Abstract
Aims
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy and impaired diastolic and systolic function. Abnormal sympathetic–parasympathetic balance is a potential stimulus for left ventricular hypertrophy in HCM patients. β-Blockers are routinely used in HCM for their strong negative inotropic effect; however, these drugs also influence the sympathetic–parasympathetic balance. This study aimed to determine the autonomic control of the cardiovascular system and the autonomic effects of β-blockers in HCM patients treated or untreated with β-blockers.
Methods
Among 51 HCM outpatients (18–70 years old; 29 men) there were 19 individuals with no medication and 32 subjects treated with a β-blocker. Fourteen age- and gender-matched (23–70 years old; nine men) healthy volunteers were enrolled in the control group. Continuous, non-invasive finger blood pressure was recorded during supine rest for 30 min. Autonomic regulation of the cardiovascular system was measured by heart rate variability and spontaneous baroreflex function (cross-correlation sequence method).
Results
The mean pulse interval, time domain and spectral measures of heart rate variability and baroreflex sensitivity were comparable between HCM patients, treated or not with β-blockers, and the control group. However, the delay of the baroreflex was significantly longer in HCM patients who were not treated with β-blockers [2.0 (1.6–2.3) s] in comparison with HCM patients receiving β-blockers [1.4 (1.1–1.8) s; P = 0.0072] or control subjects [1.2 (0.8–1.8) s; P = 0.0025]. This delay did not differ between HCM patients treated with β-blockers and the control group.
Conclusions
Hypertrophic cardiomyopathy not treated with β-blockers is accompanied by prolonged baroreflex delay. The use of β-blockers normalizes this delay.
Keywords: baroreflex delay, baroreflex sensitivity, β-blocker, heart rate variability, hypertrophic cardiomyopathy
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
β-Blockers are routinely used in pharmacological therapy in patients with hypertrophic cardiomyopathy (HCM). These drugs affect the sympathetic modulation of the cardiovascular system by blocking β-adrenergic receptors. Patients with HCM have impaired autonomic control of the heart, which can be measured by indices of heart rate variability, blood pressure variability or baroreflex function.
WHAT THIS STUDY ADDS
The baroreflex delay, which is the time necessary for the adaptive response of the sinus node to an alteration in vagal tone triggered by a preceding change in blood pressure, is prolonged in untreated HCM patients compared with healthy peers. The baroreflex delay in HCM patients who receive β-blockers is comparable to that of healthy subjects and is significantly shorter than that in untreated HCM patients. This study extends our knowledge about the specific effect of β-blockers on the baroreflex function in HCM patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disease of the cardiac sarcomere characterized by progressive, usually asymmetrical, wall thickening and left ventricular hypertrophy, impaired contractility and diastolic dysfunction of the left ventricle, with imminent development of heart failure 1–5. An unexpected drop of blood pressure during exercise, and neurological symptoms, such as repetitive fainting, dizziness or blurred vision, are found with progression of the disease. Patients with HCM are at increased risk of mortality from several causes, such as serious ventricular arrhythmia leading to sudden cardiac death, progression of heart failure or stroke 1–5.
An impaired sympathetic–parasympathetic balance is frequently involved in the pathogenesis of HCM. An increased adrenergic drive is believed to be a potential stimulus for left ventricular hypertrophy, a variety of the symptoms and premature death in HCM patients 2, 3, 6–14.
Blockers of β-adrenergic receptors, called also β-blockers, belong to the first-line pharmacological treatment in HCM patients, mainly because of their strong negative inotropic effect on the myocardium, reduction in ventricular stiffness, improvement of ventricular relaxation, slowing of the heart rate with increased time for diastolic filling, and a reduced excitability 2, 3, 15, 16. These drugs have proven beneficial effects in HCM patients with angina or dyspnoea on effort, particularly when associated with left ventricular outflow tract (LVOT) obstruction, and are often used as an antiarrhythmic treatment of ventricular arrhythmias 1–3, 6–8, 16. Although nondihydropyridine calcium channel antagonists, i.e. verapamil and diltiazem, are an alternative to the β-blocker approach in the treatment of HCM 3, 16, mainly in patients without LVOT obstruction, the evidence for their use in HCM patients is not as strong as for β-blockers. In contrast to calcium channel antagonists, β-blockers, by blocking β-adrenergic receptors, produce a number of autonomic effects in different groups of patients, such as those with hypertension, stable coronary artery disease or heart failure, including reduction of sympathetic influences or improvement of vagal effects on the heart 17–21. However, similar data on the autonomic function of the cardiovascular system in HCM patients treated or not with β-blockers are missing.
The aim of this observational study was to compare the autonomic modulation of the heart rate between consecutive HCM patients, treated or untreated with β-blockers, and healthy subjects. Heart rate variability (HRV), baroreflex sensitivity (BRS) and baroreflex delay, i.e. delay in a change in heart rate after blood pressure alteration, were used as indirect and non-invasive indices of the autonomic control of the cardiovascular system.
