Abstract
Appropriate and controlled chondrogenesis and endochondral ossification play fundamental roles in the fracture healing cascade, a regenerative process involved in highly coordinated biological events, including the Wnt/β-catenin signaling pathway. To examine the role and importance of this pathway in chondrocytes, we studied bone repair of closed tibias fractures in Col2a1-ICAT transgenic mice, in which the Wnt/β-catenin signaling pathway is specially inhibited in chondrocytes. Radiological, histological, and histomorphometric analyses at 7, 9, 12, 14, 21, and 28 days after fracture demonstrated the bone repairs were retarded in Col2a1-ICAT transgenic mice, due to reduced and delayed cartilage formation, chondrocyte hypertrophy, and bone generation. In addition, at 5 weeks, Col2a1-ICAT transgenic mice exhibited a weak mechanical tolerance to four-point bending. Furthermore, quantitative-PCR analysis revealed that the expression of genes associated specifically with cartilage extracellular matrix formation (collagen II, collagen X, and mmp13), bone remodeling (alp, collagen I, and osteocalcin), and vascular extravagation (vegf), and transcriptional activators involved in cartilage generation and ossification (sox9 and runx2) was decreased and delayed in the fracture sites of Col2a1-ICAT transgenic mice during healing. Collectively, these results suggest that Wnt/β-catenin signaling is critical for fracture healing, especially with respect to chondrogenesis and endochondral ossification. Thus, our study provides insight into the possible mechanisms of and therapeutic targets for improving normal facture repair and the healing of non-union fractures.
Keywords: Wnt/β-catenin signaling pathway, fracture healing, chondrogenesis, endochondral ossification, biomechanical strength
The biological processes involved during fracture repair recapitulate aspects of skeletal development, triggering a steady cascade of bone regeneration. Under optimal conditions, fractured bone fully returns to its innate morphological state and recovers normal biomechanical properties. This process consists of a variety of molecular and cellular events involving proliferation, differentiation, and apoptosis.1,2 Among numerous, complex cellular signaling pathways that are activated and are paramount for bone development and remodeling, a key signaling event may be credited to the Wnt/β-catenin ligand-induced intercellular pathway.3,4
During embryonic skeletogenesis, inhibition of β-catenin signaling can prevent osteoblastic differentiation, thereby inducing pluripotent mesenchymal cells to develop a chondroblastic phenotype. This consequential outcome suggests that β-catenin inhibits early differentiation of mesenchymal cells into the chondrogenic lineage.5,6 In addition, β-catenin signaling can also regulate bone mass density, as its activation results in an increased bone mass phenotype.7,8 Furthermore, numerous members of the Wnt/β-catenin pathway are activated during fracture healing in both animals and humans.9,10
Previous studies attempted to characterize the Wnt/β-catenin pathway in relation to bone regeneration using mouse models. However, results were usually inconclusive as most mice developed skeletal dysplasia and died either before or shortly after birth,11 thus hampering understanding of the role of Wnt/β-catenin signaling.
Inhibitor of β-catenin/TCF (ICAT) is an 82-aminoacid peptide, containing an N-terminal helilical domain that binds to β-catenin and an extended C-terminal region that binds to Tcfs and other β-catenin ligands. As such, ICAT disrupts the ability of β-catenin and TCF/LEF to form a complex, blocking the transcription of downstream target genes.12 Col2a1-ICAT transgenic mice, in which the ICAT transgene is under the control of the chondrocyte-specific Col2a1 promoter, are viable after birth despite lacking Wnt/β-catenin signaling.13 To better understand the role of the Wnt/β-catenin pathway in endochondral ossification during postnatal growth and development and furthermore to identify possible therapeutic targets for fracture healing, we examined fracture repair in Col2a1-ICAT transgenic mice.
MATERIALS AND METHODS
Animals
The Col2a1-ICAT transgenic mouse line was provided by Professor Di Chen (Department of Orthopedics, Center of Musculoskeletal Research, University of Rochester). All experiments were preformed according to the policies of Shanghai Jiao Tong University School of Medicine and the National Institute of Health, Shanghai, China.
