Abstract
Poly(lactic-co-glycolic acid) (PLGA) is the most often used synthetic polymer within the field of bone regeneration owing to its biocompatibility and biodegradability. As a consequence, a large number of medical devices comprising PLGA have been approved for clinical use in humans by the American Food and Drug Administration. As compared with the homopolymers of lactic acid poly(lactic acid) and poly(glycolic acid), the co-polymer PLGA is much more versatile with regard to the control over degradation rate. As a material for bone regeneration, the use of PLGA has been extensively studied for application and is included as either scaffolds, coatings, fibers, or micro- and nanospheres to meet various clinical requirements.
Introduction
Bone tissue engineering is an interdisciplinary field that applies the principles of biology and engineering for the development of bone substitutes to restore, maintain, or improve the function of diseased or damaged bone tissues.1 Different approaches have been used in reconstructive surgery to repair bone defects.2,3 Autografting refers to the use of autologous bone. Even though autografting is still the gold standard, significant limitations are associated with autografting such as the need for an extra surgical operation for the harvesting procedure, donor site morbidity at the harvest site, and limited supply of bone.4 Besides, allografting involves harvesting and processing bone from a cadaver and transplantation to the patient. It entails several problems such as rejection or disease transmission.5 As an alternative to tissue-based strategies to heal diseased or damaged bone tissue, synthetic bone substitutes can be implanted such as metals, ceramics and polymers.6 Metals are the material of choice for load-bearing applications due to their favorable mechanical properties, whereas ceramics exhibit excellent biocompatibility as a result of their chemical composition that resembles the mineral phase of bone tissue. However, both metals and ceramics are generaly poorly degradable.
In contrast, biodegradable polymers have been widely investigated and applied to fabricate scaffolds for tissue engineering. They can be classified as natural or synthetic depending on their origin. Natural polymers exhibit several benefits such as degradability and neglectable toxicity. However, a number of advantages are reported for synthetic polymers as compared with natural polymers, including the highly controlled and consistent degradation properties and excellent reproducibility. Among synthetic polymers, aliphatic polyesters such as poly(lactic acid) (PLA), poly(glycolic) acid (PGA), their co-polymer poly(lactic-co-glycolic acid) (PLGA), and poly(caprolactone) (PCL) have received considerable interest as materials for tissue engineering, and several devices comprising PLA, PGA, and PLGA have been approved for clinical use in humans by the American Food and Drug Administration.7 PGA is a hydrophilic, highly crystalline polymer with a relatively fast degradation rate. Although structurally very similar to PGA, PLA exhibits different chemical, physical, and mechanical properties because of the presence of a pendant methyl group on the alpha carbon. Generally, the co-polymer PLGA is preferred compared with its constituent homopolymers PLA and PGA for the fabrication of bone substitute constructs, as PLGA offers superior control compared with degradation properties by varying the ratio between its monomers.1 When the crystalline PGA is co-polymerized with PLA, the degree of crystallinity is reduced and, as a result, this leads to increased rates of hydration and hydrolysis.
In view of its broad application range and wide variety of application forms, the current study has reviewed the use of PLGA as structural component in bone tissue engineering. Therefore, PLGA-based bone substitutes have been categorized according to its application forms, that is, scaffolds, coatings, fibers, or spheres.
Physicochemical Properties of PLGA
Synthesis
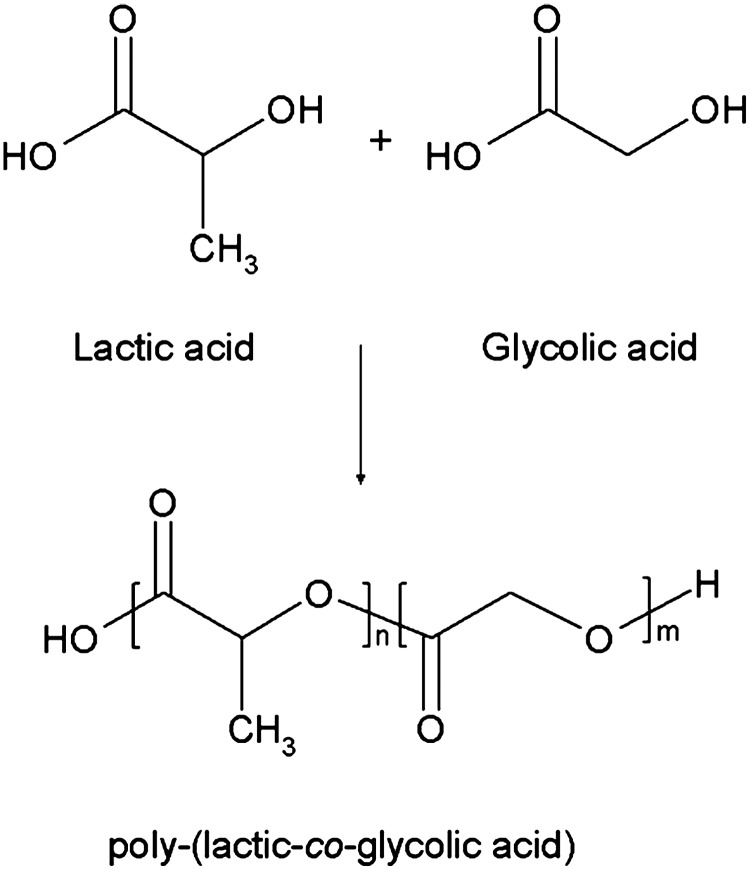
PLGA is a biocompatible and biodegradable linear co-polymer that can be prepared at different ratios between its constituent monomers lactic and glycolic acid (Fig. 1). PLGA of low molecular weight (below 10 kDa) can be obtained by a ring-opening co-polymerization of lactic and glycolic acid. PLGA of higher molecular weights can be synthesized using the same process by using catalysts or using cyclic dimers as a starting material.8 Common catalysts used in the preparation of this co-polymer include tin (II) 2-ethylhexanoate, tin (II) alkoxides, or aluminum isopropoxide. Throughout the polymerization process, successive monomeric units of glycolic or lactic acid are linked together by ester linkages, resulting in a linear, amorphous aliphatic random polyester PLGA.
FIG. 1.
Chemical structure of Poly(lactic-co-glycolic acid) (PLGA) and its monomers.
Ring-opening polymerizations limit the number and types of sequences that can be prepared. Typically, randomly distributed atactic or syndiotactic PLGAs are obtained.9 Recently, the researchers Li, Stayshich, and Meyer developed a new method for the synthesis of repeating sequence PLGA copolymers with different tacticities.10 Using 1,3 diispropylcarbodiimide and 4-(dimethylamino)pyridinium p-toluenesulfonate mediated condensation reactions, PLGAs with a high control over sequence and stereochemistry were synthesized. Nuclear magnetic resonance studies have been performed on the PLGAs, leading to the observation that the conformations of the polymers are sequence-dependent. The same researchers also found that the sequence influences the degradation rate. Sequenced PLGAs proved to degrade slower than random PLGA analogues prepared by ring-opening polymerization. Due to the uniformity of the cleavage sites in the sequenced polymers, a nearly linear molecular weight loss was obtained. Furthermore, the thermal behaviour during hydrolysis was shown to be constant, in contrast to the random PLGA analogues. These findings show the importance of control over sequence in the synthesis of PLGAs. Moreover, these results are promising for drug delivery applications, as this might give rise to stronger control over drug delivery.11
Chemistry
The various forms of PLGA are usually identified by the ratio between the two monomers. The most frequently applied type of PLGA is 50:50, which relates to a co-polymer composed of 50% of lactic 50% of glycolic acid.
