Abstract
Aims
The catechol-O-methyltransferase (COMT) Val158Met polymorphism affected pain sensitivity of healthy volunteers upon application of experimental pain stimuli. The relevance of these findings in morphine-treated postoperative cardiac patients undergoing painful healthcare procedures is unknown; therefore, the aim of this study was to investigate whether the COMT Val158Met polymorphism increases pain sensitivity in morphine-treated patients undergoing an unavoidable painful routine procedure after cardiac surgery.
Methods
One hundred and seventeen postoperative cardiac patients in the intensive care unit were genotyped for the COMT Val158Met polymorphism. All patients were treated with continuous morphine infusions for pain at rest, and received a bolus of morphine (2.5 or 7.5 mg) before a painful procedure (turning and/or chest drain removal) on the first postoperative day. Numerical rating scale (NRS) scores were evaluated at the following four time points: at baseline (at rest), and before, during and after the painful procedure.
Results
Overall mean NRS scores were significantly higher in patients carrying the Met-variant allele. During the painful procedure, the mean NRS score was significantly higher for Met/Met patients compared with Val/Met and Val/Val patients (mean NRS 3.4 ± 2.8, 2.7 ± 2.4 and 1.7 ± 1.7, respectively; P = 0.04). In Met/Met patients, the increase in NRS scores during the painful procedure compared with the baseline NRS score was clinically relevant (ΔNRS ≥ 1.3) and statistically significant and appeared to be independent of sex and the morphine bolus dose.
Conclusions
Our results show that the COMT Val158Met polymorphism contributes to variability in pain sensitivity after cardiac surgery of morphine-treated patients in the intensive care unit, because Met-allele carriers were more sensitive to overall pain and procedure-related pain.
Keywords: cardiac surgery, COMT Val158Met polymorphism, genetics, intensive care patients, numerical rating scale, pain sensitivity
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
In previous literature, polymorphisms of the catechol-O-methyltransferase (COMT) gene have been associated with changes in pain sensitivity. The relevance of these findings is unknown in intensive care patients after cardiac surgery.
WHAT THIS STUDY ADDS
In our study, we confirm these results in intensive care patients after cardiac surgery who were undergoing a painful procedure. These findings may be relevant in the analgesic treatment of these patients.
Introduction
Postoperative pain is a frequent problem in cardiac surgery 1. It is characterized by a large interindividual variability, even when the patient is at rest 2. In contrast, the numerous unavoidable painful healthcare procedures in the intensive care unit (ICU) produce pain levels that may differ greatly between patients. Turning of the patient and drain removal have been identified as the most painful procedures 3, 4. This interpatient variability in pain sensitivity may partly be explained by environmental factors, such as age, sex or anxiety 5–7. Furthermore, some candidate genes have been associated with differential pain sensitivity 2, 8.
The cathechol-O-methyltransferase (COMT) enzyme may contribute to the variability in pain sensitivity because it has a role in pain processing. Cathechol-O-methyltransferase metabolizes dopamine, adrenaline and noradrenaline, and is a key modulator of dopaminergic and adrenergic neurotransmission 9. There are two major forms of the COMT enzyme that differ by 50 amino acids at the N-terminus, namely membrane bound (MB-COMT) and soluble (S-COMT) 10. One single nucleotide polymorphism (SNP; rs2097903) is located at position 21217 in the estrogen-sensitive portion of the MB-COMT promoter region 11, 12. The second SNP (rs6269) is located in the promoter region of S-COMT 12, 13. Three SNPs (rs4633, rs4818 and rs4680) are situated within the coding region for both S- and MB-COMT (National Center for Biotechnology Information genome database; http://www.ncbi.nlm.nih.gov/gene). The SNP rs4680 is nonsynonymous and codes for a substitution of valine (Val) to methionine (Met) at codon 158 (Val158Met) 13. This substitution has been associated with a three- to fourfold decrease in COMT activity 14. Zubieta et al. 15 have suggested that lower COMT activity leads to an enhanced activation of dopaminergic neurotransmission, with lower endogenous levels of enkephalins and thus exaggerated pain sensitivity as a result 15. More specifically, in an experimental study these authors showed that healthy volunteers with the Met/Met genotype reported higher pain ratings than did those with the Val/Val genotype. In addition, subjects with the Met/Met genotype showed weaker activation of the endogenous opioid system on experimental pain stimuli than did subjects with the Val/Val genotype. These results were confirmed in two other experimental studies with healthy volunteers 16, 17 and in one study evaluating morphine consumption in patients with chronic cancer pain 9. There are, however, no reports about the relevance of this polymorphism for acute postoperative pain, such as pain after cardiac surgery, experienced either at rest or upon an unavoidable painful healthcare procedure, such as drain removal or turning of the patient, when patients are treated with intravenous morphine infusions.