To monitor fracture healing, six transgenic mice and six WT mice underwent lateral X-rays weekly (from 1 to 5 weeks after fracture). For histological and histomorphometric analysis, 36 transgenic and 36 WT mice were euthanized at 7, 9, 12, 14, 21, and 28 days after fracture (random six transgenic and six WT mice/time point). For biomechanical testing, 10 transgenic and 13 WT mice were euthanized at 5 weeks post-fracture. Finally, 42 transgenic and 42 WT control mice were euthanized at 5, 7, 9, 12, 14, 21, and 28 days after fracture (random 6 transgenic and 6 WT mice/time point); their fractured tibias were harvested for RNA extraction and gene expression test.
Fracture Model
Bone fractures were generated in 8-week-old mice as previously reported.10 Briefly, mice were anesthetized by intramuscular injection of pentobarbital sodium (0.05 mg/g, Sigma–Aldrich Co. LLC., St. Louis, Mo). In rigorous aseptic conditions, a fracture was made in each mouse by sawing the shaft of the tibia and inserting a pin, used for intramedullary fixation. The animals were allowed to move freely in their cages after recovery from anesthesia. At different time points after the fracture, mice were euthanized, and fracture calluses were harvested; ≥10 animals per time point were used for subsequent analysis.
Radiological Analysis
Bone radiographs were taken weekly after fracture, using a Faxitron MX-20 X-ray system (Faxitron X-Ray Corporation, Lincolnshire, IL), with 15 s exposure time and a voltage of 28 KVp. Three independent experienced orthopedic surgeons, blinded to the time points and groupings, analyzed and graded healing from the radiographs. Complete bridging of all four cortices on AP and lateral radiographs were considered as fracture healing.14,15
Histological Analysis
Harvested tibias were fixed in 4% paraformaldehyde, declaimed in 12.5% EDTA (pH 7.0), and embedded in paraffin. The fixed tissues were cut into 5-μm thick sections and stained with Safranin O/fast green.
Histomorphometry
Stained slices were examined under light microscopy (LEICA DM 4000B). According to previous protocols,16,17 the tissue volumes of cartilage, bone, and total callus were analyzed and calculated based on the different colors and morphology, using image analysis software (Bioquant; BIOQUANT Image Analysis Corporation, Nashville, TN). All measurements were taken from three sections of each sample.
Biomechanical Testing
Five weeks after fracture, the mice (10 Col2a1-ICAT transgenic and 13 WT controls) were euthainzed and their fractured tibias dissected. Under Loupe magnification, all soft tissue was removed from the skeleton to preserve the intact calluses formed at the fracture site. After removal of the pins, the tibias were subjected to a 4-point bending test using an Axial-Torsion Fatigue Testing System (Instron 8874), with the contra-lateral limbs as controls. The tibias were placed mediolaterally on stainless steel fixtures that were 10 mm apart and ~5 mm away from the center of the fracture site. A compression load was applied at a rate of 2.0 mm/min until failure; data points were recorded every 0.01 s. The maximum load to failure (N; ultimate force that the specimen sustained) and Young’s modulus (GPa; the slope of the stress-strain curve) were calculated from load displacement curves using materials testing software (Bluehill; Instron, Norwood, MA).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Quantitative (Q)-PCR
Total RNA was isolated from the fracture site, which encompassed the entire callus and >2 mm of adjacent bone, using the RNeasy Mini Kit (Qiagen, Hilden, Germany). According to histological images under low-magnification, this area approximately corresponded to the proximal and distal aspects of the callus. Equivalent concentrations of RNA from each tibia were reverse transcribed (RevertAid™ M-MuLV, Fermentas International Inc, Burlington, Canada) and subjected to Q-PCR using the SYBR Premix Ex Taq RT-PCR kit (TaKaRa Bio Inc., Osaka, Japan) and the ABI 7900 RT-PCR system (Applied Biosystems, Life Technologies Corporation, Carlsbad, California). RNA levels were normalized using the housekeeping gene GAPDH. Details of primers are listed in Table 1.
Table 1.