The properties of PLGA depend to a large extent on the molar ratio in which lactic acid and glycolic acid are mixed. Lactic acid is more hydrophobic than glycolic acid and is usually the most abundantly present monomer in PLGA. Degradation and drug-release rates are slower for lactic acid-rich PLGA polymers. An exception is the copolymer with an equal (50:50) ratio of lactic acid:glycolic acid, which exhibits the shortest half life and thus the fastest degradation rates.12–14 The presence of a pendant methyl group on the alpha carbon of PLA causes chirality of this molecule, which makes that PLGA is available as d, l, and dl isomers. Even though the role of stereochemistry is crucial for the final properties of the copolymer, details on the exact stereochemistry of PLGA are often lacking in many studies. Generally, PLA can be prepared from two isomers, the optically active l-lactic acid and the optically inactive racemic mixture d l-lactic acid.15 d,l-lactic acid is an amorphous and transparent material with a random distribution of the d and l units. X-ray diffraction studies revealed that d,l-lactic acid is amorphous, whereas l-lactic acid is semi-crystalline. This difference is caused by the irregular distribution of the two stereo-isomeric forms of dl-lactic acid.16,17 In contrast to lactic acid, glycolic acid lacks the methyl side group, making it highly crystalline, but this crystallinity is lost in PLGA copolymers. Gilding and Reed discussed that amorphous PLGA copolymers are preferred for applications in drug delivery, as this provides a more homogeneous dispersion of the active species in the polymer matrix. When using l-lactic acid, PLGA with a glycolic acid percentage of 25%–70% is amorphous. For the amorphous dl-lactic acid, this ratio extends to 0%–70% glycolic acid. Therefore, the use of the dl isomer is preferred compared with l-lactic acid in the composition of PLGA.16
The free carboxyl end-groups of PLGA can be used for chemical modifications to modulate its degradation rate or drug delivery properties. Among a wide range of PLGA modifications, covalent bonding between carboxyl end-group of PLGA with amine groups as present in, for example, the amino-bisphosphonate drug alendronate has been investigated as a local delivery system for treatments of metastatic bone diseases. As a result, covalent amide bonds are formed between the PLGA carrier and amine-containing drugs that allow for a more sustained drug release kinetics.
Degradation
PLGA experiences bulk or heterogeneous erosion in aqueous environments, that is, degradation is not confined to the surface of the device. Four consecutive steps can be discerned during degradation of PLGA18:
(1) Hydration; water penetrates trough the amorphous region of the polymer and disrupts the secondary forces such as van der Waals forces and hydrogen bonding, resulting in a decreased glass transition temperature.
(2) Initial degradation; cleavage of covalent bonds, the molecular weight of the polymer decreases.
(3) Progressive degradation; due to the hydrolytic reaction, carboxylic end groups autocatalyze the degradation process, and mass loss begins by massive cleavage of the backbone covalent bonds, resulting in loss of integrity.
(4) Solubilization; the fragments of the polymer are further cleaved to molecules that are soluble in the aqueous environment. After PLGA degradation, lactic and glycolic acid are formed as end products. Both acids are innocuous, as they are incorporated into the Krebs cycle and excreted in the form of carbon dioxide and water.19
Many factors can affect PLGA degradation, such as (i) molecular weight, (ii) co-polymer composition, (iii) stereochemistry, (iv) end-group functionalization, (v) chemical derivatization, (vi) geometry of the material, and (vii) characteristics of the surrounding medium.20
PLGA chain length is a very important parameter to be considered when selecting the adequate polymer properties for specific applications, as the physical strength and degradation speed of PLGA depend to a large extent on its molecular weight. By increasing the molecular weight of conventional PLGAs from 10–20 to 100 kDa, degradation rates were reported to range from several weeks to several months.20,21
The co-polymer composition (more specifically the ratio between lactic and glycolic acid) is also an important parameter that is used for controlling PLGA degradation. Lactic acid is more hydrophobic than glycolic acid, which makes that lactide-rich PLGA co-polymers are less hydrophilic, absorb less water, and, subsequently, degrade slower.
Moreover, stereochemistry plays a major role in PLGA degradation. Mixtures of d and l lactic acid monomers are most commonly used for PLGA fabrication, as the rate of water penetration is higher in amorphous d,l regions, leading to accelerated PLGA degradation.22
In addition to molecular weight and co-polymer composition, PLGA can undergo several end-terminal modifications (such ester end-capping of its free carboxylic acid end-group) that affect the final physicochemical characteristics considerably. A delay in degradation time of 4 to 6 weeks has been found in vivo for an ester end-capped PLGA in comparison with a more hydrophilic acid-terminated PLGA of a similar molecular weight and co-polymer composition.22
Additional derivatization strategies have been developed to increase the hydrophilicity of PLGA by the introduction of hydrophilic moieties such as hydroxyl groups. These hydroxyl functionalized polymers exhibit a stronger water absorption capacity than their nonfunctionalized counterparts, with, expectedly, increased degradation rates as a result.23,24 Moreover, an increased compatibility to therapeutic proteins was reported for these hydrophilic forms of PLGA, as the acidification that accompanies conventional PLGA degradation is less pronounced for hydrophilic PLGAs. Until now, several functionalized polyesters have been reported containing additional hydroxyl, amino, and carboxylic acid groups.25
Finally, physical parameters such as the geometry of the PLGA device strongly affect PLGA degradation behavior depending on the accessibility of water. In addition, the characteristics of the surrounding media affect the degradation process, that is, an acidic environment will accelerate PLGA degradation due to autocatalysis.26 Differences between the morphological characteristics of medical devices comprising PLGA such as particle size or scaffold porosity were shown to influence the process of autocatalysis of the PLGA.27 Generally, the effect of acidic degradation by-products on autocatalysis become more pronounced with decreasing area of diffusion of these products.28 With regard to tissue engineering, porosity of scaffolds greatly determines this diffusion area. For instance, porosity is known to control the flow rate of nutrients and metabolic products throughout the scaffolds, thereby facilitating the process of local vascularization, which is indispensable for tissue growth. Porosity, in turn, strongly affects the mechanical strength of scaffolds, which by itself is also influenced by the addition of surfactants such as Tween 20, polyethylene glycol, polyvinyl alcohol, or glycerol, thereby facilitating absorption of water within the PLGA matrix and increasing the degradation rate.29 This complex interlay between autocatalysis, morphology (e.g., porosity or particle size) and corresponding mechanical strength, plasticisation, and physicochemical properties of PLGA finally determine the ultimate performance of biomedical devices containing PLGA. Therefore, extensive research efforts continuously focus on identifying the optimal balance between the factors mentioned earlier. Nevertheless, this optimization process is complicated even further, as the variation in experimental conditions in terms of, for example, chemical characteristics (i.e., types of PLGA), and comparisons between these conditions can often hardly be made. Moreover, PLGA is most often used in combination other ceramic or polymeric materials that interact with PLGA, thereby enhancing the difficulty to draw unambiguous conclusions on the specific effects of physicochemical characteristics of PLGA on ultimate performance in bone regeneration. In this regard, it is important to notice that relevant information on, for example, polymer molecular weight,30–35 stereochemistry,31–34 or end-group functionalization,36–53 are often missing, which complicates scientific progress even further.
Application Forms of PLGA
To meet various clinical requirements, PLGA has been used in a wide variety of application forms such as scaffolds, coatings, fibers, or micro- and nanospheres. These application forms will be discussed in the next few sections.