Methods
Subjects
Fifty-one nonrandomized, consecutive HCM patients were recruited from our outpatient clinic. Inclusion criteria were as follows: the presence of sinus rhythm in resting ECG; no diabetes mellitus; no implanted pacemaker, with the exception of cardioverter defibrillators set on antiarrhythmic therapy and no pacing (basic rate <40 beats min−1); and no history of myocardial infarction, stroke, renal failure (defined as creatinine level > 1.0 mg dl−1) or symptoms of severe heart failure with New York Heart Association (NYHA) functional class IV. Nineteen patients were not treated (HCM BB–; mostly because they were newly diagnosed HCM patients or due to the patient's preference for no treatment or the beginning of the treatment with a nondihydropyridine calcium channel antagonist, or previous adverse effects from intolerance to β-blockers), whereas 32 were on β-blocker therapy (HCM BB+), either metoprolol succinate or bisoprolol fumarate, for at least 6 months. Fourteen healthy age- and gender-matched volunteers were enrolled in the control group. Their health status was confirmed by detailed history taking and physical examination. Both patients and control subjects underwent standard 12-lead resting ECG recording in the supine position and a transthoracic echocardiographic examination. All participants gave informed consent to participate in the study. The local ethical committee accepted the study protocol.
Echocardiography
The echocardiographic examinations were performed with the Vivid 7 scanner (GE Medical Systems, Horten, Norway) equipped with a 1.5–4.0 MHz phased array transducer. Data were acquired with the subjects at rest, in the left lateral decubitus position. For data acquisition, three complete cardiac cycles were recorded and stored in cine-loop format at sweep speeds of 100 mm s−1. All echocardiographic examinations were carried out according to the recommendations of the American Society of Echocardiography 22. For the purpose of the analysis, the mean value from three cardiac cycles was computed. Left ventricular mass (LVM) and left ventricular mass index (LVMI) were calculated using Devereux's formula and indexed for body surface area 23. Left ventricular ejection fraction was quantified by the biplane method 22.
Finger pressure waveform recording
For 30 min, the continuous finger pressure waveform was recorded non-invasively (Finometer; FMS, Amsterdam, The Netherlands) at supine rest in a quiet room with daylight and a stable temperature of 22–23°C. All recordings were obtained during the morning hours. Participants refrained from drinking coffee, smoking cigarettes and eating for at least 2 h before the measurement. With the use of a commercial software package (Beatscope version 1.1; FMS), the values of pulse intervals and systolic blood pressure for each cardiac beat were exported to ASCII files and used in further analysis.
Heart rate variability
Values of pulse intervals from the ASCII files were used to measure the mean pulse interval and HRV. For total HRV, the standard deviation of all pulse intervals (SD) was used 24. The Lomb periodogram analysis of pulse intervals was applied to calculate the power of the low frequency (LF; 0.04–0.15 Hz) and the high frequency (HF; 0.15–0.4 Hz), as well as their ratio (LF/HF) 24, 25. In addition, the powers of LF and HF were normalized to the total power (0.0–0.04 Hz) reduced by the power of very low frequency (0.0–0.04 Hz) and are shown as LFnu and HFnu, respectively.
Baroreflex function
Baroreflex function was described by two parameters, i.e. BRS and baroreflex delay, which is the delay of the sinus node response to the change in systolic blood pressure 26, 27. Both BRS and baroreflex delay were calculated from the ASCII files with the use of the cross-correlation method 28–30, which is a time-domain sequential method for baroreflex function based on spontaneous systolic blood pressure and pulse interval variability changes.
Statistical analysis
The Shapiro–Wilk test revealed that the distribution of continuous data was not normal. For this reason, the results of all continuous data are expressed as the medians and interquartile range and nonparametric tests were used for comparisons between groups (the Mann–Whitney U-test). In addition, multivariate linear regression was used to analyse the effect of β-blocker therapy adjusted to the age and gender of the patient on various autonomic measures in the HCM group. Qualitative data are expressed as numbers and percentage, and for their analysis the proportion test was applied. Statistical analysis was done with IBM® SPSS® Statistics, version 18.0 (IBM Corp., Somers, NY, USA). A value of P < 0.05 was assumed to be significant.
Results
Clinical characteristics
The clinical characteristics of the studied patients and the control group are summarized in Table 1. Age, body mass index and the proportions of men, smokers and subjects with hypercholesterolaemia (total cholesterol > 200 mg dl−1) were comparable (i.e. not statistically significant) in all studied groups.
Table 1.
