Abstract
Osteoarthritis (OA) and degenerative disc disease (DDD) are similar diseases involving the breakdown of cartilage tissue, and a better understanding of the underlying biochemical processes involved in cartilage degeneration may allow for the development of novel biologic therapies aimed at slowing the disease process. Three members of the fibroblast growth factor (FGF) family, FGF-2, FGF-18, and FGF-8, have been implicated as contributing factors in cartilage homeostasis. The role of FGF-2 is controversial in both articular and intervertebral disc (IVD) cartilage as it has been associated with species- and age-dependent anabolic or catabolic events. Recent evidence suggests that FGF-2 selectively activates FGF receptor 1 (FGFR1) to exert catabolic effects in human articular chondrocytes and IVD tissue via upregulation of matrix-degrading enzyme production, inhibition of extracellular matrix (ECM) accumulation and proteoglycan synthesis, and clustering of cells characteristic of arthritic states. FGF-18, on the other hand, most likely exerts anabolic effects in human articular chondrocytes by activating the FGFR3 pathway, inducing ECM formation and chondrogenic cell differentiation, and inhibiting cell proliferation. These changes result in dispersed chondrocytes or disc cells surrounded by abundant matrix. The role of FGF-8 has recently been identified as a catabolic mediator in rat and rabbit articular cartilage, but its precise biological impact on human adult articular cartilage or IVD tissue remains unknown. The available evidence reveals the promise of FGF-2/FGFR1 antagonists, FGF-18/FGFR3 agonists, and FGF-8 antagonists (i.e., anti-FGF-8 antibody) as potential therapies to prevent cartilage degeneration and/or promote cartilage regeneration and repair in the future.
Keywords: FIBROBLAST GROWTH FACTOR, INTERVERTEBRAL DISC, ARTICULAR CARTILAGE, HOMEOSTASIS
Osteoarthritis (OA) and degenerative disc disease (DDD) are prevalent diseases involving the degradation of cartilaginous tissues. Despite an increase in research efforts focused on understanding the pathogenesis of these two conditions, many of the underlying biochemical processes involved in cartilage degeneration remain largely unknown. Recent literature has focused on uncovering specific cell signaling cascades that positively or negatively affect cartilage homeostasis in both OA and DDD, with the intention of developing novel therapies aimed at slowing and/or reversing cartilage degradation.
The fibroblast growth factor (FGF) family has been implicated in the regulation of both articular cartilage and intervertebral disc (IVD) homeostasis. This large family of structurally related proteins binds heparin and heparan sulfate [Friedl et al., 1997] and modulates the growth, migration, differentiation, and survival of a wide variety of cell types. Specifically, three members of the FGF family, fibroblast growth factor-2 (FGF-2, also known as basic FGF), FGF-18, and more recently, FGF-8, have been implicated as pertinent contributing factors in cartilage homeostasis.
FGF-2
FGF-2 IN ARTICULAR CARTILAGE
FGF-2 is produced endogenously in cartilage and has been proposed to be sequestered by perlecan, a heparan sulfate proteoglycan (HSPG) localized in the extracellular matrix (ECM) of articular cartilage [Vincent et al., 2007]. Upon cartilage injury, FGF-2 is released from its bound matrix and subsequently activates the ERK signaling pathway [Vincent et al., 2002]. Studies on FGF-2 from a variety of species have yielded contradictory results with regards to production of ECM in articular cartilage homeostasis, and the specific role of FGF-2 on cartilage homeostasis remains controversial.
A succession of studies has determined that FGF-2 functions as a catabolic inducer in human adult articular cartilage. FGF-2 triggers proteoglycan depletion in cartilage explants, and inhibits long-term proteoglycan accumulation in articular chondrocytes in both in vitro (alginate beads) and ex vivo (organ culture of human articular cartilage explants) studies [Im et al., 2007; Yan et al., 2011]. Moreover, FGF-2 potently antagonizes bone morphogenetic protein-7 (BMP-7) and insulin-like growth factor-1 (IGF-1)-mediated proteoglycan production in human articular cartilage [Loeser et al., 2005]. In articular chondrocytes, FGF-2 elicits an array of transcriptional responses. Most notably, FGF-2 induces matrix metalloprotease-13 (MMP-13), the most potent collagen-type II degrading enzyme in articular cartilage, resulting in collagen breakdown [Wang et al., 2004; Im et al., 2007]. FGF-2 also suppresses the aggrecan gene, and promotes the expression of aggrecanases (i.e., ADAMTS-5, a disintegrin-like and metalloprotease with thrombospondin motifs), substance P, neurokinin 1 receptor, and tumor necrosis factor (TNF) receptor [Alsalameh et al., 1999; Im et al., 2008; Yan et al., 2011]. Further, the concentration of FGF-2 in synovial fluid samples of OA patients is approximately twice that of normal healthy knee joints and may contribute to upregulation of vascular endothelial growth factor (VEGF) and neovascularization, suggesting a catabolic role of FGF-2 in cartilage homeostasis and OA-induced hyperalgesia [Im et al., 2007; Yan et al., 2011].
