Abstract
Aim
To compare plasma 4β-hydroxycholesterol : cholesterol with urinary 6β-hydroxycortisol : cortisol as markers of cytochrome P4503A4 activity before and after treatment with rifampicin for 2 weeks.
Method
6β-hydroxycortisol and cortisol were determined by liquid chromatography tandem mass spectrometry and 4β-hydroxycholesterol was determined by gas chromatography–mass spectrometry in three groups of healthy volunteers.
Results
Induction ratios for 6β-hydroxycortisol : cortisol were 1.8, 3.9 and 4.5 for 20 mg day−1, 100 mg day−1 or 500 mg day−1 of rifampicin, respectively. The corresponding ratios for 4β-hydroxycholesterol : cholesterol were 1.5, 2.4 and 3.8.
Conclusions
Plasma 4β-hydroxycholesterol : cholesterol gave similar induction ratios to urinary 6β-hydroxycortisol : cortisol.
Keywords: CYP3A4, endogenous markers, induction, LC-MS/MS, rifampicin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
We have suggested that plasma 4β-hydroxycholesterol may be used as an endogenous marker for cytochrome P450 3A4/5 (CYP3A)4/5 activity. We have shown that it can be used to monitor both induction and inhibition of CYP3A activity.
WHAT THIS STUDY ADDS
Plasma 4β-hydroxycholesterol : cholesterol was compared with another marker of CYP3A activity, 6β-hydroxycortisol : cortisol in urine. Urine collections from volunteers treated with rifampicin for 2 weeks were analyzed for this ratio and compared to earlier determined plasma 4β-hydroxycholesterol : cholesterol. The two markers gave similar induction ratios.
Introduction
Cytochrome P4503A (CYP3A) is an important enzyme family metabolizing both drugs and endogenous substances such as steroids. We have shown previously that CYP3A4 converts cholesterol into 4β-hydroxycholesterol 1 and suggested that plasma 4β-hydroxycholesterol may be used as a phenotypic marker of CYP3A activity 2. CYP3A4 also metabolizes cortisol to 6β-hydroxycortisol, which has been used as an endogenous marker for CYP3A activity. Since cortisol shows a pronounced diurnal variation, the ratio of 6β-hydroxycortisol : cortisol in urine is used rather than 6β-hydroxycortisol itself 3, 4. We have previously studied 4β-hydroxycholesterol as a marker of CYP3A-activity in healthy volunteers treated with rifampicin 5. There is a weak but significant correlation between 4β-hydroxycholesterol and cholesterol. Therefore, the 4β-hydroxycholesterol to cholesterol ratio should be used rather than 4β-hydroxycholesterol itself to correct for variations in cholesterol concentration during the study period 6. In the present investigation we compared urinary 6β-hydroxycortisol : cortisol to plasma 4β-hydroxycholesterol : cholesterol determined previously 5.
Methods
Chemicals
6β-hydroxycortisol, cortisol and cortisone were from Sigma-Aldrich Sweden AB, Stockholm, Sweden. 2H4-6β-hydroxycortisol was from Toronto Research Chemicals Inc., North York, Canada and 2H4-cortisol was purchased from Larodan Fine Chemicals AB, Malmö, Sweden.
Calibration standards
Working solutions of calibrators were prepared from stock solutions (300 μmol l−1 in methanol) by dilution with water to 700 nmol l−1.
Quality control (QC) samples
Quality control samples were prepared from spiked human urine at two concentrations. The concentrations of 6β-hydroxycortisol, cortisol and cortisone were 255, 89 and 127 nmol l−1, respectively (concentration 1) and 933, 235 and 482 nmol l−1 (concentration 2).
Healthy volunteers
Twenty-four healthy Swedish volunteers were recruited to the study, 12 males and 12 females, as described previously 5. All subjects possessed the CYP3A5*3/*3 genotype 5. Urine was collected from 20.00 h to 08.00 h (12 h) before treatment and after 2 weeks of treatment with rifampicin. All volunteers gave their written informed consent to participate in the study. The study was approved by the Human Ethics Committee at Karolinska Institutet, Stockholm, Sweden.