Clinical characteristics of patients not treated (HCM BB–) or treated with β-blockers (HCM BB+) and of control subjects
| Parameter | HCM BB– (n = 19) | HCM BB+ (n = 32) | Control subjects (n = 14) |
|---|---|---|---|
| Qualitative data | n (%) | n (%) | n (%) |
| Men | 13 (68.4) | 16 (50.0) | 9 (64.3 ) |
| Smokers | 4 (21.1) | 4 (12.5) | 2 (14.3) |
| Hypercholesterolaemia | 5 (26.3) | 7 (21.9) | 4 (28.6) |
| NYHA I | 11 (57.9) | 10 (31.3) | 0 |
| NYHA II | 4 (21.1) | 16 (50) | 0 |
| NYHA III | 4 (21.1) | 6 (18.8) | 0 |
| Syncope | 3 (15.8) | 12 (37.5) | 0 |
| Aborted sudden cardiac death | 8 (42.1) | 14 (43.8) | 0 |
| Hypertension | 6 (31.6) | 8 (25.0) | 0 |
| Implanted cardioverter defibrillator | 3 (15.8) | 13 (40.6) | 0 |
| Presence of SAM | 3 (15.8) | 12 (37.5) | 0 |
| LVOT obstruction | 4 (21.1) | 15 (46.9) | 0 |
| ACE inhibitors | 2 (10.5) | 8 (25.0) | 0 |
| Calcium antagonists | 5 (26.3) | 3 (9.4) | 0 |
| Diuretics | 1 (5.3) | 3 (9.4) | 0 |
| Aldosterone antagonists | 1 (5.3) | 2 (6.3) | 0 |
| Amiodarone | 1 (5.3) | 6 (18.8) | 0 |
| Continuous data | Median (IQR) | Median (IQR) | Median (IQR) |
|---|---|---|---|
| Age (years) | 51.0 (26.0–56.0) | 47.5 (29.3–55.8) | 38 (27.5–55.5) |
| Body mass index (kg m−2) | 26.0 (22.5–30.0) | 26 (22.5–29.9) | 25.35 (22.8–27.5) |
| LVEDd (mm) | 44.0 (36.0–47.0) | 40.0 (37.0–46.0) | 43.5 (39.0–46.3) |
| IVS (mm) | 18.6 (16.0–23.2) | 20.8 (17.7–26.6) | 9.0 (9.0–9.25)†‡ |
| PWT (mm) | 11.0 (9.6–11.9) | 11.9 (10.0–13.7) | 10.0 (9.0–10.0)†‡ |
| RWT | 0.65 (0.57–0.91) | 0.82 (0.69–0.97) | 0.44 (0.41–0.48)†‡ |
| LVM (g) | 238 (198–305) | 293 (257–359)* | 116 (108–121)†‡ |
| LVMI (g m−2) | 114 (108–166) | 165 (139–185)* | 62 (57–66)†‡ |
| EF (%) | 68 (65–73) | 75 (68.3–79.8)* | 64 (61.8–67)†‡ |
Qualitative data are presented as numbers (%), while continuous data are presented as medians (IQR). Comparisons between groups were made with Fisher's exact test for qualitative data or with the Mann–Whitney U-test for continuous data. For qualitative data, only gender, smoking and hypercholesterolaemia were compared with the control group; for the remaining qualitative data, only comparisons between HCM BB– and HCM BB+ were performed. ACE, angiotensin-converting enzyme; EF, ejection fraction; IQR, interquartile range; IVS, intraventricular septum thickness; LVEDd, left ventricular end-diastolic diameter; LVM, left ventricular mass; LVMI, left ventricular mass index; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; PWT, posterior wall thickness; RWT, relative wall thickness; SAM, systolic anterior motion.
P < 0.05 for comparison of HCM BB– vs. HCM BB+;
P < 0.05 for comparison of HCM BB– vs. control group; and
P < 0.05 for comparison of HCM BB+ vs. control group.
There was no significant difference in the rate of hypertension between HCM patients with and without β-blocker treatment. Likewise, the severity of heart failure NYHA functional class, frequency of syncope, aborted sudden cardiac death, implanted cardioverter defibrillators, presence of systolic anterior motion and left ventricular outflow tract obstruction in echocardiography and the use of pharmaceutical therapy other than β-blockers were not statistically different between both HCM groups. All echocardiographic measures, with the exception of the left ventricular end-diastolic diameter, were significantly larger in both HCM groups than in healthy volunteers. The HCM patients treated with β-blockers had significantly increased left ventricular mass and its index as well as left ventricular ejection fraction in comparison to HCM patients untreated with β-blockers.
There were no statistical differences in the treatment between the HCM groups with or without β-blockers. However, the use of nondihydropyridine calcium antagonists (either verapamil or diltiazem) requires additional information. In the HCM group without β-blockers, five patients were treated with one of the mentioned calcium antagonists, one patient was persistently refusing any pharmacological treatment and the remaining 13 patients were newly diagnosed HCM cases. In the HCM group treated with β-blockers, only three patients were on calcium antagonist.
Autonomic modulation of the cardiovascular system
Table 2 shows the results of HRV and baroreflex function in the studied participants. The mean pulse interval, all measures of HRV and baroreflex sensitivity were comparable between patients with HCM, treated or not with β-blockers, and healthy volunteers. However, the delay of the baroreflex (Figure 1) was significantly longer in HCM patients untreated with β-blockers, both in comparison with HCM patients receiving β-blockers and in comparison with control subjects. This delay was not statistically different between HCM patients treated with β-blockers and the control group.
Table 2.
The results of heart rate variability and baroreflex function assessments in the studied participants
| Parameter | HCM BB– (n = 19) | HCM BB+ (n = 32) | Control subjects (n = 14) |
|---|---|---|---|
| Mean pulse interval (ms) | 907.9 (807.0–1032.6) | 983.2 (870.4–1104.7) | 963.9 (884.7–1086.8) |
| SD (ms) | 64.5 (38.2–75.1) | 56.6 (39.2–79.2) | 58.3 (48.7–90.5) |
| LF (ms2) | 813.0 (408.1–1268.8) | 596.2 (313.0–1739.7) | 638.3 (443.7–1392.4) |
| HF (ms2) | 496.6 (199.9–972.6) | 517.2 (264.5–1295.2) | 577.3 (306.9–780.7) |
| LFnu (%) | 60.3 (54.8–66.2) | 52.2 (47.4–61.0) | 57.6 (50.8–66.4) |
| HFnu (%) | 38.0 (33.5–44.2) | 47.2 (38.7–51.8) | 42.1 (33.4–48.2) |
| LF/HF | 1.6 (1.2–2.0) | 1.1 (0.9–1.6) | 1.4 (1.1–2.0) |
| BRS (ms mmHg–1) | 7.7 (5.6–13.4) | 8.5 (5.5–11.8) | 11.4 (6.6–15.7) |
| Baroreflex delay (s) | 2.0 (1.6–2.3) | 1.4 (1.1–1.8)* | 1.2 (0.8–1.8)† |
Data are presented as medians (IQR). BRS, baroreflex sensitivity; HF, power of high frequency of heart rate variability; HFnu, HF in normalized units; IQR, interquartile range; LF, power of low frequency of heart rate variability; LFnu, LF in normalized units; LF/HF, ratio of the power of LF to the power of HF; SD, standard deviation of pulse intervals.
