Abstract
Aim
To review current evidence on the effect of paracetamol on blood pressure (BP), the quality of the previous studies and the validity of the results, and to summarize these findings.
Methods
A systematic literature review was performed by searching PubMed, the Cochrane library and EMBASE for publications between the years 1963 and 2012.
Results
We identified three case reports, seven prospective observational trials, six randomized controlled trials, one commentary and two reviews. Some, but not all, of the observational studies, which included over 147 000 patients, showed an increased risk of hypertension with paracetamol use. The randomized studies were generally small and the results were inconsistent. Three studies, which included 104 patients, showed an increase of systolic BP by ∼4 mmHg, two studies, which included 27 patients, reported no change in BP and one study, which included 21 patients, reported a fall in BP although no placebo arm was included for comparison.
Conclusions
The overall effect of paracetamol on BP is unclear. Given that paracetamol is often suggested as a safer alternative to non-steroidal anti-inflammatory drugs (NSAIDs), it would seem that further prospective evidence is now needed to address the effect of paracetamol on BP. This would be best done with larger studies in relevant cohorts using BP measured by ambulatory BP monitoring as the primary endpoint.
Keywords: acetaminophen, blood pressure, hypertension, paracetamol, prostaglandins, review
Introduction
Use of non-prescription [also known as over-the-counter (OTC)] analgesic medicines allows patients to self-treat without seeking medical advice. Paracetamol (acetaminophen in the US) is a well-established OTC analgesic used to relieve mild to moderate pain, the symptoms of colds and flu, and reduce fever. The easy availability of paracetamol likely contributes to its perceived safety. Consumption of paracetamol 500 mg in the UK was reported to have increased from 1500 million tablets per year in 1967/1968 to 3500 million tablets in 2000 1. This is likely because paracetamol is sold in many forms, either alone or in combination with other analgesia such as codeine or ibuprofen, and as branded preparations for colds and flu, migraine and menstrual discomfort. As a result, consumers may not always be aware that they are taking paracetamol and more worryingly, how much. Although widely used in children, adults and during pregnancy, the mode of analgesic action of paracetamol remains poorly understood.
It is assumed that paracetamol, like the non-steroidal anti-inflammatory drugs (NSAIDs), acts through the cyclo-oxygenase (COX) pathway, reducing the production of biologically active prostanoids (PGs), such as PGE2, which mediate inflammation and pain. Two types of COX enzymes exist, commonly referred to as COX-1 and -2, referring to the specific active site that catalyzes arachidonic acid oxygenation 2. COX-1 is constitutively present in most tissues and generates PGs that regulate normal cell function, such as maintenance of gastrointestinal integrity and vascular homeostasis. COX-2, in addition to its inducible pro-inflammatory role, is expressed constitutively in several organs, such as the kidney, brain and certain other cell types, including endothelial cells 3. Furthermore, both COX-1 and -2 have a peroxidase (POX) site 2.
The analgesic and antipyretic effect of paracetamol is thought to result from inhibition of COX-2 activity, by acting as a co-substrate for the POX active site 2. In comparison, selective COX-2 inhibitors (like etoricoxib) inhibit the COX-2 isoform, but at the COX active site, and other NSAIDs (like indomethacin) may preferentially inhibit COX-1 or have a balanced effect (like fenoprofen) 4. It is often said that paracetamol has no anti-inflammatory effects. However, although its effects are much less marked than those of the NSAIDs, paracetamol does decrease post-operative swelling in both animals 5 and humans 6. The marginal effect of paracetamol on platelet function 7 indicates its limited effect on the COX-1 system. However, the very highly selective analgesic and antipyretic nature of paracetamol suggests a central mode of action, consistent with inhibition of PGE2 synthesis within the CNS during fever 8 or pain 9. This has led to the hypothesis that a paracetamol-sensitive variant of prostaglandin H synthase (PGHS) exists within the CNS, which has been designated by some investigators as COX-3 10. Alternative proposed mechanisms underpinning the analgesic action of paracetamol include inhibition of the L-arginine-nitric oxide (NO) pathway 11 mediated through substance P or N-methyl-D-aspartate 12, reinforcement of descending inhibitory serotonergic pain pathways 13 and active paracetamol metabolites that affect cannabinoid receptors 14–16. These alternative mechanisms have been comprehensively reviewed 2, 17.
Osteoarthritis (OA) and hypertension are common conditions, increasing in prevalence with age and often co-existing in the same patient. The mainstay of pharmacological treatment for OA is intermittent or regular analgesia to control joint pain 18. It has been clearly shown that both NSAIDs and selective COX-2 inhibitors increase BP in hypertensive and normotensive individuals, interfere with antihypertensive treatment 19–21 and increase the risk of serious cardiovascular events 22, 23. Although paracetamol is less effective in relieving joint pain than NSAIDs 24, 25 it is assumed to be safer and is, therefore, the recommended first line analgesia for patients with osteoarthritis 26 and cardiovascular co-morbidity 27. However, a recent study in patients with coronary artery disease (CAD) 28 has shown that paracetamol treatment is associated with a clinically significant increase in BP, and raises the question of whether paracetamol should be used with greater caution in such patients. These data prompted us to question our own clinical practice of suggesting paracetamol as a safer alternative to NSAIDs in patients with hypertension, which in turn led to this literature review.