Scaffolds consisting of pure PLGA
A scaffold is a support structure for cell growth that is composed of biocompatible materials. Porous PLGA-based scaffolds have been widely investigated in the field of bone tissue engineering.54 PLGA scaffolds can be processed into 3D porous structures (see Fig. 2) that can be designed to fit bone defects. Several processing techniques for fabrication of porous PLGA scaffolds have been developed over the past three decades.55 The most commonly used techniques to prepare PLGA scaffolds are solvent-casting particulate leaching,56–60 phase inversion particulate leaching,61 fiber bonding,62–64 gas foaming,65–68 emulsion freeze drying,69,70 and rapid prototyping.71 For applications in bone tissue engineering, PLGA scaffolds should promote cell adhesion and stimulate bone tissue ingrowth. However, despite being biocompatible, clinical application of pure PLGA scaffolds for bone regeneration is hampered by poor osteoconductivity and inflammatory responses that originate from the production of acidic by-products on PLGA degradation.72,73 Moreover, PLGA exhibits suboptimal mechanical properties for use as load-bearing applications. Besides pure PLGA-based scaffolds, PLGA is often used in combination with other materials such as polymers (section PLGA-polymer scaffolds) or ceramics (section PLGA-ceramic scaffolds) in an attempt to render PLGA scaffolds biocompatible or to modify poly(d,l-lactic-co-glycolic acid) degradation rates.74
FIG. 2.
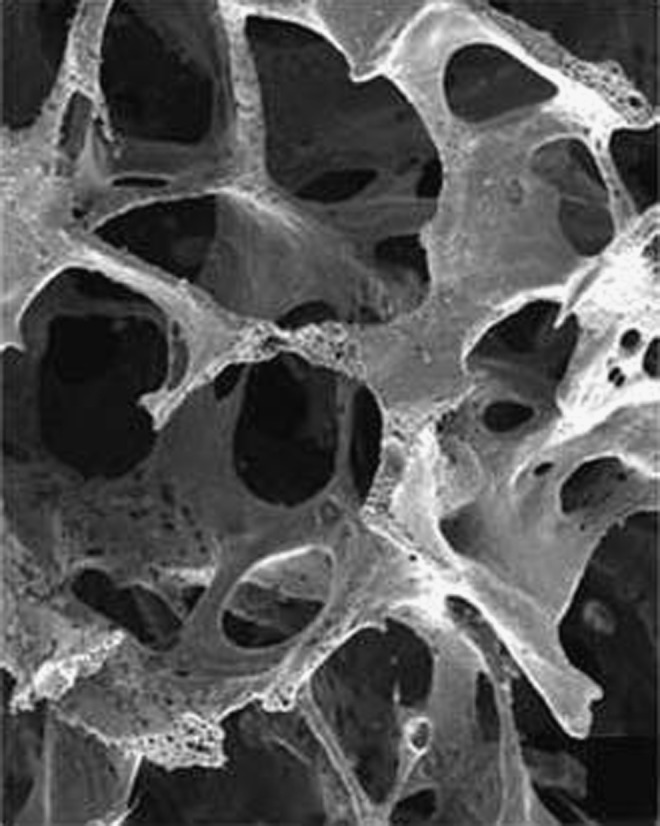
Scanning electron microscope (SEM) picture of a porous PLGA scaffold (Osteofoam™) obtained by phase inversion/particulate leaching. Reprinted from Ref. 19 with permission. Copyright 2012, John Wiley and Sons.
PLGA-polymer scaffolds
PLGA scaffolds have been developed that also contain natural or synthetic polymers to overcome the limited osteoconductivity of pure PLGA scaffolds. Natural polymers contain motifs such as RGD (Arginine-Glycine-Aspartic acid) peptide sequences, which can modulate cell adhesion improving the cellular behavior compared with other polymers that lack these cell-recognition sites. PLGA has been used in combination with several natural polymers such as collagen, chitosan, or gelatin. Collagen is an attractive natural polymer for tissue regeneration due to its excellent biocompatibility, biodegradability, and negligible immunogenicity.75,76 It represents about 90% of the proteins in the bone extracellular matrix and has been widely investigated within the field of bone tissue engineering. Since PLGA does not promote cell adhesion and proliferation due to its hydrophobic nature,77 the combination of PLGA with collagen was shown to improve the bone tissue response while maintaining the desired shape in vivo due to the mechanical strength of PLGA.78 Chitosan, a co-polymer of glucosamine and N-acetylglucosamine, is frequently used in bone tissue engineering.79 Due to its positive charge, chitosan can be used for binding and subsequent delivery of (preferably negatively charged) growth factors. The addition of chitosan to poly(d,l-lactic-co-glycolic acid) scaffolds was reported to enhance osteoblast differentiation.30 In addition, PLGA can be used in combination with gelatin, which is produced by partial hydrolysis of collagen. Gelatin is widely employed in bone tissue engineering due to its beneficial biocompatibility, controllable degradation, and strong potential for functionalization.80,81
The combination of synthetic polymers such as PCL with PLGA has also been reported for bone tissue engineering purposes in order to fabricate scaffolds with an optimal balance between porosity and mechanical strength. To this end, PLGA can be used to create a porous scaffold, while PCL is used as reinforcing material to enhance the mechanical strength of the scaffold. For instance, PLGA tubular scaffolds with a PCL nanofiber spiral structured inner core were developed that displayed improved mechanical properties.31 Pure PCL, however, does not improve the biocompatibility when compared with pure poly(d,l-lactic-co-glycolic acid), and, therefore, its combined application is limited apart from its use when a delayed degradation of the implant material is desired.82
PLGA-ceramic scaffolds
Bioceramic/polymer composites are being increasingly used in the bone regeneration field, because the complementary properties of PLGA and ceramics offer new possibilities in terms of biocompatibility and mechanical strength. Both calcium phosphate ceramics and bioactive glasses have been applied in combination with PLGA scaffolds. Ceramics or glasses can be used in combination with PLGA as a (i) reinforcing material to improve the mechanical strength of the scaffold or as a (ii) coating to improve the bone tissue response toward PLGA and to stimulate bone apposition onto the scaffold.
To improve the mechanical strength of PLGA, calcium phosphates in the form of hydroxyapatite (HA) needles or nanoparticles have been used as reinforcing material for poly(d,l-lactic-co-glycolic acid) matrices to improve the osteoconductive properties of the resulting composite.83,84 As ceramic coating, apatite has been applied onto PLGA scaffolds, enhancing their osteoconductive properties using wet-chemical deposition techniques.85 Several studies describe the use of biomimetic processes to produce apatite-coated PLGA constructs for bone tissue engineering by incubation of polymeric scaffolds in simulated body fluid (SBF) to obtain a mineralized scaffold.32–34,86 The resulting coatings were shown to enhance the osteoconductive properties of PLGA. However, this procedure has drawbacks related to the immersion of the PLGA scaffold in SBF, as the scaffold can undergo hydrolytic degradation on soaking in aqueous solutions. In addition, coating thicknesses can differ in the inner and outer parts of the scaffold due to limited fluid flow within the porous scaffold. Therefore, uniformly coated porous PLGA scaffolds have been produced by using a system that is designed to induce SBF flow throughout the pores of the scaffold.87 In parallel, PLGA scaffolds with apatite-coated pores have been developed with an alternative method to avoid immersion in SBF. This method involves inclusion of apatite-coated paraffin spheres into the PLGA followed by dissolution of the paraffin spheres.35
The basic components of bioactive glass are SiO2, Na2O, CaO, and P2O5. After implantation, the outer surface of bioactive glass forms an amorphous layer of calcium phosphate that binds to proteins, collagen, fibrin, and growth factors. Bioactive glass has been used in combination with PLGA as films, microspheres, or fibers, creating constructs that provide a good attachment and growth of osteoprogenitor cells.88–91 Moreover, bioactive glass microspheres have also been included in PLGA matrices for delivery of antibiotics.92
Coatings
A coating can be defined as a layer of a substance spread over a surface. In the field of bone tissue engineering, coatings are applied to improve the interaction between cells and the surface of biomaterials. PLGA coatings have been applied onto ceramics, metals, natural polymers, and bone allografts.
Despite the fact that bioceramics have a similar composition to natural bone, PLGA has been studied within the field of bone tissue regeneration as a coating material to improve the surface properties of several bioceramic materials. A dual porous HA/tricalcium phosphate (TCP) construct has been coated with PLGA to overcome the drawbacks of the biphasic ceramics and the polymer, that is, the low compressive strength of PLGA and the brittleness of HA/TCP ceramics (Fig. 3). These PLGA-coated HA/TCP scaffolds exhibited a higher compressive strength and compressive modulus than did bare HA/TCP scaffolds36
FIG. 3.