Determination of 6β-hydroxycortisol and cortisol in urine
6β-hydroxycortisol, cortisol and cortisone in urine were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS) using deuterium labelled 6β-hydroxycortisol (2H4-6β-hydroxycortisol) and deuterium labelled cortisol (2H4-cortisol) as internal standards.
Sample preparation
Urine (200 μl), QC samples or calibrators were diluted with an equal volume of water containing the internal standards 2H4-6β-hydroxycortisol (1000 nmol l−1) and 2H4-cortisol (400 nmol l−1). The diluted sample was applied to a 96-well solid phase extraction plate (Oasis HLB μElution Plate, Waters). After washing with 200 μl 5% methanol in water, analytes were eluted with 100 μl acetonitrile : isopropanol 2:3 (v/v) and diluted with 400 μl water. The samples were prepared on a Tecan Genesis RSP 150/TeVacS robot.
Analysis by LC-MS/MS
The samples (5 μl) were analyzed on a Waters Acquity-Quattro Premier LC-MS/MS system with a Waters UPLC BEH ShieldRP18 column (1.7 μm, 2.1 × 150 mm) at 55°C. The mobile phase was a 7 min linear gradient of 5–50% acetonitrile in water with formic acid (0.1%), flow rate 0.400 ml min−1 and total run time 12 min. Retention times for 6β-hydroxycortisol, cortisol and cortisone were 4.27, 6.27 and 6.30 min, respectively.
The mass spectrometer was operated in negative electrospray mode for 6β-hydroxycortisol and positive mode for cortisol and cortisone. The transitions used were 347→313 and 347→125 (6β-hydroxycortisol), 363→121 and 363→97 (cortisol) and 361→163 and 361→121 (cortisone). Transitions for the internal standards were 351→128 (2H4-6β-hydroxycortisol) and 367 →121 (2H4-cortisol).
Method validation
The linear range was 10–14 000 nmol l−1 for 6β-hydroxycortisol and 5–14 000 nmol l−1 for cortisol and cortisone. Urine samples spiked with 0.8–7 times the original concentration (255, 89 and 127 nmol l−1, respectively) gave recoveries of 90–97%. Urine samples spiked with 30–150 times the original concentration (933, 235 and 482 nmol l−1, respectively) gave recoveries of 105–123%. The total relative standard deviations (CV) were between 5.0 and 9.2% (n = 80, 117 and 115, respectively). The cortisol analysis is accredited according to SS-EN ISO 15189. Our laboratory participates in an external quality control programme for cortisol (UKNEQAS) on a regular basis.
Determination of 4β-hydroxycholesterol in blood plasma
The results from the determination of 4β-hydroxycholesterol in plasma were from a previous investigation 5. 4β-hydroxycholesterol was determined by combined gas chromatography-mass spectrometry as described in 1.
Determination of cholesterol in blood plasma
Cholesterol was determined by an enzymatic method, CHOD-PAPP, utilizing a Roche/Hitachi Modular instrument. The CV was 4% at 3 mmol l−1.