P < 0.05 for comparison of HCM BB– vs. HCM BB+; and
P < 0.05 for comparison of HCM BB– vs. control group.
Figure 1.
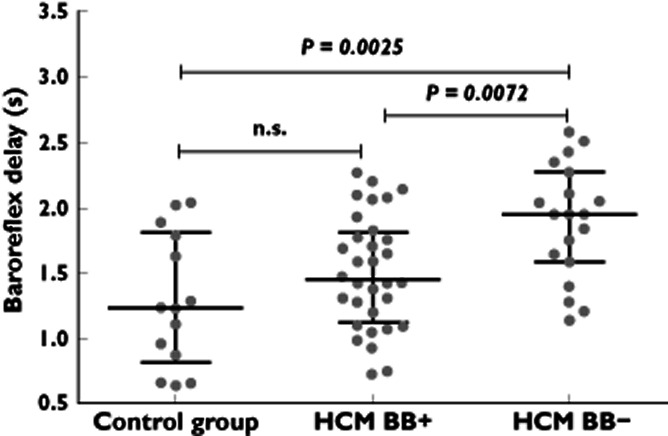
Scatterplots presenting values of baroreflex delay in healthy people (control group) and hypertrophic cardiomyopathy (HCM) patients treated (HCM BB+) or not treated with β-blockers (HCM BB–). For each group, in addition to all the individual values, the median and interquartile range are shown. The P values are derived from the Mann–Whitney U-test
Out of all studied autonomic indices, only baroreflex delay was significantly influenced by the use of β-blocker in multivariate linear regression analysis adjusted for the gender and age of the HCM patients (P = 0.0078; r2 = 0.155 for the model, n = 51 patients). The effect of β-blocker therapy on baroreflex delay remained significant (P = 0.032) even after adding to this model (r2 for the model 0.30) all other pharmaceutical agents (calcium antagonist, amiodarone, angiotensin-converting enzyme inhibitor, diuretic and aldosterone antagonist). In fact, in this model the β-blocker therapy was the only significant contributor to baroreflex delay, showing that other drugs have no effect on this variable. For this last model, however, the sample size may be insufficient for multivariate analysis; therefore, these results, albeit very interesting, should be treated as exploratory analysis of the data.
Discussion
In this study, we have observed no difference in HRV and baroreflex sensitivity between HCM patients treated and untreated with β-blockers, as well as between the HCM patients and control subjects. However, baroreflex delay is significantly prolonged in untreated HCM patients compared both with HCM individuals on β-blockers and with healthy participants. The duration of baroreflex delay in HCM patients on β-blocker therapy is not significantly different from that in healthy volunteers. In other words, HCM patients treated with β-blockers present with a normalized delay of baroreflex. This seems to be a novel, previously unreported effect of these drugs.
The autonomic regulation in HCM patients has been studied by various means, including measurement of circulating catecholamines, myocardial norepinephrine content, myocardial norepinephrine spillover or norepinephrine reuptake by sympathetic nerves 13, 14, 31, 32. It has even been reported that HCM patients have a reduced myocardial β-adrenergic receptor density 32 compared with healthy people. Looking beyond catecholamines, such non-invasive functional methods as HRV and arterial or cardiopulmonary baroreflex function analysis have also been used in HCM patients, showing not only the sympathetic agitation but also vagal withdrawal in this disease 12, 33–37.
While HRV is believed to represent mainly the vagal tonic influence on the heart, baroreflex function is a good approach for the evaluation of the reflex autonomic modulation of the whole cardiovascular system 24, 38, 39. Few papers report on the function of the cardiopulmonary or arterial baroreflex in HCM patients 36, 37. Usually, it is shown that the autonomic control of the heart is mediated by changes in blood pressure in the main or pulmonary circulation in HCM patients. An alternative hypothesis is that due to abnormal local wall strains the mechanoreceptors of the hypertrophied left ventricle may present an exaggerated response to some stimuli, e.g. exertion, and lead to some of the changes in the autonomic activity 9, 36, 37, 40.
In our study, we have analysed total HRV by means of the standard deviation of the duration of all pulse intervals and, additionally, with the use of spectral HRV analysis (Table 2) 24. There were no significant differences in HRV between the studied HCM groups, or between them and the healthy group. Also, we have not observed any significant difference in the resting spontaneous BRS 28 between the HCM patients and the healthy people, or between HCM patients with or without β-blockers. It should be mentioned, however, that baroreflex sensitivity characterizes only the magnitude of the cardiac cycle change in response to a change in blood pressure. In contrast, the baroreflex delay is an index that quantifies the latency of the heart response to a change in autonomic modulation secondary to an alteration in blood pressure 26–29, 41. In other words, baroreflex sensitivity describes the amount of response of the heart to a change in blood pressure, whereas baroreflex delay represents the dynamicity of this process.