Primer Sequences Used for Q-PCR
| Gene | Sequence | Product Size (bp) | Accession Number |
|---|---|---|---|
| GAPDH | F: GACTTCAACAGCAACTCCCAC R: TCCACCACCCTGTTGCTGTA |
125 | NM_008084 |
| Collagen II | F: ACCTTGGACGCCATGAAA R: GTGGA AGTAGACGGAGGAA |
230 | NM_001844 |
| Collagen X | F: TCTGGGATGCCGCTTGT R: CGTAGGCGTGCCGTTCTT |
261 | NM_009925 |
| MMP13 | F: TGATGAAACCTGGACAAGCA R: CCTGGGTCCTTGGAGTGAT |
102 | NM_008607 |
| Collagen I | F: TGTGTGCGATGACGTGCAAT R: GGGTCCCTCGACTCCTACA |
133 | NM_007742 |
| ALP | F: AGGGCAATGAGGTCACATCC R: AGGGCAATGAGGTCACATCC |
150 | NM_007431 |
| Osteocalcin | F: CTGACCTCACAGATCCCAAGC R: TGGTCTGATAGCTCGTCACAAG |
187 | NM_031368 |
| Sox9 | F: GAGCCGGATCTGAAGATGGA R: GCTTGACGTGTGGCTTGTTC |
151 | NM_011448 |
| Runx2 | F: TGTTCTCTGATCGCCTCAGTG R: CCTGGGATCTGTAATCTGACTCT |
146 | NM_001145920 |
| VEGF | F: CGATGAAGCCCTGGAGTG R: ATGATGGCGTGGTGGTGA |
273 | NM_001025257 |
Statistical Analysis
Statistical analysis was performed using a two-tailed Student’s t-test, with equal variance. *p < 0.05 and **p < 0.01 were considered significant.
RESULTS
Delayed Fracture Healing in Col2a1-ICAT Mice
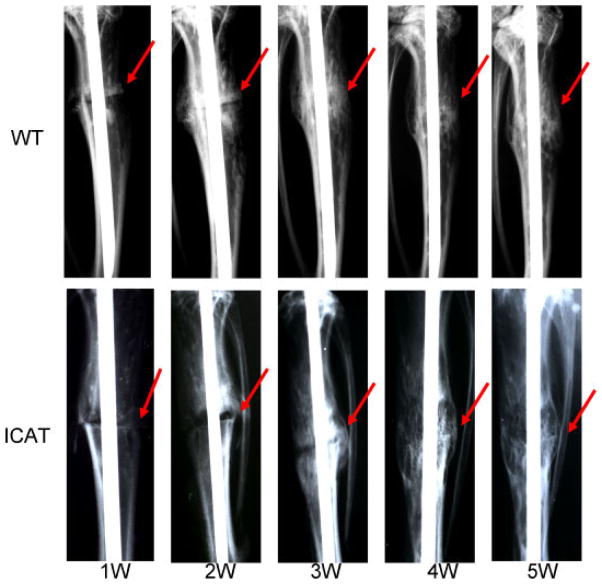
From the radiographs (Fig. 1), 1 week after fracture, we could visualize the fracture lines in the tibias of both the WT and Col2a1-ICAT mice. Two weeks post-fracture, large calluses began to form at the fracture site in tibias of WT mice. However, in Col2a1-ICAT mice, only small calluses surrounding the fracture line formed, seemingly more visible than the prior week. At 3 weeks, the fracture line became fuzzy with callus formation progressing in tibias of WT mice. In contrast, the tibias of Col2a1-ICAT mice exhibited only small increases in visible callus formation around fracture lines. By weeks 4–5, calluses in WT mice decreased, indicating the possible initiation of bone remodeling. Though fracture lines began slowly disappearing in the tibias of Col2a1-ICAT mice, enlarged calluses suggested that bone remodeling at this site had not yet been initiated.
Figure 1.
Radiological analysis of bone fracture healing at 1, 2, 3, 4, and 5 weeks after fracture in wild-type (WT) and Col2a1-ICAT mice. The red arrowheads indicate the fracture lines. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jor]
Col2a1-ICAT Mice Exhibit Abnormal Cartilage Callus Formation
A full view of the fractured callus (Fig. 2A) revealed that anormal fracture healing process in WT mice. This was confirmed by an observed increase in cartilage callus formation between 5 and 14 days post-fracture, a reduction in the size of the calluses 21 days post-fracture, and complete disappearance of the cartilage calluses by 28 days post-fracture. In contrast, callus formation between 7 and 14 days post-fracture was significantly decreased in tibias of Col2a-ICAT mice, with a visible fracture line present. The transformation from carliaginous callus to bony callus between 14 and 28 days post-fracture was delayed in the Col2a-ICAT mice, further influencing subsequent bone remodeling processes. A detailed examination of the composition of the calluses of WT and Col2a1-ICAT mice (Fig. 2B) revealed different bone healing patterns in the transgenic mice. At 7 and 9 days post-fracture, the calluses of the tibias from Col2a1-ICAT mice were comprised of a mixture of chondrocytes and undifferentiated fibroblast-like cells, whereas those of WT mice comprised only chondrocytes. At 12 days post-fracture, the cells in the calluses of WT mice exhibited signs of hypertrophy, whereas those in Col2a1-ICAT mice did not. At 14 days post-fracture, bony calluses began to form in tibias of WT mice, whereas only a small fraction of the chondrocytes in calluses of Col2a1-ICAT mice began to undergo hypertrophy. Between 21 and 28 days post-fracture, bony calluses in WT mice continued to form in a directional arrangement, the medullar cavity connected, and bone remodeling processes were initiated. In Col2a1-ICAT mice, cartilaginous calluses still surrounded the fracture sites, with few bony calluses having been formed, further suggesting that endochondral ossification was still in process.