Results
Determination of 6β-hydroxycortisol : cortisol in urine from rifampicin treated volunteers
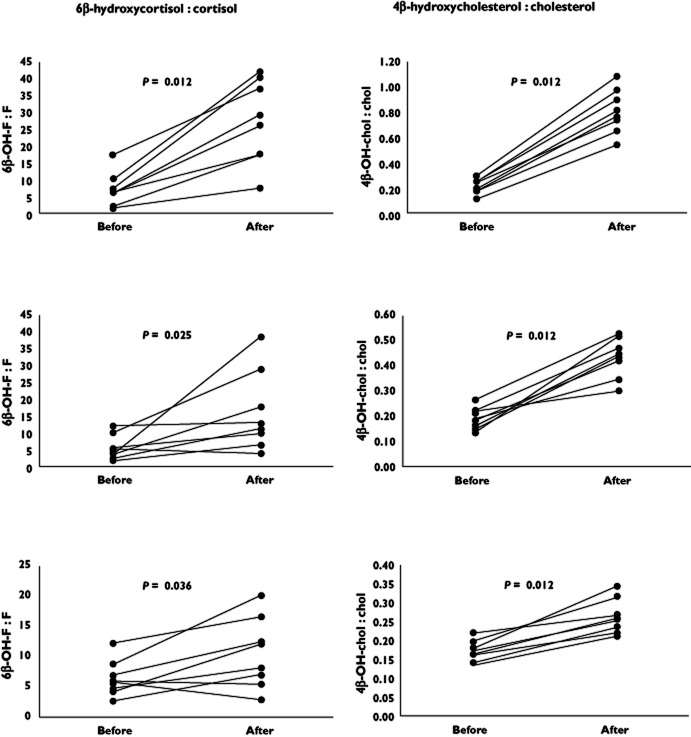
The urinary concentrations of 6β-hydroxycortisol and cortisol were determined in three groups of volunteers (eight in each group) treated with rifampicin (20 mg day−1, 100 mg day−1 or 500 mg day−1) for 14 days and the 6β-hydroxycortisol : cortisol ratios were calculated. As shown in Figure 1, the ratios before treatment were similar, 6.3, 5.4 and 7.3 and increased after 14 days treatment to 10.5, 16.2 and 27.3, respectively. Three subjects, two receiving 20 mg day−1 and one receiving 100 mg day−1, showed a reduced 6β-hydroxycortisol : cortisol ratio after 14 days of rifampicin treatment.
Figure 1.
Urinary 6β-hydroxycortisol : cortisol and plasma 4β-hydroxycholesterol : cholesterol (× 104) in adult healthy volunteers before and after 2 weeks of rifampicin treatment (500 mg day−1, upper panels; 100 mg day−1, middle panels; 20 mg day−1, lower panels). The 6β-hydroxycortisol : cortisol ratios were determined in 12 h urine collections. P values from analysis using Wilcoxon matched pairs test. 6β-OH-F: 6β-hydroxycortisol; F: cortisol; 4β-OH-chol: 4β-hydroxycholesterol; chol: cholesterol
Plasma 4β-hydroxycholesterol concentrations in rifampicin treated volunteers
The plasma 4β-hydroxycholesterol concentrations in the volunteers were published previously 5. In contrast to 6β-hydroxycortisol : cortisol, the plasma concentration of 4β-hydroxycholesterol increased in all volunteers after rifampicin treatment.
Calculation of induction ratios
The induction ratio was defined as 6β-hydroxycortisol : cortisol after treatment divided by the 6β-hydroxycortisol : cortisol before treatment. These induction ratios and the corresponding ratios for 4β-hydroxycholesterol and quinine metabolic ratio published previously 5 are shown in Table 1. The ratio of 4β-hydroxycholesterol : cholesterol should be used when the cholesterol concentration changes during a treatment period. There was a large interindividual variation in 6β-hydroxycortisol : cortisol. The ratios determined before rifampicin treatment showed a 10-fold variation and the ratios after treatment showed a 15-fold variation. The corresponding variations for 4β-hydroxycholesterol were three-fold and six-fold, respectively.
Table 1.