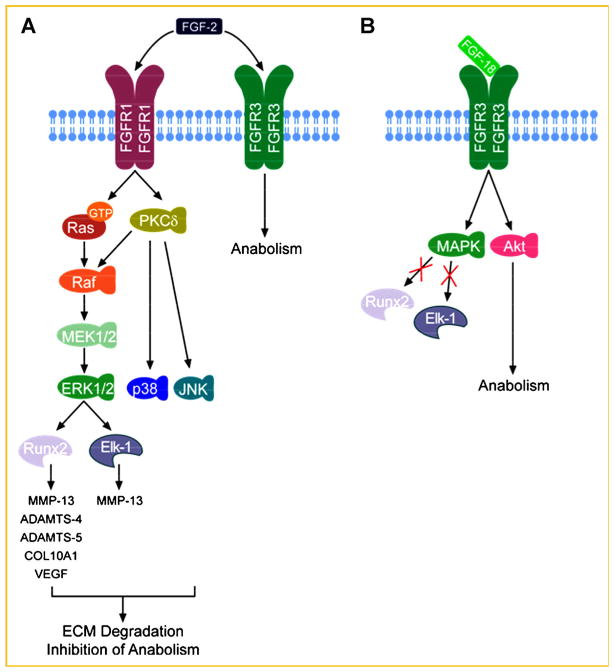
Recent studies elucidating the receptor expression profiles of FGF-2 have helped to increase our understanding of its catabolic potential in OA. Human adult articular chondrocytes express all FGF receptor (FGFR) subtypes (FGFR1–4), with significantly higher concentrations of FGFR1 and FGFR3 compared to FGFR2 and FGFR4 [Yan et al., 2011]. While FGF-2 has been found to activate both FGFR1 and FGFR3, its catabolic activities were recently found to be specifically mediated by FGFR1 [Yan et al., 2011]. The binding of FGF-2 to FGFR1 leads to receptor phosphorylation, which in turn activates two critical signaling mediators, Ras and Protein kinase C delta (PKCδ) [Yan et al., 2012]. These molecules then integrate their signaling inputs into the Raf-MEK1/2-ERK1/2 cascade to regulate target gene expression [Yan et al., 2012]. In parallel, p38, and JNK pathways are also activated by PKCδ [Im et al., 2007]. All three mitogen activated protein kinase (MAPK) subgroups (ERK, p38, and JNK) converge on the transcription factor Elk-1, which transactivates MMP-13 [Muddasani et al., 2007], ultimately promoting cartilage degradation. FGF-2 signaling also results in the activation of AP-1 and RUNX2, the latter of which may account for ADAMTS-5 induction [Wang et al., 2004; Im et al., 2007] (Fig. 1A). In human OA cells compared to healthy cells, FGFR1 expression is increased with a concomitant suppression of FGFR3 [Im et al., 2007; Yan et al., 2011], suggesting a pathological link between FGF-2, FGFR1, and OA, and a relative anabolic role of FGFR3 signaling.
Fig. 1.
Schematic model of FGF-2 and FGF-18 signaling pathways in articular cartilage and IVD. A: FGF-2 binds to both FGFR1 and FGFR3 with high affinity. The phosphorylation of FGFR1 triggers Ras and PKCδ activation, which converge on the Raf-MEK1/2–ERK1/2 axis. Active ERK1/2 utilizes at least two critical transcription factors, Elk-1 and RUNX2, to upregulate an array of genes including MMP-13, ADAMTS-4, ADAMTS-5, COL10A1, and VEGF. In parallel, PKCδ also activates p38 and JNK, which act in concert with ERK1/2 to promote ECM degeneration and inhibition of anabolic activities. The binding of FGF-2 to FGFR3 may counteract the FGF-2-induced catabolic pathway, similar to that utilized by FGF-18. Therefore, the ratio of FGFR3/FGFR1 is a key to the biological outcome of FGF-2. B: FGF-18 specifically binds to FGFR3, which in turn activates MAPK and Akt pathways. The MAPK pathway differs from those in FGF-2 signaling, because neither Elk-1 nor RUNX2 is activated as a result. The Akt pathway may enhance PG deposition and account for the anabolic activity of FGF-18.
Interestingly, the role of FGF-2 in human articular cartilage appears to contradict its role in murine cartilage. It has been reported that FGF-2 ablation leads to accelerate spontaneous and surgical-induced OA development, which can be rescued by administration of recombinant FGF-2 [Chia et al., 2009]. It was then proposed that FGF-2 acts as an intrinsic chondroprotective growth factor in murine OA. Successes in cartilage repair using FGF-2 or combinative agents including FGF-2 have also been reported in different animal models [Chuma et al., 2004; Yokoo et al., 2005; Ishii et al., 2007; Madry et al., 2010; Maehara et al., 2010]. Such discrepancies inspired the question of whether the function of FGF-2 in articular cartilage differs fundamentally between species.