Figure 2.
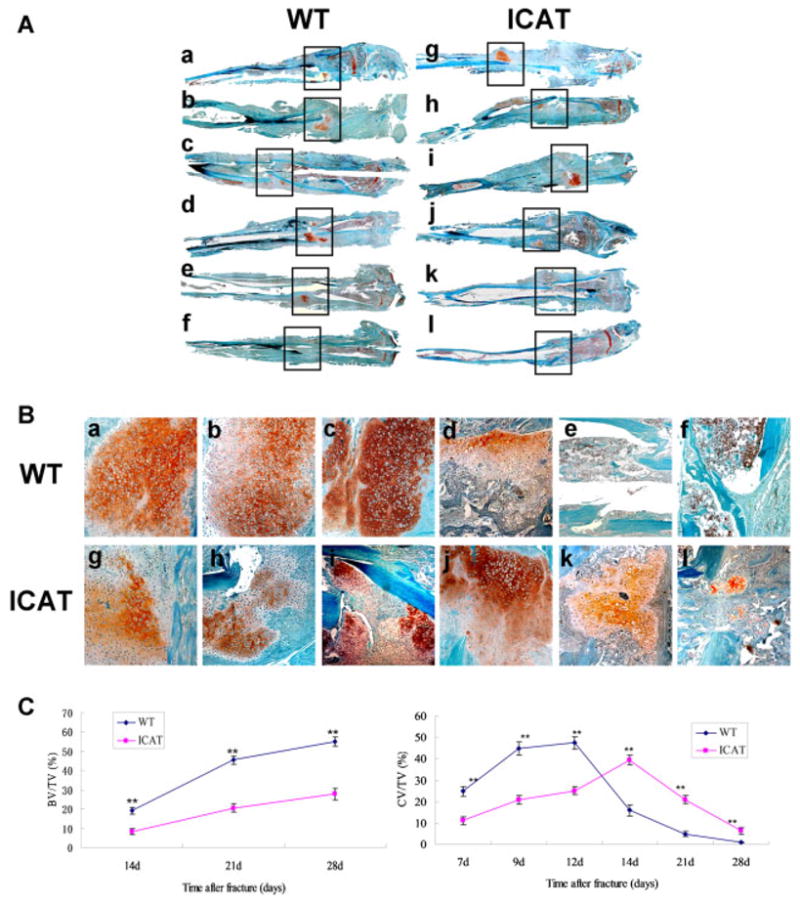
Histopathological observations of bone fracture healing at 7, 9, 12, 14, 21, and 28 days after surgery in WT (a–f) and Col2a1-ICAT (g–i) mice. The orange color indicates the cartilage calluses in the fracture sites stained with Safranin O/fast green. (A) Images of fractured tibias in WT and Col2a1-ICAT mice. The black frames demonstrate the fracture sites (original magnification ×50). (B) Enlarged view of the images shown in A (original magnification ×100). (C) Histomorphometry revealed the cartilage volume/total callus volume (CV/TV) and the bone volume/total callus volume (BV/TV) at fracture sites in WT and Col2a-ICAT mice. All the measurements were made at five different sites for each section (**p < 0.01). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jor]
Histomorphometric analysis of bone samples (Fig. 2C) revealed similar results. Before 14 days post-fracture, the cartilage volume/total callus volume (CV/TV) in WT mice was significantly higher than that observed in Col2a1-ICAT mice. When bone formation had begun at 14 days, the bone volume/total callus volume (BV/TV) detected was also higher in WT than in Col2a1-ICAT mice. Finally, a lower CV/TV was observed in WT mice than was in Col2a1-ICAT mice.
