Abstract
Imagine a medicine that is expected to have very limited effects based upon knowledge of its pharmacology and (patho)physiology and that is studied in the wrong population, with low-quality studies that use a surrogate end-point that relates to the clinical end-point in a partial manner at most. Such a medicine would surely not be recommended. The use of recombinant human erythropoietin (rHuEPO) to enhance performance in cycling is very common. A qualitative systematic review of the available literature was performed to examine the evidence for the ergogenic properties of this drug, which is normally used to treat anaemia in chronic renal failure patients. The results of this literature search show that there is no scientific basis from which to conclude that rHuEPO has performance-enhancing properties in elite cyclists. The reported studies have many shortcomings regarding translation of the results to professional cycling endurance performance. Additionally, the possibly harmful side-effects have not been adequately researched for this population but appear to be worrying, at least. The use of rHuEPO in cycling is rife but scientifically unsupported by evidence, and its use in sports is medical malpractice. What its use would have been, if the involved team physicians had been trained in clinical pharmacology and had investigated this properly, remains a matter of speculation. A single well-controlled trial in athletes in real-life circumstances would give a better indication of the real advantages and risk factors of rHuEPO use, but it would be an oversimplification to suggest that this would eradicate its use.
Keywords: athletic performance, doping in sports, erythropoietin, recombinant proteins
Sport is big business
The summer of 2012 was an intensive summer of sport. From all these events, it is clear that sport plays a very important role in our society, because it brings people together, gives pleasure, keeps people healthy and can bring professional athletes fame and honour.
Sport has grown to be so important that large amounts of money are now involved, and the will and pressure to win have steadily increased. Cheating has therefore become a threat to all sports, with some sports being more susceptible to it than others. Cheating by use of medicines has understandably taken place outside the realm of clinical pharmacology and evidence-based medicine. We question whether this is desirable, because uncontrolled use of a substance involves risks for the users, irrespective of such a substance being used legally or illegally.
In this review, we focus on the use of recombinant human erythropoietin (rHuEPO) in cycling, a sport that has had many reports of cheating, culminating in the last decade, with many suspicions and suspensions. We will address the question of whether the currently available evidence justifies the widespread use of this substance. Many of the major champions in cycling have been associated with, or suspended for, use of (blood) doping. In the Tour de France of 1998, the entire Festina team, as well as the TVM team, was taken out of the race on suspicion of rHuEPO use. This Tour was later given the name ‘Tour du Dopage’, and many confessions of systematic doping (i.e. rHuEPO) use throughout the peloton were given. In spite of this, later champions in the Tour de France, Giro d'Italia and Vuelta a España have also been suspended because of proof of blood doping, but the code of silence, also called ‘omerta’, was never broken. Seven years after the last of seven consecutive Tour de France wins, one of the most successful road cyclists ever, Lance Armstrong, has been suspended for life by the United States Anti-Doping Agency (USADA) on charges of doping (e.g. rHuEPO) use and trafficking, in the biggest doping case ever, backed by confessions of many of his teammates 1.
Knowledge of both the effects and the side-effects of rHuEPO in this population is essential, especially with so many misconceptions among the people involved. Firstly, if the effects are not pronounced, the motives for misuse will be less strong. Secondly, even if the effects are pronounced, knowledge of the potentially dangerous side-effects needs to be communicated to the cyclists, who are likely to be under severe pressure to use performance-enhancing agents, together with the coaches and physicians supervising them 1.
Physiology of erythropoietin
Erythropoietin (EPO) is a (glyco)protein that is mainly involved in erythropoiesis, the (re-)generation of erythrocytes, or red blood cells. Red blood cells are cells without a nucleus that transport oxygen through the blood. Owing to a lack of ability to repair themselves without a nucleus and other cellular machinery, erythrocytes have a lifespan of approximately 120 days in the circulation and after that need to be replaced 2. The spleen removes the old erythrocytes (2–3 million every second) and, to keep the oxygen-carrying capacity of the blood at a steady level, constant erythropoiesis is necessary 2. Erythropoiesis starts in the bone marrow, where red blood cells originate from pluripotent stem cells 3. These stem cells continuously make identical copies of themselves and, in that way, create progenitor cells for, among others, erythrocytic cells 3. These cells go through different stages, one of which is the burst-forming unit-erythroid. This cell type matures into a colony-forming unit-erythroid (CFU-E), which in turn forms the proerythroblast, which divides four times into 16 reticulocytes, later developing into mature red blood cells 3.
The first report of a factor influencing this red blood cell production was by Carnot and DeFlandre 4, who called it ‘hemopoietine’. This factor, now called erythropoietin, is a hormone of 165 amino acids with four glycosylation sites, a molecular weight of 30 400 and a carbohydrate content of 40% 5, 6. In normal (nonhypoxic) conditions, the concentration of EPO in blood is relatively constant at approximately 5 pmol l−1, essential to stimulate cells in the bone marrow to produce new erythrocytes, compensating for the physiological demise of erythrocytes 3. This level is equal to ∼20 mU ml−1 when EPO is quantified as ‘international units’ (IU), assuming a specific activity of 130 000 IU mg−1. The cells that are the main target for the hormone are the CFU-Es and proerythroblasts, containing the highest density of erythropoietin receptors (EpoRs) 7. The main effect of EPO is on CFU-Es, because it promotes survival of these cells 8. One of the pathways involved in this process, activated by EPO, is the cell proliferation pathway of Ras/mitogen-activated protein kinase 9, 10. After binding of EPO to its receptor, dimerization of two EpoR molecules occurs, and this starts the intracellular signalling that leads to proliferation of CFU-Es 11, 12.
Production and metabolism
The kidneys are the main EPO-producing organs in humans 13, 14, where peritubular interstitial cells govern its production 15, 16, which is highly regulated. Baseline EPO levels can increase up to 1000-fold in low blood oxygen content, for example in severe anaemia 3. Production of EPO is highly dependent on blood oxygen tension, with hypoxia increasing EPO production, irrespective of the cause of reduced tissue oxygen supply 3. There is a latency of approximately 1.5–2 h before EPO levels start to increase in a linear manner, reflecting the time of signal transduction and hormone synthesis and secretion. Peak EPO concentrations after hypoxia are reached within 48 h, with the concentration being dependent on the severity of hypoxia 3. However, only moderately elevated serum concentrations of EPO seem to be sufficient to maintain an increased rate of erythropoiesis 17.
The proposed oxygen-sensing mechanism that regulates EPO production involves the hypoxia-inducible factor (HIF), a transcription factor 18. Expression of HIF is seen in cells exposed to hypoxia within 30 min 19, after which the heterodimeric protein travels to the nucleus to activate the EPO enhancer 20, inducing EPO transcription. In the presence of oxygen, this factor is hydroxylated, suppressing the activity and promoting degradation 21. Another pathway involved in EPO production is the kinase C pathway, activated through adenosine, which accumulates in hypoxic conditions as a result of the sequential dephosphorylation of ATP, ADP and AMP. This non-HIF pathway also increases EPO mRNA expression 22. GATA-2, a transcription factor, inhibits the EPO promoter, and is a third pathway of EPO regulation. GATA inhibitors can therefore also enhance EPO production 23.
After hypoxia-induced EPO production, a rise in red blood cells and haematocrit (Hct) is seen after 60–70 h 24, corresponding to the time course of CFU-E differentiation into mature erythrocytes 25. The estimated half-life (t1/2) of endogenous EPO is approximately 5.2 h 26, and mechanisms of clearance are somewhat extraordinary. Clearance of EPO by the liver is, as for many other glycoproteins, rather low, mainly due to the terminal sialic acid residues, which prevent galactose receptor binding, internalization and degradation in the liver. Indeed, it has been shown that desialated EPO experiences rapid hepatic clearance 27, but this pathway is only of minor importance 28. Renal clearance also plays only a minor role, as the disappearance rate does not change markedly in the anephric state 29. The major elimination route for EPO seems to be EpoR-mediated uptake and degradation 30, and bone marrow ablation after myoablative conditioning led to a decrease in EPO elimination 31. Similar observations were made in irradiated dogs during altitude exposure 32, and the opposite was seen in patients with hyperactive marrow due to haemolytic anaemia 33. This mechanism, in turn, would indicate that elimination of EPO is related to its affinity to and residence time at the EpoR.
Recombinant erythropoietin in disease
Given that EPO plays an important role in the regulation of erythropoiesis, a major step in medicine was taken when recombinant EPO was first produced by Lin et al. 34 and Jacobs et al. 35 in Chinese hamster ovary cells, later optimized for clinical use in patients with renal anaemia. Trials with the first recombinant human EPO (rHuEPO) showed a correction of anaemia in end-stage renal disease 36, and rHuEPO was approved by the US Food and Drug Administration for human use in patients with chronic renal failure in 1989 22. These first recombinant forms of EPO (called epoetin alfa, e.g. Eprex®) are identical to endogenous human EPO with regard to the amino acid backbone and four glycosylation sites, although some differences in molecular composition of the N-glycans have been found 37. Half-lives are fairly similar to endogenous EPO (4–9 h) 38, which is also the case for second-generation rHuEPO, epoetin beta (e.g. Neorecormon®) 39. The same holds true for a later generation of recombinant EPO produced in human cells, epoetin delta (Dynepo®) 40. Other forms of EPO, darbepoetin alfa (NESP/Aranesp®) and Mircera® (CERA) have a longer half-life due to differences in amino-acid sequence, hyperglycosylation (NESP; t1/2 = 24–26 h 41) and incorporation of a large polymer chain (CERA; t1/2 = 6 days 42). All these forms of rHuEPO can help patients with chronic renal failure to overcome the insufficient production of EPO resulting from the kidney damage and maintain steady-state erythropoiesis.
… and in sport. But does it work?
The treatment immediately attracted the attention of athletes. Given that rHuEPO increases red blood cell mass and exercise capacity in anaemic patients, it might have the same effect in the athlete's body, thereby enhancing performance. With this rationale, athletes started using rHuEPO, and the use of rHuEPO was put on the International Olympic Committee's list of prohibited substances in 1990. The list has now been expanded to all erythropoiesis-stimulating agents [e.g. EPO, darbepoetin, HIF stabilizers, methoxy polyethylene glycol-epoetin beta (CERA) and peginesatide (Hematide®)] 43. The World Anti-Doping Agency defines blood doping as ‘… the misuse of certain techniques and/or substances to increase one's red blood cell mass, which allows the body to transport more oxygen to muscles and therefore increase stamina and performance’ 43.
But do rHuEPO and other erythropoiesis-stimulating agents increase red blood cell mass in world-class cyclists and does this result in increased stamina and performance? Here, we examine the factors that determine stamina and endurance performance, especially in elite cycling, and then the effects of rHuEPO on these parameters are reviewed.
What is endurance performance?
Main determining factors
The main determinants of aerobic endurance performance are the maximal oxygen uptake (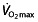 ), the lactate threshold (LT) and the work economy (C) 44. These three factors are now generally accepted as key factors in endurance performance 45–47 and are supported by findings in different studies on
), the lactate threshold (LT) and the work economy (C) 44. These three factors are now generally accepted as key factors in endurance performance 45–47 and are supported by findings in different studies on 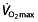 48, 49, LT 47, 48, 50, 51 and C 47, 50, 51. A fourth factor, the lactate turn point (LTP), has also received some attention 52. Here we consider briefly each factor in turn.