In a recent clinical trial by our group in postoperative cardiac patients 18, pain levels were studied around an unavoidable routine painful procedure, i.e. turning of the patients and/or drain removal. Despite continuous morphine infusions and a bolus dose of morphine before the procedure, 25% of the patients experienced unacceptable pain, rated on the numerical rating scale (NRS ≥ 4, range 0–10 19) 18. In the present study, we tested the hypothesis that the COMT Val158Met polymorphism may explain these high pain levels upon the unavoidable painful procedure in these postoperative cardiac patients. Therefore, the aim of this study was to investigate the influence of the COMT Val158Met polymorphism on pain sensitivity during an unavoidable painful routine healthcare procedure in morphine-treated adult patients after cardiac surgery in the ICU.
Methods
Design
A prospective observational study was performed in a 30-bed surgical/medical ICU in a teaching hospital, St Antonius Hospital, Nieuwegein, The Netherlands. The study was part of a randomized controlled trial (registered at ClinicalTrials.gov, identifier NCT00558090) evaluating postoperative cardiac patients' pain levels around an unavoidable painful procedure in the ICU. The clinical trial was approved by the St Antonius Hospital Ethics Committee, and written informed consent was obtained from all patients before the cardiac surgical procedure.
Patients
During a 10 month period, we considered the eligibility of all patients aged from 18 to 85 years admitted to the ICU after cardiac surgery through sternotomy, as described in a previous report 18. Within the randomized controlled trial for the prevention and treatment of procedural pain, 135 patients were considered, 129 of whom were enrolled in the study according to the eligibility criteria. Nine of those 129 patients were excluded from analysis because they underwent a second sternotomy in the first 48 h postoperatively. Two patients were excluded because the painful procedure was not executed. One other patient was excluded because genotyping failed. Hence, 117 patients were included in the analysis (Figure 1).
Figure 1.
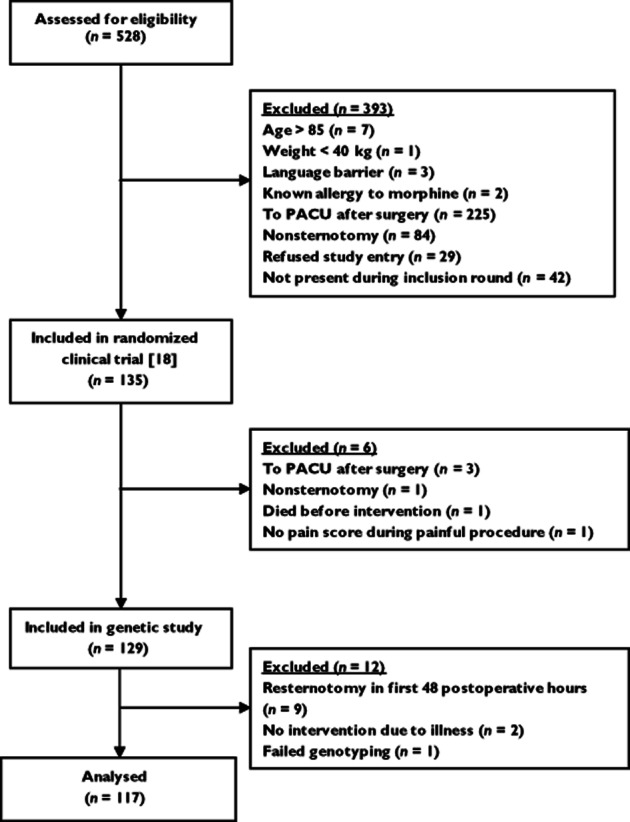
CONSORT diagram. Abbreviation: PACU, postanaesthesia care unit
Instrument for pain measurements
The patients themselves, in principle, rated their pain levels on the NRS, because this is considered as the gold standard for pain measurement 20. The NRS is based on a scale from 0 to 10; 0 represents no pain, while 10 represents worst possible pain 21, 22. An NRS score of 4 and higher is considered as unacceptable pain 19. Pain increase or decrease is considered as clinically significant when the NRS change is at least 1.3–1.5 23. The NRS was used to assess postoperative pain at rest and during procedural pain. Scoring was explained to all participating patients before cardiac surgery. If a patient was not able to self-report, the NRS was scored by the nurse, which has been proved to provide a reliable measure 24.
Standard pain titration protocol for treatment of pain at rest
For basic pain relief, a standard pain titration protocol, consisting of intravenous morphine infusions and intermittent paracetamol administration, was used in all patients, which is current practice in our ICU since 2007. Postoperatively, patients rated their pain using the NRS at three standardized times, which is routine nursing care. At NRS scores of ≥4, the attending nurse, along with the responsible physician, prescribed and administered additional analgesic medication 25.
Procedures
On day 0, patients underwent (non)elective cardiac surgery through sternotomy. Before surgery, one blood sample (10 ml) for COMT genotype determination was collected. After surgery, patients were admitted to the ICU, and were treated with continuous morphine infusions according to the standard pain titration protocol to manage pain at rest 18.