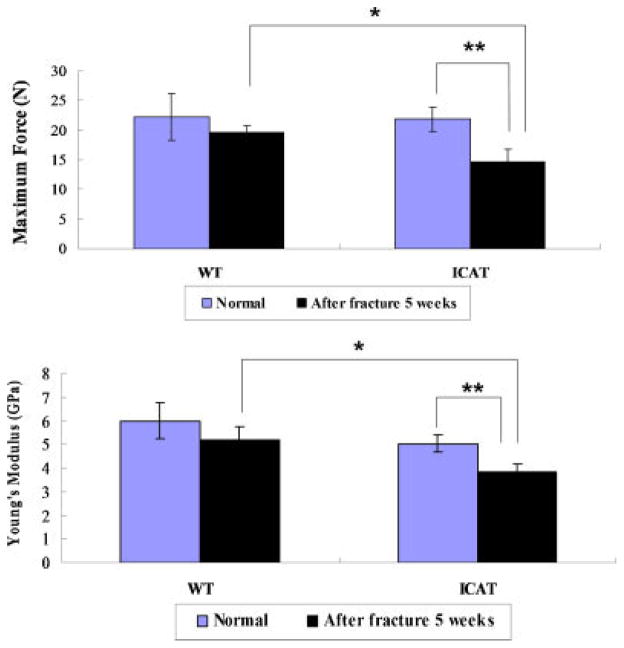
Tibias From Col2a1-ICAT Mice Exhibit Lower Biomechanical Strength
The strengths of intact and 5 weeks post-fractured tibias were examined in 4-point bending (Fig. 3). Fractured tibias from Col2a1-ICAT mice supported significantly lower load (32.6% reduction) and Young’s modulus (24% reduction) than did fractured tibias from WT mice or intact tibias from Col2a1-ICAT mice. No significant differences were observed in maximum load and modulus supported by fractured or intact tibias from WT mice, suggesting that fractured tibias can repair and recover their biomechanical strength within 5 weeks to the same standard as intact tibias in WT mice.
Figure 3.
Biomechanical analysis of intact and fractured tibias from WT (n = 13) and Col2a1-ICAT (n = 10) mice using the four-point bending test 5 weeks after surgery. The maximum full load and Young’s modulus of fractured tibias from Col2a1-ICAT mice is significantly reduced when compared to that of tibias from either intact Col2a1-ICAT or fractured WT mice (*p < 0.05, **p < 0.01).
Calluses From Col2a1-ICAT Mice Present Differential Gene Expression Patterns
During chondrogenesis in Col2a1-ICAT mice, compared with WT mice, the expression of collagen II, a marker of cartilage formation, was significantly delayed and decreased in the chondrocyte calluses during the 5–9 days post-fracture period. Similarly, when the main components of the calluse became hypertrophy chondrocytes, collagen X, as a marker of cartilage hypertrophy, reduced its expression in Col2a1-ICAT mice 12–14 days post-fracture. At the same time, levels of matrix metalloproteinase 13 (MMP13), an enzyme secreted by hypertrophic chondrocytes and involved in the degradation of extracellular matrix (ECM) cartilage, were also significantly decreased in Col2a1-ICAT mice (Fig. 4A).
Figure 4.
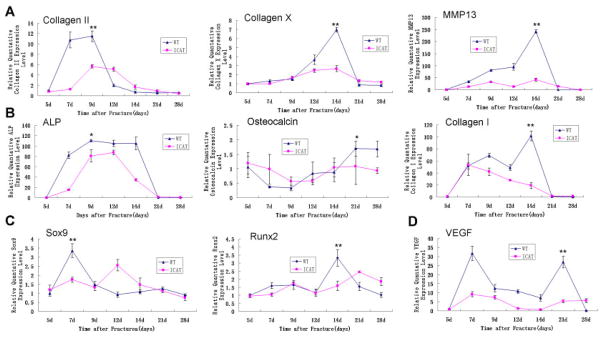
Quantitative-PCR analysis of the expression of genes associated with the genes involved in cartilage formation and hypertrophy (A), bone remodeling (B), transcription of chondrogenesis- and ossification-associated genes (C), and angiogenesis (D) in fracture calluses of WT and Col2a1-ICAT mice at 5, 7, 9, 12, 14, 21, and 28 days after surgery (*p < 0.05 for the peak values of gene expression in calluses of WT versus Col2a1-ICAT mice during the healing process). Eight mice were tested in each time point.