Methods
The literature search was conducted using PubMed, the Cochrane library and EMBASE, searching the years 1963 to 2012. The search strategy used the terms ‘blood pressure’ or ‘hypertension’ combined sequentially with ‘paracetamol’ or ‘acetaminophen’. In this review, papers were selected with the following criteria: 1) English language, 2) human subjects; 3) studies conducted in adults ≥18 years, 4) meta-analyses, randomized active or placebo-controlled trials, prospective studies, and observational studies with control groups and 5) outcome variable reporting change in BP, change in BP control or incident hypertension. Our approach 29, using these criteria, led to the inclusion of three case reports 30–32, seven prospective observational trials 33–39, six randomized controlled trials 28, 40–44, one commentary 45 and two reviews 46, 47.
Results
Observational data
The salt content of effervescent paracetamol preparations may influence BP because all effervescent formulations contain significant amounts of sodium in the form of sodium bicarbonate. The sodium content can vary widely between brands 48. The UK scientific advisory committee on nutrition suggests a maximum daily sodium intake for all adults of 100 mmol (or 6 g salt), on the basis that sodium intake is linked to BP 49. Indeed, Ubeda et al. 33 performed a non-randomized, observational study of 34 elderly hypertensive patients with uncontrolled hypertension, who were changed from an effervescent preparation of paracetamol 1 g three times per day (74 mmol of sodium in total) to paracetamol tablets (sodium-free), which resulted in the reduction of systolic and diastolic BP by 13.1 mmHg (95% CI 11.9, 14.3; P < 0.0001) and 2.5 mmHg (95% CI 2.1, 2.9; P < 0.0001), respectively, a major BP reduction, equivalent to the introduction of new drug treatment.
The first observational study on the BP effect of non-effervescent paracetamol tablets, which are sodium free, was reported in 1997 by Boyle et al. 34, and was performed in 27 intensive care patients who were given paracetamol to reduce a fever (85%) or for analgesia (15%). Following paracetamol administration, an overall fall in systolic BP (10%) was seen. However, the strength of any conclusion regarding causality is limited given the lack of a placebo group and the intensive care setting, because around half of patients were on inotropic infusions, masking the true effect of paracetamol on BP.
In 2002, the Nurses' Health Studies I 35 and II 36 were performed in 131 650 females with no history of hypertension or chronic renal disease and were followed-up for physician-diagnosed hypertension by self-report questionnaire. In both studies, the risk of hypertension was higher for paracetamol users at all use frequencies compared with non-users. Also, there was a significant trend towards an increased risk of hypertension with increasing frequency of use. However, in the Nurses' Health Study I, women who used less paracetamol (1 to 4 days a month) had lower rates of diabetes than those using more frequent paracetamol (>22 days per month). Similarly in the Nurses' Health study II, women who were taking less paracetamol (1–4 days per month) were on average younger and had a lower body mass index than those using more frequent paracetamol (>22 days per month), both of which may be important confounders.
The results of the Nurses' Health Studies led to much interest in the association between hypertension and non-narcotic analgesia. However, a major limitation was a lack of information on the indication for analgesic use, which brought concerns of further confounding, for instance with claims that analgesia may have been taken for headaches resulting from high BP. Although this association is now less clear 50, at the time this led to the assembly of two new subgroups from both of the Nurses' Health Study cohorts 37. This time more detailed information was collected, specifically regarding the indication for analgesia, and again the results showed that paracetamol remained independently associated with hypertension even in women who did not report a headache.
In 2005, the results from 8229 men from the Physicians' Health Study, (PHS) 38 were different from the previous studies in women. Here, there was no increased risk of hypertension at any cumulative paracetamol dose compared with non-users. There were initial concerns about confounding with aspirin, because the original aim of the PHS was to investigate the benefit of alternate day aspirin 325 mg in primary prevention of cardiovascular disease. However, this is unlikely, because current data suggest low dose aspirin does not affect BP 51. Nevertheless, these results were not consistent with The Health Professionals Study, performed in 16 031 men 39 (designed to complement the Nurses' Health Study). These results showed that men who used paracetamol 6 to 7 days per week had an increased risk for incident hypertension compared with non-users. Unlike some of the observational studies performed in women, the association between paracetamol use and risk of incident hypertension in this study was greater among men with a lower BMI and those younger than 60 years old.
Despite strong suggestions of an association between paracetamol use and an increased risk of hypertension, any causal interpretation of these observational data (Table 1) is risky because observed differences in BP may have resulted from many confounders. It is well known that lifestyle factors and including diet, exercise and alcohol intake- affect BP and are hard to account for especially in studies that rely on self-report questionnaires. In addition, it is plausible that analgesic users take several analgesics so failure to consider or adjust for other analgesics may influence the outcome. Furthermore, it is possible that more frequent analgesic users may have more general practitioner contact and be more likely to have their BP measured and hypertension diagnosed. Indeed, cause and effect can only be firmly established by methodologically sound prospective, randomized, interventional studies.
Table 1.