It has been shown that baroreflex delay increases after atropine-induced vagal blockade, i.e. the conditions in which the vagally mediated control of sinus node activity are stopped, while the sympathetic effects become unopposed 29, 41. Westerhof et al. 29 have also observed that the baroreflex delay is prolonged during head-up tilting, and the magnitude of this prolongation depends both on the duration of the tilting and the tilting angle. Head-up tilting is a physiological challenge accompanied by fast, persistent vagal withdrawal and sympathetic agitation 29, 41–43. These studies clearly show that the baroreflex delay is a sensitive marker of changes in the sympathetic activity 29, 41, 43.
To the best of our knowledge, the baroreflex delay has never before been analysed in HCM patients, and thus reporting on the delay in these patients is one of the novelties of this study. We have observed that the baroreflex delay in HCM patients without β-blockers is significantly longer, by ∼0.8 s, than in healthy people. This suggests that the dynamicity of the whole baroreflex arc between arterial baroreceptors loaded or unloaded by any change in systolic blood pressure (afferent part of this reflex) and the evoked sinus node response in the form of an altered duration of the cardiac cycle is postponed (efferent part of the reflex). This finding is no longer surprising if we connect the following two aforementioned aspects: (i) the adrenergic predominance in HCM patients compared with healthy people 13, 14, 31–37; and (ii) the change in the baroreflex delay elicited by changes in the sympathetic and parasympathetic activity 29, 41. Taking into account both these aspects, it starts to become obvious that the increased adrenergic drive in HCM patients must increase the baroreflex delay in them.
The observed prolongation of the baroreflex delay is due to the untreated adrenergic effects in HCM patients without β-blockers. This situation seems to be treatable and reversible, because the baroreflex delay in HCM patients on β-blockers is approximately 0.5 s shorter than in HCM patients without these drugs, and it becomes comparably long as in healthy people. The shorter and normalized baroreflex delay in HCM patients on β-blockers is another novelty of our study, because a similar effect has not been studied or demonstrated before.
β-Blockers represent the mainstay of the most contemporary pharmacological therapy in HCM patients, because they reduce symptoms by a number of potential mechanisms (e.g. strong negative inotropic effects, improvement of ventricular filling and reduction in heart rate, oxygen consumption and angina pain) 3, 6, 16. Our additional statistical analysis shows that only β-blockers influence baroreflex delay, and their effect is independent of age and gender in HCM patients. Most probably, it is also independent of the remaining pharmacological treatment in our patients. If so, a natural consequence of our findings are questions about the mechanisms of the observed beneficial effects of β-blockers on the baroreflex delay in HCM patients, and whether such an effect might be predicted or expected?
Regarding the mechanisms of action of β-blockers, we need to refer to the aforementioned effect of catecholamines, i.e. the downregulation of myocardial β-adrenergic receptors in HCM patients 1–3, 6–8, 16. If we consider β-blockers, these drugs reduce or completely prevent the influence of catecholamines on β-adrenergic receptors within the cardiovascular system in different diseases, including HCM. It has been observed that the use of β-blockers in patients with heart failure due to dilated cardiomyopathy was associated with upregulation of the density of myocardial β-adrenergic receptors 44. Although a similar study in HCM has not yet been performed, we may assume that these drugs can induce similar adaptation of the adrenergic system. It is thus plausible that in HCM patients receiving long-term treatment with β-blockers (in our study, metoprolol or bisoprolol for at least 6 months), there was sufficient time for such upregulation of the β-adrenergic receptors to take place. A higher density of receptors usually requires less agonist to induce a comparable effect than during conditions in which the receptor density is lower. This might provide new conditions for the sympathetic–parasympathetic balance and improve the dynamicity for the blood pressure–heart rate interactions, i.e. shortening the prolonged baroreflex delay. However, this explanation is based not only on the results from other studies but also on some extrapolations of these results and it therefore remains only a speculation.
The answer to the question of whether the observed effect of β-blocker on baroreflex delay might be expected or not is not so obvious. On the one hand, β-blockers directly slow down the rate of spontaneous depolarization of the sinus node; therefore, the later response of the sinus node to a change in blood pressure with a further prolongation of the already longer baroreflex delay might be expected. Of our HCM patients, those on β-blockers had a slightly longer mean resting pulse interval of 983 ms (i.e. a heart rate of 61 beats min−1) than patients without β-blockers, whose pulse interval was 908 ms (or a heart rate of 66 beats min−1). These mean pulse intervals did not differ significantly between both HCM groups (Table 2), whereas their baroreflex delays were significantly different. For this reason, it is probably not the effect of β-blockers on the heart rate that is responsible for our findings. Another potential explanation is the beneficial action of β-blockers on the sympathetic–parasympathetic balance. The β-blocker-induced shift of this balance towards the restoration of vagal control over the heart might increase the dynamicity of such control. It has been reported that vagal effects on the sinus node take no more than 0.3–0.5 s in the resting supine position, and that this time is prolonged during sympathetic stimulation triggered by head-up tilt (to ∼0.9 s) or atropine blockade (to >1.2 s) 41. If HCM without β-blockade translates into more adrenergic activity and HCM with β-blockers means less sympathetic tone, then the above argument seems to be in agreement with the expectation that β-blockers shorten the baroreflex delay.