We assessed the gene expression levels of key markers involved in the process of osteogenesis: (i) the early osteogenous marker, ALP; (ii) the osteoblast matrix marker, collagen I; and (iii) the matrix mineralization marker, osteocalcin (Fig. 4B). Compared with that in WT mice, the expressions of these three genes were significantly reduced or delayed in Col2a1-ICAT mice.
The expressions of two key transcription factors involved in bone generation, Sox9 and Runx2 (Fig. 4C), were also significantly reduced and delayed during fracture healing in Col2a1-ICAT mice. As expected, the expression of VEGF (Fig. 4D) was also significant-ly reduced during early and late chondrogenesis and osteogenesis in Col2a1-ICAT mice.
DISCUSSION
Known as a recapitulation of bone development, fracture healing is a well-defined process involving many signaling pathways.18 The investigation of these pathways may lead scientists to an understanding of the key regulators of this process and the development of pharmacologic interventions that are aimed at enhancing the fracture healing of bone.
Previous studies demonstrated that Wnt/β-catenin signaling is precisely regulated to facilitate early pluripotent mesenchymal stem cell differentiation and bone formation during the process of facture repair.3,10 However, few researchers focused on the effects of this pathway in cartilage callus formation and endochodral ossification, two important stages of bone healing. In our study, we observed the critical role of β-catenin in chondrocytes during cartilage formation and endochondral ossification within bone healing, using the Col2a1-ICAT transgenic mice in which the Wnt/β-catenin signaling is specially inhibited in chondrocytes.
We compared the repair of tibia fractures in WT and Col2a1-ICAT mice. Examination by radiography revealed that the fractures healed in WT and Col2a1-ICAT mice in a similar fashion 1 week post-fracture, suggesting that an initial inflammatory response was not elicited in the transgenic mice. However, upon examination of cartilage callus formation and endochondral ossification, 2–3 weeks post-fracture, the fracture line and the callus size in Col2a1-ICAT mice were markedly different and led to the observed delay in the remodeling of bone. Histological analyses of the fractured tibias revealed that when Wnt/β-catenin signaling was inhibited in the chondrocytes of Col2a1-ICAT mice, retardation of cartilage formation, hypertrophy, endochondral ossification, and bone remodeling resulted, further delaying overall healing. Moreover, biomechanical testing confirmed that fracture repair at the later stages of the healing process was hindered in Col2a1-ICAT mice when compared with WT mice.
We also examined the expression of several key genes and factors that play a regulatory role in the fracture healing process to identify the mechanisms involved in delayed healing when Wnt/β-catenin signaling is inhibited. Collagen II and X, ECM markers of proliferation and hypertrophy in chondrocytes, respectively,19,20 had decreased expression throughout the process of fracture healing. The delayed expression of collagen II indicates that the inhibition of Wnt/β-catenine signaling is maintained longer in Col2a1-ICAT mice than in WT mice. In addition, the inhibition of Wnt/β-catenin signaling in Col2a1-ICAT mice resulted in a lower frequency of hypertrophic chondrocytes and a decreased secretion of MMP13, characteristic of chondrocytes and osteoblasts and essential to the degradation of ECM components.21 The observed decrease in MMP13 expression by chondrocytes may also account for the persistence of cartilage callus in Col2a1-ICAT mice. Similarly, the expressions of ALP, collagen I, and osteocalcin were reduced during late stage healing, indicating that ossification and bone remodeling are also affected by inhibition of Wnt/β-catenin signaling in chondrocytes.
Fracture repair occurs through a number of different stages. The process begins by formation of a thrombus callus, and then proceeds to the induction of endochondral bone formation and an extended period of bone remodeling that eventually results in the healings of each fracture side giving way to lamellar bone formation.22 During this process, progress from one stage to another is controlled by several transcription factors. Sox9 and Runx2, two of the most important factors involved in chondrification and ossification,23,24 are direct, highly relevant targets of canonical Wnt-β-catenin signaling.25,26 Therefore, when this signaling was inhibited, the delayed peak of Sox9 and Runx2 expression in Col2a1-ICAT mice suggested that the chondrification and ossification processes were postponed. Furthermore, the critical role of Sox9 in chondrocyte differentiation and the stimulation of Col2a1 expression has been demonstrated.27 Runx2 is colocalized with Col10a1 or MMP13 and directly affects their expression.28,29 Therefore, we suspect that the inhibition of Wnt/β-catenin signaling may alter the expression of Sox9 and Runx2, and thus exert an effect on the stages of healing.