The effects of paracetamol on BP: observational studies
| Author | n | Duration | Paracetamol use | Cases of HTN | Relative risk |
|---|---|---|---|---|---|
| Dedier et al. 35 | 51 630 | 8 years | Days per month | ||
| None | 4037 | 1.00 | |||
| 1–4 | 2959 | 1.07 | |||
| 5–14 | 1033 | 1.22 | |||
| 15–21 | 317 | 1.31 | |||
| >22 | 457 | 1.2 | |||
| Curhan et al. 36 | 80 020 | 2 years | Days per month | ||
| None | 369 | 1.00 | |||
| 1–4 | 661 | 1.19 | |||
| 5–14 | 229 | 1.37 | |||
| 15–21 | 62 | 1.62 | |||
| ≥22 | 72 | 2.00 | |||
| Kurth et al. 38 | 8 229 | 14 years | Cumulative use over 14 years | ||
| <12 | 1204 | 1.00* | |||
| 12–1499 | 607 | 0.86 (0.77–0.95)* | |||
| 1500–2499 | 87 | 1.17 (0.93–1.46)* | |||
| ≥2500 | 97 | 1.08 (0.87–1.34)* | |||
| Forman et al. 39 | 16 031 | 2 years | Days per week | ||
| 0 | 1743 | 1.00 | |||
| 1 | 47 | 1.00 | |||
| 2–3 | 69 | 1.00 | |||
| 4–5 | 36 | 1.59 | |||
| 6–7 | 50 | 1.34 |
Hazard ratio. HTN, hypertension.
Interventional studies
Few prospective, randomized controlled trials have examined the effect of paracetamol on BP; and the results have been inconsistent, reporting a reduction or no change in BP, or a small but potentially clinically significant increase in BP (Table 2).
Table 2.
The effects of paracetamol on BP: randomised controlled trials
| Study | Design | Patient | n | Age (years) | Paracetamol dose | Duration | Baseline systolic BP (mmHg) | End systolic BP (mmHg) | Change in systolic BP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Chalmers et al. 40 | Randomized, double-blind, two phase, crossover, placebo- controlled | HTN OA | 22 | – | 1 g three times daily | 4 weeks | – | – | 4 |
| Lewis et al. 41 | Unblinded, three phase, crossover | HTN OA | 21 | 62 | 1 g four times daily | 2 weeks | 110.3 (MAP) | 103.8 (MAP) | −6.5 (MAP) |
| Radack et al. 42 | Randomized, double-blind, parallel groups, placebo controlled | HTN | 15 | 53 | 1 g four times daily | 3 weeks | 123 | – | 0.2 |
| Chau et al. 43 | Randomized, double-blind, three phase, crossover | HTN | 12 | 31–71 | 650 mg | Once | – | 121 ± 12 | 1.2 ± 6.0 |
| Pavlicevic et al. 44 | Randomized, single-blind, three phase, parallel groups | HTN | 49 | 70 | 1 g three times daily | 1 month | 139.3L | 133.9L/I/Pa | −5.4 |
| 133.3L | 132.9L/P/Pa | −0.4 | |||||||
| 144.8A | 142.0A/I/Pa | −2.8 | |||||||
| 130.2A | 131.4A/P/Pa | 1.2 | |||||||
| Sudano et al. 28 | Randomized, double-blind, two phase, crossover, placebo controlled | CAD | 33 | 61 | 1 g three times daily | 2 weeks | 122 | 125 | 3 |
A, amlodipine; CAD, coronary artery disease; HTN, hypertension; I, ibuprofen; L, lisinopril/hydrochlorothiazide; MAP, mean arterial pressure; OA, osteoarthritis; P, piroxicam; Pa, paracetamol.
The interventional study in 1984 by Chalmers et al. 40 was the first to explore the association between paracetamol use and the risk of hypertension. It was a randomized, double-blind, two phase placebo-controlled cross-over trial comparing non-effervescent paracetamol (sodium free) 1 g every 8 h with placebo, and recruited 22 treated hypertensive patients who were taking, or had recently taken, NSAIDs for degenerative joint disease or musculoskeletal pain. After 4 weeks there was a significant 4 mmHg increase in supine and standing systolic pressure with paracetamol compared with placebo (P < 0.05). However, only treated hypertensive patients were recruited and with no normotensive adults for comparison, it would be incorrect to generalize these results to the broader adult population, as at present it is not clear whether paracetamol directly increases BP, interferes with the BP lowering effect of antihypertensive medications or indeed, both. In addition, it is also possible that the diminished analgesic effect of paracetamol may have led to a rise in BP, given that nociception and BP are intrinsically linked 52.
The second study by Lewis et al. 41, published in 1986, was an unblinded, three phase, crossover study using indomethacin 50 mg twice daily, sulindac 200 mg twice daily and paracetamol 1 g four times daily for 6 weeks, recruiting 21 hypertensive patients who were taking regular NSAIDs for joint pain. This study was designed to determine the effect of indomethacin and sulindac on BP, rather than paracetamol, which was used in place of placebo as all patients required regular analgesia for musculoskeletal disease. After 2 weeks, mean arterial pressure (range) was significantly higher with indomethacin 117.8 mmHg (102.6–152.0) than sulindac 109.9 mmHg (87.0–138.3) and paracetamol 103.8 mmHg (81.0–120.0) (P < 0.001), compared with baseline (110.3 mmHg). Here, it is difficult to draw any conclusions on the effect of paracetamol on BP because no placebo phase was included. However, this study did expose the inadequacy of paracetamol as a suitable analgesic in musculoskeletal disease, because 90% of the patients stopped paracetamol after 2 weeks due to poor symptom relief.
In 1987, a randomized, double-blind, placebo-controlled, parallel study by Radack et al. 42 was performed with ibuprofen 400 mg 8 hourly, paracetamol 1 g 8 hourly and matched placebo for 3 weeks, recruiting 15 hypertensive patients on at least two antihypertensive drugs. The mean change in supine and sitting systolic BP in the paracetamol group (0.2 mmHg ± 2.9 mmHg and −1.7 ± 3.1 mmHg respectively) was not significant compared with the initial baseline reading. In addition, no difference in BP was observed with paracetamol amongst a variety of antihypertensive agents. However, a notable finding of the participants recruited was that 80% were African American and, given the known ethnic influences on the pathophysiology of hypertension, these results may not be applicable to other patient groups.