Initial insight into the species-dependent role of FGF-2 was recently provided by findings from our group. Using a progressive OA murine model, we demonstrated that FGF-2 promotes proteoglycan deposition in both femoral and tibial cartilage, which is consistent with previous reports [Chia et al., 2009; Li et al., 2012]. The molecular basis for FGF-2-mediated anabolic effects apparently involves the FGFR expression profile in murine cartilage. In contrast with the human profile (predominant expression of FGFR1 and FGFR3), murine FGFR2 and FGFR4 are predominantly expressed, and FGFR3 is barely detectable in healthy knee joint articular cartilage [Li et al., 2012]. Surgical induction of OA in murine cartilage reduces the expression of all FGFR subtypes, but FGF-2 local injection markedly induces FGFR3 expression, which is opposite to the human scenario [Yan et al., 2011; Li et al., 2012]. The anabolic role of FGFR3 in cartilage has also been demonstrated in the context of FGF-18 signaling [Davidson et al., 2005; Moore et al., 2005]. Moreover, a recent study using FGFR1 conditional knockout mice demonstrated that FGFR1 deficiency attenuated cartilage degeneration [Weng et al., 2012]. The protective effect of FGFR1 blockade on articular cartilage was associated with reduction of MMP-13 expression [Weng et al., 2012]. Together with our findings, these data suggest that FGF-2 probably mediates PG production via FGFR3 signaling in murine models.
Taken together, it is possible that, via upregulating FGFR3, FGF-2 activates anabolic processes in murine cartilage, in contrast to the FGF-2-mediated catabolic activation of FGFR1 in human cartilage. In the murine model, despite the increased proteoglycan deposition, FGF-2 does not significantly alleviate joint pain, while it does appear to promote angiogenic activity and infiltration of inflammatory cells [Li et al., 2012]. In addition to its species-dependent effects, the chondroprotective activity of FGF-2 in animal models seems appears to be age-dependent as well, suggesting its proposed potential in cartilage repair may be more restricted [Yamamoto et al., 2004]. Based on these findings, we argue contend that FGF-2 should not be deemed a worthy biological treatment candidate in human OA therapy.
FGF-2 IN THE INTERVERTEBRAL DISC
Similar to its role in articular cartilage, the role of FGF-2 in the IVD has yet to be clearly elucidated as the literature has yielded contradictory results depending on tissue type. FGF-2 has been identified as an anabolic mediator of disc homeostasis via an FGF-2-mediated stimulation of proteoglycan synthesis in a canine IVD tissue culture system [Thompson et al., 1991] and upregulation of cell proliferation in rat discs [Nagano et al., 1995]. Tsai et al. [2007] analyzed the effects of FGF-2 on bovine nucleus pulposus (NP) cell growth and differentiation cultured in monolayer and alginate and reported FGF-stimulated increases in sulfated proteoglycan synthesis, lower aggrecan turnover, and differentiation of NP cell phenotype by maintaining responsiveness to transforming growth factor-β (TGF-β).
In contrast, findings from our laboratory [Li et al., 2008] and others [Peng et al., 2006], suggest that FGF-2 serves primarily a catabolic role in disc homeostasis. Peng et al. [2006] first demonstrated highly upregulated FGF-2 and FGFR1 expression in painful degenerated human spine disc cells compared to normal cells, providing a striking similarity to our findings on FGFR expression profiles in articular cartilage [Im et al., 2007; Yan et al., 2011]. In the bovine IVD, FGF-2 released by chondrocytes after mechanical injury induces catabolism by stimulating MMP-13 production, inhibiting proteoglycan synthesis, and antagonizing the well-known anabolic activity of BMP-7 [Li et al., 2008]. Our results also suggest that FGF-2 stimulates an overall increase in proteoglycan synthesis in bovine NP tissue [Li et al., 2008], similar to results reported by Tsai et al. [2007]. However, after normalization to cell number, both dimethylethylene blue (DMMB) and 35S-sulfate incorporation results suggested that, per cell, total proteoglycan accumulation and synthesis significantly decreased with FGF-2 stimulation in a dose-dependent manner, revealing a negative regulatory function of FGF-2 in spine cartilage homeostasis.
