte A. Peyman A. Studies on the mechanism of stabilization of partially phosphorothioated oligonucleotides against nucleolytic degradation. Antisense Nucleic Acid Drug Dev. 1997;7:345. doi: 10.1089/oli.1.1997.7.345. [DOI] [PubMed] [Google Scholar]
- 92.Suuronen E.J. Zhang P. Kuraitis D. Cao X. Melhuish A. McKee D., et al. An acellular matrix-bound ligand enhances the mobilization, recruitment and therapeutic effects of circulating progenitor cells in a hindlimb ischemia model. FASEB J. 2009;23:1447. doi: 10.1096/fj.08-111054. [DOI] [PubMed] [Google Scholar]
- 93.Chuang T.-W. Masters K.S. Regulation of polyurethane hemocompatibility and endothelialization by tethered hyaluronic acid oligosaccharides. Biomaterials. 2009;30:5341. doi: 10.1016/j.biomaterials.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 94.Soletti L. Nieponice A. Hong Y. Ye S.-H. Stankus J.J. Wagner W.R., et al. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res Part A. 2011;96:436. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tardif K. Cloutier I. Miao Z. Lemieux C. St-Denis C. Winnik F.M., et al. A phosphorylcholine-modified chitosan polymer as an endothelial progenitor cell supporting matrix. Biomaterials. 2011;32:5046. doi: 10.1016/j.biomaterials.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 96.Perea H. Aigner J. Hopfner U. Wintermantel E. Direct magnetic tubular cell seeding: a novel approach for vascular tissue engineering. Cells Tissues Organs. 2006;183:156. doi: 10.1159/000095989. [DOI] [PubMed] [Google Scholar]
- 97.Tiwari A. Punshon G. Kidane A. Hamilton G. Seifalian A. Magnetic beads (Dynabead) toxicity to endothelial cells at high bead concentration: implication for tissue engineering of vascular prosthesis. Cell Biol Toxicol. 2003;19:265. doi: 10.1023/b:cbto.0000004929.37511.ed. [DOI] [PubMed] [Google Scholar]
- 98.Kim J.A. Lee H.J. Kang H.-J. Park T.H. The targeting of endothelial progenitor cells to a specific location within a microfluidic channel using magnetic nanoparticles. Biomed Microdevices. 2009;11:287. doi: 10.1007/s10544-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 99.Kang H.-J. Kim J.-Y. Lee H.-J. Kim K.-H. Kim T.-Y. Lee C.-S., et al. Magnetic bionanoparticle enhances homing of endothelial progenitor cells in mouse hindlimb ischemia. Korean Circ J. 2012;42:390. doi: 10.4070/kcj.2012.42.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roach P. Eglin D. Rohde K. Perry C.C. Modern biomaterials: a review—bulk properties and implications of surface modifications. J Mater Sci Mater Med. 2007;18:1263. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 101.Simon C. Palmaz J.C. Sprague E.A. Influence of topography on endothelialization of stents: clues for new designs. J Long Term Eff Med Implants. 2000;10:143. doi: 10.1615/jlongtermeffmedimplants.v10.i12.120. [DOI] [PubMed] [Google Scholar]
- 102.Miller D.C. Thapa A. Haberstroh K.M. Webster T.J. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004;25:53. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 103.Hubbi M.E. Ahn E.H. Downey J. Afzal J. Kim D.H. Rey S., et al. Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Sci Signal. 2012;5:ra41. doi: 10.1126/scisignal.2003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vaz C.M. Van Tuijl S. Bouten C.V.C. Baaijens F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005;1:575. doi: 10.1016/j.actbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 105.Baker B.M. Mauck R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurpinski K.T. Stephenson J.T. Janairo R.R.R. Lee H. Li S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials. 2010;31:3536. doi: 10.1016/j.biomaterials.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shalumon K.T. Sreerekha P.R. Sathish D. Tamura H. Nair S.V. Chennazhi K.P., et al. Hierarchically designed electrospun tubular scaffolds for cardiovascular applications. J Biomed Nanotechnol. 2011;7:609. doi: 10.1166/jbn.2011.1337. [DOI] [PubMed] [Google Scholar]