In 1991, a randomized, double-blind, placebo controlled, three phase crossover study by Chua et al. 43 was performed to compare the BP effect of two ‘cold’ medications, pseudoephedrine 60 mg or chlorpheniramine combined with paracetamol 4/650 mg, against placebo. The study was performed in 12 hypertensive patients known to have a pressor response to pseudoephedrine 53. The results showed that the effect of chlorpheniramine/paracetamol on BP was not significantly different from that of placebo, with a mean change (±SD) from baseline in systolic BP of 1.2 mmHg ± 6.0 mmHg, compared with 2.4 mmHg ± 3.3 mmHg for placebo. However, pseudoephedrine produced a significant increase in systolic BP with a mean change in systolic BP of 6.9 mmHg ± 5.9 mmHg from the baseline value. Although some antihistamines do not effect BP 54, 55, the effect of chlorpheniramine on BP has not been studied. Therefore, it is very difficult to draw any conclusions on the direct effect of paracetamol on BP in this study.
In 2008, a randomized, single-blind, three phase, parallel study by Pavličević et al. 44 was performed to compare the effect on BP of ibuprofen 400–600 mg three times daily or piroxicam 10–20 mg once daily followed by paracetamol 1 g three times daily. Each treatment phase lasted 1 month. Forty-nine controlled hypertensive patients on long term analgesia for osteoarthritis and 39 hypertensive controls were recruited, and each was taking either a lisinopril/hydrochlorothiazide combination or amlodipine. In the lisinopril/hydrochlorothiazide subgroup, ibuprofen increased systolic BP to 144.4 ± 17.1 mmHg (from a baseline of 139. ± 16.1 mmHg), which decreased to 133.9 ± 20.8 mmHg during the paracetamol phase. Similarly, piroxicam increased systolic BP to 149.4 ± 21.1 mmHg (from a baseline of 133.3 ± 16.5 mmHg) which decreased to 132.9 ± 18.4 mmHg during the paracetamol phase. Although these results suggest that paracetamol may have a hypotensive effect, the control group of hypertensive patients on lisinopril/hydrochlorthiazide or amlodipine (but not taking analgesia) showed an even larger reduction in systolic BP from the baseline reading (138.0 ± 21.1 mmHg to 129.6 ± 15.7 mmHg in the lisinopril/hydrochlorthiazide group and 138.1 ± 10.1 to 135.3 ± 11.6 mmHg in the amlodipine group). It is likely that the higher baseline BP at the start of the study is due to the ‘white coat effect’. In the amlodipine subgroup, ibuprofen, piroxicam and paracetamol did not significantly increase BP, similar to findings in other studies 56, 57, showing that the BP lowering effect of calcium channel blockers may be less affected by NSAIDs than other antihypertensives.
The most recent interventional study by Sudano et al. 28 was published in 2010. A randomized, double blind, two phase crossover study, was performed in 33 patients with established CAD (documented by coronary angiography, nuclear imaging or positive stress test). Paracetamol 1g (sodium free) or placebo was taken three times daily in addition to standard cardiovascular therapy. Two weeks' treatment with paracetamol significantly increased mean systolic (from 122.4 ± 11.9 to 125.3 ± 12.0 mmHg P < 0.02 vs. placebo) and diastolic ambulatory BP (from 73.2 ± 6.9 to 75.4 ± 7.9 mmHg P < 0.02 vs. placebo), similar to the change in BP observed with NSAIDs. However, only patients with CAD were recruited and it would be incorrect to generalize these results to the broader population. Also, this was the only study to use ambulatory BP monitoring (ABPM) to assess BP response, which may be why the effect was identified in a relatively small study.
Biological plausibility
The likely biological mechanism underlying the hypertensive effect of paracetamol, apart from the sodium loading that can occur with effervescent tablets, is through inhibition of renal PG synthesis (Figure 1). Although in health under basal conditions both COX-1 and -2 pathways are responsible for the biosynthesis of prostanoids 3, PGs are widely considered to be unimportant in the maintenance of renal function. However, in patients with an apparent decreased effective circulatory volume or decreased renal perfusion, PGE2 plays a critical role in maintaining renal blood flow by vasodilating renal vascular beds, mainly through the COX-2 pathway 3. In addition, PGE2 directly stimulates natriuresis, inhibiting absorption of sodium in the thick ascending limb and collecting ducts 3 and inhibits renal water absorption induced by anti-diuretic hormone 58. Like PGE2, prostacylin (PGI2) is thought to play an equally important role in maintaining renal vasodilatation under stress 3 and both furthermore, mediate renin release from the macula densa 59.
Figure 1.