48, 49, LT 47, 48, 50, 51 and C 47, 50, 51. A fourth factor, the lactate turn point (LTP), has also received some attention 52. Here we consider briefly each factor in turn.
Maximal oxygen uptake is a prerequisite but not a sole determining factor
The maximal oxygen uptake has traditionally been regarded as the most important measure in endurance performance. According to Fick's Law, it is dependent on cardiac output and the arteriovenous oxygen difference. These, in turn, are mainly dependent on total blood volume, which is the main limiting factor of stroke volume, and total body haemoglobin. However, lung diffusing capacity, heart rate, distribution of the blood volume to working skeletal muscles and arterial O2 extraction contribute to 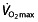 as well, as reviewed by Joyner and Coyle 45 and Bassett and Howley 53 and reported by other researchers 54, 55. Heinicke et al. 54 demonstrated the relationship between
as well, as reviewed by Joyner and Coyle 45 and Bassett and Howley 53 and reported by other researchers 54, 55. Heinicke et al. 54 demonstrated the relationship between 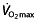 and blood volume and total body haemoglobin in endurance disciplines. Training can improve many of the mentioned factors to increase
and blood volume and total body haemoglobin in endurance disciplines. Training can improve many of the mentioned factors to increase 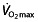 , such as increasing blood volume 56, and indeed, the
, such as increasing blood volume 56, and indeed, the 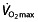 values of champion endurance athletes are 50–100% greater than those observed in normally active healthy young subjects 45. That an increase in
values of champion endurance athletes are 50–100% greater than those observed in normally active healthy young subjects 45. That an increase in 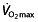 has a great potential to increase endurance performance was already shown by Buick et al. 57 and Brien and Simon 58. After autologous red blood cell reinfusion to elevate haemoglobin and haematocrit levels in well-trained runners, running performance was significantly increased. Ekblom 59 cites another article by Celsing et al. 60 to show that an increase in haemoglobin, irrespective of baseline haemoglobin levels, will increase maximal aerobic power and therefore performance. However, the last statement in this paper by Ekblom is at least as important, where the author warns against extrapolating this finding to the physically fit athlete, because in these subjects factors other than haemoglobin and maximal aerobic power may play a limiting role in performance. Later research emphasized this warning, because
has a great potential to increase endurance performance was already shown by Buick et al. 57 and Brien and Simon 58. After autologous red blood cell reinfusion to elevate haemoglobin and haematocrit levels in well-trained runners, running performance was significantly increased. Ekblom 59 cites another article by Celsing et al. 60 to show that an increase in haemoglobin, irrespective of baseline haemoglobin levels, will increase maximal aerobic power and therefore performance. However, the last statement in this paper by Ekblom is at least as important, where the author warns against extrapolating this finding to the physically fit athlete, because in these subjects factors other than haemoglobin and maximal aerobic power may play a limiting role in performance. Later research emphasized this warning, because 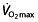 was found not to be the only determinant of endurance performance, and more emphasis has recently come to the other two factors described by Pate and Kriska 44. The
was found not to be the only determinant of endurance performance, and more emphasis has recently come to the other two factors described by Pate and Kriska 44. The 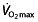 , although a prerequisite to perform at a high level 48, has a very limited predictive value for endurance performance within a group of high-performance athletes 61–67. Also, although successful endurance athletes reached a high
, although a prerequisite to perform at a high level 48, has a very limited predictive value for endurance performance within a group of high-performance athletes 61–67. Also, although successful endurance athletes reached a high 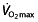 after initial years of training, they subsequently maintain a plateau in their
after initial years of training, they subsequently maintain a plateau in their 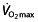 but continue to improve their performance further 47, 68, 69 (note that one of these reports 69 is about Lance Armstrong). Research into training for endurance performance shows the same trend; moderately trained athletes are able to improve
but continue to improve their performance further 47, 68, 69 (note that one of these reports 69 is about Lance Armstrong). Research into training for endurance performance shows the same trend; moderately trained athletes are able to improve 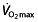 (as well as LT and C) by interval and/or intensive training 70, 71, whereas these training regimens do not improve
(as well as LT and C) by interval and/or intensive training 70, 71, whereas these training regimens do not improve 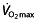 in well-trained athletes, but mainly improve the LT and C 50, 72, possibly by improving buffering capacity 73.
in well-trained athletes, but mainly improve the LT and C 50, 72, possibly by improving buffering capacity 73.
It is more than the 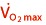
It is not 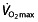 , but power output at submaximal intensities, such as the first (VT1 or VT) and second ventilation threshold (VT2), or the respiratory compensation point (RCP) that differ significantly between elite amateur and professional cyclists 64, 74. Thus, factors other than
, but power output at submaximal intensities, such as the first (VT1 or VT) and second ventilation threshold (VT2), or the respiratory compensation point (RCP) that differ significantly between elite amateur and professional cyclists 64, 74. Thus, factors other than 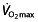 play an important role in determining performance in professional and world-class cyclists. For example, when a published model 75 for predicting endurance performance is used to predict the 1 h cycling world record, as described by Padilla et al. 76, its predictions are far from the observed results. Based on the
play an important role in determining performance in professional and world-class cyclists. For example, when a published model 75 for predicting endurance performance is used to predict the 1 h cycling world record, as described by Padilla et al. 76, its predictions are far from the observed results. Based on the 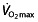 and body mass of Miguel Indurain, a professional cyclist, the distance covered in 1 h predicted by the model would have been 43.645 km, whereas the world record he set was 53.040 km h−1. Calculating back from this record, the model would predict an impossible
and body mass of Miguel Indurain, a professional cyclist, the distance covered in 1 h predicted by the model would have been 43.645 km, whereas the world record he set was 53.040 km h−1. Calculating back from this record, the model would predict an impossible 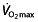 of 10.3 l min−1, whereas ranges for world-class athletes are 5–6 l min−1
67, 77, 78. This and another model 79 both rely on
of 10.3 l min−1, whereas ranges for world-class athletes are 5–6 l min−1
67, 77, 78. This and another model 79 both rely on 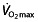 as the most important determinant for endurance performance and describe the relationship as being proportionally curvilinear, meaning that the better the athlete is trained, a similar increase in
as the most important determinant for endurance performance and describe the relationship as being proportionally curvilinear, meaning that the better the athlete is trained, a similar increase in 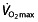 leads to a proportionally smaller increase in performance. This also demonstrates that in world-class athletes, an increase in
leads to a proportionally smaller increase in performance. This also demonstrates that in world-class athletes, an increase in 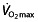 will have only limited effect on performance. The failure of such a model 75 to predict 1 h performance 76 suggests that factors other than
will have only limited effect on performance. The failure of such a model 75 to predict 1 h performance 76 suggests that factors other than 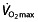 are limiting in endurance performance at this level of performance.
are limiting in endurance performance at this level of performance.
Lactate threshold
We therefore now address the importance of LT in determining the performance of endurance athletes. Lactate threshold, similar to VT1, is the intensity of work or oxygen uptake (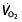 ) at which the blood lactate concentration gradually starts to increase 80. Aerobic enzyme activity is a major determinant of LT, reflected by a decline in activity during a period of detraining accompanying a reduction in LT 81. Given that LT reflects an onset of anaerobic metabolism and the coinciding metabolic alterations 45, 53, LT in turn determines the fraction of maximal aerobic power that can be sustained for an extended period. Several studies show that the
) at which the blood lactate concentration gradually starts to increase 80. Aerobic enzyme activity is a major determinant of LT, reflected by a decline in activity during a period of detraining accompanying a reduction in LT 81. Given that LT reflects an onset of anaerobic metabolism and the coinciding metabolic alterations 45, 53, LT in turn determines the fraction of maximal aerobic power that can be sustained for an extended period. Several studies show that the 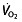 at this LT is highly related to performance, more so than
at this LT is highly related to performance, more so than 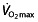 45, 47, 63–65, 67, 68, 82. Elite cyclists are reported to be able to reach LTs between 300 and 400 W 63, 77, 83, or 70–85%
45, 47, 63–65, 67, 68, 82. Elite cyclists are reported to be able to reach LTs between 300 and 400 W 63, 77, 83, or 70–85% 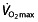 (3.5–4.7 l min−1) 65, 67. The LT reflects a balance between the rate of lactate production in the muscles (hence, the rate of lactate influx to the blood) and clearance from the blood.
(3.5–4.7 l min−1) 65, 67. The LT reflects a balance between the rate of lactate production in the muscles (hence, the rate of lactate influx to the blood) and clearance from the blood.
In this balance, another independent factor appears to play a role in endurance performance; the difference in performance (time to fatigue) in cyclists with similar values of 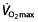 can be explained by
can be explained by 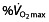 at LT, but an additional increase in performance in some athletes seems to be related to a high muscle capillary density 45, 65. A similar correlation between endurance performance and capillary density was found in another study by Coyle et al. 67, and Anderson and Henriksson 84 found that capillary density increases with training. This might indicate that these athletes have a higher capacity to remove and recycle muscle-fatiguing metabolites, thereby allowing muscles to tolerate lactic acid production and anaerobic metabolism better 85, or to maintain/elongate mean transit time of the blood to increase oxygen extraction 86.
at LT, but an additional increase in performance in some athletes seems to be related to a high muscle capillary density 45, 65. A similar correlation between endurance performance and capillary density was found in another study by Coyle et al. 67, and Anderson and Henriksson 84 found that capillary density increases with training. This might indicate that these athletes have a higher capacity to remove and recycle muscle-fatiguing metabolites, thereby allowing muscles to tolerate lactic acid production and anaerobic metabolism better 85, or to maintain/elongate mean transit time of the blood to increase oxygen extraction 86.
Lactate turn point
Lactate turn point (LTP) is a distinct factor that is also related to lactate 52. The RCP 87, VT2 or the onset of blood lactate accumulation (OBLA) 88 are related measures. These factors represent a level of high work intensity, at which lactate concentrations show a sudden and sustained rise and hypocapnic hyperventilation occurs 63, 68. This threshold is notably high in professional cyclists and is an important factor during extreme endurance events 64, 83. A relationship between RCP and endurance performance has been reported 63, 89, with world-class cyclists having values up to 430–505 W 63, 76, 83, 90, or 90% of 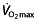 .
.
Economy
The third main factor contributing to endurance performance is assumed to be completely independent of 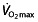 and lactate-related factors and is called work economy or efficiency (C). It is the ratio between work output (speed or power) and oxygen cost. Running economy is commonly defined as the steady-rate
and lactate-related factors and is called work economy or efficiency (C). It is the ratio between work output (speed or power) and oxygen cost. Running economy is commonly defined as the steady-rate 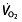 in millilitres per kilogram per minute at a standard velocity, cycling economy as the caloric expenditure at a given work rate. Several physiological and biomechanical factors influence C in trained or elite athletes. These include metabolic adaptations within the muscle, such as increased mitochondria and oxidative enzymes, the ability of the muscles to store and release elastic energy by altering the mechanical properties of the muscles, and more efficient mechanics, leading to less energy being wasted on braking forces and excessive vertical oscillation 44. The work efficiency is a discriminator of endurance performance independently of
in millilitres per kilogram per minute at a standard velocity, cycling economy as the caloric expenditure at a given work rate. Several physiological and biomechanical factors influence C in trained or elite athletes. These include metabolic adaptations within the muscle, such as increased mitochondria and oxidative enzymes, the ability of the muscles to store and release elastic energy by altering the mechanical properties of the muscles, and more efficient mechanics, leading to less energy being wasted on braking forces and excessive vertical oscillation 44. The work efficiency is a discriminator of endurance performance independently of 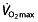 in runners 48, 68, 91–93 and cyclists 63, 69, 78, becoming more important than
in runners 48, 68, 91–93 and cyclists 63, 69, 78, becoming more important than 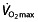 once a certain level of fitness is reached 63. A possible explanation for differences in C between individuals is the composition of the working muscles, where higher economy implies an improved efficiency of ATP turnover within muscle fibres during contraction 94. Different muscle fibre types have different efficiencies; type I fibres (slow twitch) are most efficient, then type IIa fibres are recruited and lastly type IIb fibres (fast twitch). The work efficiency (hence, endurance performance) is related to the percentage of type I fibres 67, 94, 95. Training can induce changes from type IIb to IIa and from type IIa to type I in animals 96, and possibly in humans 67, 97.
once a certain level of fitness is reached 63. A possible explanation for differences in C between individuals is the composition of the working muscles, where higher economy implies an improved efficiency of ATP turnover within muscle fibres during contraction 94. Different muscle fibre types have different efficiencies; type I fibres (slow twitch) are most efficient, then type IIa fibres are recruited and lastly type IIb fibres (fast twitch). The work efficiency (hence, endurance performance) is related to the percentage of type I fibres 67, 94, 95. Training can induce changes from type IIb to IIa and from type IIa to type I in animals 96, and possibly in humans 67, 97.