Interestingly, FGF-2 may play an important role in the spontaneous resorption process of degenerative or herniated IVD tissue via stimulation of angiogenesis and/or inflammatory cytokines that aid in cartilage destruction [Minamide et al., 1999; Melrose et al., 2002; Walsh et al., 2004; Tolonen et al., 2006]. Minamide’s group used a rabbit disc sequestration-type model to emulate IVD herniation in vivo and reported that epidural injection of FGF-2 stimulates increased angiogenesis, increased speed of disc resorption, and increased number of inflammatory cells compared to control (saline) [Minamide et al., 1999]. Tolonen et al. [2006] postulated that FGF-2 contributes to the resorption of herniated disc tissue by regulating matrix-degrading enzyme expression such as collagenase, stromelysin, and plasminogen activator. Melrose et al. [2002] further emphasized the role of FGF-2 in the repair process after IVD injury. In an ovine annular injury model, immunoreactivity for FGF-2 and TGF-β was positive in the outer third of the annulus fibrosus (AF), corresponding to the region of trauma, which reached maximum levels 12 months after injury and diminished by 26 months. The presence of FGF-2 was associated with angiogenesis and fibroblast infiltration around the defect, and immunoreactivity was strongly associated with regions of the annular lesions undergoing matrix reorganization, consistent with an active repair response mediated in part by FGF-2.
Based on these findings, one could suggest multiple roles of FGF-2 in disc homeostasis depending on the stage of degeneration and type of disease process. In normal or recently injured disc tissue, FGF-2 may act as a catabolic mediator, stimulating MMP-13 expression, suppressing proteoglycan synthesis, and inducing disc degeneration. However, these same properties may be beneficial after disc herniation, stimulating degradation of herniated tissue and encouraging spontaneous disc resorption. Indeed, it is possible that FGF-2 exhibits similar activities in articular cartilage and the IVD, and the lack of current evidence supporting beneficial effects of FGF-2 in human cartilage prevents the authors from supporting its use as a biological treatment strategy for DDD.
FGF-18
In contrast to the controversial role of FGF-2 in articular cartilage and the IVD, FGF-18 is a well-known anabolic growth factor involved in chondrogenesis as well as osteogenesis depending on cell types, in addition to articular cartilage repair [Liu et al., 2002; Ohbayashi et al., 2002; Ellsworth et al., 2002; Davidson et al., 2005; Moore et al., 2005]. Local delivery of adenovirus expressing FGF-18 into the pinnae of nude mice induced the formation of auricular cartilage, type II collagen, proteoglycan accumulation, and chondrocyte proliferation [Ellsworth et al., 2002]. Systemic delivery of pharmacologic doses of FGF-18 to rats via a single intravenous injection stimulated expansion of various cartilage depots, including the rib-sternum junction, trachea, spine, and articular cartilage within a 2-week period [Ellsworth et al., 2002]. Similarly, overexpression of FGFR-18 induced a dramatic enlargement of bronchial cartilage expressing type II collagen in lung tissue [Whitsett et al., 2002].
Moore et al. [2005] were the first to study the potential for in vivo cartilage repair by FGF-18 via intra-articular injection in a rat meniscal tear model of OA. A series of FGF-18 injections starting 21 days after surgical damage induced a dose-dependent increase in de novo cartilage formation and a reduction in cartilage degeneration in the tibial plateau of OA rats in vivo, demonstrating potent anabolic effects of FGF-18. Further, FGF-18 has been suggested to facilitate the chondrogenic activity of BMPs via suppression of noggin expression, a naturally occurring inhibitor of BMP signaling [Reinhold et al., 2004]. Noggin expression may play a vital role in helping to explain the opposite roles of FGF-18 and FGF-2 in human cartilage. Li et al. [2008] reported that stimulation of bovine IVD cells with FGF-2 induced a dose-dependent increase in noggin gene expression, suggesting that the increase in noggin may be one mechanism by which FGF-2 antagonizes the effects of the well-known anabolic factor BMP-7 (otherwise known as OP-1). Therefore, the stimulation (via FGF-2) or suppression (via FGF-18) of noggin may serve as one potential mechanism for the contrasting effects mediated by these two growth factors in cartilage homeostasis.
Previous evidence revealed that FGF-18 activates FGFR2–4 in mature articular cartilage and murine pinnae [Chang et al., 2000; Ellsworth et al., 2002]. More recent data from our laboratory [Im et al., 2007; Muddasani et al., 2007; Yan et al., 2011] and those from others [Davidson et al., 2005] indicate that FGF-18 signals selectively through the activation of FGFR3 in human articular chondrocytes and mice. The FGFR3–FGF-18 interaction suppresses cellular proliferation, promotes limb mesenchymal cell differentiation, and exerts anabolism in articular cartilage, as opposed to the FGFR1–FGF2 interaction. Both FGFR1 and FGFR3 activate the MAPK signaling cascades in human adult articular chondrocytes. Interestingly, FGFR1-mediated MAPK activation leads to the activation of RUNX2 and Elk-1, two critical transcription factors involved in the expression of multiple MMPs, aggrecanases and chondrocyte hypertrophy, whereas FGFR3-mediated MAPK signaling appears to activate a different set of downstream transcription factors that lead to chondroprotective outcomes (Fig. 1B). Previously, we demonstrated that the FGFR1–FGF2 pathway antagonizes BMP7 and/or IGF-1 in articular cartilage regeneration, whereas the FGF-18–FGFR3 axis promotes BMP7-induced cartilage regeneration, in part, by suppression of noggin, a naturally occurring inhibitor of BMP7 signaling that is stimulated by BMP7 itself as a negative feedback [Im et al., unpublished data].