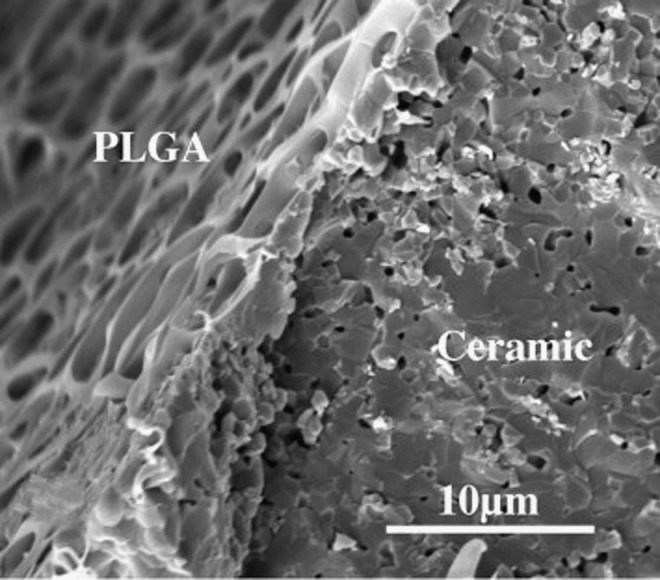
SEM image of a ceramic scaffold covered by a PLGA coating. Adapted from Ref. 66 with permission. Copyright 2012, Elsevier.
Patterned PLGA coatings have been developed to modify the surface morphology of titanium composites to improve cell-material interactions, thereby increasing osteoblast proliferation and adhesion.37,38
The addition of a PLGA coating to a chitosan scaffold resulted in a more controlled degradation process. Still, a decreased osteoblast proliferation was observed in vitro when compared with apatite-coated chitosan. Nevertheless, PLGA-coated chitosan scaffolds were shown to enhance bone formation in comparison to plain chitosan scaffolds after implantation in a rat calvarial defect.39,40
PLGA coatings have been applied even into cortical bone allografts in an attempt to maintain bone structural architecture immediately after implantation. Despite the biocompatibility of bone allografts, an improved subsequent host bone response was reported.41
Fibers
Though less popular than PLGA scaffolds, electrospun PLGA fibers are increasingly studied for bone tissue engineering purposes. Polymer fibers are used for several applications in bone tissue engineering. Commonly, they are either used for scaffold fabrication by the fiber bonding technique or as the dispersed phase when combined with other materials.
Electrospinning is a widely used method to prepare polymer fibers. It uses an electrical charge to draw very fine fibers (typically at micro- or nanoscale) from a polymer solution. Electrospinning shares characteristics of both electrospraying and conventional solution dry spinning of fibers. During this process, high temperatures are not needed to fabricate solid threads from precursor solutions. This procedure is particularly suitable for the production of fibers using large and complex molecules such as enzymes. Depending on the composition of the initial PLGA solution, micro- or nanofibers can be obtained by electrospinning. Fibers exhibit a very high surface-to-volume ratio and a relatively smooth structure at the molecular level. Similar to other types of PLGA structures, fibers exhibit a controllable degradability, biocompatibility, and favorable mechanical strength.
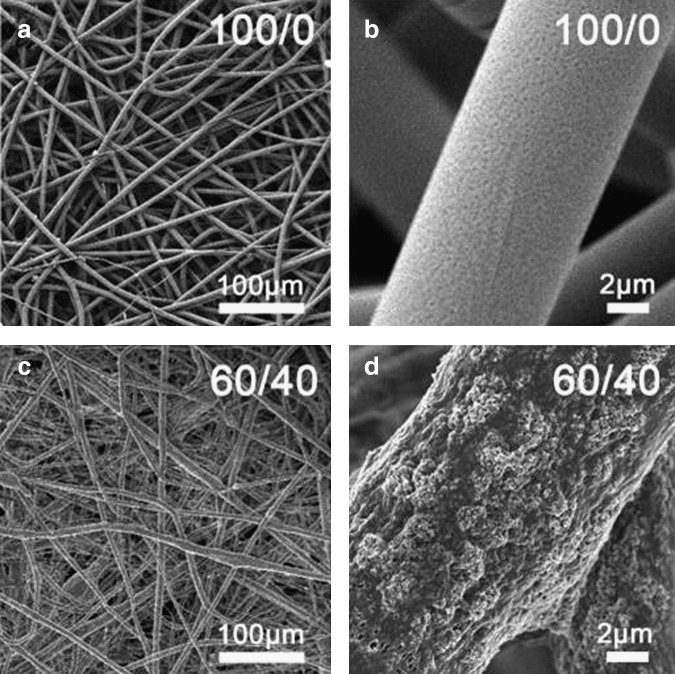
PLGA fibers have been employed for the fabrication of composites with bioceramics in order to reinforce calcium phosphate cements.42 Promising results were shown for electrospun PLGA fibers when used in combination with calcium phosphate nanoparticles for enhanced bone tissue formation43–45 and hydroxyapatite nanoparticles to improve the mechanical properties46,47 (Fig. 4).
FIG. 4.
Scanning electron microscopy images of electrospun scaffolds: Pure PLGA (a, b) and PLGA containing 60% of amorphous tricalcium phosphate (c, d). Reprinted from Ref. 75 with permission. Copyright 2012, John Wiley and Sons.
Similar to PLGA scaffolds, PLGA electrospun fibers can also be used in bone tissue engineering in blends with natural polymers such as elastin, gelatin, and collagen,48–50 as well as in combination with synthetic materials such as poly (3-hydroxybutyrate-co-3-hydroxyvalerate)51 or diamond nanoparticles52 to form composite fibers of improved functionality.
PLGA spheres
PLGA spheres can be classified into nanospheres or microspheres depending on their diameter. Several methods such as water/oil/water emulsion, oil/water emulsion, coacervation, or spray drying are used to fabricate PLGA microspheres. During fabrication, the sphere size can be optimized by varying parameters such as solvent type, surfactant, viscosity emulsification speeds, emulsification time, polymer concentration, and/or by sieving. Novel approaches have been developed over the past decades to achieve PLGA spheres with a more narrow size distribution and consequently controlled porosity (when used as porogen) or geometry of scaffolds fabricated by microsphere fusion. Berkland et al. fabricated PLGA spheres with controlled particle size by spraying the polymer solution through a nozzle with acoustic excitation to produce droplets and an annular, nonsolvent carrier stream to allow further control of the particle size.93 Microspheres with controlled diameters from 5 to 500 μm were produced by this method. PLGA spheres smaller than 10 μm were produced by laser ablation (Fig. 5) in which an ultra-short pulse of a laser beam strikes periodically into a liquid jet to create highly monodisperse microspheres.94 Porous membranes are also employed for controlled fabrication of monodisperse PLGA spheres ranging in diameter from 1 to 100 μm. Using this method, the polymer solution is permeated through the uniform pores of a membrane into an aqueous solution containing an emulsifier, allowing excellent control over particle diameter and polydispersity.95,96
FIG. 5.