Other factors
Besides these main determinants, several other factors have also been reported to influence endurance performance. Heart rate, for example, shows a rightward shift in its relationship with running speed 68 as a consequence of chronic endurance training, although values corresponding to physiological markers such as LT and VT2 remain stable 68, 83. This could be related to an increase in cardiac volume due to endurance training 98, 99, leading to an increase in stroke volume and allowing a reduced heart rate for the same cardiac output. Breathing pattern is another factor that influences cycling performance, because professional cyclists have been reported to lack a tachypnoeic shift at high workloads, possibly indicating a more efficient use of their respiratory muscles 100. Also, the quantity of muscle mass recruited for sustained power production can influence performance, because elite cyclists can use 20–25% more muscle mass in endurance tests, therefore reducing the stress and power production per fibre 65, 101. Additionally, peak power output has been shown to be a predictor of performance in a time trial 102, and power-to-weight ratios contribute to climbing performance in cycling 103. Lastly, two world-class endurance performance athletes have been shown to have extremely low peak blood lactate concentrations, which might indicate a mechanism for their outstanding performances 68, 69 (note that one of these reports is about Lance Armstrong 69).
In summary, endurance performance mainly depends on an athlete's 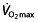 , LT, LTP and C;
, LT, LTP and C; 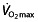 and LT/LTP interact to determine how long a rate of aerobic and anaerobic metabolism can be sustained, and C then determines how much speed or power is achieved for a given level of energy consumption. The relative importance of each of these factors differs at different levels of training. Moderately trained athletes can easily improve all factors, whereas increasing performance in elite athletes is mainly governed by changes in LT, LTP and C. Additional factors, including capillary density, heart rate and heart volume, muscle mass and breathing pattern, can also influence endurance performance.
and LT/LTP interact to determine how long a rate of aerobic and anaerobic metabolism can be sustained, and C then determines how much speed or power is achieved for a given level of energy consumption. The relative importance of each of these factors differs at different levels of training. Moderately trained athletes can easily improve all factors, whereas increasing performance in elite athletes is mainly governed by changes in LT, LTP and C. Additional factors, including capillary density, heart rate and heart volume, muscle mass and breathing pattern, can also influence endurance performance.
Studying the effects of rHuEPO on endurance performance
Search strategy
Several studies have addressed the effects of rHuEPO with regard to endurance performance in subjects other than patients. A literature search was conducted in PubMed to identify these papers, using combinations of the key words ‘erythropoietin’, ‘athletic performance’, ‘physical endurance’, ‘doping in sports’ and ‘athletes’ for the primary search. Literature references in key papers were examined manually to identify additional papers. We did not attempt to derive quantitative systematic conclusions from a meta-analysis; therefore, this could be termed a qualitative systematic review.
Study population mismatch with professional cyclists
Some of the identified studies included ‘(endurance trained) recreational athletes’ or ‘well-trained individuals’, others ‘healthy normal subjects’. This raises a problem when interpreting these studies. No standard, such as that proposed by Jeukendrup et al. 77, has been used to classify the cycling abilities of the subjects, and included subjects vary in baseline endurance performance and fitness level within a study and between studies. The level of training of the subjects is poorly reported, but when trying to classify cycling ability 77 using the scarce information reported, based on maximal power output and 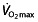 (absolute and per kilogram of body weight), subjects in these studies would be either ‘untrained cyclists’ 104–110 or ‘trained cyclists’ 111–115 or ‘unclassifiable’ 116. It is, however, clear that in no study reported subjects are at a ‘competing’ level of cycling performance. This highlights a very problematic aspect, which is that the studies do not use well-trained cyclists, still less elite or world-class cyclists, who would be expected 77 to have
(absolute and per kilogram of body weight), subjects in these studies would be either ‘untrained cyclists’ 104–110 or ‘trained cyclists’ 111–115 or ‘unclassifiable’ 116. It is, however, clear that in no study reported subjects are at a ‘competing’ level of cycling performance. This highlights a very problematic aspect, which is that the studies do not use well-trained cyclists, still less elite or world-class cyclists, who would be expected 77 to have 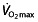 values above 70 ml kg−1 min−1 (5 l min−1) and power outputs above 5 W kg−1. Figure 1 compares the studied subjects with these reference values. In the only study using subjects with mean power outputs above 5 W kg−1, the mean
values above 70 ml kg−1 min−1 (5 l min−1) and power outputs above 5 W kg−1. Figure 1 compares the studied subjects with these reference values. In the only study using subjects with mean power outputs above 5 W kg−1, the mean 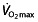 is only ∼64 ml kg min−1
111. The studied subjects may not have reached a plateau in
is only ∼64 ml kg min−1
111. The studied subjects may not have reached a plateau in 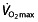 , confounding the interpretation as explained above. Cyclists classified as ‘well trained’ or higher differ in factors contributing to endurance performance from ‘trained’ or ‘untrained’ cyclists 45, 77, 117. The
, confounding the interpretation as explained above. Cyclists classified as ‘well trained’ or higher differ in factors contributing to endurance performance from ‘trained’ or ‘untrained’ cyclists 45, 77, 117. The 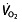 kinetics are very different even between ‘well-trained’ and ‘world-class’ cyclists 63. Additionally, this classification shows that there are major discrepancies between the groups in training status, which makes comparison difficult. Hopkins et al. 118 state that: ‘the results of a research study apply with reasonable certainty only to populations that have similar characteristics to the sample under study. Elite athletes almost certainly have genetic endowment, training history, and training programs that differ from those of subelite athletes. A treatment may therefore produce different effects on performance in these two groups. It follows that the subjects in a study have to be elite athletes for the results to apply convincingly to elite athletes.’ Therefore, it cannot be assumed that effects found in these rHuEPO studies on healthy untrained or trained individuals automatically apply to well-trained, elite and world-class cyclists.
kinetics are very different even between ‘well-trained’ and ‘world-class’ cyclists 63. Additionally, this classification shows that there are major discrepancies between the groups in training status, which makes comparison difficult. Hopkins et al. 118 state that: ‘the results of a research study apply with reasonable certainty only to populations that have similar characteristics to the sample under study. Elite athletes almost certainly have genetic endowment, training history, and training programs that differ from those of subelite athletes. A treatment may therefore produce different effects on performance in these two groups. It follows that the subjects in a study have to be elite athletes for the results to apply convincingly to elite athletes.’ Therefore, it cannot be assumed that effects found in these rHuEPO studies on healthy untrained or trained individuals automatically apply to well-trained, elite and world-class cyclists.
Figure 1.
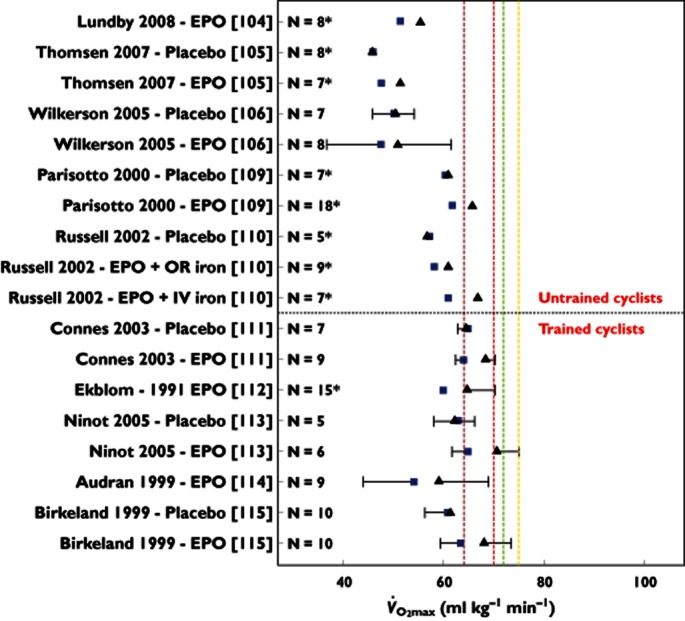
Maximal oxygen uptake (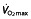 ) before (
) before ( ) and after (▴) treatment with rHuEPO in the different studies per treated group (bars representing SD). N is the number of subjects in each group, with an asterisk indicating that the article reported
) and after (▴) treatment with rHuEPO in the different studies per treated group (bars representing SD). N is the number of subjects in each group, with an asterisk indicating that the article reported 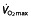 values only in litres per minute, which has been converted to millilitres per kilogram per minute by dividing this value by mean weight of the group for comparison purposes (no SD is given for these studies because of this conversion). Studies above the horizontal red dotted line were performed using subjects classified as untrained, while below the horizontal line the subjects were classified as trained cyclists. Vertical dashed lines represent minimal values of
values only in litres per minute, which has been converted to millilitres per kilogram per minute by dividing this value by mean weight of the group for comparison purposes (no SD is given for these studies because of this conversion). Studies above the horizontal red dotted line were performed using subjects classified as untrained, while below the horizontal line the subjects were classified as trained cyclists. Vertical dashed lines represent minimal values of 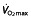 for different classifications of cyclists as suggested by Jeukendrup et al. 77 (brown dashed line, trained; red dashed line, well trained; green dashed line, elite; and yellow dashed line, world class).
for different classifications of cyclists as suggested by Jeukendrup et al. 77 (brown dashed line, trained; red dashed line, well trained; green dashed line, elite; and yellow dashed line, world class).  ,
, 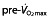 ; ▴,
; ▴, 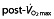 ;
;  , trained;
, trained;  , well-trained;
, well-trained;  , elite; and
, elite; and  , world class
, world class
Recombinant human erythropoietin dosing
The doses of rHuEPO in all studies vary, but all are subcutaneous injections, most in a similar range of 150 IU kg−1 week−1 (Table 1). Almost all studies used forms of rHuEPO with half-lives similar to endogenous EPO, namely Eprex® 109–111, 113, 114, 116 or Neorecormon® 104–107, Recormon® 115, or it was not reported 112. Only one study used rHuEPO with a longer half-life, NESP 108. Another problem with evaluating the results of these studies is that only eight 105, 106, 109–111, 113, 115, 116 of the 13 studies were placebo controlled. As endurance performance can change significantly, for example, due to training, it is crucial to control for these effects with a placebo-treated group. Moreover, unfortunately only five 106, 109, 111, 113, 115 of these studies were reported to be double blinded, controlling for any bias due to expectation of a positive treatment effect, which is potentially of major influence on the exercise tests performed in the studies, the outcome of which depends on perseverance, hence on motivation. Given that the study using NESP as rHuEPO treatment was not placebo controlled and did not measure any performance parameters during normoxia, it is difficult to draw conclusions about the effects of this form of rHuEPO on endurance performance. Moreover, the newest form of rHuEPO, CERA, to our knowledge has not yet been studied for effects on endurance performance in athletes at all.