Mutation studies have further revealed the importance of FGFR3–FGF-18 signaling. Mice lacking FGF-18 exhibit malformations in cartilage and bone, including delayed closure of calvarial sutures, enlargement of the proliferating and hypertrophic zones in the growth plate of long bones, defects in joint development, and delays in osteogenic differentiation [Liu et al., 2002; Ohbayashi et al., 2002]. Many of the common forms of dwarfism are caused by activating mutations in FGFR3 [Naski et al., 1998], suggesting that in the growth plate of long bones, FGFR3 is a negative regulator of chondrocyte proliferation. However, Iwata et al. [2000] reported that signaling through FGFR3 could both promote and inhibit chondrocyte proliferation depending on the stage of development. FGF-18 signaling through FGFR3 may enhance chondrocyte proliferation in immature committed chondrocytes, even though it is well established that signaling through FGFR3 inhibits chondrocyte proliferation and differentiation in the mature proliferating chondrocyte zone of the growth plate [Ellsworth et al., 2002; Liu et al., 2002]. This suggests that signaling through FGFR3 has a biphasic role during chondrocyte development: first promoting chondrocyte proliferation at early embryonic stages; and later acting to suppress chondrocyte proliferation. This paradoxical effect on proliferation supports a model in which chondrocytes at different stages of development may switch their cellular responsiveness to FGFR3–FGF-18 signaling from a mitogenic response early in development to a non-mitogenic response later in development [Liu et al., 2002, 2007].
Previous work has already identified FGFR3 signaling as a key regulator of chondrocyte function in chondrogenesis in the developmental stage. Nevertheless, the role of FGFR3 in adult articular cartilage and cartilage degeneration remains largely unknown and highly understudied area. Ellsworth et al. [2002] reported significant anabolic effects of FGF-18 on human articular cartilage homeostasis via increased chondrocyte proliferation and ECM production both in vivo and in vitro, and found that proliferation of cells expressing FGFR3-(IIIc) or FGFR2-(IIIc) was increased after incubation with FGF-18. The expression of FGFR18, FGFR3-(IIIc) and FGFR2-(IIIc) mRNA was localized to chondrocytes of human articular cartilage by in situ hybridization, suggesting a potential role of FGFR2 or FGFR3 in FGF-18-mediated human articular cartilage homeostasis. Valverde-Franco et al. [2006] noted that the absence of signaling through FGFR3 in chondrocytes in vivo leads to a similar degeneration of articular cartilage in mice as that seen in human OA. In the joints of FGFR3−/− mice, the absence of signaling leads to premature cartilage degeneration and early arthritis, demonstrated by excessive breakdown of aggrecan and type II collagen, increased expression of MMP-13, cellular hypertrophy, and increased loss of proteoglycan at the articular surface compared to control. Their results identified FGFR3 as a critical anabolic regulator of articular cartilage metabolism and a potential pathway for early intervention in degenerative joint disease. Similar findings from our laboratory reveal that treatment of human adult articular chondrocytes with FGF-18 for 21 days in alginate beads stimulates the activation of FGFR3 rather than FGFR1 and leads to increased PG deposition compared to cells treated with FGF-2 [Yan et al., 2011], and similar results were observed using bovine spine disc cells [Im et al., unpublished data].
Collectively, these studies suggest that activation of FGFR1 exerts anti-anabolic and catabolic biological effects in human adult articular cartilage, represented by fibroblast-like cell proliferation, inhibition of ECM production, and upregulation of matrix-degrading enzyme production. On the other hand, activation of FGFR3 via, for example, stimulation with FGF-18 exerts anabolic effects in human articular chondrocytes as reflected by increased cell-associated matrix formation and promotion of cell viability, leading to dispersed chondrocytes surrounded by abundant ECM instead of clusters of cells seen after stimulation with FGF-2 (Fig. 1). To date, the precise signaling pathway and molecular mechanisms mediated by FGFR3–FGF-18 axis in human adult articular cartilage and IVD homeostasis has yet to be elucidated. More recently, Su et al. [2010] successfully generated FGFR3 conditional knockout mice. This knockout mouse is expected to be valuable for the studies on the functional role of FGFR3 in adult articular cartilage in vivo.