- 108.Du F. Zhao W. Zhang M. Mao H. Kong D. Yang J. The synergistic effect of aligned nanofibers and hyaluronic acid modification on endothelial cell behavior for vascular tissue engineering. J Nanosci Nanotechnol. 2011;11:6718. doi: 10.1166/jnn.2011.4750. [DOI] [PubMed] [Google Scholar]
- 109.Chung T.W. Liu D.Z. Wang S.Y. Wang S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24:4655. doi: 10.1016/s0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 110.Miller D.C. Haberstroh K.M. Webster T.J. PLGA nanometer surface features manipulate fibronectin interactions for improved vascular cell adhesion. J Biomed Mater Res A. 2007;81:678. doi: 10.1002/jbm.a.31093. [DOI] [PubMed] [Google Scholar]
- 111.Ranjan A. Webster T.J. Increased endothelial cell adhesion and elongation on micron-patterned nano-rough poly(dimethylsiloxane) films. Nanotechnology. 2009;20:305102. doi: 10.1088/0957-4484/20/30/305102. [DOI] [PubMed] [Google Scholar]
- 112.Dickinson L.E. Rand D.R. Tsao J. Eberle W. Gerecht S. Endothelial cell responses to micropillar substrates of varying dimensions and stiffness. J Bomed Mater Res Part A. 2012;100:1457. doi: 10.1002/jbm.a.34059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Der Lei B. Wildevuur C.R. From a synthetic, microporous, compliant, biodegradable small-caliber vascular graft to a new artery. Thorac Cardiovasc Surg. 1989;37:337. doi: 10.1055/s-2007-1020349. [DOI] [PubMed] [Google Scholar]
- 114.Salem A.K. Stevens R. Pearson R.G. Davies M.C. Tendler S.J.B. Roberts C.J., et al. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J Biomed Mater Res. 2002;61:212. doi: 10.1002/jbm.10195. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z. Wang Z. Liu S. Kodama M. Pore size, tissue ingrowth, and endothelialization of small-diameter microporous polyurethane vascular prostheses. Biomaterials. 2004;25:177. doi: 10.1016/s0142-9612(03)00478-2. [DOI] [PubMed] [Google Scholar]
- 116.Maheshwari G. Brown G. Lauffenburger D.A. Wells A. Griffith L.G. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 117.Koo L.Y. Irvine D.J. Mayes A.M. Lauffenburger D.A. Griffith L.G. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci. 2002;115(Pt 7):1423. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- 118.Huang J. Grater S.V. Corbellini F. Rinck S. Bock E. Kemkemer R., et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9:1111. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le Saux G. Magenau A. Böcking T. Gaus K. Gooding J.J. The relative importance of topography and RGD ligand density for endothelial cell adhesion. PLoS One. 2011;6:e21869. doi: 10.1371/journal.pone.0021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vartanian K.B. Kirkpatrick S.J. McCarty O.J.T. Vu T.Q. Hanson S.R. Hinds M.T. Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J Biomed Mater Res Part A. 2009;91:528. doi: 10.1002/jbm.a.32225. [DOI] [PubMed] [Google Scholar]
- 121.Dickinson L.E. Moura M.E. Gerecht S. Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter. 2010;6:5109. [Google Scholar]
- 122.Ito Y. Hasuda H. Terai H. Kitajima T. Culture of human umbilical vein endothelial cells on immobilized vascular endothelial growth factor. J Biomed Mater Res Part A. 2005;74:659. doi: 10.1002/jbm.a.30360. [DOI] [PubMed] [Google Scholar]
- 123.Wang R. Yang Y. Qin M. Wang L. Yu L. Shao B., et al. Biocompatible hydrophilic modifications of poly(dimethylsiloxane) using self-assembled hydrophobins. Chem Mater. 2007;19:3227. [Google Scholar]
- 124.Bilewicz R. Witomski J. Van Der Heyden A. Tagu D. Palin B. Rogalska E. Modification of electrodes with self-assembled hydrophobin layers. J Phys Chem B. 2001;105:9772. [Google Scholar]