On the first postoperative day, 30 min prior to the unavoidable routine care painful procedure, i.e. turning of the patient and/or chest drain removal between 07.30 and 09.30 h, patients received an intravenous bolus dose of morphine of either 2.5 or 7.5 mg 18. Pain levels were assessed using the NRS at four time points, i.e. at baseline (immediately before administration of the morphine bolus dose), and 5 min before, during and 5 min after the procedure. Patients' characteristics and perioperative data were collected.
Genotype analysis
Genomic DNA was isolated from 200 μl ethylenediaminetetraacetic acid-treated whole blood using a MagnaPure LC (Roche Diagnostics GmbH, Mannheim, Germany). The COMT rs4680G>A polymorphism (Val158Met) was determined through allelic discrimination analysis using TaqMan® (Applied Biosystems, Carlsbad, CA, USA) genotyping assays (C_25746809_50) on the ABI PRISM 7500® Fast real-time PCR Systems (Applied Biosystems). The PCR cycle consisted of an initial step of 1 min at 60°C, followed by a denaturation step at 95°C for 30 sand 40 cycles with 95°C for 3 sand 60°C for 30 s. The final post-PCR read was made in 1 min at 60°C. The volume for each reaction was 12 μl, consisting of 5 μl TaqMan® GTXpress™ Master Mix, 0.125 μl of TaqMan® SNP genotyping assay (80×), containing the primers (64 μm) and the probes (16 μm), and 20 ng genomic DNA.
Statistical analysis
Statistical analyses were made with the SPSS statistical package (version 18.0 for Windows; SPSS Inc., Chicago, IL, USA). Continuous data are expressed as means ± SD or medians (range) where appropriate. To estimate the effect of genotype on the outcome variables (NRS scores), the NRS scores were compared between genotypes by a linear mixed-model analysis based on the maximal likelihood ratio, with the patient genotype status as the fixed factor and the time point of pain assessment as the repeated measurement. No structure was imposed on variances and covariances between and within times of the repeated measurements.
Pain sensitivity and response to opioids is different between male and females 26, 27. Furthermore, the estrogenic regulation of COMT transcription is partly responsible for sex differences in COMT activity 12. Moreover, women have lower baseline levels of COMT compared with men 28. To adjust for the potential confounding effect of the patient's sex, linear mixed-model analysis was also performed by adding sex as a covariate. Furthermore, we added the dose of morphine as a covariate, to adjust for the potential confounding effect of the two morphine doses, which were received by the patients before the painful procedure.
One-way ANOVA was performed to compare means between the three genotype groups (Val/Val, Val/Met and Met/Met genotype) at a single time point under the null hypothesis that the means of the compared groups were equal. Potentially confounding effects of patient's sex and the morphine dose (2.5 vs. 7.5 mg) were assessed with multiple linear regression analyses. For these models, the homozygous genotype for the most frequent allele was set as the reference. Student's paired t-tests served to test the significance of the change in NRS scores during the painful procedure (third time point) when compared with baseline (i.e. before the morphine bolus dose; first time point), either for the entire population or for subgroups stratified by COMT genotype. Categorical data are expressed as a percentage of total in each category, and the associations were tested by Pearson's χ2 test. A P value of ≤0.05 was considered as statistically significant.
Results
Patient characteristics
Thirty-two women and eighty-five men were included in the study, all of whom were Caucasian except for two of Asian and African American origin. Thirty patients were homozygous for the wild-type allele (Val/Val) with regard to the COMT Val158Met polymorphism, 66 were heterozygous (Val/Met) and 21 were homozygous for the variant allele (Met/Met). This yielded a 46.2% frequency of the Met-allele, in line with previous data 17, while the genotype distribution did not deviate from the Hardy–Weinberg equilibrium (χ2 = 2.1, P = 0.14). Patient characteristics are presented in Table 1; there were no differences between the three genotype groups with respect to sex, age, type of surgery, intraoperative use of analgesics or cumulative morphine consumption before the painful procedure. Finally, the proportion of patients randomized to receive either a 2.5 or 7.5 mg bolus dose of morphine did not differ between the three genotype groups (χ2 = 3.6, P = 0.16; Table 1).
Table 1.