Table 1.
Overview of characteristics and outcomes of the studies investigating the effects of recombinant human erythropoietin on endurance performance in subjects other than patients
| Study | Types of subjects | Study set-up | Product | Dosing | Max. Hb increase (%) | Max. Hct increase (%) | Max. 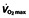 increase (%) increase (%) |
|---|---|---|---|---|---|---|---|
| Lundby et al. (2008) 104 | Untrained | Uncontrolled | Neorecormon® | 5000 IU (∼65 IU kg−1) on alternating days for 14 days followed by once a week for 2 weeks | 10.2 | 11.2 | 7.9 |
| Thomsen et al. (2007) 105 | Untrained | Placebo | Neorecormon® | 5000 IU (∼60 IU kg−1) on alternating days for 2 weeks, a dose on three consecutive days for 1 week and one dose a week for 12 weeks | 11.1 | 10.7 | 9.1 |
| Wilkerson et al. (2005) 106 | Untrained | Placebo + blinded | Neorecormon® | 150 IU kg−1 once a week for 4 weeks | 7 | 12 | 7 |
| Rasmussen et al. (2010) 107 | Untrained | Uncontrolled | Neorecormon® | 5000 IU (∼60 IU kg−1) on alternating days for 2 weeks, a dose on three consecutive days for 1 week and one dose a week for 12 weeks | NA | 12 | NA |
| Lundby & Damsgaard (2006) 108 | Untrained | Uncontrolled | NESP | 144 IU kg−1 (0.72 μg kg−1) once a week for 4 weeks | 17.4 | 16.4 | NA |
| Parisotto et al. (2000) 109 | Untrained | Placebo + blinded | Eprex® | 50 IU kg−1 three times a week over 25 days (in combination with ∼100 mg iron either intramuscularly or orally) | 7.4/12 | NA | 6.3/6.9 |
| Russell et al. (2002) 110 | Untrained | Placebo | Eprex® | 50 IU kg−1 three times a week for 3 weeks and 20 IU kg−1 for 5 weeks | NA | 15 | 9.7 |
| Connes et al. (2003) 111 | Trained | Placebo + blinded | Eprex® | 50 IU kg−1 three times a week for 4 weeks | 9.6 | 8.3 | 7 |
| Ekblom & Berglund (1991) 112 | Trained | Uncontrolled | NA | 20 IU kg−1 three times a week for 6 weeks (or 4 weeks, and 40 IU kg−1 for the remaining 2 weeks) | NA | 11.7 | 8 |
| Ninot et al. (2006) 113 | Trained | Placebo + blinded | Eprex® | 50 IU kg−1 three times a week for 4 weeks, followed by 20 IU kg−1 three times a week for 2 weeks | 9.5 | 10.2 | 7 |
| Audran et al. (1999) 114 | Trained | Uncontrolled | Eprex® | 50 IU kg−1 daily for 26 days | 9.3 | 11.5 | 9.3 |
| Birkeland et al. (2000) 115 | Trained | Placebo + blinded | Recormon® | 5000 IU (181–232 IU kg−1 week−1) three times a week | 11.2 | 19 | 7 |
| Souillard et al. (1996) 116 | Unknown | Placebo | Eprex® | 200 IU kg−1 five times in 11 days | 4.6 | 8.9 | NA |
All effects are calculated based on the greatest difference found in the parameter when multiple measuring time points were reported. Abbreviations are as follows: Hb, haemoglobin; Hct, haematocrit; NA, not applicable; NEPS, novel erythropoiesis stimulating protein (darbepoietin-alfa);  , maximal oxygen uptake.
, maximal oxygen uptake.
Haematological effects of rHuEPO
Although doses differ somewhat across the studies, most studies report similar effects on haematological parameters albeit with a suggestion of dose-related effect. Reticulocyte numbers approximately doubled with the lower doses 109, 110 and tripled with the higher doses 114, 116, and declined to below baseline approximately 7–14 days after rHuEPO treatment ceased 109, 110, 114, 116. Erythropoietin concentrations also drop below baseline after rHuEPO treatment is stopped 109, 116. Increases of 4.6–17.4 and 8.3–19% are reported for haemoglobin concentration and Hct, respectively (Table 1), with no obvious differences between athletes with different training statuses. These levels are reported to return to baseline within 1 month after cessation of treatment 109.
An increase in Hct could lead to an increase in oxygen-carrying capacity, but does this enhance performance? Haematocrit is not a good marker of performance, because endurance athletes usually have lower Hct values than untrained subjects owing to plasma volume expansion 119. Additionally, it is a very variable measure and is affected by different circumstances 120. Increases of Hct cause an increase in viscosity of the blood 121, 122, which might hamper performance owing to reductions in blood flow and increased cardiac work. Decreased plasma volume during exercise exaggerates increased Hct 120, as may dehydration, hyperthermia and altitude, so it is not obvious what effects a rise in Hct will have in professional cyclists. The rHuEPO treatment not only increases haemoglobin concentration and Hct, but at the same time decreases plasma volume, thereby resulting in almost no effect on, or a slight decrease in, blood volume 123. Recombinant human erythropoietin could therefore counteract the plasma volume expansion of endurance training 56. Nevertheless, the combination of effects seems to increase the performance parameter 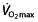 , at least in the studied subjects in laboratory conditions.
, at least in the studied subjects in laboratory conditions.
Effects on 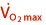
The most important question then is whether these effects on haematological parameters translate into an effect on performance. The different parameters that determine endurance performance have been discussed in the foregoing sections, but unfortunately most studies examine only one of these parameters, namely 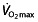 . In the reported studies, this parameter is increased in the rHuEPO-treated subjects, with a relatively constant value for all studies, independent of the training status of the subjects, between 7 and 9.7% (Table 1). Absolute values of
. In the reported studies, this parameter is increased in the rHuEPO-treated subjects, with a relatively constant value for all studies, independent of the training status of the subjects, between 7 and 9.7% (Table 1). Absolute values of 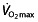 and treatment effects can be seen in Figure 1. This increase in
and treatment effects can be seen in Figure 1. This increase in 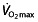 has been reported to be accompanied by an increase in power output 105, 106, 110, 111, 114. This, in turn, resulted in an increase in performance estimated by a time-to-exhaustion test of 22 106 and 54.3% 105 in untrained subjects and a smaller increase of 9.4 (vs. 1.5% in placebo-treated subjects) 115 and 16.6% 112 in trained subjects. Importantly, this surrogate parameter (time to exhaustion) is measured in a test lasting about 20 min and leading to exhaustion, entirely different from the required ∼5 h performance in a cycling race.
has been reported to be accompanied by an increase in power output 105, 106, 110, 111, 114. This, in turn, resulted in an increase in performance estimated by a time-to-exhaustion test of 22 106 and 54.3% 105 in untrained subjects and a smaller increase of 9.4 (vs. 1.5% in placebo-treated subjects) 115 and 16.6% 112 in trained subjects. Importantly, this surrogate parameter (time to exhaustion) is measured in a test lasting about 20 min and leading to exhaustion, entirely different from the required ∼5 h performance in a cycling race.
Does it translate to cycling performance?
As mentioned earlier, 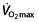 is poorly related to cycle performance 64, 74, and Lucia et al. 124 even questions whether
is poorly related to cycle performance 64, 74, and Lucia et al. 124 even questions whether 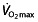 is the limiting factor for maximal endurance performance in some 50% of professional cyclists, owing to a lack of plateau in
is the limiting factor for maximal endurance performance in some 50% of professional cyclists, owing to a lack of plateau in 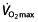 during an exercise-to-exhaustion test. Additionally, time-to-exhaustion protocols like the ones used here are subject to high variability and poor reproducibility 125, 126, whereas time-trial protocols would give a better performance evaluation 125, also eliminating the influence of wrongly extrapolating results from a laboratory test setting to race events 118. The use of rHuEPO in these subjects clearly has an effect on
during an exercise-to-exhaustion test. Additionally, time-to-exhaustion protocols like the ones used here are subject to high variability and poor reproducibility 125, 126, whereas time-trial protocols would give a better performance evaluation 125, also eliminating the influence of wrongly extrapolating results from a laboratory test setting to race events 118. The use of rHuEPO in these subjects clearly has an effect on 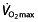 , which might improve performance at peak intensity during severe exercise, although evidence for this is rather ‘soft’. Apart from the uncertainty concerning whether these same effects can be observed in well-trained or elite cyclists, surprisingly little is known from these studies about effects on submaximal intensities. This might be of major importance when looking at the nature of cycling. Long exercise times during consecutive days, with the finish line as a known end-point (contrary to the ‘open end’ of time-to-exhaustion tests) makes it crucial for cyclists to distribute their power during a race. This, combined with (team) tactics, the terrain and the effects of drag force, means that cyclists work for only a small amount of time at their peak intensities, or even above intensities where lactate accumulation occurs. Investigations in world-class cyclists show that during 3 week races the subjects' heart rate is above such an intensity (HROBLA) for only 3.6% (119 s) of the time climbing a ‘Hors catégorie’ climb (hardest climb), even less so during first and second category climbs, 2.6% (45 s) and 2.5% (22 s), respectively 90. Similar low percentages were reported by Lucia et al. 127 for total race time with heart rate above the RCP (at 90%
, which might improve performance at peak intensity during severe exercise, although evidence for this is rather ‘soft’. Apart from the uncertainty concerning whether these same effects can be observed in well-trained or elite cyclists, surprisingly little is known from these studies about effects on submaximal intensities. This might be of major importance when looking at the nature of cycling. Long exercise times during consecutive days, with the finish line as a known end-point (contrary to the ‘open end’ of time-to-exhaustion tests) makes it crucial for cyclists to distribute their power during a race. This, combined with (team) tactics, the terrain and the effects of drag force, means that cyclists work for only a small amount of time at their peak intensities, or even above intensities where lactate accumulation occurs. Investigations in world-class cyclists show that during 3 week races the subjects' heart rate is above such an intensity (HROBLA) for only 3.6% (119 s) of the time climbing a ‘Hors catégorie’ climb (hardest climb), even less so during first and second category climbs, 2.6% (45 s) and 2.5% (22 s), respectively 90. Similar low percentages were reported by Lucia et al. 127 for total race time with heart rate above the RCP (at 90% 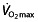 ) during the Tour de France or Vuelta a España, 2.7% (149 min) and 3.3% (166 min), respectively. For time trials, a difference in time spent with a heart rate above OBLA was found between different types of time trials, with prologue, short, long and uphill time trials recording 59, 38, 3.5 and 0% for cyclists going all-out 128. Heart rate values corresponding to physiological markers of performance (e.g. LT and VT2) are stable during a training year in professional cyclists 83.
) during the Tour de France or Vuelta a España, 2.7% (149 min) and 3.3% (166 min), respectively. For time trials, a difference in time spent with a heart rate above OBLA was found between different types of time trials, with prologue, short, long and uphill time trials recording 59, 38, 3.5 and 0% for cyclists going all-out 128. Heart rate values corresponding to physiological markers of performance (e.g. LT and VT2) are stable during a training year in professional cyclists 83.