- 125.Corvis Y. Walcarius A. Rink R. Mrabet N.T. Rogalska E. Preparing catalytic surfaces for sensing applications by immobilizing enzymes via hydrophobin layers. Anal Chem. 2005;77:1622. doi: 10.1021/ac048897w. [DOI] [PubMed] [Google Scholar]
- 126.Hou S. Li X. Li X. Feng X. Coating of hydrophobins on three-dimensional electrospun poly(lactic-co-glycolic acid) scaffolds for cell adhesion. Biofabrication. 2009;1:035004. doi: 10.1088/1758-5082/1/3/035004. [DOI] [PubMed] [Google Scholar]
- 127.Solouk A. Cousins B.G. Mirzadeh H. Seifalian A.M. Application of plasma surface modification techniques to improve hemocompatibility of vascular grafts: a review. Biotechnol Appl Biochem. 2011;58:311. doi: 10.1002/bab.50. [DOI] [PubMed] [Google Scholar]
- 128.Siow K.S. Britcher L. Kumar S. Griesser H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—a review. Plasma Proc Polym. 2006;3:392. [Google Scholar]
- 129.Liu H. Li X. Niu X. Zhou G. Li P. Fan Y. Improved hemocompatibility and endothelialization of vascular grafts by covalent immobilization of sulfated silk fibroin on poly(lactic-co-glycolic acid) scaffolds. Biomacromolecules. 2011;12:2914. doi: 10.1021/bm200479f. [DOI] [PubMed] [Google Scholar]
- 130.Wulf K. Teske M. Lo M. Luderer F. Schmitz K-P. Löbler M., et al. Surface functionalization of poly(ɛ-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J Biomed Res Part B Appl Biomater. 2011;98:89. doi: 10.1002/jbm.b.31836. [DOI] [PubMed] [Google Scholar]
- 131.Sin D. Miao X. Liu G. Wei F. Chadwick G. Yan C., et al. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater Sci Eng C. 2010;30:78. [Google Scholar]
- 132.Wu H. Fan J. Chu C.C. Wu J. Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts. J Mater Sci Mater Med. 2010;21:3207. doi: 10.1007/s10856-010-4164-8. [DOI] [PubMed] [Google Scholar]
- 133.Ziabari M. Mottaghitalab V. Haghi A.K. A new approach for optimization of electrospun nanofiber formation process. Korean J Chem Eng. 2010;27:340. [Google Scholar]
- 134.Soliman S. Sant S. Nichol J.W. Khabiry M. Traversa E. Khademhosseini A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J Biomed Mater Res Part A. 2011;96:566. doi: 10.1002/jbm.a.33010. [DOI] [PubMed] [Google Scholar]
- 135.Fridrikh S.V. Yu J.H. Brenner M.P. Rutledge G.C. Controlling the fiber diameter during electrospinning. Phys Rev Lett. 2003;90:144502. doi: 10.1103/PhysRevLett.90.144502. [DOI] [PubMed] [Google Scholar]
- 136.Subbiah T. Bhat G.S. Tock R.W. Parameswaran S. Ramkumar S.S. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96:557. [Google Scholar]
- 137.Lee J.W. Ahn G. Kim J.Y. Cho D.-W. Evaluating cell proliferation based on internal pore size and 3D scaffold architecture fabricated using solid freeform fabrication technology. J Mater Sci Mater Med. 2010;21:3195. doi: 10.1007/s10856-010-4173-7. [DOI] [PubMed] [Google Scholar]
- 138.Zhang A.P. Qu X. Soman P. Hribar K.C. Lee J.W. Chen S., et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater. 2012;24:4266. doi: 10.1002/adma.201202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baudis S. Nehl F. Ligon S.C. Nigisch A. Bergmeister H. Bernhard D., et al. Elastomeric degradable biomaterials by photopolymerization-based CAD-CAM for vascular tissue engineering. Biomed Mater. 2011;6:055003. doi: 10.1088/1748-6041/6/5/055003. [DOI] [PubMed] [Google Scholar]
- 140.Pareta R.A. Reising A.B. Miller T. Storey D. Webster T.J. Increased endothelial cell adhesion on plasma modified nanostructured polymeric and metallic surfaces for vascular stent applications. Biotechnol Bioeng. 2009;103:459. doi: 10.1002/bit.22276. [DOI] [PubMed] [Google Scholar]
- 141.Lu J. Khang D. Webster T.J. Greater endothelial cell responses on submicron and nanometer rough titanium surfaces. J Biomed Mater Res Part A. 2010;94:1042. doi: 10.1002/jbm.a.32778. [DOI] [PubMed] [Google Scholar]