Characteristics of patients
| Characteristic | Val/Val | Val/Met | Met/Met | P value |
|---|---|---|---|---|
| Number of patients | 30 | 66 | 21 | – |
| Age (years) | 71 ± 11 | 69 ± 11 | 66 ± 12 | 0.45 |
| Sex (male/female; n) | 22/8 | 49/17 | 14/7 | 0.79 |
| Body mass index (kg m−2) | 27.7 ± 4.3 | 27.5 ± 4.2 | 26.6 ± 3.9 | 0.59 |
| Preoperative use of analgesics* | 6 (20%) | 11 (17%) | 2 (10%) | 0.64 |
| Type of cardiothoracic surgery [n (%)] | 0.41 | |||
| CABG | 7 (23%) | 16 (24%) | 2 (10%) | – |
| Valve surgery | 5 (17%) | 16 (24%) | 7 (33%) | – |
| CABG and valve surgery | 14 (47%) | 23 (35%) | 6 (29%) | – |
| Aortic surgery | 4 (13%) | 11 (17%) | 6 (29%) | – |
| Mean duration of anaesthesia (h) | 4.2 ± 1.2 | 4.2 ± 1.0 | 3.7 ± 1.1 | 0.24 |
| Mean intraoperative dose of fentanyl (mg) | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.7 ± 0.6 | 0.68 |
| Mean intraoperative dose of remifentanil (mg) | 1.0 ± 1.0 | 1.2 ± 1.3 | 1.3 ± 1.3 | 0.71 |
| Cumulative postoperative dose of morphine (mg) before morphine bolus | 12.6 ± 6.8 | 13.6 ± 6.7 | 14.7 ± 6.6 | 0.55 |
| Morphine bolus dose 2.5 mg/7.5 mg (n)† | 14/13 | 30/33 | 15/6 | 0.16 |
| Unavoidable routine care procedure, i.e. turning/turning & drain removal | 11/19 | 36/30 | 8/13 | 0.18 |
| Mean ICU stay (days) | 2.1 ± 1.6 | 2.3 ± 2.2 | 2.4 ± 1.5 | 0.83 |
All values are expressed as means ± SD or as number (%). Abbreviations are as follows: CABG, coronary artery bypass graft; COMT, catechol-O-methyltransferase; and ICU, intensive care unit.
Includes paracetamol, nonsteroidal anti-inflammatory drugs and tramadol.
There were six missing values.
Numerical rating scale scores around the painful procedure
Figure 2 illustrates the NRS pain scores at the four time points for the three genotype groups. In a linear mixed-model considering all four time points, analysis of repeated measurements demonstrated that the overall mean NRS scores estimated from the model were significantly different across COMT genotype clusters, being on average 1.2 ± 0.20, 1.6 ± 0.12 and 1.9 ± 0.17 for Val/Val, Val/Met and Met/Met patients, respectively (ANOVA, P = 0.03; Figure 2). Pairwise analyses revealed that the mean difference in NRS score differed significantly between patients with the Met/Met genotype and patients with the Val/Val genotype (ΔNRS = 0.7 ± 0.3; P = 0.01). This difference was not significant between Val/Met patients vs. Met/Met patients (ΔNRS = −0.3 ± 0.2; P = 0.19) and vs. Val/Val patients (ΔNRS = 0.4 ± 0.2, P = 0.07; Figure 2). When sex was introduced as a covariate in the linear mixed-model analysis, adjusted mean NRS scores were 1.1 ± 0.23, 1.5 ± 0.15 and 1.8 ± 0.20 for Val/Val, Val/Met and Met/Met patients, respectively (ANOVA, P = 0.03), indicating that the effect of COMT genotype was independent of sex.
Figure 2.
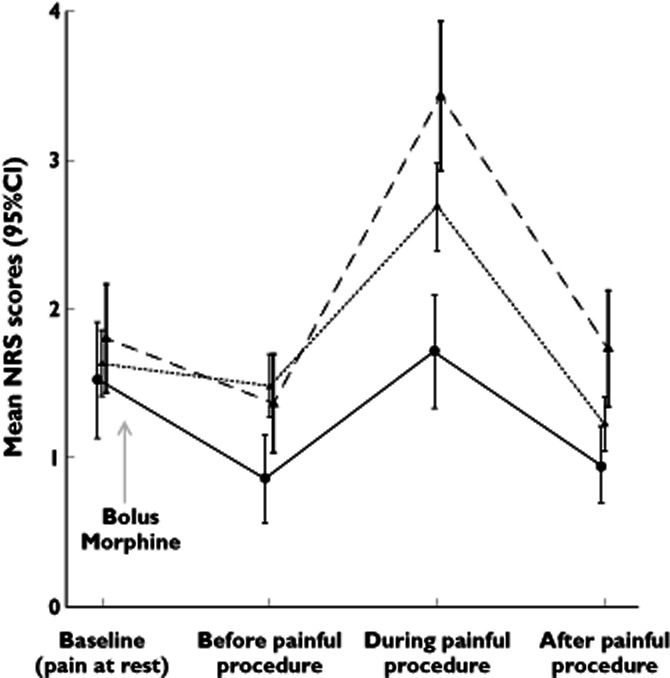
Mean numerical rating scale (NRS) scores around the painful procedure. Pain was rated at four individual times using the NRS. A bolus morphine was administered after time point ‘at baseline’. Linear mixed-model analysis revealed a significantly higher overall NRS score in patients with the Met/Met genotype compared with the Val/Met patients and Val/Val patients. The COMT genotypes are represented as follows: •, Val/Val; ▴, Val/Met; ▴, Met/Met;  , Val/Val;
, Val/Val;  , Val/Met;
, Val/Met;  , Met/Met
, Met/Met
Table 2 reports mean NRS scores for the four time points separately. Only the mean NRS scores during the painful procedure were significantly different between the genotype groups (P = 0.04). The highest pain score was recorded for patients with the Met/Met genotype (mean NRS 3.4 ± 2.8), followed by heterozygous patients with the Val/Met genotype (mean NRS 2.7 ± 2.4) and patients with the Val/Val genotype (mean NRS 1.7 ± 1.7). At this time point, the difference in mean NRS scores between Val/Val and Met/Met patients was thus 1.7, which is considered clinically relevant (ΔNRS ≥ 1.3–1.5). In an additional analysis, we adjusted for potential confounding by sex. In a multiple linear regression model, the difference in mean NRS score between the genotype groups during the painful procedure remained significant (P = 0.014). In this model, it was observed that the COMT allelic status explained more than 5% of the variability in NRS scoring during painful intervention (r2 = 0.053, P = 0.01).