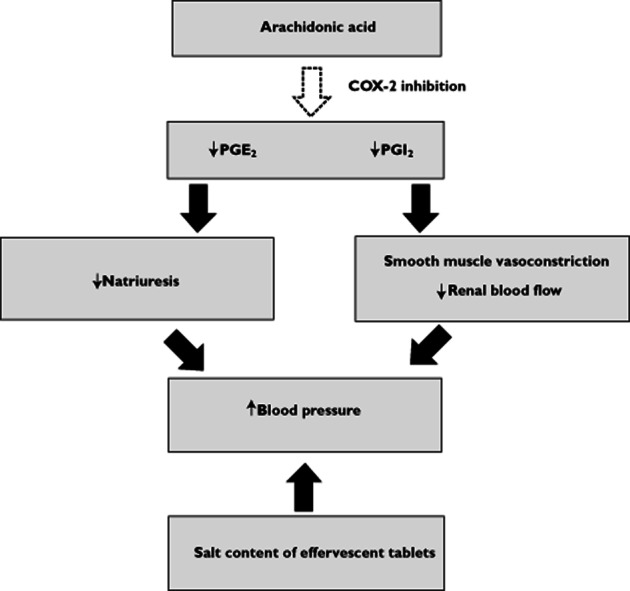
Potential biological mechanisms underlying the BP raising effect of paracetamol
Recent clinical studies have consistently shown that the administration of COX-2 selective inhibitors is complicated by sodium retention, oedema and development of hypertension 21. An association between reduction in urinary PG metabolites and reduced urinary sodium excretion with COX-2 inhibitors has been reported 60–63, 64 suggesting the likely mechanism. In women, urinary PGE2 and 6-keto-PGF1α (the renal metabolite of PGI2) is due to renal synthesis, but in men it is excreted from both the kidney and prostate gland 65. Thus, studies in women provide a more reliable indicator of the effect of drugs on renal PG synthesis. Like the COX-2 inhibitors, one study using paracetamol 1 g four times daily for 3 days 66 showed a significant reduction in urinary PGE2 and 6-keto-PGF1α, associated with a statistically highly significant reduction in mean urinary sodium excretion. Although this study did not examine the effect of paracetamol on BP, it did show paracetamol had no effect on plasma renin activity. One paper 67 has recently demonstrated that PGI2 production is driven by COX-1, rather than COX-2, and therefore, paracetamol should have no effect on PGI2 although this requires further supportive evidence. Other paracetamol studies performed in women have shown either a reduction in urinary PGs 68 or urinary sodium excretion 69. In contrast, one study also performed in women showed no reduction of urinary PGs 70 and the cause for this conflicting result is unclear, but may in part be due to the different assays used.
The effect of paracetamol on BP in hypertensive patients on various classes of anti-hypertensive agents has yet to be defined. However, using the data from clinical trials performed in hypertensive patients with NSAIDs, have repeatedly shown BP elevation in patients on β-adrenoceptor blockers, vasodilators, diuretics, ACE inhibitors (ACEI), methydopa and angiotensin receptor blockers (ARBs) 19, 20, 40, 71–73. Calcium channel blockers appear to be less affected, with some studies showing no significant change in BP 56, 57. As yet there are few similar data for paracetamol. However, it would seem plausible that paracetamol, like the NSAIDs, has the potential to increase BP by blocking the synthesis of PGE2 and PGI2, reducing natriuresis and vasodilatation, and thereby, also potentially attenuating the BP lowering effects of many of the major antihypertensive medications.
Conclusions
Although paracetamol has been presented as a relatively safe drug, except for the hepatotoxicity seen with overdose, the general safety of paracetamol in therapeutic, licensed doses has now also come into question because several studies have showed asymptomatic elevations in alanine aminotransferase 74–76 with more than 5 days of therapeutic dosing. Whilst the probability of developing significant liver injury seems very unlikely, given the very widespread use of paracetamol and no reports of significant liver injury to date, there have been no published prospective studies. In addition, one study has further questioned the rationale of suggesting paracetamol over NSAIDs to patients at risk of peptic ulcer disease, after results showed similar degrees of blood loss with paracetamol and ibuprofen following therapeutic use 77.
Here, we are concerned that sodium-free paracetamol may cause a clinically important increase in BP. The results from the observational and interventional studies are sometimes conflicting, and overall the effect of paracetamol on BP is unclear. Some of the clinical trial data suggest short term paracetamol use has a negligible effect on BP and others show an increase of around 3 mmHg in CAD 28 and 4 mmHg in treated hypertension 40. To put this into context, the increase in systolic BP with NSAIDs in patients with controlled hypertension is around 3–6 mmHg 19, 20. Even these small increases in BP have major clinical implications on a population basis, because a 2 mmHg rise in systolic BP is associated with a 7% and 10% increased risk of mortality from ischaemic heart disease and stroke, respectively 49. This potential increase in BP with paracetamol may be a major concern for patients with hypertension. However, the small number of participants and the narrow patient cohorts previously studied limit the generalizability of these results. What we need is methodologically sound randomized placebo and active control trials, studying the effect of paracetamol on BP in a larger number of adults. ABPM has several distinct advantages over conventional clinic BP which include; little or no ‘white coat’ effect, negligible placebo response, better reproducibility, provision of a 24 h profile, assessment of BP variability and is a better predictor of cardiovascular mortality 78. Thus, ABPM greatly outweighs the limitations arising from clinic BP measurements and we suggest ABPM should be used in all pharmacological trials evaluating BP response. These trials should include patients with hypertension, on a variety of anti-hypertensive agents, and patients with renal impairment. Indeed, it may be useful to assess carefully the effect of paracetamol on BP in the broader adult population without hypertension.