There is one additional aspect of our study which requires another explanation. The HCM patients on β-blocker therapy had a significantly increased LVM, LVMI and left ventricular ejection fraction compared with HCM patients who were not treated with β-blockers. These findings might be explained by the incidence of the obstruction of the LVOT in HCM patients with or without β-blockers. In the group of 19 HCM patients without β-blockers, there were only four patients with the LVOT obstruction. In contrast, in the group of 32 HCM patients treated with β-blockers, nearly half of them (15 cases) presented with LVOT obstruction (Table 1). Although the proportion test did not show any statistical difference in the incidence of LVOT obstruction, its presence is always associated with much greater left ventricular hypertrophy, with more increased LVM and LVMI and with more dynamic myocardial contraction, shown here by the higher ejection fraction 1–3, 7, 8.
Limitations of the study must be recognized. First, only indirect measures for the evaluation of autonomic modulation of the cardiovascular system were applied. However, various methods for HRV and baroreflex function are commonly used, both in research and in clinical practice 24, 39, 45–49. A second limitation is the use of a spontaneous method for baroreflex function and not, for example, phenylephrine or nitroprusside challenges. However, with HCM patients even short-lasting but dramatic changes in afterload and preload can cause serious clinical problems (e.g. arrhythmia, angina or syncope). This raises both safety and ethical issues, for which reason we have decided not to perform phenylephrine/nitroprusside tests. We are also aware that the use of eight parameters in a multivariate linear regression model for baroreflex delay with only 51 HCM patients may raise some questions about the reliability of the results. We used this analysis only as an exploratory data-mining tool to look for new ideas and suggestions for future and larger studies. Finally, we have limited detailed considerations of the various mechanisms leading to sympathetic–parasympathetic imbalance in HCM patients, because they would only be speculative and far beyond the scope of this paper.
In summary, we have observed that the baroreflex delay is longer in HCM patients untreated with β-blockers in comparison to both HCM patients on β-blocker therapy and healthy subjects. This prolongation of the delay may suggest a change in the dynamicity of the sinus node response to a change in blood pressure caused by an increased sympathetic predominance in HCM patients. We have also observed that this delay is normalized in HCM patients who are treated with β-blockers, and that the reduction in the baroreflex delay caused by β-blockers is independent of the age and gender of the patient. As this study appears to provide the first analysis of baroreflex delay in HCM patients, we believe that our results add important information regarding the pathomechanisms of HCM. Furthermore, we believe that the reported effect of β-blockers on baroreflex delay in HCM patients provides some new insights into the mechanisms of action of these medications in HCM patients.
We are aware, however, that more extensive clinical studies in HCM patients are needed to explore the clinical value of baroreflex delay and the effects of other treatment options (e.g. calcium antagonists, septal myotomy or alcohol ablation, or coil embolization of the septal coronary arteries 3, 16) on the autonomic regulation of the cardiovascular system.
Acknowledgments
This study was supported by an internal research grant from Poznan University of Medical Sciences. This study has no relationship with industry.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Maron BJ, Towbin AJ, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. An American Heart Association Scientific Statement From the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention of American Heart Association: contemporary Definitions and Classifications of the Cardiomyopathies. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, 3rd, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and European Society of Cardiology Committee for Practice Guidelines. Eur Heart J. 2003;24:1965–1991. doi: 10.1016/s0195-668x(03)00479-2. [DOI] [PubMed] [Google Scholar]
- 3.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy; executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761–2796. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 4.Elliott PE, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;6:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 5.Nunez DJ, Clifford CP, al-Mahdawi S, Dutka D. Hypertensive cardiac hypertrophy-is genetic variance the missing link? Br J Clin Pharmacol. 1996;42:107–117. doi: 10.1046/j.1365-2125.1996.37315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DC, Braunwald E, Glick G, Mason D, Chidsey C, Ross J. Effects of beta-adrenergic blockade on the circulation, with particular reference to observation in patients with hypertrophic subaortic stenosis. Circulation. 1964;29:84–98. doi: 10.1161/01.cir.29.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Bonow RO, Canzon RO, Leon MB, Epstein SE. Hypertrophic cardiomyopathy: interrelations of clinical manifestations, pathophysiology, and therapy (1) N Engl J Med. 1987;316:780–789. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Bonow RO, Cannon RO, 3rd, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (2) N Engl J Med. 1987;316:844–852. doi: 10.1056/NEJM198704023161405. [DOI] [PubMed] [Google Scholar]