Table 2.
Numerical rating scale scores at individual time points
| Numerical rating scale score | ||||
|---|---|---|---|---|
| Baseline (pain at rest) | Before painful procedure | During painful procedure | After painful procedure | |
| Genotype | ||||
| Val/Val | 1.56 ± 1.8 | 0.96 ± 1.4 | 1.7 ± 1.7 | 1.0 ± 1.2 |
| Val/Met | 1.6 ± 1.8 | 1.5 ± 1.7 | 2.7 ± 2.4 | 1.2 ± 1.5 |
| Met/Met | 1.8 ± 2.0 | 1.4 ± 1.8 | 3.4 ± 2.8 | 1.7 ± 2.1 |
| P value | 0.86 | 0.34 | 0.04 | 0.20 |
All values are expressed as means ± SD.
Finally, unacceptable pain scores (NRS ≥ 4) during the painful procedure were considered. In total, 26% of the 117 patients experienced unacceptable pain (NRS ≥ 4) during the painful procedure. The proportion of patients reporting such high scores did not differ between the three genotype groups (P = 0.14).
Increase in NRS scores upon the painful procedure compared with baseline
For all patients, the NRS increase (ΔNRS), corresponding to the NRS score recorded during the painful procedure minus the baseline NRS score recorded before the bolus administration of morphine, was a mean of 1.1 ± 2.3, indicating a statistically significant increase (P < 0.001), albeit not a clinically significant increase (ΔNRS < 1.3).
Regarding the subgroups, the Met/Met homozygote group showed a statistically significant and clinically relevant increase in the NRS score during the painful procedure compared with baseline, with a mean ΔNRS of 1.6 ± 2.5 (P = 0.001). The mean ΔNRS for the Val/Met group was 1.1 ± 2.2 (P < 0.001), which is not considered clinically relevant, while that for the Val/Val homozygote group was 0.2 ± 1.8 (P = 0.63), thus neither statistically significant nor clinically relevant. In a multiple linear regression model to adjust for potential confounding by sex, the ΔNRS score was significantly correlated to the COMT allelic status (P = 0.03), and this relationship was not modified when sex was introduced as an independent variable. In this model, it was observed that the COMT allelic status explained more than 4% of the variability in ΔNRS scoring. Similar results were observed after stratification according to the bolus dose of morphine (i.e. 2.5 or 7.5 mg), indicating that the change in NRS scores was independent of the bolus morphine dose (Table 3).
Table 3.
Change in numerical rating scale score in relation to the COMT allelic status stratified to the bolus dose of morphine (i.e. 2.5 or 7.5 mg)
| Genotype | Change in numerical rating scale score | P value |
|---|---|---|
| Morphine dose 2.5 mg | ||
| Val/Val | +0.1 ± 1.9 | 0.79 |
| Val/Met | −1.3 ± 1.6 | <0.001 |
| Met/Met | −2.0 ± 3.2 | 0.04 |
| Morphine dose 7.5 mg | ||
| Val/Val | −1.0 ± 1.3 | 0.11 |
| Val/Met | −1.0 ± 2.7 | 0.04 |
| Met/Met | −1.3 ± 1.7 | 0.02 |
All values are expressed as means ± SD.
Overall, 29 patients (24%) who reported acceptable pain (NRS < 3) at baseline, reported unacceptable pain (NRS ≥ 4) during the painful procedure; the proportion of these patients did not differ between the genotype groups (P = 0.20).
Discussion
We evaluated the influence of the COMT Val158Met polymorphism in postoperative cardiac patients treated with continuous morphine infusions to manage pain at rest and with an additional bolus dose of morphine before an unavoidable postoperative painful procedure (drain removal and/or turning of the patient) in the ICU. The main finding was that patients carrying the Met-variant allele experienced both significantly increased overall pain and significantly increased pain during the painful procedure, in comparison to patients with the Val/Val genotype. More specifically, patients with the Met/Met genotype showed a statistically significant and clinically relevant increase in pain scores during the painful procedure, unlike patients with the Val/Val genotype. To our knowledge, this is the first study to evaluate the impact of the COMT Val158Met polymorphism on pain sensitivity within a clinical research design in morphine-treated postoperative ICU patients undergoing an unavoidable painful stimulus.