Other endurance performance parameters are unstudied
For the major part of a race, cyclists therefore exercise well below their 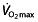 levels, but this parameter has nevertheless attracted the most attention when looking at rHuEPO effects. Some studies that evaluated other parameters found no change in the
levels, but this parameter has nevertheless attracted the most attention when looking at rHuEPO effects. Some studies that evaluated other parameters found no change in the 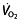 kinetics 105, 106, 110, 129 or
kinetics 105, 106, 110, 129 or 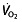 at submaximal exercise 112, despite the increased oxygen-carrying capacity due to the increase in haemoglobin concentration and Hct. This would mean that the oxygen-carrying capacity of the blood does not determine
at submaximal exercise 112, despite the increased oxygen-carrying capacity due to the increase in haemoglobin concentration and Hct. This would mean that the oxygen-carrying capacity of the blood does not determine 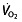 kinetics, but that this is regulated and limited by factors in the muscles rather than oxygen supply. This would also indicate that there is no change in LT in these subjects resulting from rHuEPO treatment, as shown by Wilkerson et al. 106, who found no effect on gas exchange threshold, a measure closely related to LT, due to the rHuEPO treatment. However, other researchers did find an increase in VT of 14.3% 114, although this trial was not placebo controlled, so training and placebo effects cannot be accounted for. Another group 111, with a placebo-controlled, blinded study, also found an increase in VT of 12.6%. No conclusive evidence for effects of rHuEPO on LT/VT is therefore available, with evidence on another important lactate parameter, LTP/OBLA/VT2/RCP, completely absent. It is important to elucidate the effects of rHuEPO on these parameters, because performance in cycling is much better related to these factors 64, 74. Time-trial world-record performance (1 h world record), for example, seems to be best correlated to and predicted by the speed or power output at OBLA 76. Other groups also report that performance in longer time trials is highly correlated to power output at OBLA 130 or power output at LT 131, or with
kinetics, but that this is regulated and limited by factors in the muscles rather than oxygen supply. This would also indicate that there is no change in LT in these subjects resulting from rHuEPO treatment, as shown by Wilkerson et al. 106, who found no effect on gas exchange threshold, a measure closely related to LT, due to the rHuEPO treatment. However, other researchers did find an increase in VT of 14.3% 114, although this trial was not placebo controlled, so training and placebo effects cannot be accounted for. Another group 111, with a placebo-controlled, blinded study, also found an increase in VT of 12.6%. No conclusive evidence for effects of rHuEPO on LT/VT is therefore available, with evidence on another important lactate parameter, LTP/OBLA/VT2/RCP, completely absent. It is important to elucidate the effects of rHuEPO on these parameters, because performance in cycling is much better related to these factors 64, 74. Time-trial world-record performance (1 h world record), for example, seems to be best correlated to and predicted by the speed or power output at OBLA 76. Other groups also report that performance in longer time trials is highly correlated to power output at OBLA 130 or power output at LT 131, or with 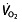 at VT1 132 or LT 67. In >50 km time trials during the Tour the France, performance was correlated with power output at VT1 133. In these time trials,
at VT1 132 or LT 67. In >50 km time trials during the Tour the France, performance was correlated with power output at VT1 133. In these time trials, 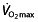 is not related to performance, which was only demonstrated in shorter time trials (20 min) 131. Lastly, uphill cycling also has been correlated best to power outputs at LT or OBLA 130. This means that the most determining disciplines for the general classification in stage races in professional cycling are correlated to submaximal exercise parameters.
is not related to performance, which was only demonstrated in shorter time trials (20 min) 131. Lastly, uphill cycling also has been correlated best to power outputs at LT or OBLA 130. This means that the most determining disciplines for the general classification in stage races in professional cycling are correlated to submaximal exercise parameters.
In the reviewed rHuEPO studies, economy (C), was measured by only one group 105 and did not change after rHuEPO treatment. This would be expected from the nonhaematological, biomechanical factors that determine C, as discussed in foregoing sections. There is, however, some evidence that prolonged exposure to rHuEPO in healthy subjects may induce changes in the skeletal muscle, with an increase in the relative amount of the slow myosin light chain (type I fibres) and decreased fast myosin light chain (type II) fibres, possibly leading to an improvement in C 134. More evidence is needed to draw conclusions about effects of rHuEPO on C, especially given that Lance Armstrong, accused of having the biggest doping (e.g. rHuEPO) network in the history of sports, was reported to have a high muscular efficiency that partly contributed to his world-class performance 69.
Some other parameters, such as blood lactate, end-exercise heart rate and heart rate kinetics, were investigated and reported not to be altered by rHuEPO treatment 106, although other studies indicate a nonsignificant reduction in in blood lactate 110 and heart rate 110, 111 or a significant reduction in in heart rate 114, although only at submaximal exercise 112. A significant drop in blood lactate at rest and 10 min into a time-to-exhaustion test, but not at exhaustion 105, was seen. Blood volume was also not affected 112, 123. One blinded study investgated the effect of rHuEPO on perception of physical well-being and reported a positive effect on perceived physical condition and strength 113 which, on the basis of evidence, is unlikely to be related to an increased muscular mass or improved vascularization. In a publication 135 from the same study performed by Thomsen et al. 105, no effects of prolonged rHuEPO treatment on capillarization or muscle fibre hypertrophy in healthy volunteers were reported; although this is contrasted by an animal study showing that overexpression of EPO resulted in 14% greater muscle volume and 25% increase in muscle vascularization, even these effects did not translate to increased muscle force or stamina 136.
Alternative mechanisms by which EPO works?
It may be argued that focusing on direct endurance measures does not take into account possible mechanisms by which rHuEPO causes better recovery after exercise. Recombinant human erythropoietin may have anti-inflammatory effects and may mitigate ischaemia – reperfusion-related damage 137–140, which could potentially improve recovery. It has been suggested that EPO and its receptor function as a paracrine/autocrine system to mediate the protection of tissues subjected to (metabolic) stress 141. However, these effects have not been confirmed in properly designed clinical trials. In fact, most clinical trials focusing on postulated tissue-protective effects of rHuEPO have shown adverse rather than beneficial effects. Serious untoward effects have also been shown in rHuEPO-treated patients with stroke, myocardial infarction, acute kidney injury and surgery 142, compatible with a procoagulant state and/or an augmentation of acute inflammation 143. The data therefore do not suggest substantial beneficial effects on recovery of muscle injury during exercise.
Thus, except on 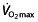 , no coherent or reproducible findings have been reported for both erythropoietic and non-erythropoietic effects of rHuEPO, rendering the evidence too weak to support any conclusion about effects on performance in professional cyclists.
, no coherent or reproducible findings have been reported for both erythropoietic and non-erythropoietic effects of rHuEPO, rendering the evidence too weak to support any conclusion about effects on performance in professional cyclists.
Lack of scientific evidence
Given that (i) most of the research with rHuEPO on endurance performance has focused on a parameter for maximal exercise, 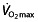 ; (ii) the factors that make professional and world-class cyclists unique are not
; (ii) the factors that make professional and world-class cyclists unique are not 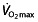 , but LT, RCP and C; (iii) endurance performance in professional cycling, such as in time trials, is best correlated with submaximal exercise factors (e.g. LT, VT1, OBLA and RCP); (iv) only small parts of professional cycling races are cycled at severe or maximal intensities (above OBLA/RCP); and (v) the characteristics of the study populations differed from the population suspected of rHuEPO abuse, it cannot be concluded that rHuEPO use enhances performance in professional or elite cyclists.
, but LT, RCP and C; (iii) endurance performance in professional cycling, such as in time trials, is best correlated with submaximal exercise factors (e.g. LT, VT1, OBLA and RCP); (iv) only small parts of professional cycling races are cycled at severe or maximal intensities (above OBLA/RCP); and (v) the characteristics of the study populations differed from the population suspected of rHuEPO abuse, it cannot be concluded that rHuEPO use enhances performance in professional or elite cyclists.
A more scientific approach is needed
In summary, the available literature lacks the appropriate information, validity and robustness to conclude that rHuEPO enhances world-class cycling performance. To be able to make such statements, more thorough research needs to be conducted to investigate the effects of rHuEPO on submaximal performance parameters and the cycling economy, preferably in a population with cycling performance abilities as close as possible to those of professional cyclists and in conditions closely resembling racing conditions and the required performance duration. It can be argued that putting the treatment on the prohibited list falsely implies a proven beneficial effect on performance in professional cycling and unintentionally stimulates its abuse 144, although it should also be recognized that there is no convincing evidence that any drug works in this context.
Adverse effects of rHuEPO in athletes
Apart from creating a level playground for all athletes by banning and trying to prevent doping use, doping is also forbidden to protect the athletes from using possibly harmful substances. The presented rHuEPO studies in healthy or trained subjects do not focus on adverse effects. A significant rise in systolic blood pressure at rest or during submaximal exercise 112, 129 was reported. The number of subjects and treatment times in the presented studies are too small to detect rare adverse events. Larger studies, namely patient studies, must be consulted for this, although it must be kept in mind that results of these studies do not per se translate to well-trained athletes. One patient study was prematurely discontinued owing to an increased incidence of thrombotic events in rHuEPO-treated metastatic breast cancer patients 145. Other trials and meta-analyses showed a similar trend in different groups of patients treated with rHuEPO compared with placebo 146–148. These studies used approximately four times higher doses of rHuEPO (usually in the range of 40 000 IU or 600 IU kg−1 week−1) compared with the endurance performance studies in healthy subjects. The increased blood viscosity in treated anaemic patients 122, 149, the previously described rise in blood pressure and enhanced coagulation 150, endothelial activation and platelet reactivity 151 and inflammation 152 after rHuEPO treatment have been evoked to explain these thrombotic events. Acute exercise per se also enhances coagulation 153, although this is less pronounced in trained than in untrained subjects. Given that plasma volume and blood volume are reduced in acute exercise, Hct is increased 120. This is even more pronounced in dehydrated and hyperthermic exercise conditions 154, 155. This combination of factors might increase the risk of thrombotic events in endurance performance athletes using rHuEPO. Increased Hct may lower cerebral blood flow and limit oxygen supply to the brain, predisposing to cerebral infarction 156. Thrombotic risks are underlined by a case report by Lage et al. 157 that describes how a professional cyclist presented with cerebral sinus thrombosis, thereafter confessing to 3 months of 2000 IU rHuEPO use every 2 days, in combination with 15 days of growth hormone and continuous high doses of vitamins A and E. High Hct also predisposes to heart failure, myocardial infarction, seizures and pulmonary embolism 158, 159. Another association is hypertensive posterior encephalopathy 160. Red cell aplasia, a rare adverse effect, mainly linked to anti-erythropoietin antibody formation due to Eprex® use 161, is another dire adverse effect of rHuEPO treatment. The improper handling and storage of rHuEPO preparations associated with illicit use in sport might be expected to increase the risks of this and possibly also of other immunogenic complications 162, 163. Finally, rHuEPO use may promote tumour growth and angiogenesis in tumours, although this is contested 164.
To summarize, published case reports have linked adverse effects to rHuEPO use in cyclists. Patient-based studies indicate that rHuEPO has several cardiovascular effects, raising the risk of thrombotic events, encephalopathy and other complications. These risks might plausibly be higher in cyclists because the circumstances in this sport could compound these risks. Also, secrecy due to illicit use in sports might lead to bad handling and storage of the rHuEPO, plausibly elevating the risks of adverse effects, such as red cell aplasia.