Angiogenesis is a key event in the process of fracture healing, from the initial stages of hematoma formation to the final stage of bone remodeling.30 Chen et al.13 showed that Col2a1-ICAT mice have defects in cartilage angiogenesis because the β-catenin can directly activate VEGF gene transcription. In our study, we found reduced VEGF expression during fracture healing in Col2a1-ICAT mice, which may also account for delayed fracture healing.
In summary, our results demonstrate that the inhibition of Wnt/β-catenin signaling in chondrocytes significantly alters several stages of the fracture repair process and delays the healing by affecting vascularization, early cartilage callus formation, endochondral ossification, late stage remodeling, and the recovery of mechanical strength. This study highlights the crucial role of Wnt/β-catenin signaling in postnatal bone regeneration, and provides new insights for therapeutic intervention and the treatment of bone fractures.
Acknowledgments
This research was supported by the National Basic Research Program (2009ZX09503-024 and 2010CB945600), the National Natural Science Foundation of China (No. 30811120440 and No. 30871435), the knowledge innovation program of the Chinese Academy of Sciences (KSCX2-YW-R-245), the Shanghai International Collaboration Foundation (No. 08410701800 and 09540703600), and Key Disciplines of Shanghai Municipal Education Commission (J50206).
References
- 1.Schindeler A, McDonald MM, Bokko P, et al. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: Which are the important molecules? Injury. 2007;38 (Suppl 1):S11–S25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Alman BA. Wnt pathway, an essential role in bone regeneration. J Cell Biochem. 2009;106(3):353–362. doi: 10.1002/jcb.22020. [DOI] [PubMed] [Google Scholar]
- 4.Silkstone D, Hong H, Alman BA. Beta-catenin in the race to fracture repair: In it to Wnt. Nat Clin Pract Rheumatol. 2008;4(8):413–419. doi: 10.1038/ncprheum0838. [DOI] [PubMed] [Google Scholar]
- 5.Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Hill TP, Spater D, Taketo MM, et al. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 8.Glass DA, II, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Hadjiargyrou M, Lombardo F, Zhao S, et al. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277(33):30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: Implications for therapy to improve bone healing. PLoS Med. 2007;4(7):e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haegel H, Larue L, Ohsugi M, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 12.Tago K, Nakamura T, Nishita M, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14(14):1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Zhu M, Awad H, et al. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J Cell Sci. 2008;12:1455–1465. doi: 10.1242/jcs.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann M, Peter Schmidt J, Umanskaya N, et al. The role of hyperhomocysteinemia as well as folate, vitamin B (6) and B (12) deficiencies in osteoporosis: A systematic review. Clin Chem Lab Med. 2007;45(12):1621–1632. doi: 10.1515/CCLM.2007.362. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham BW, Lowery GL, Serhan HA, et al. Total disc replacement arthroplasty using the AcroFlex lumbar disc: A non-human primate model. Eur Spine J. 2002;11 (Suppl 2):S115–S123. doi: 10.1007/s00586-002-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baharav H, Kupershmit I, Oman M, et al. Comparison between incisal embrasures of natural and prosthetically restored maxillary anterior teeth. J Prosthet Dent. 2009;101(3):200–204. doi: 10.1016/S0022-3913(09)60030-5. [DOI] [PubMed] [Google Scholar]
- 17.Röntgen V, Blakytny R, Matthys R, et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J Orthop Res. 2010;28(11):1456–1462. doi: 10.1002/jor.21148. [DOI] [PubMed] [Google Scholar]
- 18.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90 (Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 19.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37(1–2):109–116. [PubMed] [Google Scholar]
- 21.Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131(23):5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozen N, Lewinson D, Bick T, et al. Role of bone regeneration and turnover modulators in control of fracture. Gene Expr. 2007;17(3):197–213. doi: 10.1615/critreveukargeneexpr.v17.i3.30. [DOI] [PubMed] [Google Scholar]
- 23.Mackie EJ, Ahmed YA, Tatarczuch L, et al. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 25.Topol L, Chen W, Song H, et al. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komori T. Regulation of bone development and extra-cellular matrix protein genes by RUNX2. Cell Tissue Res. 2008;339(1):189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Manner PA, Horner A, et al. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12(12):963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34(4):680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]