So far, studies of healthy volunteers have evaluated this polymorphism in relation to pain sensitivity by applying experimental pain stimuli, with similar results to those in the present study (Table 4). Loggia et al. 29 demonstrated that individuals with the Met/Met genotype exhibit stronger pain signals in numerous cortical and subcortical structures after repeated noxious stimulation when compared with other genotype groups. This is also consistent with other studies evaluating the impact of the COMT Val158Met polymorphism on pain sensitivity. Zubieta et al. 15 demonstrated that Met/Met subjects were characterized by higher sensory and affective pain ratings; two other studies 16, 17 found that individuals with the Met/Met genotype were more susceptible to pain after repeated thermal stimuli. Jensen et al. 17 showed that, after remifentanil bolus injection and repeated heat pain stimuli, Met/Met subjects reported higher pain scores than did Val/Val subjects, while heterozygous subjects reported intermediate scores.
Table 4.
Overview of studies that investigated the COMT Val158Met polymorphism in relation to pain sensitivity
| Study design | Study population | Intervention | Main outcome | Reference |
|---|---|---|---|---|
| Experimental | 29 healthy volunteers | Binding potential of the μ receptor and muscle pain was measured twice: during intensity-controlled sustained pain induced by infusion of 5% hypertonic saline into the masseter muscle and during the infusion of nonpainful 0.9% isotonic saline. | Subjects with the Met/Met genotype of the COMT Val158Met polymorphism showed diminished regional μ-opioid system responses to pain compared with Met/Val and Val/Val subjects. These effects were accompanied by higher sensory and affective ratings of pain. | 15 |
| Experimental | 202 female healthy volunteers | Threshold and tolerance to thermal, ischaemic and mechanical stimuli, as well as temporal summation to heat pain, were determined. | The Val158Met polymorphism was associated with sensitivity to painful heat stimuli, which suggests that the Val158Met polymorphism plays a primary role in variation in temporal summation of pain. | 16 |
| Experimental | 43 healthy volunteers | Five applications of thermal heat pain were made to the hand. After each stimulus, subjects rated pain on a VAS. Before the second and the fourth stimulus, respectively, an intravenous injection of remifentanil (0.08 mg kg−1) and placebo was administered. | Met/Met subjects reported significantly more pain compared with Val/Val subjects in the case of repeated pain stimuli, although not during an initial response of the descending pain system. The opioid intervention induced analgesia without a separating effect for genotype. | 17 |
| Experimental | 54 healthy volunteers | Subjects received two heat pain stimuli on the right forearm during functional magnetic resonance imaging. After each stimulus, subjects rated their pain intensity using the Gracely Sensory Scale. | Met/Met subjects showed stronger pain-related functional magnetic resonance imaging signals than Val/Val subjects in several brain structures, only for high-intensity pain stimuli after repeated administration. | 29 |
| Experimental | 500 healthy volunteers | Subjects received a painful thermal and cold stimulus, measured on the hand up to the wrist, with VAS ratings and temperature dimensions of personality. In total, three single nucleotide polymorphisms were evaluated. | In a classification and regression tree analysis, the Val158Met polymorphism did not provide a strong predictive value for heat or cold pain sensitivity. | 33 |
| Clinical research design | 207 patients treated with morphine for chronic cancer pain | Between the genotype groups, morphine doses, serum concentrations of morphine and morphine metabolites were compared. | After a mean treatment period of 3.5 months, patients with the Val/Val genotype needed more morphine when compared with the Val/Met and the Met/Met genotype groups. Pain scores were not reported. | 9 |
| Clinical research design | 221 acute postsurgical patients | Clinically induced pain was recorded using the VAS after oral surgery every 20 min until subjects requested analgesic medication (ketorolac), followed by VAS scores at 15 min intervals for 180 min. | Although the COMT enzyme showed significant associations with the maximal postoperative pain, this association was not sustained after correcting for multiple comparisons. | 31 |
| Clinical research design | 2294 cancer patients | Among other things, pain intensity, time on opiods and 112 single nucleotide polymorphisms that may predict opioid dose were included as covariates in a regression model. | None of the 112 single nucleotide polymorphisms showed significant associations with opioid dose. | 32 |
Abbreviations are as follows: COMT, catechol-O-methyltransferase; and VAS, visual analog scale, range 0–10.
In contrast, there are reports in which it was not possible to identify a significant association between COMT Val158Met polymorphism and pain sensitivity, when studying patients with postoperative pain or cancer pain, or healthy subjects with experimental pain 30. Kim et al. 31 concluded that in 221 acute postsurgical patients, genetic polymorphisms, including those of the COMT enzyme, show only a weak association with clinically induced acute injury. In a large study by Klepstad et al. 32, the evaluation of 112 SNPs in 23 candidate genes proposed to influence opioid efficacy did not predict the need for opioids in cancer patients with pain. Finally, a study in which experimental pain was observed in healthy volunteers did not find associations between the genotypes of the COMT enzyme and pain sensitivity, measuring painful thermal and cold stimuli 33.