Cyclists and rHuEPO: a risky choice to what advantage?
As the case of the United States Anti Doping Agency versus Armstrong proves again, rHuEPO has been used by many professional (including champion) cyclists. Given that it increases Hct, it is thought to enhance performance in professional cycling and has been put on the list of prohibited substances of the International Olympic Committee. As rHuEPO is on this list, cyclists caught were breaking the rules and should be punished for doing so. However, this review shows that only very weak scientific evidence exists about the effects of rHuEPO on cycling performance in professional or even well-trained cyclists. Sport physicians and cyclists should be informed about the dangers of the use of such a substance, as already proposed by Kuipers about doping in general 144. Neither a scientific basis for performance-enhancing properties nor possible harmful side-effects have been provided for athletes or trainees.
The situation with rHuEPO use in athletes is analogous to the many forms of non-evidence-based treatments that exist in medical practice and which, by common opinion, should be refuted or confirmed by good clinical trials with real-life end-points. A single well-controlled trial in athletes during real-life circumstances would give a better indication of the real advantages and risk factors of rHuEPO use, but it would be an oversimplification to suppose that this would eradicate its use, even if no benefit were to be seen with increased biomarkers of risk.
High-quality scientific evidence is always preferable to the current situation, in which athletes risk their career and health with irrational use of a substance. If the size of the athletic benefit could be shown to be large (which, on the basis of the evidence presented in this review, is unlikely), it would support the enormous and apparently largely ineffective efforts currently made to detect and prevent the use of rHuEPO. If the effect were to be small, these efforts could be directed elsewhere.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.United States Anti-Doping Agency. 2012. Reasoned decision of the united states anti-doping agency on disqualification and ineligibility – united states anti-doping agency, claimant, v. lance armstrong, Respondent.
- 2.Sackmann E. Biological membranes architecture and function. In: Lipowsky R, Sackmann E, editors. Handbook of Biological Physics. Amsterdam: Elsevier; 1995. pp. 1–62. [Google Scholar]
- 3.Ratcliffe P, Eckardt K, Bauer C. Hypoxia, erythropoietin gene expression, and erythropoiesis. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology-Environmental Physiology. New York: Published for the American Physiological Society by Oxford University Press; 1996. pp. 1125–1153. [Google Scholar]
- 4.Carnot P, DeFlandre C. Sur l'activite hemopoietique de serum au cours de la regeneration du sang. C R Acad Sci (Paris) 1906;143:384–386. [Google Scholar]
- 5.Imai N, Kawamura A, Higuchi M, Oh-Eda M, Orita T, Kawaguchi T, Ochi N. Physicochemical and biological comparison of recombinant human erythropoietin with human urinary erythropoietin. J Biochem. 1990;107:352–359. doi: 10.1093/oxfordjournals.jbchem.a123050. [DOI] [PubMed] [Google Scholar]
- 6.Recny MA, Scoble HA, Kim Y. Structural characterization of natural human urinary and recombinant DNA-derived erythropoietin. Identification of des-arginine 166 erythropoietin. J Biol Chem. 1987;262:17156–17163. [PubMed] [Google Scholar]
- 7.Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77:2583–2590. [PubMed] [Google Scholar]
- 8.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 9.Gobert S, Duprez V, Lacombe C, Gisselbrecht S, Mayeux P. The signal transduction pathway of erythropoietin involves three forms of mitogen-activated protein (MAP) kinase in UT7 erythroleukemia cells. Eur J Biochem. 1995;234:75–83. doi: 10.1111/j.1432-1033.1995.075_c.x. [DOI] [PubMed] [Google Scholar]
- 10.Miura Y, Miura O, Ihle JN, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 11.Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson LO, Goldwasser E, Fried W, Plzak L. Role of the kidney in erythropoiesis. 1957. J Am Soc Nephrol. 2000;11:589–590. [PubMed] [Google Scholar]
- 14.Kuratowska Z, Lewartowski B, Michalski E. Studies on the production of erythropoietin by isolated organs. Blood. 1961;18:527–534. [PubMed] [Google Scholar]
- 15.Fisher JW, Koury S, Ducey T, Mendel S. Erythropoietin production by interstitial cells of hypoxic monkey kidneys. Br J Haematol. 1996;95:27–32. doi: 10.1046/j.1365-2141.1996.d01-1864.x. [DOI] [PubMed] [Google Scholar]
- 16.Koury ST, Bondurant MC, Koury MJ, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991;77:2497–2503. [PubMed] [Google Scholar]
- 17.Cotes PM, Dore CJ, Yin JA, Lewis SM, Messinezy M, Pearson TC, Reid C. Determination of serum immunoreactive erythropoietin in the investigation of erythrocytosis. N Engl J Med. 1986;315:283–287. doi: 10.1056/NEJM198607313150503. [DOI] [PubMed] [Google Scholar]
- 18.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 19.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 22.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 23.Imagawa S, Nakano Y, Obara N, Suzuki N, Doi T, Kodama T, Nagasawa T, Yamamoto M. A GATA-specific inhibitor (K-7174) rescues anemia induced by IL-1beta, TNF-alpha, or L-NMMA. FASEB J. 2003;17:1742–1744. doi: 10.1096/fj.02-1134fje. [DOI] [PubMed] [Google Scholar]
- 24.Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- 25.Koury MJ, Sawyer ST, Bondurant MC. Splenic erythroblasts in anemia-inducing Friend disease: a source of cells for studies of erythropoietin-mediated differentiation. J Cell Physiol. 1984;121:526–532. doi: 10.1002/jcp.1041210311. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol. 1989;66:1785–1788. doi: 10.1152/jappl.1989.66.4.1785. [DOI] [PubMed] [Google Scholar]
- 27.Goldwasser E, Kung CK, Eliason J. On the mechanism of erythropoietin-induced differentiation. 13. The role of sialic acid in erythropoietin action. J Biol Chem. 1974;249:4202–4206. [PubMed] [Google Scholar]
- 28.Dinkelaar RB, Engels EY, Hart AA, Schoemaker LP, Bosch E, Chamuleau RA. Metabolic studies on erythropoietin (EP): II. The role of liver and kidney in the metabolism of Ep. Exp Hematol. 1981;9:796–803. [PubMed] [Google Scholar]
- 29.Steinberg SE, Garcia JF, Matzke GR, Mladenovic J. Erythropoietin kinetics in rats: generation and clearance. Blood. 1986;67:646–649. [PubMed] [Google Scholar]
- 30.Nalbant D, Saleh M, Goldman FD, Widness JA, Veng-Pedersen P. Evidence of receptor-mediated elimination of erythropoietin by analysis of erythropoietin receptor mRNA expression in bone marrow and erythropoietin clearance during anemia. J Pharmacol Exp Ther. 2010;333:528–532. doi: 10.1124/jpet.109.163568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widness JA, Schmidt RL, Hohl RJ, Goldman FD, Al-Huniti NH, Freise KJ, Veng-Pedersen P. Change in erythropoietin pharmacokinetics following hematopoietic transplantation. Clin Pharmacol Ther. 2007;81:873–879. doi: 10.1038/sj.clpt.6100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stohlman F., Jr Observations on the physiology of erythropoietin and its role in the regulation of red cell production. Ann NY Acad Sci. 1959;77:710–724. doi: 10.1111/j.1749-6632.1959.tb36935.x. [DOI] [PubMed] [Google Scholar]
- 33.Alexanian R. Erythropoietin excretion in bone marrow failure and hemolytic anemia. J Lab Clin Med. 1973;82:438–445. [PubMed] [Google Scholar]
- 34.Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 36.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Bothner B, Dell A, Fukuda M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem. 1987;262:12059–12076. [PubMed] [Google Scholar]
- 38.Egrie J, Eschbach JW, Adamson JW, McGuire T. Pharmacokinetics of recombinant human erythropoietin administered to hemodialysis (HD) patients. Kidney Int. 1988;33:262. [Google Scholar]
- 39.European Medicines Agency. 2004. NeoRecormon, INN-Epoetin beta.
- 40.European Medicines Agency. 2002. Dynepo is epoetin delta.