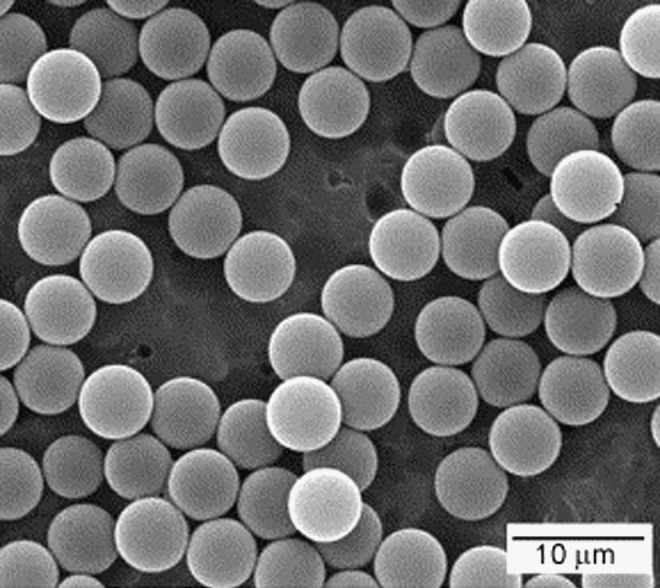
Representative SEM image of PLGA microspheres fabricated by the laser ablation technique. Adapted from Ref. 85 with permission. Copyright 2012, Elsevier.
PLGA microspheres are used as the main component of sphere-based scaffolds, as porogens for scaffolds, or as a drug delivery vehicle. Both PLGA fibers and PLGA spheres can be sintered by melting the PLGA at temperatures between 170°C and 230°C depending on their initial chemical composition to create scaffolds for bone regeneration.97,98 These sphere-based scaffolds can be coated or reinforced with calcium phosphate to enhance the osteoconductive properties of the construct.99–101 Moreover, PLGA spheres have been combined with apatitic nanoparticles102,85 to reduce fibrous encapsulation of PLGA, promoting new bone ingrowth while enhancing direct bone contact.103–111
Porosity can be created in tissue-engineered scaffolds by the incorporation of PLGA spheres, as a porous scaffold is gradually formed on degradation of PLGA spheres. Degradation of injectable calcium phosphate cements has been accelerated considerably by the incorporation of PLGA microspheres into the injectable paste. After PLGA degradation, a porous calcium phosphate cement scaffold was formed, which undergoes accelerated degradation due to acid production by PLGA porogens, thereby allowing for enhanced bone tissue formation within the scaffold112–114 (Fig. 6).
FIG. 6.
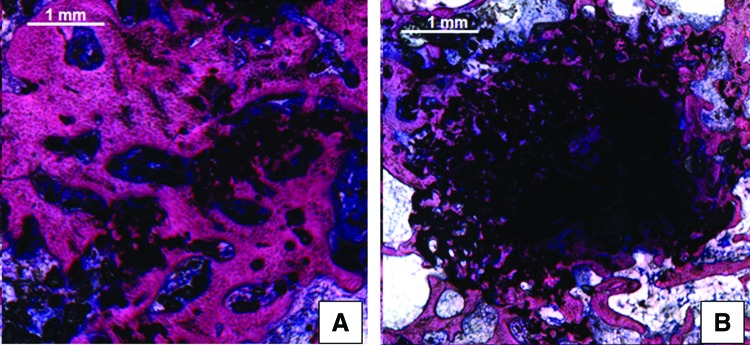
Representative histological sections of calcium phosphate cement containing (A) acid-terminated or (B) end-capped PLGA microspheres after 12 weeks of implantation in a rabbit femoral condyle defect. Accelerated material degradation and bone tissue ingrowth can be observed when acid-terminated PLGA microspheres are used. Adapted from Ref. 105 with permission. Copyright 2012, Elsevier. Color images available online at www.liebertpub.com
Finally, oppositely charged PLGA nanospheres can be employed for the fabrication of cohesive colloidal gels, which can be applied as injectable drug-loaded filler to promote healing in bone defects.115 To this end, PLGA nanoparticles were derivatized to increase their electrical charge, and colloidal gels were formed by mixing different ratios of positively and negatively charged PLGA particles.
Conclusions
The potential of PLGA for bone tissue engineering has been explored for several decades due to its biological safety and tunable degradation properties. The current study has reviewed the use of PLGA for bone regeneration by categorizing PLGA according to its application form, that is, scaffolds, coatings, fibers, or microspheres.
Acknowledgments
The authors gratefully acknowledge the support of the SmartMix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture, and Science.
Disclosure Statement
No competing financial interests exist.
References
- 1.Porter J.R. Ruckh T.T. Popat K.C. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 2.Ferrone M.L. Raut C.P. Modern surgical therapy: limb salvage and the role of amputation for extremity soft-tissue sarcomas. Surg Oncol Clin N Am. 2012;21:201. doi: 10.1016/j.soc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Martou G. Antonyshyn O.M. Advances in surgical approaches to the upper facial skeleton. Curr Opin Otolaryngol Head Neck Surg. 2011;19:242. doi: 10.1097/MOO.0b013e328347f895. [DOI] [PubMed] [Google Scholar]
- 4.Dimitriou R. Jones E. McGonagle D. Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;31:9. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman J.A. Greenwald M.A. Grossi P.A. Transmission of infection with human allografts: essential considerations in donor screening. Clin Infect Dis. 2012;55:720. doi: 10.1093/cid/cis519. [DOI] [PubMed] [Google Scholar]
- 6.Cabraja M. Kroppenstedt S. Bone grafting and substitutes in spine surgery. J Neurosurg Sci. 2012;56:87. [PubMed] [Google Scholar]
- 7.Hammouche S. Hammouche D. McNicholas M. Biodegradable bone regeneration synthetic scaffolds: in tissue engineering. Curr Stem Cell Res Ther. 2012;7:134. doi: 10.2174/157488812799219018. [DOI] [PubMed] [Google Scholar]
- 8.Lü J.M. Wang X. Marin-Muller C. Wang H. Lin P.H. Yao Q. Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dechy-Cabaret O. Martin-Vaca B. Bourissou D. Controlled ring-opening polymerization of lactide and glycolide. Chem Rev. 2004;104:6147. doi: 10.1021/cr040002s. [DOI] [PubMed] [Google Scholar]
- 10.Li J. Stayshich R.M. Meyer T.Y. Exploiting sequence to control the hydrolysis behavior of biodegradable PLGA copolymers. J Am Chem Soc. 2011;133:6910. doi: 10.1021/ja200895s. [DOI] [PubMed] [Google Scholar]
- 11.Stayshich R.M. Meyer T.Y. New insights into poly(lactic-co-glycolic acid) microstructure: using repeating sequence copolymers to decipher complex NMR and thermal behavior. J Am Chem Soc. 2010;132:10920. doi: 10.1021/ja102670n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S. Alonso M.J. Langer R. Novel approaches to controlled-release antigen delivery. Int J Technol Assess Health Care. 1994;10:121. doi: 10.1017/s0266462300014045. [DOI] [PubMed] [Google Scholar]
- 13.Miller R.A. Brady J.M. Cutright D.E. Degradation rates of oral resorbable implants (Polylactates and Polyglycolates): rate modification with changes in PLA/PGA copolymer ratios. J Biomed Mater Res. 1977;11:711. doi: 10.1002/jbm.820110507. [DOI] [PubMed] [Google Scholar]
- 14.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym Int. 2005;54:36. [Google Scholar]
- 15.Jalil R. Nixon J.R. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J Microencapsulation. 1990;7:297. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
- 16.Gilding D.K. Reed A.M. Biodegradable polymers for use in surgery polyglycolic/poly(lactic acid) homo- and copolymers:1. Polymer. 1979;20:1459. [Google Scholar]
- 17.Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 18.Wu X.S. Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J Biomater Sci Polym Ed. 2001;2:21. doi: 10.1163/156856201744425. [DOI] [PubMed] [Google Scholar]
- 19.Van Blintterswijk C. London, UK: Elsevier; 2008. Tissue Engineering. [Google Scholar]
- 20.Yoshioka T. Kawazoe N. Tateishi T. Chen G. In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials. 2008;29:3438. doi: 10.1016/j.biomaterials.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Félix Lanao R.P. Leeuwenburgh S.C. Wolke J.G. Jansen J.A. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011;7:3459. doi: 10.1016/j.actbio.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Wise D.L. Tratolo D.T. Lewandrowski K. Gresser J.D. Cattaneo M.V. Yaszemski M.J. Totow, NJ: Humana Press; 2000. Biomaterials engineering and devices, human applications. [Google Scholar]
- 23.Leemhuis M. van Nostrum F. Kruijtzer J.A.W. Zhong Z.Y. Breteler ten M.R. Dijkstra P.J. Feijen J. Hennink W.E. Functionalized poly(α-hydroxy acid)s via ring-opening polymerization: toward hydrophilic polyesters with pendant hydroxyl groups. Macromolecules. 2006;39:3500. [Google Scholar]