FGF-8
FGF-8 was discovered from a conditioned medium of an androgen-dependent mouse mammary tumor cell line. Based on its structural similarities to other FGF gene products, it was classified as a member of the FGF family [Tanaka et al., 1992]. Four different isoforms of FGF-8 have been discovered in humans (FGF-8a, FGF-8b, FGF-8e, and FGF-8f), and FGF-8 can bind to three distinct transmembrane tyrosine kinase receptors (FGFR2-IIIC, FGFR3-IIIc, and FGFR4) [Blunt et al., 1997]. FGF-8 has been shown to play a pivotal role in embryogenesis and morphogenesis [Ohuchi et al., 1994]. In particular, FGF-8 expression during gastrulation is involved in limb and face morphogenesis in mice [Mahmood et al., 1995] and chicks [Crossley et al., 1996]. It has also been found to be a regulator of ectopic bone and cartilage formation by breast cancer cells [Valta et al., 2006] and synovial sarcoma cells [Ishibe et al., 2005].
In mature cartilage, however, the role of FGF-8 has only recently been published in the literature. Uchii et al. [2008] studied the effects of FGF-8 in both a rabbit and rat joint OA model using in vitro and in vivo techniques. In contrast to absent or minimal expression of FGF-8 in cartilage of healthy rabbit knees (sham control animals), the authors noted significantly increased expression of FGF-8 in proliferative fibroblast-like synovial cells of partially meniscectomized rabbit knees, suggesting a pathological role of FGF-8 in experimental OA conditions. Using in vitro analyses in the same model, they reported that degradation of the ECM was promoted in the presence of FGF-8 and this degradation was enhanced when combined with interleukin-1 (IL-1). FGF-8 also induced the production of MMP-3 and prostaglandin E2 (PGE2) in rabbit articular chondrocytes [Uchii et al., 2008], both critical regulators of ECM degradation and OA [Li et al., 2009]. Importantly, FGF-8-mediated catabolic effects were dose-dependently suppressed by the addition of neutralizing anti-FGF-8 antibody.
Further, using two different in vivo OA models in rat knee joints (intra-articular FGF-8 injection model, and monoiodoacetic acid-induced arthritis (MIA) injection model), FGF-8 induced a dose-dependent increase in sulfated glycosaminoglycan (S-GAG) in synovial fluid via cartilage degradation, and again these results were suppressed by the addition of anti-FGF-8 antibody [Uchii et al., 2008]. The authors postulated that targeting FGF-8 (e.g., using anti-FGF-8 antibody) might be a potential therapeutic approach in the treatment of OA in the future. Additional studies are warranted to further elucidate the pathological role of FGF-8 in human adult articular cartilage as well as IVD homeostasis, including the downstream signaling cascades and FGFR profile utilized by FGF-8.
FUTURE DIRECTIONS
Decades of research have implicated important, yet at times controversial, roles of FGF family members in cartilage and IVD homeostasis. In particular, future investigations are warranted to resolve the confusion over the role of FGF-2 in cartilage degeneration. Although it has been shown in murine models that FGF-2 stimulates PG deposition with a concurrent increase in FGFR3 expression, direct evidence is needed to substantiate the link between FGFR3 and FGF-2-mediated anabolic action in this model. A feasible way to address this question is to utilize a conditional FGFR3 knockout model to examine whether FGF-2 exerts the same effect on PG production in FGFR3−/− mice. Results from such experiments would shed further light on the mechanistic basis for species-dependent FGF-2 action in articular cartilage.
In addition, our current understanding of the interplay between FGF-2 and BMP7/IGF-1 remains inadequate. One of the prospective foci on this topic is to delineate the signaling crosstalk between the FGF-2 and BMP7/IGF-1 pathways. In chondrocytes, FGF-2 activates multiple signaling axes, including all three MAPK subgroups (ERK, p38, JNK), and NFκB [Im et al., 2007; Muddasani et al., 2007]. These pathways may interfere with BMP7-induced Smad signaling via (i) alteration of sumoylation level of Smad4; (ii) phosphorylation of Smad1 linker region; or (iii) induction of negative regulators of BMP7 signaling, such as inhibitory Smad (Im et al., unpublished data). However, the interaction between FGF-2 and IGF-1 seems more puzzling. Raf-1 (activated by FGF-2) has been identified as a substrate negatively regulated by Akt (activated by IGF-1) [Zimmermann and Moelling, 1999], whereas our findings suggest that FGF-2 signaling can override IGF-1-mediated PI3K/Akt signaling [Loeser et al., 2005]. Therefore, it remains to be determined whether FGF-2 antagonizes IGF-1 through delayed induction of negative regulators, rather than direct crosstalk between immediate signaling pathways.
In contrast to the controversial status of FGF-2 biology, FGF-18 may be a promising candidate in OA therapy. A phase II trial using recombinant FGF-18 is currently underway [Beenken and Mohammadi, 2009]. Much anticipation surrounds this trial, because current OA therapeutic regimens lack disease-modifying drugs that interfere with cartilage degradation at the most basic biochemical level. The potential success of FGF-18 therapy, therefore, may demonstrate the feasibility and potential therapeutic capacity of biological therapy in OA and DDD.