- 41.Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L, Chang D, Fuller J, Grant J, Hernday N, Hokum M, Hu S, Knudten A, Levin N, Komorowski R, Martin F, Navarro R, Osslund T, Rogers G, Rogers N, Trail G, Egrie J. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21:414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]
- 42.Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- 43. World Anti-Doping Agency. [online]. Available at http://www.wada-ama.org/en (last accessed June 2012)
- 44.Pate RR, Kriska A. Physiological basis of the sex difference in cardiorespiratory endurance. Sports Med. 1984;1:87–98. doi: 10.2165/00007256-198401020-00001. [DOI] [PubMed] [Google Scholar]
- 45.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coyle EF. Physiological determinants of endurance exercise performance. J Sci Med Sport. 1999;2:181–189. doi: 10.1016/s1440-2440(99)80172-8. [DOI] [PubMed] [Google Scholar]
- 47.Jones AM. A five year physiological case study of an Olympic runner. Br J Sports Med. 1998;32:39–43. doi: 10.1136/bjsm.32.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part I: cardiorespiratory aspects. Ann NY Acad Sci. 1977;301:310–322. doi: 10.1111/j.1749-6632.1977.tb38209.x. [DOI] [PubMed] [Google Scholar]
- 49.Saltin B, Astrand PO. Maximal oxygen uptake in athletes. J Appl Physiol. 1967;23:353–358. doi: 10.1152/jappl.1967.23.3.353. [DOI] [PubMed] [Google Scholar]
- 50.Enoksen E, Shalfawi SA, Tonnessen E. The effect of high- vs. low-intensity training on aerobic capacity in well-trained male middle-distance runners. J Strength Cond Res. 2011;25:812–818. doi: 10.1519/JSC.0b013e3181cc2291. [DOI] [PubMed] [Google Scholar]
- 51.Helgerud J, Engen LC, Wisloff U, Hoff J. Aerobic endurance training improves soccer performance. Med Sci Sports Exerc. 2001;33:1925–1931. doi: 10.1097/00005768-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Davis HA, Bassett J, Hughes P, Gass GC. Anaerobic threshold and lactate turnpoint. Eur J Appl Physiol Occup Physiol. 1983;50:383–392. doi: 10.1007/BF00423244. [DOI] [PubMed] [Google Scholar]
- 53.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmid A, Huber G, Friedmann B, Schmidt W. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22:504–512. doi: 10.1055/s-2001-17613. [DOI] [PubMed] [Google Scholar]
- 55.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- 56.Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23:1338–1348. [PubMed] [Google Scholar]
- 57.Buick FJ, Gledhill N, Froese AB, Spriet L, Meyers EC. Effect of induced erythrocythemia on aerobic work capacity. J Appl Physiol. 1980;48:636–642. doi: 10.1152/jappl.1980.48.4.636. [DOI] [PubMed] [Google Scholar]
- 58.Brien AJ, Simon TL. The effects of red blood cell infusion on 10-km race time. JAMA. 1987;257:2761–2765. [PubMed] [Google Scholar]
- 59.Ekblom B. Blood doping and erythropoietin. The effects of variation in hemoglobin concentration and other related factors on physical performance. Am J Sports Med. 1996;24:S40–S42. [PubMed] [Google Scholar]
- 60.Celsing F, Svedenhag J, Pihlstedt P, Ekblom B. Effects of anaemia and stepwise-induced polycythaemia on maximal aerobic power in individuals with high and low haemoglobin concentrations 1. Acta Physiol Scand. 1987;129:47–54. doi: 10.1111/j.1748-1716.1987.tb08038.x. [DOI] [PubMed] [Google Scholar]
- 61.Sjodin B, Svedenhag J. Applied physiology of marathon running. Sports Med. 1985;2:83–99. doi: 10.2165/00007256-198502020-00002. [DOI] [PubMed] [Google Scholar]
- 62.Londeree BR. The use of laboratory test results with long distance runners. Sports Med. 1986;3:201–213. doi: 10.2165/00007256-198603030-00004. [DOI] [PubMed] [Google Scholar]
- 63.Lucia A, Hoyos J, Santalla A, Perez M, Chicharro JL. Kinetics of VO(2) in professional cyclists. Med Sci Sports Exerc. 2002;34:320–325. doi: 10.1097/00005768-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 64.Lucia A, Pardo J, Durantez A, Hoyos J, Chicharro JL. Physiological differences between professional and elite road cyclists. Int J Sports Med. 1998;19:342–348. doi: 10.1055/s-2007-971928. [DOI] [PubMed] [Google Scholar]
- 65.Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol. 1988;64:2622–2630. doi: 10.1152/jappl.1988.64.6.2622. [DOI] [PubMed] [Google Scholar]
- 66.Wilber RL, Zawadzki KM, Kearney JT, Shannon MP, Disalvo D. Physiological profiles of elite off-road and road cyclists. Med Sci Sports Exerc. 1997;29:1090–1094. doi: 10.1097/00005768-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Coyle EF, Feltner ME, Kautz SA, Hamilton MT, Montain SJ, Baylor AM, Abraham LD, Petrek GW. Physiological and biomechanical factors associated with elite endurance cycling performance. Med Sci Sports Exerc. 1991;23:93–107. [PubMed] [Google Scholar]
- 68.Jones AM. The physiology of the Wolrd Record Holder for the Women's Marathon. Int J Sports Sci Coach. 2006;1:101–116. [Google Scholar]
- 69.Coyle EF. Improved muscular efficiency displayed as Tour de France champion matures. J Appl Physiol. 2005;98:2191–2196. doi: 10.1152/japplphysiol.00216.2005. [DOI] [PubMed] [Google Scholar]
- 70.Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 71.Esfarjani F, Laursen PB. Manipulating high-intensity interval training: effects on VO2max, the lactate threshold and 3000 m running performance in moderately trained males. J Sci Med Sport. 2007;10:27–35. doi: 10.1016/j.jsams.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Billat VL, Flechet B, Petit B, Muriaux G, Koralsztein JP. Interval training at VO2max: effects on aerobic performance and overtraining markers. Med Sci Sports Exerc. 1999;31:156–163. doi: 10.1097/00005768-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 73.Weston AR, Myburgh KH, Lindsay FH, Dennis SC, Noakes TD, Hawley JA. Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. Eur J Appl Physiol Occup Physiol. 1997;75:7–13. doi: 10.1007/s004210050119. [DOI] [PubMed] [Google Scholar]
- 74.Chicharro JL, Hoyos J, Lucia A. Effects of endurance training on the isocapnic buffering and hypocapnic hyperventilation phases in professional cyclists. Br J Sports Med. 2000;34:450–455. doi: 10.1136/bjsm.34.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nevill AM, Jobson SA, Palmer GS, Olds TS. Scaling maximal oxygen uptake to predict cycling time-trial performance in the field: a non-linear approach. Eur J Appl Physiol. 2005;94:705–710. doi: 10.1007/s00421-005-1321-8. [DOI] [PubMed] [Google Scholar]
- 76.Padilla S, Mujika I, Angulo F, Goiriena JJ. Scientific approach to the 1-h cycling world record: a case study. J Appl Physiol. 2000;89:1522–1527. doi: 10.1152/jappl.2000.89.4.1522. [DOI] [PubMed] [Google Scholar]
- 77.Jeukendrup AE, Craig NP, Hawley JA. The bioenergetics of World Class Cycling. J Sci Med Sport. 2000;3:414–433. doi: 10.1016/s1440-2440(00)80008-0. [DOI] [PubMed] [Google Scholar]
- 78.Lucia A, Hoyos J, Perez M, Santalla A, Chicharro JL. Inverse relationship between VO2max and economy/efficiency in world-class cyclists. Med Sci Sports Exerc. 2002;34:2079–2084. doi: 10.1249/01.MSS.0000039306.92778.DF. [DOI] [PubMed] [Google Scholar]
- 79.Ingham SA, Whyte GP, Pedlar C, Bailey DM, Dunman N, Nevill AM. Determinants of 800-m and 1500-m running performance using allometric models. Med Sci Sports Exerc. 2008;40:345–350. doi: 10.1249/mss.0b013e31815a83dc. [DOI] [PubMed] [Google Scholar]
- 80.di Prampero PE, Atchou G, Bruckner JC, Moia C. The energetics of endurance running. Eur J Appl Physiol Occup Physiol. 1986;55:259–266. doi: 10.1007/BF02343797. [DOI] [PubMed] [Google Scholar]
- 81.Coyle EF, Martin WH, III, Bloomfield SA, Lowry OH, Holloszy JO. Effects of detraining on responses to submaximal exercise. J Appl Physiol. 1985;59:853–859. doi: 10.1152/jappl.1985.59.3.853. [DOI] [PubMed] [Google Scholar]
- 82.Kumagai S, Tanaka K, Matsuura Y, Matsuzaka A, Hirakoba K, Asano K. Relationships of the anaerobic threshold with the 5 km, 10 km, and 10 mile races. Eur J Appl Physiol Occup Physiol. 1982;49:13–23. doi: 10.1007/BF00428959. [DOI] [PubMed] [Google Scholar]
- 83.Lucia A, Hoyos J, Perez M, Chicharro JL. Heart rate and performance parameters in elite cyclists: a longitudinal study. Med Sci Sports Exerc. 2000;32:1777–1782. doi: 10.1097/00005768-200010000-00018. [DOI] [PubMed] [Google Scholar]
- 84.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tesch PA, Wright JE. Recovery from short term intense exercise: its relation to capillary supply and blood lactate concentration. Eur J Appl Physiol Occup Physiol. 1983;52:98–103. doi: 10.1007/BF00429033. [DOI] [PubMed] [Google Scholar]
- 86.Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42D–47. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- 87.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 88.Sjodin B, Jacobs I. Onset of blood lactate accumulation and marathon running performance. Int J Sports Med. 1981;2:23–26. doi: 10.1055/s-2008-1034579. [DOI] [PubMed] [Google Scholar]
- 89.Lucia A, Hoyos J, Chicharro JL. Physiology of professional road cycling. Sports Med. 2001;31:325–337. doi: 10.2165/00007256-200131050-00004. [DOI] [PubMed] [Google Scholar]
- 90.Padilla S, Mujika I, Santisteban J, Impellizzeri FM, Goiriena JJ. Exercise intensity and load during uphill cycling in professional 3-week races. Eur J Appl Physiol. 2008;102:431–438. doi: 10.1007/s00421-007-0602-9. [DOI] [PubMed] [Google Scholar]
- 91.Conley DL, Krahenbuhl GS. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc. 1980;12:357–360. [PubMed] [Google Scholar]
- 92.Saltin B, Kim CK, Terrados N, Larsen H, Svedenhag J, Rolf CJ. Morphology, enzyme activities and buffer capacity in leg muscles of Kenyan and Scandinavian runners. Scand J Med Sci Sports. 1995;5:222–230. doi: 10.1111/j.1600-0838.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 93.Bassett DR, Jr, Howley ET. Maximal oxygen uptake: ‘classical’ versus ‘contemporary’ viewpoints. Med Sci Sports Exerc. 1997;29:591–603. doi: 10.1097/00005768-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- 95.Horowitz JF, Sidossis LS, Coyle EF. High efficiency of type I muscle fibers improves performance. Int J Sports Med. 1994;15:152–157. doi: 10.1055/s-2007-1021038. [DOI] [PubMed] [Google Scholar]
- 96.Green HJ, Klug GA, Reichmann H, Seedorf U, Wiehrer W, Pette D. Exercise-induced fibre type transitions with regard to myosin, parvalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflugers Arch. 1984;400:432–438. doi: 10.1007/BF00587545. [DOI] [PubMed] [Google Scholar]
- 97.Tesch PA, Karlsson J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J Appl Physiol. 1985;59:1716–1720. doi: 10.1152/jappl.1985.59.6.1716. [DOI] [PubMed] [Google Scholar]
- 98.Longhurst JC, Kelly AR, Gonyea WJ, Mitchell JH. Cardiovascular responses to static exercise in distance runners and weight lifters. J Appl Physiol. 1980;49:676–683. doi: 10.1152/jappl.1980.49.4.676. [DOI] [PubMed] [Google Scholar]
- 99.Roskamm H. Optimum patterns of exercise for healthy adults. Can Med Assoc J. 1967;96:895–900. [PMC free article] [PubMed] [Google Scholar]
- 100.Lucia A, Carvajal A, Calderon FJ, Alfonso A, Chicharro JL. Breathing pattern in highly competitive cyclists during incremental exercise. Eur J Appl Physiol Occup Physiol. 1999;79:512–521. doi: 10.1007/s004210050546. [DOI] [PubMed] [Google Scholar]
- 101.Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev. 1995;23:25–63. [PubMed] [Google Scholar]
- 102.Hawley JA, Noakes TD. Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol Occup Physiol. 1992;65:79–83. doi: 10.1007/BF01466278. [DOI] [PubMed] [Google Scholar]
- 103.Swain DP. The influence of body mass in endurance bicycling. Med Sci Sports Exerc. 1994;26:58–63. [PubMed] [Google Scholar]
- 104.Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P. Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol. 2008;105:417–419. doi: 10.1152/japplphysiol.90529.2008. [DOI] [PubMed] [Google Scholar]
- 105.Thomsen JJ, Rentsch RL, Robach P, Calbet JA, Boushel R, Rasmussen P, Juel C, Lundby C. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol. 2007;101:481–486. doi: 10.1007/s00421-007-0522-8. [DOI] [PubMed] [Google Scholar]