- 24.Leemhuis M. Kruijtzer J.A. Nostrum C.F. Hennink W.E. In vitro hydrolytic degradation of hydroxyl-functionalized poly(alpha-hydroxy acid)s. Biomacromolecules. 2007;8:2943. doi: 10.1021/bm700476h. [DOI] [PubMed] [Google Scholar]
- 25.Ghassemi A.H. van Steenbergen M.J. Talsma H. van Nostrum C.F. Jiskoot W. Crommelin D.J. Hennink W.E. Preparation and characterization of protein loaded microspheres based on a hydroxylated aliphatic polyester, poly(lactic-co-hydroxymethyl glycolic acid) J Control Release. 2009;138:57. doi: 10.1016/j.jconrel.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Pamula E. Menaszek E. In vitro and in vivo degradation of poly(lactide-co-glycolide) films and scaffolds. J Mater Sci Mater Med. 2008;19:2063. doi: 10.1007/s10856-007-3292-2. [DOI] [PubMed] [Google Scholar]
- 27.Barbanti S.H. Santos A.R. Zavaglia C.A.C. Duek E.A.R. Porous and dense poly(l-lactic acid) and poly(d,l-lactic acid-co-glycolic acid) scaffolds: in vitro degradation in culture medium and osteoblasts culture. J Mater Sci Mater Med. 2004;14:1315. doi: 10.1007/s10856-004-5740-6. [DOI] [PubMed] [Google Scholar]
- 28.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res. 1999;48:342. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed F. van der Walle C.F. PLGA microcapsules with novel dimpled surfaces for pulmonary delivery of DNA. Int J Pharm. 2006;27:97. doi: 10.1016/j.ijpharm.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y.C. Shaw S.Y. Lin H.R. Lee T.M. Yang C.Y. Bone tissue engineering evaluation based on rat calvaria stromal cells cultured on modified PLGA scaffolds. Biomaterials. 2006;27:896. doi: 10.1016/j.biomaterials.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang J. Yu X. Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering. Acta Biomater. 2010;6:3004. doi: 10.1016/j.actbio.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Segvich S. Smith H.C. Luong L.N. Kohn D.H. Uniform deposition of protein incorporated mineral layer on three-dimensional porous polymer scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84:340. doi: 10.1002/jbm.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy W.L. Kohn D.H. Mooney D.J. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50:50. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Chou Y.F. Dunn J.C. Wu B.M. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75:81. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- 35.Yu S. Hariram K.P. Kumar R. Cheang P. Aik K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials. 2005;26:2343. doi: 10.1016/j.biomaterials.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Miao X. Tan D.M. Li J. Xiao Y. Crawford R. Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly(lactic-co-glycolic acid) Acta Biomater. 2008;4:638. doi: 10.1016/j.actbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Liu H. Slamovich E.B. Webster T.J. Increased osteoblast functions among nanophase titania/poly(lactide-co-glycolide) composites of the highest nanometer surface roughness. J Biomed Mater Res A. 2006;78:798. doi: 10.1002/jbm.a.30734. [DOI] [PubMed] [Google Scholar]
- 38.Sato M. Slamovich E.B. Webster T.J. Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol-gel titanium coatings. Biomaterials. 2005;26:1349. doi: 10.1016/j.biomaterials.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 39.Yokota S. Sonohara S. Yoshida M. Murai M. Shimokawa S. Fujimoto R. Fukushima S. Kokubo S. Nozaki K. Takahashi K. Uchida T. Yokohama S. Sonobe T. A new recombinant human bone morphogenetic protein-2 carrier for bone regeneration. Int J Pharm. 2001;223:69. doi: 10.1016/s0378-5173(01)00728-1. [DOI] [PubMed] [Google Scholar]
- 40.Jung U.W. Song K.Y. Kim C.S. Lee Y.K. Cho K.S. Kim C.K. Choi S.H. Effects of a chitosan membrane coated with polylactic and polyglycolic acid on bone regeneration in a rat calvarial defect. Biomed Mater. 2007;2:101. doi: 10.1088/1748-6041/2/3/S03. [DOI] [PubMed] [Google Scholar]
- 41.Lewandrowski K.U. Bondre S.P. Gresser J.D. Wise D.L. Tomford W.W. Trantolo D.J. Improved osteoconduction of cortical bone grafts by biodegradable foam coating. Biomed Mater Eng. 1999;9:265. [PubMed] [Google Scholar]
- 42.Dagang G. Haoliang S. Kewei X. Yong H. Long-term variations in mechanical properties and in vivo degradability of CPC/PLGA composite. J Biomed Mater Res B Appl Biomater. 2007;82:533. doi: 10.1002/jbm.b.30759. [DOI] [PubMed] [Google Scholar]
- 43.Schneider O.D. Weber F. Brunner T.J. Loher S. Ehrbar M. Schmidlin P.R. Stark W.J. In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 2009;5:1775. doi: 10.1016/j.actbio.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 44.Schneider O.D. Loher S. Brunner T.J. Uebersax L. Simonet M. Grass R.N. Merkle H.P. Stark W.J. Cotton wool-like nanocomposite biomaterials prepared by electrospinning: in vitro bioactivity and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2008;84:350. doi: 10.1002/jbm.b.30878. [DOI] [PubMed] [Google Scholar]
- 45.Wang C. Wang M. Dual-source dual-power electrospinning and characteristics of multifunctional scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2012;23:2381. doi: 10.1007/s10856-012-4669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jose M.V. Thomas V. Johnson K.T. Dean D.R. Nyairo E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009;5:305. doi: 10.1016/j.actbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Lao L. Wang Y. Zhu Y. Zhang Y. Gao C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J Mater Sci Mater Med. 2011;22:1873. doi: 10.1007/s10856-011-4374-8. [DOI] [PubMed] [Google Scholar]
- 48.Mondrinos M.J. Chen X. Lelkes P.I. Electrospun blends of natural and synthetic polymers as scaffolds for tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2005;6:5858. doi: 10.1109/IEMBS.2005.1615822. [DOI] [PubMed] [Google Scholar]
- 49.Li M. Mondrinos M.J. Chen X. Gandhi M.R. Ko F.K. Lelkes P.I. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79:963. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 50.Ravichandran R. Ng C.C. Liao S. Pliszka D. Raghunath M. Ramakrishna S. Chan C.K. Biomimetic surface modification of titanium surfaces for early cell capture by advanced electrospinning. Biomed Mater. 2012;7:1. doi: 10.1088/1748-6041/7/1/015001. [DOI] [PubMed] [Google Scholar]
- 51.Ndreu A. Nikkola L. Ylikauppila H. Ashammakhi N. Hasirci V. Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomed. 2008;3:45. doi: 10.2217/17435889.3.1.45. [DOI] [PubMed] [Google Scholar]
- 52.Parizek M. Douglas T.E. Novotna K. Kromka A. Brady M.A. Renzing A. Voss E. Jarosova M. Palatinus L. Tesarek P. Ryparova P. Lisa V. Dos Santos A.M. Bacakova L. Nanofibrous poly(lactide-co-glycolide) membranes loaded with diamond nanoparticles as promising substrates for bone tissue engineering. Int J Nanomedicine. 2012;7:1931. doi: 10.2147/IJN.S26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber F.E. Eyrich G. Gratz K.W. Maly F.E. Sailer H.F. Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int J Oral Maxillofac Surg. 2002;31:60. doi: 10.1054/ijom.2001.0154. [DOI] [PubMed] [Google Scholar]