- 9.Counihan PJ, Frennaux P, Webb DJ, McKenna WJ. Abnormal vascular responses during supine exercise in hypertrophic cardiomyopathy. Circulation. 1991;84:686–696. doi: 10.1161/01.cir.84.2.686. [DOI] [PubMed] [Google Scholar]
- 10.Smith OI, Wettrell G, Riesenfeld T. A cohort study of childhood hypertrophic cardiomyopathy: improved survival following high-dose beta-adrenoreceptor antagonist treatment. J Am Coll Cardiol. 1999;34:1813–1822. doi: 10.1016/s0735-1097(99)00421-0. [DOI] [PubMed] [Google Scholar]
- 11.Carl I, Ong H, Donnelly R, Riley M, Nicholls DP. Exercise in hypertrophic cardiomyopathy is associated with sympatho-adrenal imbalance. Int J Cardiol. 2007;116:124–125. doi: 10.1016/j.ijcard.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Gilligan DM, Chan WL, Sbarouni E, Nihoyannopoulos P, Oakley CM. Autonomic function in hypertrophic cardiomyopathy. Br Heart J. 1993;69:525–529. doi: 10.1136/hrt.69.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brush JE, Jr, Eisenhofer G, Garty M, Stull R, Maron BJ, Cannon RO, 3rd, Panza JA, Epstein SE, Goldstein DS. Cardiac norepinephrine kinetics in hypertrophic cardiomyopathy. Circulation. 1993;79:836–844. doi: 10.1161/01.cir.79.4.836. [DOI] [PubMed] [Google Scholar]
- 14.Kawai C, Yui Y, Hoshino T, Sasayama S, Matsumori A. Myocardial catecholamines in hypertrophic and dilated (congestive) cardiomyopathy: a biopsy study. J Am Coll Cardiol. 1983;2:834–840. doi: 10.1016/s0735-1097(83)80229-0. [DOI] [PubMed] [Google Scholar]
- 15.Thompson DS, Naqvi N, Juul SM, Swanton RH, Coltart DJ, Jenkins BS, Webb-Peploe MM. Effects of propranolol on myocardial oxygen consumption, substrate extraction, and haemodynamics in hypertrophic obstructive cardiomyopathy. Br Heart J. 1980;44:488–498. doi: 10.1136/hrt.44.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spoladore R, Maron MS, D'Amato R, Camici PG, Olivotto I. Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J. 2012;33:1724–1733. doi: 10.1093/eurheartj/ehs150. [DOI] [PubMed] [Google Scholar]
- 17.Lin JL, Chan HL, Du CC, Lin IN, Lai CW, Lin KT, Wu CP, Tseng YZ, Lien WP. Long-term beta-blocker therapy improves autonomic nervous regulation in advanced congestive heart failure: a longitudinal heart rate variability study. Am Heart J. 1999;137:658–665. doi: 10.1016/s0002-8703(99)70219-x. [DOI] [PubMed] [Google Scholar]
- 18.Airaksinen KE, Niemelä MJ, Huikuri HV. Effect of beta-blockade on baroreflex sensitivity and cardiovascular autonomic function tests in patients with coronary artery disease. Eur Heart J. 1994;15:1482–1485. doi: 10.1093/oxfordjournals.eurheartj.a060418. [DOI] [PubMed] [Google Scholar]
- 19.Niemelä MJ, Airaksinen KE, Huikuri HV. Effect of beta-blockade on heart rate variability in patients with coronary artery disease. J Am Coll Cardiol. 1994;23:1370–1377. doi: 10.1016/0735-1097(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 20.Lucini D, Pagani M, Mela GS, Malliani A. Sympathetic restraint of baroreflex control of heart period in normotensive and hypertensive subjects. Clin Sci (Lond) 1994;86:547–556. doi: 10.1042/cs0860547. [DOI] [PubMed] [Google Scholar]
- 21.Mortara A, La Rovere MT, Pinna GD, Maestri R, Capomolla S, Cobelli F. Nonselective beta-adrenergic blocking agent, carvedilol, improves arterial baroflex gain and heart rate variability in patients with stable chronic heart failure. J Am Coll Cardiol. 2000;36:1612–1618. doi: 10.1016/s0735-1097(00)00900-1. [DOI] [PubMed] [Google Scholar]
- 22.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, Sahn DJ, Schiller NB, Tajik A, Teichholz LE, Weyman AE. Report of American Society of Echocardiography Committee on Nomenclature and Standards in 2-D Echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB. Left ventricular mass in children and adolescents. J Am Coll Cardiol. 1988;12:709–714. doi: 10.1016/s0735-1097(88)80061-5. [DOI] [PubMed] [Google Scholar]
- 24.Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurements, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 25.Lomb NR. Least squares frequency analysis unequally spaced data. Astrophys Space Sci. 1975;39:447–462. [Google Scholar]
- 26.Borst C, Karemaker JM. Time delays in the human baroreceptor reflex. J Auton Nerv Syst. 1983;9:399–409. doi: 10.1016/0165-1838(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 27.Eckberg DL. Temporal response patterns of the human sinus node to brief carotid baroreceptor stimuli. J Physiol. 1976;258:769–782. doi: 10.1113/jphysiol.1976.sp011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM. Time-domain cross -correlation baroreflex sensitivity: performance on the EUROBAVAR data set. J Hypertens. 2004;22:1371–1380. doi: 10.1097/01.hjh.0000125439.28861.ed. [DOI] [PubMed] [Google Scholar]
- 29.Westerhof BE, Gisolf J, Karemaker JM, Wesseling KH, Secher NH, van Lieshout JJ. Time course analysis of baroreflex sensitivity during postural stress. Am J Physiol Heart Circ Physiol. 2006;291:2864–2874. doi: 10.1152/ajpheart.01024.2005. [DOI] [PubMed] [Google Scholar]