Thus, although there is some inconsistent literature, the results of our clinical study confirm the results of the experimental pain studies in healthy volunteers, where the pain sensitivity of patients carrying the Met-allele was higher than that of patients with the Val/Val genotype. We showed that this genotype-related difference in pain sensitivity is clinically relevant in morphine-treated patients after cardiac surgery undergoing an unavoidable (instead of experimental) pain stimulus in the ICU.
Although we found a clinically relevant increase in NRS score in patients with the Met/Met genotype, the proportion of patients whose pain increased from an acceptable level (NRS < 4) at baseline to an unacceptable level (NRS ≥ 4) during the painful procedure did not differ between the genotype groups. We realize that in our study the mean NRS scores generally were low (NRS < 4), and that in particular the increase to unacceptable pain during the painful procedure should be prevented. However, the knowledge that patients with the Met/Met genotype are at higher risk for a clinically relevant increase in pain during a painful intervention may be of value for procedural-related pain management.
The results of our study suggest that patients carrying the Met-allele may benefit from a more individualized therapy in the event of a second surgery. Higher morphine doses may be anticipated, although only a small increase in efficacy may be expected at an increased incidence of adverse events. In this respect, we hypothesized that non-opioid analgesics may be preferable, because they probably act independently from endogenous enkephalin levels and the μ receptor density. However, agents such as nonsteroidal anti-inflammatory drugs may be disadvantageous in postoperative cardiac patients because they carry the risk of cardiovascular side-effects. Thus, further research should focus on these and other aspects of individualized pain management.
In our study, patients received continuous morphine infusions for pain at rest, with a bolus dose of morphine before a painful procedure. The morphine requirement did not differ between the genotype groups during the ICU stay and during the painful procedure. Interestingly, at first sight our results deviate from the observation made by Rakvåg et al. 9 that Met/Met homozygous cancer patients required less morphine compared with patients who had the Val/Val variant. The authors suggest that this may be explained by an increase in μ-opioid receptor density in Met/Met patients, which causes morphine to be more effective. However, those patients were treated for prolonged cancer pain for approximately 3.5 months 9, in contrast to our patients, who were treated for postoperative acute pain (maximum of 48 h). It is possible that an increase in μ-opioid receptor density may occur after a more prolonged period of opioid treatment. Moreover, the study of Rakvåg et al. 9 concerned a heterogeneous group of patients, with differences in disease severity and progression, and thus several nociceptive stimuli, whereas in our study all patients underwent the same type of painful procedure.
Considering the bolus morphine dose (2.5 vs. 7.5 mg), we observed no significant effect of the genotype on the analgesic response to morphine. These results are supported by the study of Jensen et al. (Table 4, 17), in which a single dose of remifentanil before a heat stimulus induced analgesia, without a separating effect for genotype. They also found that Met/Met subjects reported significantly more pain than did Val/Val subjects in the case of repeated pain stimuli, except after the initial pain stimulus. Given that our patients underwent cardiac surgery the day before the painful procedure, it is likely that the pain system was already triggered at the time of the painful procedure. Therefore, we suggest that Met/Met patients receiving the same dose as Val/Val patients are more sensitive to pain as a result of repeated painful stimuli, which is independent of a morphine bolus dose.
Two limitations of our pilot study should be addressed. Firstly, our study was in fact underpowered, because this analysis was conducted within a randomized controlled trial and was thus not designed primarily as a pharmacogenetic study. However, even with these low numbers of patients, we found a significant effect of genotype on both overall pain levels and pain during the painful procedure. Secondly, pain sensitivity is influenced by many factors, probably including genetic contributions of more than one gene. Therefore, this study cannot determine the relative importance of the Val158Met polymorphism in comparison to other genes involved in pain.
In conclusion, the results of the present clinical study suggest that the COMT Val158Met polymorphism is correlated to pain sensitivity in morphine-treated patients undergoing a painful healthcare procedure after cardiac surgery, showing that Met-allele carriers are more sensitive to overall pain and procedural pain.
Acknowledgments
The authors thank the staff and nurses of the Department of Anaesthesiology and Intensive Care and the staff of the Department of Clinical Pharmacy of the St Antonius Hospital for their contribution to this study. Ko Hagoort is thanked for editorial assistance. Laure Elens is a research fellow with the Wallonie-Bruxelles International (WBI.WORLD) program and Fonds Spécial de Recherche (FSR 10). There was no financial or other support.