- 54.Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 55.Liu X. Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 56.Douglas T. Pamula E. Hauk D. Wiltfang J. Sivananthan S. Sherry E. Warnke P.H. Porous polymer/hydroxyapatite scaffolds: characterization and biocompatibility investigations. J Mater Sci Mater Med. 2009;20:1909. doi: 10.1007/s10856-009-3756-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P. Hong Z. Yu T. Chen X. Jing X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide) Biomaterials. 2009;30:58. doi: 10.1016/j.biomaterials.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 58.Yoon S.J. Park K.S. Kim M.S. Rhee J.M. Khang G. Lee H.B. Repair of diaphyseal bone defects with calcitriol-loaded PLGA scaffolds and marrow stromal cells. Tissue Eng. 2007;13:1125. doi: 10.1089/ten.2006.0287. [DOI] [PubMed] [Google Scholar]
- 59.Widmer M.S. Gupta P.K. Lu L. Meszlenyi R.K. Evans G.R. Brandt K. Savel T. Gurlek A. Patrick C.W., Jr. Mikos A.G. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19:1945. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 60.Thomson R.C. Mikos A.G. Beahm E. Lemon J.C. Satterfield W.C. Aufdemorte T.B. Miller M.J. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials. 1999;20:2007. doi: 10.1016/s0142-9612(99)00103-9. [DOI] [PubMed] [Google Scholar]
- 61.Ellis M.J. Chaudhuri J.B. Human bone derived cell culture on PLGA flat sheet membranes of different lactide:glycolide ratio. Biotechnol Bioeng. 2008;101:369. doi: 10.1002/bit.21902. [DOI] [PubMed] [Google Scholar]
- 62.Xu C.Y. Inai R. Kotaki M. Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 63.Yang F. Murugan R. Wang S. Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 64.Yin Z. Chen X. Chen J.L. Shen W.L. Hieu Nguyen T.M. Gao L. Ouyang H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 65.Kim S.S. Park M. Jeon O. Yong Choi C. Kim B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Murphy W.L. Peters M.C. Kohn D.H. Mooney D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 67.Yoon J.J. Song S.H. Lee D.S. Park T.G. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25:5613. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Kim S.S. Ahn K.M. Park M.S. Lee J.H. Choi C.Y. Kim B.S. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A. 2007;50:206. doi: 10.1002/jbm.a.30836. [DOI] [PubMed] [Google Scholar]
- 69.Whang K. Goldstick T.K. Healy K.E. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21:2545. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 70.Whang K. Healy K.E. Elenz D.R. Nam E.K. Tsai D.C. Thomas C.H. Nuber G.W. Glorieux F.H. Travers R. Sprague S.M. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5:35. doi: 10.1089/ten.1999.5.35. [DOI] [PubMed] [Google Scholar]
- 71.Ge Z. Tian X. Heng B.C. Fan V. Yeo J.F. Cao T. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed Mater. 2009;4:21001. doi: 10.1088/1748-6041/4/2/021001. [DOI] [PubMed] [Google Scholar]
- 72.Kitsugi T. Yamamuro T. Nakamura T. Kokubo T. The bonding of glass ceramics to bone. Int Orthop. 1989;13:199. doi: 10.1007/BF00268048. [DOI] [PubMed] [Google Scholar]
- 73.Kitsugi T. Yamamuro T. Nakamura T. Kokubo T. Bone bonding behavior of MgO-CaO-SiO2-P2O5-CaF2 glass (mother glass of A.W-glass-ceramics) J Biomed Mater Res. 1989;23:631. doi: 10.1002/jbm.820230607. [DOI] [PubMed] [Google Scholar]
- 74.Ochi K. Chen G. Ushida T. Gojo S. Segawa K. Tai H. Ueno K. Ohkawa H. Mori T. Yamaguchi A. Toyama Y. Hata J. Umezawa A. Use of isolated mature osteoblasts in abundance acts as desired-shaped bone regeneration in combination with a modified poly-DL-lactic-co-glycolic acid (PLGA)-collagen sponge. J Cell Physiol. 2003;194:45. doi: 10.1002/jcp.10185. [DOI] [PubMed] [Google Scholar]
- 75.Lee C.H. Singla A. Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 76.Li S.T. Biologic biomaterials: tissue-derived biomaterials (collagen) In: Park J.B., editor; Bronzino J.D., editor. Biomaterials: Principles and Applications. Boca Roton, FL: CRC Press; 2003. [Google Scholar]
- 77.Lee S.J. Khang G. Lee Y.M. Lee H.B. Interaction of human chondrocytes and NIH/3T3 fibroblasts on chloric acid-treated biodegradable polymer surfaces. J Biomater Sci Polym Ed. 2002;13:197. doi: 10.1163/156856202317414375. [DOI] [PubMed] [Google Scholar]
- 78.Liu H.W. Chen C.H. Tsai C.L. Hsiue G.H. Targeted delivery system for juxtacrine signaling growth factor based on rhBMP-2-mediated carrier-protein conjugation. Bone. 2006;39:825. doi: 10.1016/j.bone.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 79.Shi S. Cheng X. Wang J. Zhang W. Peng L. Zhang Y. RhBMP-2 microspheres-loaded chitosan/collagen scaffold enhanced osseointegration: an experiment in dog. J Biomater Appl. 2009;23:331. doi: 10.1177/0885328208090013. [DOI] [PubMed] [Google Scholar]
- 80.Young S. Wong M. Tabata Y. Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 81.Schrieber R. Gareis H. Weinheim: Wiley-VCH Verlag; 2007. Gelatine Handbook: Theory and Industrial Practice. [Google Scholar]
- 82.Barbanti S.H. Santos A.R., Jr. Zavaglia C.A. Duek E.A. Poly(ɛ-caprolactone) and poly(D,L-lactic acid-co-glycolic acid) scaffolds used in bone tissue engineering prepared by melt compression-particulate leaching method. J Mater Sci Mater Med. 2011;22:2377. doi: 10.1007/s10856-011-4398-0. [DOI] [PubMed] [Google Scholar]
- 83.Thomson R.C. Yaszemski M.J. Powers J.M. Mikos A.G. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19:1935. doi: 10.1016/s0142-9612(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 84.Wilberforce S.I. Finlayson C.E. Best S.M. Cameron R.E. The influence of the compounding process and testing conditions on the compressive mechanical properties of poly(D,L-lactide-co-glycolide)/α-tricalcium phosphate nanocomposites. J Mech Behav Biomed Mater. 2011;4:1081. doi: 10.1016/j.jmbbm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Kang S.W. Yang H.S. Seo S.W. Han D.K. Kim B.S. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res A. 2008;85:747. doi: 10.1002/jbm.a.31572. [DOI] [PubMed] [Google Scholar]
- 86.Auclair-Daigle C. Bureau M.N. Legoux J.G. Yahia L. Bioactive hydroxyapatite coatings on polymer composites for orthopedic implants. J Biomed Mater Res A. 2005;73:398. doi: 10.1002/jbm.a.30284. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y. Mak A.F. Li J. Wang M. Shum A.W. Formation of apatite on poly(alpha-hydroxy acid) in an accelerated biomimetic process. J Biomed Mater Res B Appl Biomater. 2005;73:68. doi: 10.1002/jbm.b.30178. [DOI] [PubMed] [Google Scholar]