- 30.Guzik P, Wykretowicz A, Krauze T, Piskorski J, Adamska A, Milewska A, Wesseling KH, Wysocki H. Add-on therapy with a nighttime dose of doxazosin in patients with uncontrolled hypertension: effects on autonomic modulation of the cardiovascular system. Hypertens Res. 2008;31:443–453. doi: 10.1291/hypres.31.443. [DOI] [PubMed] [Google Scholar]
- 31.Schäfers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O, Camici PO. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ Res. 1998;82:57–62. doi: 10.1161/01.res.82.1.57. [DOI] [PubMed] [Google Scholar]
- 32.Lefroy DC, de Silva R, Choudhury L, Uren NG, Crake T, Rhodes CG, Lammertsma AA, Boyd H, Patsalos PN, Nihoyannopoulos P, Oakley CM, Jones T, Camici PG. Diffuse reduction of myocardial beta-adrenoceptors in hypertrophic cardiomyopathy: a study with positron emission tomography. J Am Coll Cardiol. 1993;22:1653–1660. doi: 10.1016/0735-1097(93)90591-n. [DOI] [PubMed] [Google Scholar]
- 33.Limbruno U, Strata G, Mengozzi G, Baglini R, Vincenzo A, Leoncini GP, Mariani M. Spectrum analysis of heart rate variability in obstructive hypertrophic myocardiopathy. Evidence of altered autonomic function. Cardiologia. 1992;37:847–852. [PubMed] [Google Scholar]
- 34.Counihan PJ, Fei L, Bashir Y, Farrell TG, Haywood GA, McKenna WJ. Assessment of heart rate variability in hypertrophic cardiomyopathy. Association with clinical and prognosis features. Circulation. 1993;88:1682–1690. doi: 10.1161/01.cir.88.4.1682. [DOI] [PubMed] [Google Scholar]
- 35.Döven O, Sayin T, Guldal M Karaoguz R, Oral D. Heart rate variability in hypertrophic obstructive cardiomyopathy: association with functional classification and left ventricular outflow gradients. Int J Cardiol. 2001;77:281–286. doi: 10.1016/s0167-5273(00)00447-2. [DOI] [PubMed] [Google Scholar]
- 36.Thomson HL, Morris-Thurgood J, Atherton J, Frenneaux M. Reduced cardiopulmonary baroreflex sensitivity in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1998;31:1377–1382. doi: 10.1016/s0735-1097(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 37.Thaman R, Elliott PM, Shah JS, Williams L, Murphy RT, McKenna MJ, Frenneaux MP. Reversal of inappropriate peripheral vascular responses in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:883–892. doi: 10.1016/j.jacc.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 38.Bauer A, Barthel P, Schneider R, Ulm K, Müller A, Joeinig A, Stich R, Kiviniemi A, Hnatkova K, Huikuri H, Schömig A, Malik M, Schmidt G. Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk) Eur Heart J. 2009;30:576–583. doi: 10.1093/eurheartj/ehn540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, Guzik P, Lombardi F, Müller A, Oto A, Schneider R, Watanabe M, Wichterle D, Zareba W. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: international Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008;52:1353–1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Frenneaux MP, Counihan PJ, Caforio AC, Chikamori T, McKenna WJ. Abnormal blood pressure responses during exercise in hypertrophic cardiomyopathy. Circulation. 1990;82:1995–2002. doi: 10.1161/01.cir.82.6.1995. [DOI] [PubMed] [Google Scholar]
- 41.Keyl C, Schneider A, Dambacher M, Bernardi L. Time delay of vagally mediated cardiac baroreflex response varies with autonomic cardiovascular control. J Appl Physiol. 2001;91:283–289. doi: 10.1152/jappl.2001.91.1.283. [DOI] [PubMed] [Google Scholar]
- 42.Gilligan DM, Nihoyannopoulos P, Chan WL, Oakley CM. Investigation of a hemodynamic basis for syncope in hypertrophic cardiomyopathy. Use of a head-up tilt test. Circulation. 1992;85:2140–2148. doi: 10.1161/01.cir.85.6.2140. [DOI] [PubMed] [Google Scholar]
- 43.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heilbrunn SM, Shah P, Bristow MR, Valantine HA, Ginsburg R, Fowler MB. Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation. 1989;79:483–490. doi: 10.1161/01.cir.79.3.483. [DOI] [PubMed] [Google Scholar]
- 45.Hohnloser S, Klingenheben T, Zabel M, Li Y. Heart rate variability used as an arrhythmia risk stratifier after myocardial infarction. PACE. 1997;20:2594–2601. doi: 10.1111/j.1540-8159.1997.tb06109.x. [DOI] [PubMed] [Google Scholar]
- 46.La Rovere MT, Schwarz PJ. Baroreflex sensitivity as a cardiac and arrhythmia mortality risk stratifier. PACE. 1997;20:2602–2613. doi: 10.1111/j.1540-8159.1997.tb06110.x. [DOI] [PubMed] [Google Scholar]
- 47.Guzik P, Piskorski J, Barthel P, Bauer A, Müller A, Junk N, Ulm K, Malik M, Schmidt G. Heart rate deceleration runs for postinfarction risk prediction. J Electrocardiol. 2012;45:70–76. doi: 10.1016/j.jelectrocard.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Mussalo H, Vanninen E, Ikaheimo R, Latinen T, Laakso M, Lansimies E, Hartikainen J. Baroreflex sensitivity in essential and secondary hypertension. Clin Auton Res. 2002;12:465–471. doi: 10.1007/s10286-002-0069-z. [DOI] [PubMed] [Google Scholar]
- 49.Johnson P, Shore A, Potter J, Penerai R, James M. Baroreflex sensitivity measured by spectral and sequence analysis in cerebrovascular disease: methodological consideration. Clin Auton Res. 2006;16:270–275. doi: 10.1007/s10286-006-0351-6. [DOI] [PubMed] [Google Scholar]