- 106.Wilkerson DP, Rittweger J, Berger NJ, Naish PF, Jones AM. Influence of recombinant human erythropoietin treatment on pulmonary O2 uptake kinetics during exercise in humans. J Physiol. 2005;568:639–652. doi: 10.1113/jphysiol.2005.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rasmussen P, Foged EM, Krogh-Madsen R, Nielsen J, Nielsen TR, Olsen NV, Petersen NC, Sorensen TA, Secher NH, Lundby C. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol. 2010;109:476–483. doi: 10.1152/japplphysiol.00234.2010. [DOI] [PubMed] [Google Scholar]
- 108.Lundby C, Damsgaard R. Exercise performance in hypoxia after novel erythropoiesis stimulating protein treatment. Scand J Med Sci Sports. 2006;16:35–40. doi: 10.1111/j.1600-0838.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 109.Parisotto R, Gore CJ, Emslie KR, Ashenden MJ, Brugnara C, Howe C, Martin DT, Trout GJ, Hahn AG. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica. 2000;85:564–572. [PubMed] [Google Scholar]
- 110.Russell G, Gore CJ, Ashenden MJ, Parisotto R, Hahn AG. Effects of prolonged low doses of recombinant human erythropoietin during submaximal and maximal exercise 1. Eur J Appl Physiol. 2002;86:442–449. doi: 10.1007/s00421-001-0560-6. [DOI] [PubMed] [Google Scholar]
- 111.Connes P, Perrey S, Varray A, Prefaut C, Caillaud C. Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch. 2003;447:231–238. doi: 10.1007/s00424-003-1174-0. [DOI] [PubMed] [Google Scholar]
- 112.Ekblom B, Berglund B. Effect of erythropoietin administration on maximal aerobic power. Scand J Med Sci Sports. 1991;1:88–93. [Google Scholar]
- 113.Ninot G, Connes P, Caillaud C. Effects of recombinant human erythropoietin injections on physical self in endurance athletes 1. J Sports Sci. 2006;24:383–391. doi: 10.1080/02640410500131340. [DOI] [PubMed] [Google Scholar]
- 114.Audran M, Gareau R, Matecki S, Durand F, Chenard C, Sicart MT, Marion B, Bressolle F. Effects of erythropoietin administration in training athletes and possible indirect detection in doping control. Med Sci Sports Exerc. 1999;31:639–645. doi: 10.1097/00005768-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32:1238–1243. doi: 10.1097/00005768-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Souillard A, Audran M, Bressolle F, Gareau R, Duvallet A, Chanal JL. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in athletes. Blood sampling and doping control. Br J Clin Pharmacol. 1996;42:355–364. doi: 10.1046/j.1365-2125.1996.41911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faria EW, Parker DL, Faria IE. The science of cycling: physiology and training – part 1. Sports Med. 2005;35:285–312. doi: 10.2165/00007256-200535040-00002. [DOI] [PubMed] [Google Scholar]
- 118.Hopkins WG, Hawley JA, Burke LM. Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc. 1999;31:472–485. doi: 10.1097/00005768-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 119.Eichner ER. Sports anemia, iron supplements, and blood doping. Med Sci Sports Exerc. 1992;24:S315–S318. [PubMed] [Google Scholar]
- 120.Schmidt W, Biermann B, Winchenbach P, Lison S, Boning D. How valid is the determination of hematocrit values to detect blood manipulations? Int J Sports Med. 2000;21:133–138. doi: 10.1055/s-2000-8871. [DOI] [PubMed] [Google Scholar]
- 121.PIROFSKY B. The determination of blood viscosity in man by a method based on Poiseuille's law. J Clin Invest. 1953;32:292–298. doi: 10.1172/JCI102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shand BI, Buttimore AL, Lynn KL, Bailey RR, Robson RA. Effect of hemodialysis and recombinant human erythropoietin on determinants of blood viscosity. Ren Fail. 1994;16:407–413. doi: 10.3109/08860229409044880. [DOI] [PubMed] [Google Scholar]
- 123.Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, Calbet JA, Robach P. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol. 2007;578:309–314. doi: 10.1113/jphysiol.2006.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lucia A, Rabadan M, Hoyos J, Hernandez-Capilla M, Perez M, San Juan AF, Earnest CP, Chicharro JL. Frequency of the VO2max plateau phenomenon in world-class cyclists. Int J Sports Med. 2006;27:984–992. doi: 10.1055/s-2006-923833. [DOI] [PubMed] [Google Scholar]
- 125.Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc. 1996;28:266–270. doi: 10.1097/00005768-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 126.Krebs PS, Powers SK. Reliability of laboratory endurance tests. Med Sci Sports Exerc. 1989;21:S10. [Google Scholar]
- 127.Lucia A, Hoyos J, Santalla A, Earnest C, Chicharro JL. Tour de France versus Vuelta a Espana: which is harder? Med Sci Sports Exerc. 2003;35:872–878. doi: 10.1249/01.MSS.0000064999.82036.B4. [DOI] [PubMed] [Google Scholar]
- 128.Padilla S, Mujika I, Orbananos J, Angulo F. Exercise intensity during competition time trials in professional road cycling. Med Sci Sports Exerc. 2000;32:850–856. doi: 10.1097/00005768-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 129.Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JA. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- 130.Padilla S, Mujika I, Cuesta G, Goiriena JJ. Level ground and uphill cycling ability in professional road cycling. Med Sci Sports Exerc. 1999;31:878–885. doi: 10.1097/00005768-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 131.Bentley DJ, McNaughton LR, Thompson D, Vleck VE, Batterham AM. Peak power output, the lactate threshold, and time trial performance in cyclists. Med Sci Sports Exerc. 2001;33:2077–2081. doi: 10.1097/00005768-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 132.Hoogeveen AR, Hoogsteen GS. The ventilatory threshold, heart rate, and endurance performance: relationships in elite cyclists. Int J Sports Med. 1999;20:114–117. doi: 10.1055/s-2007-971103. [DOI] [PubMed] [Google Scholar]
- 133.Lucia A, Hoyos J, Perez M, Santalla A, Earnest CP, Chicharro JL. Which laboratory variable is related with time trial performance time in the Tour de France? Br J Sports Med. 2004;38:636–640. doi: 10.1136/bjsm.2003.008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christensen B, Lundby C, Jessen N, Nielsen TS, Vestergaard PF, Moller N, Pilegaard H, Pedersen SB, Kopchick JJ, Jorgensen JO. Evaluation of functional erythropoietin receptor status in skeletal muscle in vivo: acute and prolonged studies in healthy human subjects. PLoS ONE. 2012;7:e31857. doi: 10.1371/journal.pone.0031857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lundby C, Hellsten Y, Jensen MB, Munch AS, Pilegaard H. Erythropoietin receptor in human skeletal muscle and the effects of acute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol. 2008;104:1154–1160. doi: 10.1152/japplphysiol.01211.2007. [DOI] [PubMed] [Google Scholar]
- 136.Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS ONE. 2009;4:e5894. doi: 10.1371/journal.pone.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 138.Brines M. The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors. Blood Purif. 2010;29:86–92. doi: 10.1159/000245630. [DOI] [PubMed] [Google Scholar]
- 139.Rathod DB, Salahudeen AK. Nonerythropoietic properties of erythropoietin: implication for tissue protection. J Investig Med. 2011;59:1083–1085. doi: 10.2310/JIM.0b013e31822cf86e. [DOI] [PubMed] [Google Scholar]
- 140.Vogel J, Gassmann M. Erythropoietic and non-erythropoietic functions of erythropoietin in mouse models. J Physiol. 2011;589:1259–1264. doi: 10.1113/jphysiol.2010.196147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Erbayraktar S, Yilmaz O, Gokmen N, Brines M. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep. 2003;2:465–470. [PubMed] [Google Scholar]
- 142.Patel NS, Collino M, Yaqoob MM, Thiemermann C. Erythropoietin in the intensive care unit: beyond treatment of anemia. Ann Intensive Care. 2011;1:40. doi: 10.1186/2110-5820-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hojman P, Taudorf S, Lundby C, Pedersen BK. Erythropoietin augments the cytokine response to acute endotoxin-induced inflammation in humans. Cytokine. 2009;45:154–157. doi: 10.1016/j.cyto.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 144.Kuipers H. Doping in sport–exercise scientists have to take responsibility. Int J Sports Med. 2001;22:545. doi: 10.1055/s-2001-18529. [DOI] [PubMed] [Google Scholar]
- 145.Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage. 2004;27:185–190. doi: 10.1016/j.jpainsymman.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 146.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 147.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Djulbegovic B, Bennett CL, Langensiepen S, Hyde C, Engert A. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 148.Stowell CP, Jones SC, Enny C, Langholff W, Leitz G. An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: safety analysis. Spine. 2009;34:2479–2485. doi: 10.1097/BRS.0b013e3181bd163f. [DOI] [PubMed] [Google Scholar]
- 149.Linde T, Sandhagen B, Danielson BG, Wikstrom B. Impaired erythrocyte fluidity during treatment of renal anaemia with erythropoietin. J Intern Med. 1992;231:601–606. doi: 10.1111/j.1365-2796.1992.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 150.Clyne N, Berglund B, Egberg N. Treatment with recombinant human erythropoietin induces a moderate rise in hematocrit and thrombin antithrombin in healthy subjects. Thromb Res. 1995;79:125–129. doi: 10.1016/0049-3848(95)91520-u. [DOI] [PubMed] [Google Scholar]
- 151.Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, Kabrna E, Geissler K, Jilma B. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95:2983–2989. [PubMed] [Google Scholar]
- 152.Tobu M, Iqbal O, Fareed D, Chatha M, Hoppensteadt D, Bansal V, Fareed J. Erythropoietin-induced thrombosis as a result of increased inflammation and thrombin activatable fibrinolytic inhibitor. Clin Appl Thromb Hemost. 2004;10:225–232. doi: 10.1177/107602960401000304. [DOI] [PubMed] [Google Scholar]
- 153.Sinzinger H, Virgolini I. Effects of exercise on parameters of blood coagulation, platelet function and the prostaglandin system. Sports Med. 1988;6:238–245. doi: 10.2165/00007256-198806040-00005. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- 155.Gonzalez-Alonso J, Mora-Rodriguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol Heart Circ Physiol. 2000;278:H321–H330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- 156.Wade JP. Transport of oxygen to the brain in patients with elevated haematocrit values before and after venesection. Brain. 1983;106(Pt 2):513–523. doi: 10.1093/brain/106.2.513. [DOI] [PubMed] [Google Scholar]
- 157.Lage JM, Panizo C, Masdeu J, Rocha E. Cyclist's doping associated with cerebral sinus thrombosis. Neurology. 2002;58:665. doi: 10.1212/wnl.58.4.665. [DOI] [PubMed] [Google Scholar]
- 158.Jelkmann W. Use of recombinant human erythropoietin as an antianemic and performance enhancing drug. Curr Pharm Biotechnol. 2000;1:11–31. doi: 10.2174/1389201003379068. [DOI] [PubMed] [Google Scholar]
- 159.Burch GE, DePasquale NP. Hematocrit, blood viscosity and myocardial infarction. Am J Med. 1962;32:161–163. [Google Scholar]
- 160.Delanty N, Vaughan C, Frucht S, Stubgen P. Erythropoietin-associated hypertensive posterior leukoencephalopathy. Neurology. 1997;49:686–689. doi: 10.1212/wnl.49.3.686. [DOI] [PubMed] [Google Scholar]
- 161.Cazzola M. Erythropoietin therapy: need for rationality and active surveillance. Haematologica. 2003;88:601–605. [PubMed] [Google Scholar]
- 162.Casadevall N, Cournoyer D, Marsh J, Messner H, Pallister C, Parker-Williams J, Rossert J. Recommendations on haematological criteria for the diagnosis of epoetin-induced pure red cell aplasia. Eur J Haematol. 2004;73:389–396. doi: 10.1111/j.1600-0609.2004.00348.x. [DOI] [PubMed] [Google Scholar]
- 163.Smalling R, Foote M, Molineux G, Swanson SJ, Elliott S. Drug-induced and antibody-mediated pure red cell aplasia: a review of literature and current knowledge. Biotechnol Annu Rev. 2004;10:237–250. doi: 10.1016/S1387-2656(04)10008-2. [DOI] [PubMed] [Google Scholar]
- 164.Fandrey J, Dicato M. Examining the involvement of erythropoiesis-stimulating agents in tumor proliferation (erythropoietin receptors, receptor binding, signal transduction), angiogenesis, and venous thromboembolic events. Oncologist. 2009;14(Suppl. 1):34–42. doi: 10.1634/theoncologist.2009-S1-34. [DOI] [PubMed] [Google Scholar]