To date, research on the roles of FGFs in cartilage and IVD have been limited to three well-characterized members, namely FGF-2, FGF-18, and FGF-8. The FGF family is now comprised of 23 recognized members, many of which remain functionally obscure. Recent discoveries regarding the bioactivities of FGF-8 piloted our exploration into this uncharted area. A preliminary step to globally appreciate the FGF biology in cartilage and IVD tissue would be to perform expression profiling in these cell populations under healthy and diseased conditions, which will likely generate a shortlist of members for further characterization.
CONCLUSION
Current clinical treatment strategies for cartilage degenerative diseases such as OA and DDD fail to interfere with underlying biochemical processes involved in disease progression, and recent literature has begun to elucidate the potential for disease-modifying biological agents to slow or reverse cartilage degradation. Three members of the FGF family, FGF-2, FGF-18, and FGF-8, have been implicated as contributing factors in cartilage homeostasis. FGF-2 and FGF-8 have been found to induce catabolic effects in human cartilage, while FGF-18 demonstrates anabolic effects in similar tissues. Differences in activity may be due to receptor expression, or other unknown signaling pathways, in a variety of tissues. Nevertheless, the available evidence reveals the promise of FGF-2/FGFR1 antagonists, FGF-18/FGFR3 agonists, and FGF-8 antagonists (i.e., anti-FGF-8 antibody) as potential therapies to prevent cartilage degeneration and/or promote cartilage regeneration and repair in the future.
Acknowledgments
Grant sponsor: NIH; Grant numbers: R01 AR053220, R01 AR062136.
Abbreviations used
- ADAMTS
a disintegrin-like and metalloprotease with thrombospondin motifs
- AF
annulus fibrosus
- BMP
bone morphogenetic protein
- DDD
degenerative disc disease
- DMMB
dimethylmethylene blue
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HSPG
heparan sulfate proteoglycan
- IGF-1
insulin-like growth factor-1
- IL-1
interleukin-1
- IVD
intervertebral disc
- MAPK
mitogen activated protein kinase
- MIA
mono-iodoacetate
- MMP
matrix metalloprotease
- NP
nucleus pulposus
- OA
osteoarthritis
- OP-1
osteogenic protein-1
- PGE2
prostaglandin E2
- PKCδ
protein kinase C delta
- S-GAG
sulfated-glycosaminoglycan
- TGF-β
transforming growth factor-beta
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
The authors declared they have no conflict of interest.
References
- Alsalameh S, Mattka B, Al-Ward R, Lorenz HM, Manger B, Pfizenmaier K, Grell M, Kalden JR. Preferential expression of tumor necrosis factor receptor 55 (TNF-R55) on human articular chondrocytes: Selective transcriptional upregulation of TNF-R75 by proinflammatory cytokines interleukin 1beta, tumor necrosis factor-alpha, and basis fibroblast growth factor. J Rheumatol. 1999;26:645–653. [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt AG, Lawshe A, Cunningham ML, Seto ML, Ornitz DM, MacArthur CA. Overlapping expression and redundant activation of mesenchymal fibroblast growth factor (FGF) receptors by alternatively spliced FGF-8 ligands. J Biol Chem. 1997;272:3733–3738. doi: 10.1074/jbc.272.6.3733. [DOI] [PubMed] [Google Scholar]
- Chang Z, Meyer K, Rapraeger AC, Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 2000;14:137–144. doi: 10.1096/fasebj.14.1.137. [DOI] [PubMed] [Google Scholar]
- Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- Chuma H, Mizuta H, Kudo S, Takagi K, Hiraki Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage. 2004;12:834–842. doi: 10.1016/j.joca.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280:20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren H, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- Friedl A, Chang Z, Tierney A, Rapraeger AC. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: Comparison of normal and abnormal human tissues. Am J Pathol. 1997;150:1443–1455. [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase C delta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Li X, Muddasani P, Kim GH, Davis F, Rangan J, Forsyth CB, Ellman M, Thonar EJ. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol. 2008;215:452–463. doi: 10.1002/jcp.21317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR, Shima Y, Nagayama S, Katagiri T, Nakamura Y, Nakamura T, Toguchida J. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: Potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–2712. doi: 10.1158/1078-0432.CCR-04-2057. [DOI] [PubMed] [Google Scholar]
- Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007;89:693–700. doi: 10.1302/0301-620X.89B5.18450. [DOI] [PubMed] [Google Scholar]