Induction ratios for 6β-hydroxycortisol : cortisol, 4β-hydroxycholesterol, 4β-hydroxycholesterol : cholesterol and quinine metabolic ratio (MR) in volunteers treated for 2 weeks with 20, 100 or 500 mg rifampicin day−1 (mean ± SD, n = 8). Data on 4β-hydroxycholesterol and quinine metabolic ratio (MR) are from a previous publication 5
| Rifampicin (mg day−1) | 6β-OH-cortisol : cortisol | 4β-OH-cholesterol | 4β-OH-cholesterol : cholesterol | Quinine MR |
|---|---|---|---|---|
| 20 | 1.77 ± 0.82 | 1.46 ± 0.24 | 1.53 ± 0.20 | 1.57 ± 0.33 |
| 100 | 3.92 ± 3.28 | 2.49 ± 0.66 | 2.40 ± 0.75 | 2.95 ± 1.10 |
| 500 | 4.46 ± 1.81 | 4.06 ± 0.85 | 3.76 ± 0.67 | 4.25 ± 2.07 |
Discussion
The induction of CYP3A4 could be monitored by 6β-hydroxycortisol : cortisol at all three doses of rifampicin. Compared with 4β-hydroxycholesterol : cholesterol, the P values for the differences in the 6β-hydroxycortisol : cortisol before and after treatment were higher 5 and in contrast to 4β-hydroxycholesterol : cholesterol some individuals had lower ratios after treatment than before treatment (Figure 1). However, the induction ratios for 6β-hydroxycortisol : cortisol and 4β-hydroxycholesterol : cholesterol are similar and also similar to the metabolic ratio of the exogenous CYP3A marker quinine (Table 1, data on 4β-hydroxycholesterol and quinine are from 5). An advantage with 4β-hydroxycholesterol is that only a single blood sample is required for the determination while a urine collection over at least 12 h is necessary when using 6β-hydroxycortisol : cortisol. The long half-life of 4β-hydroxycholesterol 7 results in small variations in concentration, but excludes this marker in short term studies.
In conclusion, similar estimates for the induction of CYP3A4 following rifampicin treatment were obtained using either urinary 6β-hydroxycortisol : cortisol or plasma 4β-hydroxycholesterol : cholesterol.
Acknowledgments
This work was supported by grants from AstraZeneca, the Swedish Research Council, Medicine (3902), Torsten and Ragnar Söderbergs Foundation and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (SLL 582107).
Competing Interests
K.P. Kanebratt is an employee of AstraZeneca. Unrestricted financial support was provided by AstraZeneca. This has not influenced the design or the interpretation of results of this study. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work (YMA, HN, AL-S, KPK, KW, UD had support (grant) from AstraZeneca for the submitted work and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years (YMA, HN, AL-S, KPK, KW). UD received support (grant, consultancy) from AstraZeneca in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work (YMA, HN, AL-S, KPK, KW, UD).
References
- 1.Bodin K, Bretillon L, Aden Y, Bertilsson L, Broomé U, Einarsson C, Diczfalusy U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. Evidence for involvement of cytochrome P450 3A4. J Biol Chem. 2001;276:38685–38689. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- 2.Diczfalusy U, Miura J, Roh H-K, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allqvist A, Jande M, Kim J-W, Aklillu E, Gustafsson LL, Bertilsson L. 4β-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genom. 2008;18:201–208. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]
- 3.Galteau MM, Shamsa F. Urinary 6β-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur J Clin Pharmacol. 2003;59:713–733. doi: 10.1007/s00228-003-0690-3. [DOI] [PubMed] [Google Scholar]
- 4.Peng CC, Templeton I, Thummel KE, Davis C, Kunze KL, Isoherranen N. Evaluation of 6β-hydroxycortisol, 6β-hydroxycortisone, and a combination of the two as endogenous probes for inhibition of CYP3A4 in vivo. Clin Pharmacol Ther. 2011;89:888–895. doi: 10.1038/clpt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanebratt KP, Diczfalusy U, Bäckström T, Sparve E, Bredberg E, Böttiger Y, Andersson TB, Bertilsson L. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4β-hydroxycholesterol. Clin Pharmacol Ther. 2008;84:589–594. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- 6.Diczfalusy U, Nylén H, Elander P, Bertilsson L. 4β-hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol. 2011;71:183–189. doi: 10.1111/j.1365-2125.2010.03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diczfalusy U, Kanebratt KP, Bredberg E, Andersson TB, Böttiger Y, Bertilsson L. 4β-Hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br J Clin Pharmacol. 2009;67:38–43. doi: 10.1111/j.1365-2125.2008.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]