The recent evidence suggests that paracetamol should be used with caution in patients with established CAD. Having performed a systematic review of the literature there appears to be additional clinical trial data supporting the association between paracetmol use and BP elevation in patients with hypertension. Given that there is a plausible biological mechanism for an increase in BP with paracetamol, it may be that we have a misplaced confidence in the cardiovascular safety of paracetamol. Indeed, it would seem to us that further prospective evidence is now needed to address the safety of paracetamol on BP in larger studies in relevant cohorts using BP measured by ABPM as the primary endpoint.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Sheen CL, Dillon JF, Bateman DN, Simpson KJ, MacDonald TM. Paracetamol toxicity: epidemiology, prevention and costs to the health-care system. Q J Med. 2002;95:609–619. doi: 10.1093/qjmed/95.9.609. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 5.Mburu DN, Mbugua SW, Skoglund L, Lokken L. Effects of paracetamol and acetylsalicylic acid on the post-operative course after experimental orthopaedic surgery in dogs. J Vet Pharmacol Ther. 1998;11:163–171. doi: 10.1111/j.1365-2885.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 6.Lokken P, Skjelbred P. Analgesic and anti-inflammatory effect of paracetamol evaluated by bilateral oral surgery. Br J Clin Pharmacol. 1980;10:253S–260. doi: 10.1111/j.1365-2125.1980.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour RA, Williams FM, Oxley A, Ward A, Fearns M, Brighan K, Rawlins MD, Jones PM. A comparative study of the effects of aspirin and paracetamol (acetaminophen) on platelet aggregation and bleeding time. Eur J Clin Pharmacol. 1984;26:567–571. doi: 10.1007/BF00543486. [DOI] [PubMed] [Google Scholar]
- 8.Feldberg W, Gupta K, Milton A, Wendland S. Effect of pyrogen and antipyretics on prostaglandin activity in cisternal CSF of anaesthetised cats. J Physiol. 1973;234:279–303. doi: 10.1113/jphysiol.1973.sp010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferarri RA, Ward SJ, Zobre CM, Van Liew DK, Perrone MH, Connell MJ, Haubrich DR. Estimation of the in vivo effect of cyclooxygenase inhibitors on prostaglandin E2 levels in mouse brain. Eur J Pharmacol. 1990;179:25–34. doi: 10.1016/0014-2999(90)90398-p. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekharan NV, Dai H, Lamar Turepu Roos K, Evanson NK, Tomsik J, Elton TS. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorkman R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anaesthesiol Scand Suppl. 1995;103:1–44. [PubMed] [Google Scholar]
- 12.Hunskaar S, Fasmer OB, Hole K. Acetylsalicylic acid, paracetamol and morphine inhibit behavioral responses to intrathecally administered substance P or capsaicin. Life Sci. 1985;37:1835–1841. doi: 10.1016/0024-3205(85)90227-9. [DOI] [PubMed] [Google Scholar]
- 13.Pickering G, Esteve V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84:47–51. doi: 10.1038/sj.clpt.6100403. [DOI] [PubMed] [Google Scholar]
- 14.Hogestatt ED, Jonsson BA, Ermund A, Anderson DA, Bjork H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 15.Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Högestätt ED, Zygmunt PM. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat Commun. 2011;22:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- 17.Graham G, Scott K. Mechanisms of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Bijlsma JW, Berenbaum F, Lafeber F. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 19.Pope J, Anderson J, Felson D. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–484. [PubMed] [Google Scholar]
- 20.Johnston A, Nguyen M, Day R. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Aw T, Haas S, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–496. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 22.Schjerning Olsen AM, Fosbøl EL, Lindhardsen J, Folke F, Charlot M, Selmer C, Lamberts M, Bjerring Olesen J, Køber L, Hansen PR, Torp-Pedersen C, Gislason GH. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226–2235. doi: 10.1161/CIRCULATIONAHA.110.004671. [DOI] [PubMed] [Google Scholar]
- 23.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis?: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63:901–907. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother. 2010;44:489–506. doi: 10.1345/aph.1M332. [DOI] [PubMed] [Google Scholar]
- 26.NICE clinical guideline 59; The care and management of osteoarthritis in adults. February 2008. Available at http://www.nice.org.uk/guidance/CG59 (last accessed 29 October 2012)
- 27.Antman EM, Bennet JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal anti-inflammatory drugs. An update for clinicians. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 28.Sudano I, Flammer AJ, Periat D, Enseleit F, Hermann M, Wolfrum M, Hirt A, Kaiser P, Hurlimann D, Neidhart M, Gay S, Holzmeister J, Nussberger J, Mocharla P, Landmesser U, Haile S, Corti R, Vanhoutte PM, Luscher TF, Noll G, Ruschitzka F. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122:1789–1796. doi: 10.1161/CIRCULATIONAHA.110.956490. [DOI] [PubMed] [Google Scholar]
- 29.Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ. 2005;330:68. doi: 10.1136/bmj.38336.804167.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas L, Akil M. Sodium in soluble paracetamol may be linked to raised blood pressure. BMJ. 2006;332:1133. doi: 10.1136/bmj.332.7550.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery B. Does paracetamol cause hypertension? BMJ. 2008;336:1190–1191. doi: 10.1136/bmj.39526.654016.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaillard M, Mathiaux F, Aldigier J, Laroche M, Nouaille Y, Merle L. Paracetamol use and risk of hypertension: Case report and literature review. Fundamental and Clinical Pharmacology. Conference Publication. 2011.