- Iwata T, Chen L, Li C, Ovchinnikov DA, Behringer RR, Francomano CA, Deng CX. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:1603–1613. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- Li X, An HS, Ellman M, Phillips F, Thonar EJ, Park DK, Udayakumar RK, Im HJ. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10:R48. doi: 10.1186/ar2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–523. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ellman MB, Kroin JS, Chen D, Yan D, Mikecz K, Ranjan KC, Xiao G, Stein GS, Kim SG, Cole B, van Wijnen AJ, Im HJ. Species-specific biological effects of FGF-2 in articular cartilage: Implication for distinct roles within the FGF receptor family. J Cell Biochem. 2012;113:2532–2542. doi: 10.1002/jcb.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol. 2007;302:80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005;52:3910–3917. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry H, Orth P, Kaul G, Zurakowski D, Menger MD, Kohn D, Cucchiarini M. Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch Orthop Trauma Surg. 2010;130:1311–1322. doi: 10.1007/s00402-010-1130-3. [DOI] [PubMed] [Google Scholar]
- Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, Yuasa M, Hirano M, Mochizuki N, Kikuchi M, Shinomiya K, Okawa A. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2) J Orthop Res. 2010;28:677–686. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Bresnick J, Hornbruch A, Mahony C, Morton N, Colquhoun K, Martin P, Lumsden A, Dickson C, Mason I. A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr Biol. 1995;5:797–806. doi: 10.1016/s0960-9822(95)00157-6. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: Implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- Minamide A, Hashizume H, Yoshida M, Kawakami M, Hayashi N, Tamaki T. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine. 1999;24:940–945. doi: 10.1097/00007632-199905150-00003. [DOI] [PubMed] [Google Scholar]
- Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Muddasani P, Norman JC, Ellman M, van Wijnen AJ, Im HJ. Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J Biol Chem. 2007;282:31409–31421. doi: 10.1074/jbc.M706508200. [DOI] [PubMed] [Google Scholar]
- Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- Naski MC, Colvin JS, Coffin JD, Ornitz DM. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–4988. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Yoshioka H, Tanaka A, Kawakami Y, Nohno T, Noji S. Involvement of androgen-induced growth factor (FGF-8) gene in mouse embryogenesis and morphogenesis. Biochem Biophys Res Commun. 1994;204:882–888. doi: 10.1006/bbrc.1994.2542. [DOI] [PubMed] [Google Scholar]
- Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Abe M, Kapadia RM, Liao Z, Naski MC. FGF18 represses noggin expression and is induced by calcineurin. J Biol Chem. 2004;279:38209–38219. doi: 10.1074/jbc.M404855200. [DOI] [PubMed] [Google Scholar]
- Su N, Xu X, Li C, He Q, Zhao L, Chen S, Luo F, Yi L, Du X, Huang H, Deng C, Chen L. Generation of Fgfr3 conditional knockout mice. Int J Biol Sci. 2010;6:327–332. doi: 10.7150/ijbs.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Miyamoto K, Minamino N, Takeda M, Sato B, Matsuo H, Matsumoto K. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci USA. 1992;89:8928–8932. doi: 10.1073/pnas.89.19.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, Shapiro IM, Risbud MV. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: Implications for cell-based transplantation therapy. Spine. 2007;32:495–502. doi: 10.1097/01.brs.0000257341.88880.f1. [DOI] [PubMed] [Google Scholar]
- Uchii M, Tamura T, Suda T, Kakuni M, Tanaka A, Miki I. Role of fibroblast growth factor 8 (FGF8) in animal models of osteoarthritis. Arthritis Res Ther. 2008;10:R90. doi: 10.1186/ar2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valta MP, Hentunen T, Qu Q, Valve EM, Harjula A, Seppanen JA, Vaananen HK, Harkonen PL. Regulation of osteoblast differentiation: A novel function for fibroblast growth factor 8. Endocrinology. 2006;147:2171–2182. doi: 10.1210/en.2005-1502. [DOI] [PubMed] [Google Scholar]
- Valverde-Franco G, Binette JS, Li W, Wang H, Chai S, Laflamme F, Tran-Khanh N, Quenneville E, Meijers T, Poole AR, Mort JS, Buschmann MD, Henderson JE. Defects in articular cartilage metabolism and early arthritis in fibroblast growth factor receptor 3 deficient mice. Hum Mol Genet. 2006;15:1783–1792. doi: 10.1093/hmg/ddl100. [DOI] [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Weng T, Yi L, Huang J, Luo F, Wen X, Du X, Chen Q, Deng C, Chen D, Chen L. Genetic inhibition of FGFR1 in cartilage attenuates articular cartilage degeneration in adult mice. Arthritis Rheum. 2012 doi: 10.1002/art.34645. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Clark JC, Picard L, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT. Fibroblast growth factor 18 influences proximal programming during lung morphogenesis. J Biol Chem. 2002;277:22743–22749. doi: 10.1074/jbc.M202253200. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Wakitani S, Imoto K, Hattori T, Nakaya H, Saito M, Yonenobu K. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthritis Cartilage. 2004;12:636–641. doi: 10.1016/j.joca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, Im HJ. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther. 2011;13:R130. doi: 10.1186/ar3441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yan D, Chen D, Im HJ. Fibroblast growth factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis that coordinates with the PKCdelta pathway in human articular chondrocytes. J Cell Biochem. 2012;113(9):2856–2865. doi: 10.1002/jcb.24160. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T. Repair of articular cartilage defect by auto-logous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]