Conflict of Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Gelinas C. Management of pain in cardiac surgery ICU patients: have we improved over time? Intensive Crit Care Nurs. 2007;23:298–303. doi: 10.1016/j.iccn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Allegri M, De Gregori M, Niebel T, Minella C, Tinelli C, Govoni S, Regazzi M, Braschi A. Pharmacogenetics and postoperative pain: a new approach to improve acute pain management. Minerva Anestesiol. 2010;76:937–944. [PubMed] [Google Scholar]
- 3.Puntillo KA, White C, Morris AB, Perdue ST, Stanik-Hutt J, Thompson CL, Wild LR. Patients' perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care. 2001;10:238–251. [PubMed] [Google Scholar]
- 4.Siffleet J, Young J, Nikoletti S, Shaw T. Patients' self-report of procedural pain in the intensive care unit. J Clin Nurs. 2007;16:2142–2148. doi: 10.1111/j.1365-2702.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 5.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657–677. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 6.Rudin A, Wolner-Hanssen P, Hellbom M, Werner MU. Prediction of post-operative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesiol Scand. 2008;52:938–945. doi: 10.1111/j.1399-6576.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Sommer M, de Rijke JM, van Kleef M, Kessels AG, Peters ML, Geurts JW, Patijn J, Gramke HF, Marcus MA. Predictors of acute postoperative pain after elective surgery. Clin J Pain. 2010;26:87–94. doi: 10.1097/AJP.0b013e3181b43d68. [DOI] [PubMed] [Google Scholar]
- 8.Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: recent advances. J Med Genet. 2012;49:1–9. doi: 10.1136/jmedgenet-2011-100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakvåg TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, Krokan HE, Skorpen F. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain. 2005;116:73–78. doi: 10.1016/j.pain.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 11.DeMille MM, Kidd JR, Ruggeri V, Palmatier MA, Goldman D, Odunsi A, Okonofua F, Grigorenko E, Schulz LO, Bonne-Tamir B, Lu RB, Parnas J, Pakstis AJ, Kidd KK. Population variation in linkage disequilibrium across the COMT gene considering promoter region and coding region variation. Hum Genet. 2002;111:521–537. doi: 10.1007/s00439-002-0809-0. [DOI] [PubMed] [Google Scholar]
- 12.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamer UM, Stuber F. Genetic factors in pain and its treatment. Curr Opin Anaesthesiol. 2007;20:478–484. doi: 10.1097/ACO.0b013e3282ef6b2c. [DOI] [PubMed] [Google Scholar]
- 15.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 16.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS ONE. 2009;4:e6016. doi: 10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlers SJ, van Gulik L, van Dongen HP, Bruins P, van de Garde EMW, Van Boven WJ, Tibboel D, Knibbe CA. Efficacy of an intravenous bolus of morphine 2.5 versus morphine 7.5 mg for procedural pain relief in postoperative cardiothoracic patients in the intensive care unit: a randomized double-blind controlled trial. Anaesth Intensive Care. 2012;40:417–426. doi: 10.1177/0310057X1204000306. [DOI] [PubMed] [Google Scholar]
- 19.Hamill-Ruth RJ, Marohn ML. Evaluation of pain in the critically ill patient. Crit Care Clin. 1999;15:35–54. doi: 10.1016/s0749-0704(05)70038-5. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA, Jr, Murray MJ, Peruzzi WT, Lumb PD. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 22.Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain. 1981;10:241–248. doi: 10.1016/0304-3959(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 23.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 24.Ahlers SJ, van Gulik L, van der Veen AM, van Dongen HP, Bruins P, Belitser SV, de Boer A, Tibboel D, Knibbe CA. Comparison of different pain scoring systems in critically ill patients in a general ICU. Crit Care. 2008;12:R15. doi: 10.1186/cc6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Gulik L, Ahlers SJ, Brkic Z, Belitser SV, van Boven WJ, van Dongen EP, Knibbe CA, Bruins P. Improved analgesia after the realisation of a pain management programme in ICU patients after cardiac surgery. Eur J Anaesthesiol. 2010;27:900–905. doi: 10.1097/eja.0b013e32833d91c3. [DOI] [PubMed] [Google Scholar]
- 26.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- 29.Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The Catechol-O-Methyltransferase (COMT) valmet Polymorphism Affects Brain Responses to Repeated Painful Stimuli. PLoS ONE. 2011;6:e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Lee H, Rowan J, Brahim J, Dionne RA. Genetic polymorphisms in monoamine neurotransmitter systems show only weak association with acute post-surgical pain in humans. Mol Pain. 2006;2:1–10. doi: 10.1186/1744-8069-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klepstad P, Fladvad T, Skorpen F, Bjordal K, Caraceni A, Dale O, Davies A, Kloke M, Lundstrom S, Maltoni M, Radbruch L, Sabatowski R, Sigurdardottir V, Strasser F, Fayers PM, Kaasa S. Influence from genetic variability on opioid use for cancer pain: a European genetic association study of 2294 cancer pain patients. Pain. 2011;152:1139–1145. doi: 10.1016/j.pain.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]