- 33.Ubeda A, Llopico J, Sanchez MT. Blood pressure reduction in hypertensive patients after withdrawal of effervescent medication. Pharmacoepidemiol Drug Saf. 2009;18:417–419. doi: 10.1002/pds.1701. [DOI] [PubMed] [Google Scholar]
- 34.Boyle M, Hundy S, Torda TA. Paracetamol administration is associated with hypotension in the critically ill. Aust Crit Care. 1997;10:120–122. doi: 10.1016/s1036-7314(97)70414-4. [DOI] [PubMed] [Google Scholar]
- 35.Dedier J, Stampfer MJ, Hankinson SE, Willet WC, Speizer FE, Curham GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension. 2002;40:604–608. doi: 10.1161/01.hyp.0000035856.77718.da. [DOI] [PubMed] [Google Scholar]
- 36.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204–2208. doi: 10.1001/archinte.162.19.2204. [DOI] [PubMed] [Google Scholar]
- 37.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500–507. doi: 10.1161/01.HYP.0000177437.07240.70. [DOI] [PubMed] [Google Scholar]
- 38.Kurth T, Hennekens CH, Stürmer TH, Sesso HD, Glynn RJ, Buring JE, Gaziano M. Analgesic use and risk of subsequent hypertension in apparently healthy men. Arch Intern Med. 2005;165:1903–1909. doi: 10.1001/archinte.165.16.1903. [DOI] [PubMed] [Google Scholar]
- 39.Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167:394–399. doi: 10.1001/archinte.167.4.394. [DOI] [PubMed] [Google Scholar]
- 40.Chalmers JP, West MJ, Wing LMH, Bune AJC, Graham JR. Effects of indomethacin, sulindac, naproxen, aspirin and paracetamol in treated hypertensive patients. Clin Exp Hypertens. 1984;6:1077–1093. doi: 10.3109/10641968409039582. [DOI] [PubMed] [Google Scholar]
- 41.Lewis RV, Toner JM, Jackson PR, Ramsay LE. Effects of indomethacin and sulindac on blood pressure of hypertensive patients. BMJ. 1986;292:934–935. doi: 10.1136/bmj.292.6525.934-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radack K, Deck C, Bloomfield S. Ibuprofen interferes with the efficacy of antihypertensive drugs. A randomized, double-blind, placebo-controlled trial of ibuprofen compared with acetaminophen. Ann Intern Med. 1987;107:628–635. doi: 10.7326/0003-4819-107-5-628. [DOI] [PubMed] [Google Scholar]
- 43.Chua SS, Benrimoj SI, Gordon RD, Williams G. Cardiovascular effects of a chlorpheniramine/paracetamol combination in hypertensive patients who were sensitive to the pressor effect of pseudoephedrine. Br J Clin Pharmacol. 1991;31:360–362. doi: 10.1111/j.1365-2125.1991.tb05545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavličević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol. 2008;15:372–382. [PubMed] [Google Scholar]
- 45.Brotman DJ. Acetaminophen and hypertension: a causal association or pain mediated? Arch Intern Med. 2003;163:1115–1116. doi: 10.1001/archinte.163.9.1113-b. [DOI] [PubMed] [Google Scholar]
- 46.Wilson SL, Poulter NR. The effect of non-steroidal anti-inflammatory drugs and other commonly used non-narcotic analgesics on blood pressure level in adults. J Hypertens. 2006;24:1457–1469. doi: 10.1097/01.hjh.0000239278.82196.a5. [DOI] [PubMed] [Google Scholar]
- 47.Sudano I, Flammer AJ, Roas S, Enseleit F, Noll G, Ruschitzka F. Nonsteroidal antiinflammatory drugs, acetaminophen, and hypertension. Curr Hypertens Rep. 2012;14:304–309. doi: 10.1007/s11906-012-0274-7. [DOI] [PubMed] [Google Scholar]
- 48.Friedrich I, Bonatz A, Schwenke K. Sodium content of effervescent analgesics. February 2011. Available at http://www.industrialpharmacy.eipg.eu (last accessed 21 September 2012)
- 49.NICE clinical guideline 127; Clinical management of hypertension in adults. August 2011. Available at http://www.nice.org.uk/guidance/CG127 (last accessed 29 October 2012)
- 50.Tronvik E, Stovner LJ, Hagan K, Holmen J, Zwart JA. High pulse pressure protects against headache: prospective and cross sectional data (HUNT Study) Neurology. 2008;70:1329–1336. doi: 10.1212/01.wnl.0000309222.79376.57. [DOI] [PubMed] [Google Scholar]
- 51.Zanchetti A, Hansson L, Leonetti G, Rahn KH, Ruilope L, Warnold I, Wedel H. Low-dose aspirin does not interfere with the blood pressure lowering effects of antihypertensive therapy. J Hypertens. 2002;20:1015–1022. doi: 10.1097/00004872-200205000-00038. [DOI] [PubMed] [Google Scholar]
- 52.Zamir N, Maixner W. The relationship between cardiovascular and pain regulatory systems. Ann N Y Acad Sci. 1986;467:371–384. doi: 10.1111/j.1749-6632.1986.tb14641.x. [DOI] [PubMed] [Google Scholar]
- 53.Chua SS, Benrimoj SI, Gordon RD, Williams G. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369–372. doi: 10.1111/j.1365-2125.1989.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Britton MG, Empey DW, John GC, McDonnell KA, Hughes DT. Histamine challenge and anterior nasal rhinometry: their use in the assessment of pseudoephedrine and triprolidine as nasal decongestants in subjects with hayfever. Br J Clin Pharmacol. 1978;6:51–58. doi: 10.1111/j.1365-2125.1978.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Empey DW, Frosolono MF, Hughes DT, Perkins JG. Comparison of pseudoephedrine and triprolidine, alone and in combination in preventing nasal congestion in subjects with allergic rhinitis using nasal histamine challenge. Br J Clin Pharmacol. 1984;18:86–89. doi: 10.1111/j.1365-2125.1984.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled in amlodipine or enalapril. Am J Hypertens. 2000;13:1161–1167. doi: 10.1016/s0895-7061(00)01204-8. [DOI] [PubMed] [Google Scholar]