- 88.Pamula E. Kokoszka J. Cholewa-Kowalska K. Laczka M. Kantor L. Niedzwiedzki L. Reilly G.C. Filipowska J. Madej W. Kolodziejczyk M. Tylko G. Osyczka A.M. Degradation, bioactivity, and osteogenic potential of composites made of PLGA and two different sol-gel bioactive glasses. Ann Biomed Eng. 2011;39:2114. doi: 10.1007/s10439-011-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu C. Ramaswamy Y. Zhu Y. Zheng R. Appleyard R. Howard A. Zreiqat H. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(DL-lactide-co-glycolide) films. Biomaterials. 2009;30:2199. doi: 10.1016/j.biomaterials.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 90.Keshaw H. Georgiou G. Blaker J.J. Forbes A. Knowles J.C. Day R.M. Assessment of polymer/bioactive glass-composite microporous spheres for tissue regeneration applications. Tissue Eng Part A. 2009;15:1451. doi: 10.1089/ten.tea.2008.0203. [DOI] [PubMed] [Google Scholar]
- 91.Liu G. Wu C. Fan W. Miao X. Sin D.C. Crawford R. Xiao Y. The effects of bioactive akermanite on physiochemical, drug-delivery, and biological properties of poly(lactide-co-glycolide) beads. J Biomed Mater Res B Appl Biomater. 2011;96:360. doi: 10.1002/jbm.b.31779. [DOI] [PubMed] [Google Scholar]
- 92.Makinen T.J. Veiranto M. Lankinen P. Moritz N. Jalava J. Tormala P. Aro H.T. In vitro and in vivo release of ciprofloxacin from osteoconductive bone defect filler. J Antimicrob Chemother. 2005;56:1063. doi: 10.1093/jac/dki366. [DOI] [PubMed] [Google Scholar]
- 93.Berkland C. Kim K. Pack D.W. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73:59. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 94.Xie B. Preparation of uniform biodegradable microparticles using laser ablation. Int J Pharm. 2006;325:194. doi: 10.1016/j.ijpharm.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 95.Liu R. Huang S.S. Wan Y.H. Ma G.H. Su Z.G. Preparation of insulin-loaded PLA/PLGA microcapsules by a novel membrane emulsification method and its release in vitro. Colloids Surf B Biointerfaces. 2006;51:30. doi: 10.1016/j.colsurfb.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 96.Ateh D.D. Leinster V.H. Lambert S.R. Shah A. Khan A. Walklin H.J. Johnstone J.V. Ibrahim N.I. Kadam M.M. Malik Z. Gironès M. Veldhuis G.J. Warnes G. Marino S. McNeish I.A. Martin J.E. The intracellular uptake of CD95 modified paclitaxel-loaded poly(lactic-co-glycolic acid) microparticles. Biomaterials. 2011;32:8538. doi: 10.1016/j.biomaterials.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 97.Boschetti F. Tomei A.A. Turri S. Swartz M.A. Levi M. Design, fabrication, and characterization of a composite scaffold for bone tissue engineering. Int J Artif Organs. 2008;31:697. doi: 10.1177/039139880803100803. [DOI] [PubMed] [Google Scholar]
- 98.Sosnowski S. Wozniak P. Lewandowska-Szumiel M. Polyester scaffolds with bimodal pore size distribution for tissue engineering. Macromol Biosci. 2006;6:425. doi: 10.1002/mabi.200600003. [DOI] [PubMed] [Google Scholar]
- 99.Kofron M.D. Cooper J.A., Jr. Kumbar S.G. Laurencin C.T. Novel tubular composite matrix for bone repair. J Biomed Mater Res A. 2007;82:415. doi: 10.1002/jbm.a.31148. [DOI] [PubMed] [Google Scholar]
- 100.Devin J.E. Attawia M.A. Laurencin C.T. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. J Biomater Sci Polym Ed. 1996;7:661. doi: 10.1163/156856296x00435. [DOI] [PubMed] [Google Scholar]
- 101.Jabbarzadeh E. Nair L.S. Khan Y.M. Deng M. Laurencin C.T. Apatite nano-crystalline surface modification of poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering: implications for protein adsorption. J Biomater Sci Polym Ed. 2007;18:1141. doi: 10.1163/156856207781554073. [DOI] [PubMed] [Google Scholar]
- 102.Shi X. Wang Y. Ren L. Zhao N. Gong Y. Wang D.A. Novel mesoporous silica-based antibiotic releasing scaffold for bone repair. Acta Biomater. 2009;5:1697. doi: 10.1016/j.actbio.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 103.Nagano M. Kitsugi T. Nakamura T. Kokubo T. Tanahashi M. Bone bonding ability of an apatite-coated polymer produced using a biomimetic method: a mechanical and histological study in vivo. J Biomed Mater Res. 1996;31:487. doi: 10.1002/(SICI)1097-4636(199608)31:4<487::AID-JBM8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 104.Yan W.Q. Nakamura T. Kawanabe K. Nishigochi S. Oka M. Kokubo T. Apatite layer-coated titanium for use as bone bonding implants. Biomaterials. 1997;18:1185. doi: 10.1016/s0142-9612(97)00057-4. [DOI] [PubMed] [Google Scholar]
- 105.Barrere F. van der Valk C.M. Meijer G. Dalmeijer R.A. de Groot K. Layrolle P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J Biomed Mater Res B Appl Biomater. 2003;67:655. doi: 10.1002/jbm.b.10057. [DOI] [PubMed] [Google Scholar]
- 106.Ohgushi H. Caplan A.I. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48:913. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 107.Vallet-Regi M. Romero A.M. Ragel C.V. LeGeros R.Z. XRD, SEM-EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol-gel glasses. J Biomed Mater Res. 1999;44:416. doi: 10.1002/(sici)1097-4636(19990315)44:4<416::aid-jbm7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 108.Li P. Nakanishi K. Kokubo T. de Groot K. Induction and morphology of hydroxyapatite, precipitated from metastable simulated body fluids on sol-gel prepared silica. Biomaterials. 1993;14:963. doi: 10.1016/0142-9612(93)90186-6. [DOI] [PubMed] [Google Scholar]
- 109.Campbell A.A. Fryxell G.E. Linehan J.C. Graff G.L. Surface-induced mineralization: a new method for producing calcium phosphate coatings. J Biomed Mater Res. 1996;32:111. doi: 10.1002/(SICI)1097-4636(199609)32:1<111::AID-JBM13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 110.Wen H.B. de Wijn J.R. Cui F.Z. de Groot K. Preparation of calcium phosphate coatings on titanium implant materials by simple chemistry. J Biomed Mater Res. 1998;41:227. doi: 10.1002/(sici)1097-4636(199808)41:2<227::aid-jbm7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 111.Tretinnikov O.N. Kato K. Ikada Y. In vitro hydroxyapatite deposition onto a film surface-grated with organophosphate polymer. J Biomed Mater Res. 1994;28:1365. doi: 10.1002/jbm.820281115. [DOI] [PubMed] [Google Scholar]
- 112.Bodde E.W. Habraken W.J. Mikos A.G. Spauwen P.H. Jansen J.A. Effect of polymer molecular weight on the bone biological activity of biodegradable polymer/calcium phosphate cement composites. Tissue Eng Part A. 2009;15:3183. doi: 10.1089/ten.TEA.2008.0694. [DOI] [PubMed] [Google Scholar]
- 113.Félix Lanao R.P. Leeuwenburgh S.C. Wolke J.G. Jansen J.A. Bone response to fast-degrading, injectable calcium phosphate cements containing PLGA microparticles. Biomaterials. 2011;32:8839. doi: 10.1016/j.biomaterials.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Simon C.G., Jr. Khatri C.A. Wight S.A. Wang F.W. Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly(lactide-co-glycolide) microspheres. J Orthop Res. 2002;20:473. doi: 10.1016/S0736-0266(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q. Wang L. Detamore M.S. Berkland C. Biodegradable colloidal gels as moldable tissu e engineering scaffolds. Adv Mater. 2008;20:236. [Google Scholar]