- 142.Desmet T. Morent R. De Geyter N. Leys C. Schacht E. Dubruel P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromolecules. 2009;10:2351. doi: 10.1021/bm900186s. [DOI] [PubMed] [Google Scholar]
- 143.Xu H. Deshmukh R. Timmons R. Nguyen K.T. Enhanced endothelialization on surface modified poly(L-lactic acid) substrates. Tissue Eng Part A. 2011;17:865. doi: 10.1089/ten.tea.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ando J. Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 145.Obi S. Yamamoto K. Shimizu N. Kumagaya S. Masumura T. Sokabe T., et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106:203. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 146.Obi S. Masuda H. Shizuno T. Sato A. Yamamoto K. Ando J., et al. Fluid shear stress induces differentiation of circulating phenotype endothelial progenitor cells. J Appl Physiol. 2012;303:C595. doi: 10.1152/ajpcell.00133.2012. [DOI] [PubMed] [Google Scholar]
- 147.Xiao Q. Kiechl S. Patel S. Oberhollenzer F. Weger S. Mayr A., et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis–results from a large population-based study. PLoS One. 2007;2:9. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Falco E. Porcelli D. Torella A.R. Straino S. Iachininoto M.G. Orlandi A., et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 149.Fong G.H. Rossant J. Gertsenstein M. Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 2002;376:376. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 150.Fong G.H. Klingensmith J. Wood C.R. Rossant J. Breitman M.L. Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn. 1996;207:1. doi: 10.1002/(SICI)1097-0177(199609)207:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 151.Bahlmann F.H. DeGroot K. Duckert T. Niemczyk E. Bahlmann E. Boehm S.M., et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 152.Li A. Dubey S. Varney M.L. Dave B.J. Singh R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 153.Kermani P. Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamamoto K. Takahashi T. Asahara T. Ohura N. Sokabe T. Kamiya A., et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 155.Suzuki Y. Yamamoto K. Ando J. Matsumoto K. Matsuda T. Arterial shear stress augments the differentiation of endothelial progenitor cells adhered to VEGF-bound surfaces. Biochem Biophys Res Commun. 2012;423:91. doi: 10.1016/j.bbrc.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 156.Barsotti M.C. Magera A. Armani C. Chiellini F. Felice F. Dinucci D., et al. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell Prolif. 2011;44:33. doi: 10.1111/j.1365-2184.2010.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ziebart T. Schnell A. Walter C. Kämmerer P.W. Pabst A. Lehmann K.M., et al. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin Oral Investig. 2012;17:301. doi: 10.1007/s00784-012-0691-7. [DOI] [PubMed] [Google Scholar]
- 158.Chai C. Leong K.W. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15:467. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi Z. Neoh K.G. Kang E.T. Poh C.K. Wang W. Enhanced endothelial differentiation of adipose-derived stem cells by substrate nanotopography. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1496. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 160.Yoder M.C. Human endothelial progenitor cells. Cold Spring Harbor Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hagensen M.K. Vanhoutte P.M. Bentzon J.F. Arterial endothelial cells: still the craftsmen of regenerated endothelium. Cardiovasc Res. 2012;95:1. doi: 10.1093/cvr/cvs182. [DOI] [PubMed] [Google Scholar]
- 162.Yoder M.C. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(Suppl 1):49. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]