- 57.Houston MC, Weir M, Gray J, Ginsberg D, Szeto C, Kaihlenen PM, Sugimoto D, Runde M, Lefkowitz M. The effect of nonsteroidal anti-inflammatory drugs on blood pressures of patients with hypertension controlled by verapamil. Arch Intern Med. 1995;155:1049–1054. [PubMed] [Google Scholar]
- 58.Dunn MJ, Hood VL. Prostaglandins and the kidney. Am J Physiol. 1977;233:169–184. doi: 10.1152/ajprenal.1977.233.3.F169. [DOI] [PubMed] [Google Scholar]
- 59.Gerber JG, Olson RD, Nies AS. Interrelationship between prostaglandins and renin release. Kidney Int. 1981;19:816–821. doi: 10.1038/ki.1981.85. [DOI] [PubMed] [Google Scholar]
- 60.Whelton A, Schulman G, Wallemark C, Drower EJ, Isakson PC, Verburg KM, Geis S. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med. 2000;160:1465–1470. doi: 10.1001/archinte.160.10.1465. [DOI] [PubMed] [Google Scholar]
- 61.McAdam BF, Catella-Lawson F, Mardini SK, Kapoor S, Lawson JA, Fitzgerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catella-Lawson F, McAdam B, Morrison BW, Kapoor S, Kujubu D, Antes L, Lasseter KC, Quan H, Gertz BJ, Fitzgerald G. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- 63.Swan SK, Rudy DW, Lasseter KC, Ryan CF, Buechel KL, Lambrecht LJ, Pinto MB, Dilzer SC, Obrda O, Sundblad KJ, Gumbs CP, Ebel DL, Quan H, Larson PJ, Schwartz JI, Musliner TA, Gertz BJ, Brater C, Yao SL. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low salt diet. Ann Intern Med. 2000;133:1–9. doi: 10.7326/0003-4819-133-1-200007040-00002. [DOI] [PubMed] [Google Scholar]
- 64.Rossat J, Maillard M, Nussberger J, Brunner HR, Burnier M. Renal effects of selective cyclooxygenase-2 inhibition in normotensive salt-depleted subjects. Clin Pharmacol Ther. 1999;66:76–84. doi: 10.1016/S0009-9236(99)70056-1. [DOI] [PubMed] [Google Scholar]
- 65.Patrono C, Wennmalm A, Ciabattoni G, Nowak J, Pugliese F, Cinotti GA. Evidence for extra-renal origin of urinary prostaglandins in healthy men. Prostaglandins. 1979;18:623–629. doi: 10.1016/0090-6980(79)90029-7. [DOI] [PubMed] [Google Scholar]
- 66.Prescott L, Mattison P, Menzies G, Manson LM. The comparative effects of paracetamol and indomethacin on renal function in healthy female volunteers. Br J Clin Pharmacol. 1990;29:403–412. doi: 10.1111/j.1365-2125.1990.tb03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirkby NS, Lundberg MH, Harrington LS, Leadbeater PD, Milne GL, Potter CM, Al-Yamani M, Adeyemi O, Warner TD, Mitchell JA. Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proc Natl Acad Sci U S A. 2012;109:17597–17602. doi: 10.1073/pnas.1209192109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green K, Drvota V, Vesterqvist O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen. Prostaglandins. 1989;37:311–315. doi: 10.1016/0090-6980(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 69.Haylor J. Prostaglandin synthesis and renal function in man. J Physiol. 1980;298:383–396. doi: 10.1113/jphysiol.1980.sp013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bippi H, Frolich JC. Effects of acetylsalicylic acid and paracetamol alone and in combination on prostanoid synthesis in man. Br J Clin Pharmacol. 1990;29:305–310. doi: 10.1111/j.1365-2125.1990.tb03640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izhar M, Alausa T, Folker A, Hung E, Bakris B. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated Blacks and Hispanics. Hypertension. 2004;43:573–577. doi: 10.1161/01.HYP.0000115921.55353.e0. [DOI] [PubMed] [Google Scholar]
- 72.Conlin PR, Moore TJ, Swartz SL, Barr E, Gazdick L, Fletcher C, DeLucca P, Demopoulos L. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–465. doi: 10.1161/01.hyp.36.3.461. [DOI] [PubMed] [Google Scholar]
- 73.Fogari R, Zoppi A, Carretta C. Effect of indomethacin on the antihypertensive efficacy of valsartan and lisinopril: a multicentre study. J Hypertens. 2002;20:1007–1014. doi: 10.1097/00004872-200205000-00037. [DOI] [PubMed] [Google Scholar]
- 74.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 75.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 76.Heard KJ, Green JL, Dart RC. Serum alanine aminotransferase elevation during 10 days of acetaminophen administration in non-drinkers. Pharmacotherapy. 2010;30:818–822. doi: 10.1592/phco.30.8.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doherty M, Hawkey C, Goulder M, Gibb I, Hill N, Aspley S, Reader S. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community derived people with knee pain. Ann Rheum Dis. 2011;70:1534–1541. doi: 10.1136/ard.2011.154047. [DOI] [PubMed] [Google Scholar]
- 78.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]


